
Bacteriocin Harvested from the Synbiotic Culture of Selected Lactic Acid Bacteria with Various Vegetables, Cereals, Fruits, Medicinal and Tuber Plants: Inhibition of Vibrio parahaemolyticus
Siriwoot Sookkhee*, Yothin Kumsang, and Sathian BoongumPublished Date : 2022-07-11
DOI : https://doi.org/10.12982/CMUJNS.2022.050
Journal Issues : Number 3, July-September 2022
Abstract The present study was to investigate and identify the antimicrobial lactic acid bacteria against some gastrointestinal pathogens, to compare the activity between their synbiotic culture and bacteria culture alone, and to characterize the bacteriocins produced by the synbiotic culture of these isolates. Among 600 isolates of selected lactic acid bacteria, three isolates of Lactocaseibