
Effects of Modulated Concentration of ZnO Nanoparticles on Enhancing Biosynthesis of Metabolites and Protecting Plant Membrane. Wilailack Chayaprasert and Kanokporn Sompornpailin*
Published Date : 2019-04-1
DOI : https://doi.org/10.12982/CMUJNS.2019.0013
Journal Issues :
Number 2 , April-June 2019
ABSTRACT
Nowadays, nanoparticles are raising their applications around the world. ZnO nanoparticles are extensively used in the industrial and agricultural processes because of low price and less toxicity. Nevertheless, the pros and cons, effects of ZnO nanoparticles are still under discussing. This research aimed to study the protective effects of ZnO nanoparticles on plant physiology at the cellular level by analyzing biomaterial levels in relation to the stability of cellular membranes. Shoots of wild type and tt8 transgenic tobaccos were grown under tissue culture condition in the medium, adding 0, 10, 20 mg/L ZnO nanoparticles. Under media conditions adding 10, 20 mg/L ZnO nanoparticles, tobacco increased accumulation of plant metabolites (total soluble sugar and flavonoids). In these ZnO conditions, wild type and transgenic tobaccos enhanced total soluble sugar about 30-50% and 20-30%, respectively. The highest content of total soluble sugar was found in 20 mg/L ZnO nanoparticle condition. In similarity to the content of total soluble sugar, tobacco plants showed the highest average contents of flavone, flavonol and anthocyanin after growing in 20 mg/L ZnO nanoparticle conditions, 92.8%, 61.39% and 48.81% in wild type and 73.49%, 52.39% and 81.68% in transgenics, respectively. Tobaccos under 10 mg/L ZnO media condition increased the accumulation of total soluble sugar and flavonoid contents less than those under 20 mg/L ZnO media condition. However, these plants showed the lowest average percentage of cell membrane injury, 19.5% in wild type and 10-18% in transgenic tobaccos, following with plant under 20 mg/L ZnO nanoparticle and non ZnO nanoparticle conditions. Therefore, 10-20 mg/L ZnO nanoparticles in the media showed the induction of plant metabolites, especially in transgenics, and the enhancements of plant protection when compared with the same line tobacco under condition of non ZnO nanoparticles. Ten mg/L ZnO nanoparticle media is the best condition in protecting cellular membrane of tobacco.
Keywords: Nanoparticle, ZnO, Plant, Cell membrane, Flavonoid, Sugar, Protection
INTRODUCTION
The rapid development of nanotechnology influences on the applications of nanoparticles in multilaterally fields (Ov, 2004; Srinivas et al., 2010). Nanoparticle usages are increasing in the processes of industry and agriculture for the decade. However, toxicology awareness is also rising. ZnO nanoparticles are the important nanoparticle that have been used around the world. These nanoparticles showed the impacts on inducing plant growth and biosynthesis much better than bulk ZnO did (Prasad et al., 2012; Bandyopadhyay et al., 2015). ZnO nanoparticles has been proven to have the effects on enhancing plant development, enzymatic activity and biomaterial synthesis (Mahajan et al., 2011; Mukherjee et al., 2014; Wang et al., 2016). Since, some researcher reported the toxicological impacts of ZnO nanoparticles on plant growth when a high concentration of ZnO nanoparticles was added into the planting material (Mukherjee et al., 2014; Bandyopadhyay, 2015; Wang et al., 2016). Pros and cons, effects of ZnO nanoparticles on plant growth and development are under discussing. The majority effects of ZnO nanoparticles on plant depended on the concentration of ZnO nanoparticles, stage and species of plants (Mahajan et al., 2011; Mukherjee, 2014). Plant synthesizes various types of plant metabolite in responding to environmental conditions. The level of these metabolites implies cellular status of plants. In this experimental work, the contents of total soluble sugar and flavonoids were quantified. Sugars are known as fundamental signaling in several signal transduction processes while flavonoids are groups of cellular protective metabolites which contain an antioxidant function.
This work had aimed to examine the ZnO nanoparticles effect on plant physiology at the cellular level. The plant was treated with an attenuating concentration of ZnO nanoparticles and analyzed the levels of biomaterials in responding to protect the cellular membrane. In this experiment, wild typeand transgenic tobaccos over expressing flavonoid regulatory gene were used to identify the obvious results of the plant responses.
MATERIALS AND METHODS
Materials
Wild type and transgenic plants carrying the flavonoid regulatory gene (transparent testa8, tt8) line No. 1-5, obtained from previous work of our laboratory, were used in this experiment. Samples were grown in Murashige and Skoog medium (Murashige and Skoog, 1962) adding 0, 10, 20 mg/L ZnO nanoparticles. After 4 weeks under 16/8 hour lighten and darkness at 25±2°C condition, leaf from each sample was prepared for analysis.
Total soluble sugar content
Total soluble sugar of each sample was measured by extracting 0.5 mg leaf in 2 ml 80% ethanol, 80 °C 15 min, then the samples were agitated at room temperature for 1 hour and kept overnight at 4 °C. Four hundred microliters of the solution was mixed with 400 µl deionized water and chloroform. The solution was diluted with deionized water. This solution was detected total soluble sugar by following phenol-sulfuric acid method (Shou et al., 2003). The diluted solution was added an equal volume of 5% phenol and 2 ml of sulfuric acid, then placed at room temperature for 10 min. The sample was measured the absorbance value at wavelength 490 nm and calculated the content of total soluble sugar (µg/ml) from the standard curve.
Flavonoid content
All leaf samples were ground to powder with liquid nitrogen and extracted with methanol-acid solution (3:2 of water: 1% HCI in Methanol) in proportion of a 1 g sample per 4 ml extract solution (Harborne, 1998) then agitated at 250 rpm for 2 hours. Samples were added 1 ml chloroform and centrifuged at 10,000 rpm for 10 min. Supernatant was separated and measured the contents of flavone, flavonol and anthocyanin at a specific absorbance of each flavonoid subgroup.
Plant cell membrane injury analysis
Level of plant injury was estimated using the modified method of Bajji, 2002. The percentage of cell membrane injury was calculated from the electrical conductivity (EC) of plant cells by following method. An equal gram of tobacco samples was cut into the same size, then immersed in deionized water for 20 min and measured an initial electrical conductivity (ECi). The sample was left for 4 hours and measured the final electrical conductivity (ECf). Then all samples were boiled for 2 min and measured the total electrical conductivity (ECt). The percentage of cell membrane injury was calculated using the following equation:
% cell membrane injury = [(ECf-ECi)/(ECt-ECi)] x100
The lipid peroxidation was determined by the amount of malondialdehyde (MDA). The ground leaves (0.15 g) were added 1.5 ml 1% trichloroacetic acid (TCA). After incubated for 1 hour at room temperature, 500 µl of supernatants were separated into two new tubes. The 1st tube was added 500 µl of 20% TCA. The 2nd tube was added 500 µl of mixed solution (20% TCA and 0.5% thiobarbituric acid (TBA)). All samples were boiled at 95°C for 30 min. Absorbance values at the specific wavelength of each sample were measured and calculated the malondialdehyde level according to the method of Hodges et al. (1999). The first tube was measured absorbance value at 532 nm and 600 nm and the second tube was measured absorbance value at 532 nm, 600 nm and 440 nm. The concentration of malondialdehyde was calculated using the following formula:
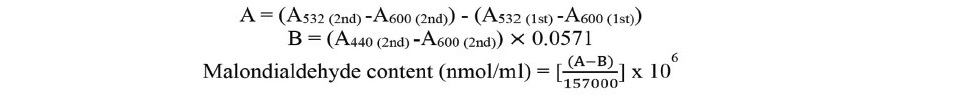
Statistical analysis
All treatments were performed at least 3 duplicates in completely randomized design (CRD) then using one-way analysis of variance (ANOVA) and Duncan’s multiple range test (DMRT) for statistical analysis. The resulting data are presented as means ± standard deviation (SD). The differences among means were determined by LSD test, significant at a P value ≤ 0.05
RESULTS
The effect of ZnO nanoparticles on plant biomaterials
The contents of signaling metabolite (total soluble sugar) of both wild type and transgenics are increased when tobaccos were grown in Murashige and Skoog media containing ZnO nanoparticles (Figure 1). The wild type tobacco grown in ZnO media showed the content of total soluble sugar about 30-50%, which was higher than wild type in normal Murashige and Skoog media did. Transgenic tobaccos increased their total soluble sugar content about 20-30% when were grown in the treatment of ZnO media. Transgenic tobaccos had total soluble sugar content in their tissue higher than wild type tobacco. The highest amount of total soluble sugar for both wild type and transgenic tobaccos was founded in 20 mg/L ZnO nanoparticle condition.
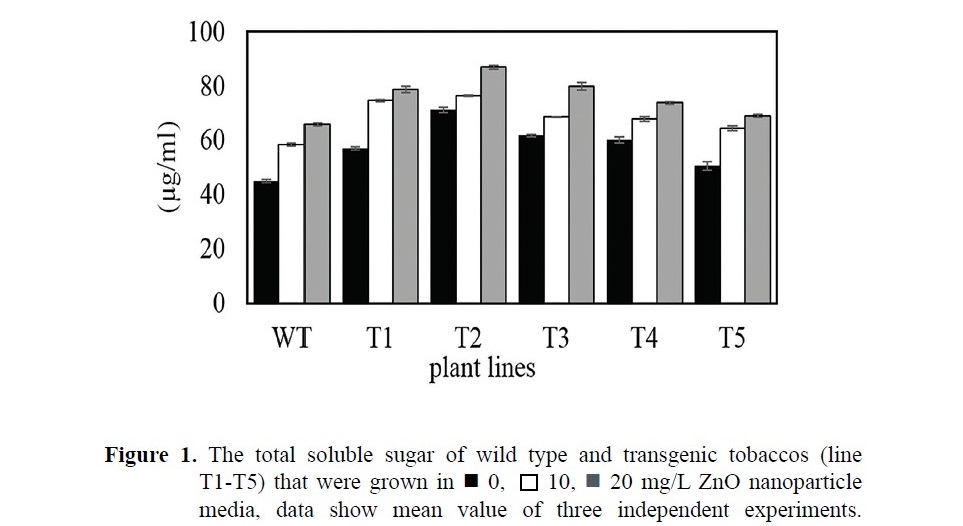
Table 1. Flavonoid contents (µg/g Fresh weight) of wild type and transgenic tobaccos (T1-T5) that were grown in 0, 10, 20 mg/L ZnO nanoparticle media.
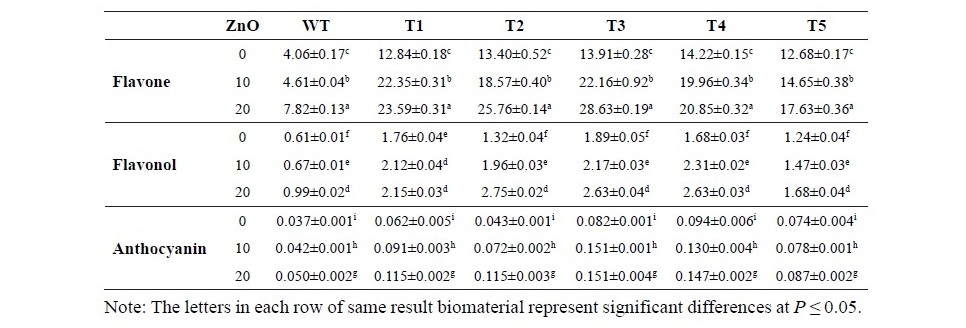
The protective metabolites of flavonoid synthesized in plant tissue from different concentrations of ZnO nanoparticle conditions are presented in Table 1.
All lines of transgenic tobaccos had the content of flavonoid subgroups higher than wild type tobacco. In normal condition, transgenic tobaccos contained flavone (2.3 times), flavonol (1.5 times) and anthocyanin (1.1 times) more than wild type did. Under this experimental condition, ZnO nanoparticles in media significantly enhanced the contents of all flavonoid subgroups in plant tissue. Under the condition of 10 mg/L ZnO in Murashige and Skoog media, wild type plant increased the contents of flavone, flavonol and anthocyanin, 13.68%, 8.83% and 24.60%, respectively, while transgenics increased the contents of flavone, flavonol and anthocyanin, 45.58 %, 27.96% and 42.33%, respectively, in comparing to same line that were grown in non ZnO nanoparticle condition. The highest concentration of ZnO nanoparticles in this experiment (20 mg/L) was marked as the highest inducing of flavonoid content. In this treatment, wild type increased flavone, flavonol and anthocyanin, 92.8%, 61.39% and 48.81%, while transgenic tobaccos increased 73.49%, 52.39% and 81.68%, respectively in comparing to plant under normal media condition.
Effects of ZnO nanoparticles on plant cell membrane
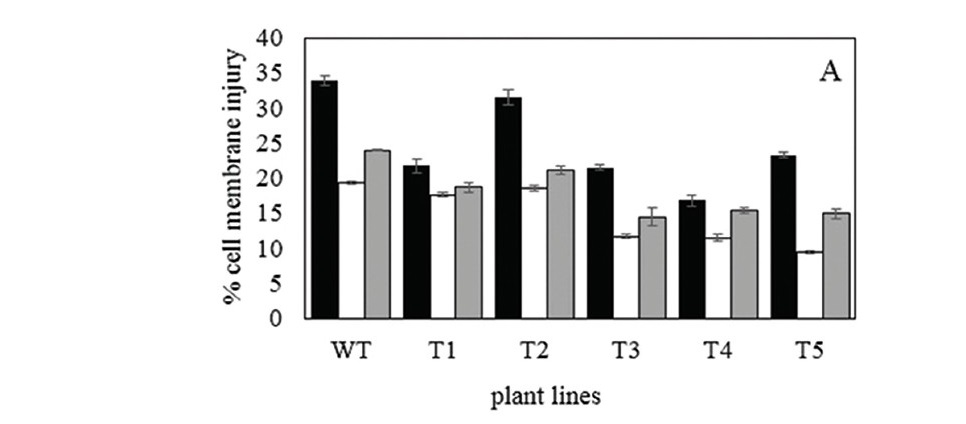
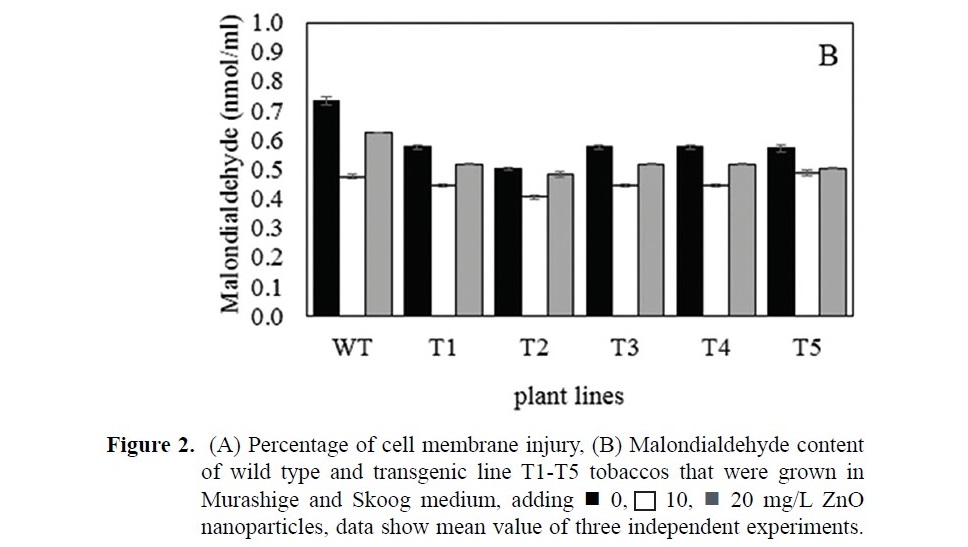
The quality of cell membrane indicates a plant cellular condition. Under experimental study, an attenuating concentration of ZnO nanoparticles (10- 20 mg/L) in media reduced the percentage of cell membrane injury of tobacco (Figure 2A). Result data of tobacco in 10 mg/L ZnO nanoparticle media showed the least percentage of cell membrane injury (19.43% in wild type and about 10-18% in transgenics). These percentages were less than that in 20 mg/L ZnO nanoparticle condition (23.96% in wild type and about 14-19% in transgenics). Plants grown in media without ZnO nanoparticles showed the highest percentage of cell membrane injury (33.91% in wild type and about 17-24% in transgenics). Under media without ZnO nanoparticle condition, transgenic tobacco tissues showed lower percentages of cell membrane injury than wild type tissues. The lowest percentage of cell membrane injury was found in transgenic plants grown under the condition of media with 10 mg/L ZnO nanoparticles. Level of malondialdehyde, a byproduct of the lipid peroxidation reaction, are presented in Figure 2B. Samples that were grown in media adding 10 and 20 mg/L ZnO nanoparticles showed less membrane damage detecting in compared to those in normal media. The lowest contents of malondialdehyde were found in tissue of plant grown under 10 mg/L ZnO nanoparticle condition in both wild type and transgenic plants, following with those under 20 mg/L ZnO nanoparticle condition.
DISCUSSION
Normal composition of Murashige and Skoog media contains Zn2+ in the form of ZnSO4.7H2O at 1.95 mg/L or 29.9 µM in their chemical formula. In this experiment, the concentration of Zn in the medium was enhanced in ZnO nanoparticle form, at 10-20 mg/L concentration. Our results showed that wild type and transgenic plants enhancing an accumulation of total soluble sugar and protective biomaterial of flavonoid subgroups at different levels. The addition of 10 mg/L ZnO nanoparticles into medium also had positive effects on protecting cell membrane because this concentration of zinc may be used up for valuable metabolic processes of plants. So, in this ZnO condition, wild type and transgenic plants increased their cellular rigidity and protecting effects. These effects presented as the better results of percentage of cell membrane injury and lipid peroxidation.
When the concentration of ZnO nanoparticles in the medium was increased to 20 mg/L, the contents of total soluble sugar and flavonoid subgroups in plant tissue were induced higher than plant under 10 mg/L ZnO nanoparticle condition did. This 20 mg/L ZnO nanoparticle condition also showed reduced membrane damage in plant better than the control condition. However, plant under this condition have slightly higher the percentage of cell membrane injury and the malondialdehyde content than 10 mg/L ZnO nanoparticle condition. Plant uptake ZnO nanoparticles and may convert into a Zn2+ ions form (Lv et al., 2015). Over concentration of zinc metal may increase toxicity to plant (Reichman, 2002; Gyana and Premananda, 2003).
Zn2+ is an essential micronutrient element which is a component of several proteins in the plant cell system. This element also acts as a co-factor that regulate the activities of enzymes in various biosynthetic pathways (Keith et al., 2000; Hafeez et al., 2013). So that, adding ZnO nanoparticles in medium may enhance biosynthesis of plant metabolites (total soluble sugar and flavonoids) and increase an efficiency of plant physiology presented as a cell membrane stability. In similar to previous researches, various plant increased their growth rate and contents of importance biomaterials when they were treated with ZnO nanoparticles (Prasad et al., 2012; Mukherjee et al., 2014; Bandyopadhyay, 2015; Wang et al., 2016). The transgenic tobaccos had levels of total soluble sugar and flavonoid biomaterials higher than wild type tobacco in all conditions. The accumulation levels of biomaterials (total soluble sugar and flavonoids) in transgenic tobacco under 20 mg/L ZnO nanoparticle condition were higher than wild type. These levels of biomaterials were different depending on tt8 transgenic lines. Under the membrane injury experiments, all transgenic tobaccos had lower percentages of cell membrane injury than normal plant (Figure 2).
The zinc metal element has a potential of oxidative harmful for the cellular system. Plants enhanced flavonoid biosynthesis in responding to oxidative stresses have been reported (Fini et al., 2011; Nakabayashi et al., 2014). However, the enhancing mechanisms are affected by various factors and are under investigation. Flavonoids have a plenty function inside the plant cell, such as reducing overused injury of metal elements and attenuating the severity of metal effects (Oyvind and Kenneth, 2006; Katharina and Jutta, 2009; Marzena, and Mateusz, 2012). Therefore, the higher amount of flavonoid contents in transgenic may protect plant cell from lipid peroxidation reaction and cell membrane damages. The similar results were found in our previous work that the transgenics carry another flavonoid regulatory gene named production of anthocyanin pigment 1 (pap1) synthesized biomaterials in responding to the concentration of ZnO nanoparticles containing media (Chayaprasert and Sompornpailin, 2017). However, tt8 transgenic plants enhanced flavonoid subgroup higher than pap1 transgenic plants and slightly differed in the specific flavonoid group. Moreover, tt8 transgenic plant are better than pap1 transgenic plant in cellular membrane protection.
CONCLUSION
Plants grown in Murashige and Skoog media adding ZnO nanoparticles enhanced the number of biomaterials (total soluble sugar and flavonoids) in their plant tissues depend on ZnO nanoparticle concentration. Under these experimental conditions, both wild type and transgenic plants maximized enhancements the contents of total soluble sugar and flavonoids under the condition of 20 mg/L ZnO media. Although, plant grown in media with 10 mg/L ZnO nanoparticles had the accumulation of total soluble sugar and flavonoids in their tissue less than those in media with 20 mg/L ZnO nanoparticles did. However, plant under 10 mg/L ZnO nanoparticle condition showed the best quality of membrane presented as the percentage of cell membrane injury and lipid peroxidation. Moreover, transgenic plants containing tt8 gene are highly responsive to ZnO nanoparticles in synthesizing flavonoid subgroups content and increase cell protection.
REFERENCES
Bajji, M., Kinet, J.M., and Lutts, S. 2002. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regulation. 36: 61-70.
Bandyopadhyay, S., Plascencia, V.G., Mukherjee, A., Rico, M.C., Yacamán, J.M.,Videa, P.R.J., and Torresdey, G.L.J. 2015. Comparative phytotoxicity of ZnO nanoparticles, bulk ZnO, and ionic zinc on to the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Science of the Total Environment. 515-516: 60-69. https://doi.org/10.1016/j.scitotenv.2015.02.014
Chayaprasert, W., and Sompornpailin, K. 2017. Suitable concentration of ZnO nanoparticles enhanced biomaterial synthesis and plant cell protection. Applied Mechanics and Materials. 866: 21-24. https://doi.org/10.4028/ www.scientific.net/AMM.866.21
Fini, A., Brunetti, C., Ferdinando, M.D., Ferrini, F., and Tattini, M. 2011. Stress- induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signaling and Behavior. 6(5): 709-711. https://doi.org/10.4161/psb. 6.5.15069
Gyana, R., and Premananda, D. 2003. Effect of metal toxicity on plant growth and metabolism: I. Zinc. Agronomie, EDP Sciences. 23(1): 3-11.
Hafeez, B., Khanif, Y.M. and Saleem, M. 2013. Role of zinc in plant nutrition. American Journal of Experimental Agriculture. 3(2): 374-391. https:// doi.org/10.9734/AJEA/2013/2746
Harborne, J.В. 1998. Phytochemical methods a guide to modern techniques of plant analysis. Chapman and Hall Ltd, London.33-80 p.
Hodges, D.M., Delong, J.M., Forney, C.F., and Prange, R.K. 1999. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 207: 604-611. https://doi.org/10.1007/s004250050524
Katharina, K., and Jutta, L.M. 2009. Effect of flavonoids on heavy metal tolerance in Arabidopsis thaliana seedlings. Botanical Studies. 50: 311-318.
Keith, A.M., Chih-chin, H., and Carol, A.F. 2000. Function and mechanism of zinc metalloenzymes. The Journal of Nutrition. 130:1437-1446. https://doi. org/10.1093/jn/130.5.1437S
Lv, J., Zhang, S., Luo, L., Zhang J., Yang, K., and Christie, P. 2015. Accumulation speciation and uptake pathway of ZnO nanoparticles in maize. Environmental Science Nano. 2: 68-77.
Mahajan, P., Dhoke, S.K., and Khanna, A.S. 2011. Effect of nano-Zno particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. Journal of Nanotechnology. 2011: 696535. https://doi.org/10.1155/2011/696535
Marzena, S., and Mateusz, K. 2012. Flavonoids and their properties to form chelate complexes. Biotechnology and Food Sciences. 76(1): 35-41. Mukherjee, A. 2014. Impact of zinc oxide nanoparticles on green pea plant & seed quality and effects on physiological traits of green peas, corn, and zucchini
by silver nanoparticles. ProQuest Dissertations Publishing, Texas.
Mukherjee, A., Peralta, V.J.R., Bandyopadhyay, S., Rico, M.C., Zhao, L., and Gardea, T.J.L. 2014. Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics. 6: 132-138. https://doi.org/10.1039/C3MT00064H
Murashige, T., and Skoog, F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 15: 473- 497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nakabayashi, R., Yonekura, K.S., Urano, K., Suzuki, M., Yamada, Y., Nishizawa, T., Matsuda, F., Kojima, M., Sakakibara, H., Shinozaki, K., et al. 2014. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. The Plant Journal. 77: 367-379. https://doi.org/10.1111/tpj.12388
Ov, S. 2004. Applications of nanoparticles in biology and medicine. Journal of Nanobiotechnology. 2: 3. https://doi.org/10.1186/1477-3155-2-3
Oyvind, M.A., and Kenneth, R.M. 2006. Flavonoids: chemistry, biochemistry and applications. Stress Protection. CRC Press Taylor & Francis Group, Florida. 408-412 p.
Prasad, T.N.V.K.V., Sudhakar, P., Sreenivasulu, Y., Latha, P., Munaswamy, V., Raja, R.K., Sreeprasad, T.S., Sajanlal, P.R., and Pradeep, T. 2012. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. Journal of Plant Nutrition. 35: 905–927. https://doi.org/10.1080/01 904167.2012.663443
Reichman, S.M. 2002. The responses of plants to metal toxicity: a review focusing on Copper, Manganese and Zinc. Australian Minerals & Energy Environment Foundation, Melbourne. 16-37 p.
Shou, H., Bordallo, P., Fan, J.B., Yeakley, J.M., Bibikova, M., Sheen, J., and Wang, K. 2003. Expression of an active tobacco mitogen-activated protein kinase enhances freezing tolerance in transgenic maize. Proceeding of the National Academy of Science of the United States of America. 9: 3298-3303.
Srinivas, R.P., Philbert, M., Vu, Q.T., Huang, Q., Kokini, L.J., Saos, E., Chen, H., Peterson, M.C., Friedl, E.K., Mcdade, N.C., et al. 2010. Nanotechnology research applications in nutritional sciences. American Society for Nutrition. 140: 119-124. https://doi.org/10.3945/jn.109.115048
Wang, X., Yang, X., Chen, S., Li, Q., Wang, W., Hou, C., Gao, X., Wang, L., and Wang, S. 2016. Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Plant Science. 6: 1243. https://doi.org/ 10.3389/fpls.2015.01243
Wilailack Chayaprasert and Kanokporn Sompornpailin*
College of Nanotechnology, King Mongkut’s Institute of Technology Ladkrabang, Bangkok 10520, Thailand
*Corresponding author. E-mail: kanokporn.so@kmitl.ac.th
Total Article Views
Article history:
Received: July 16, 2018;
Revised: October 24, 2018;
Accepted: November 1, 2018

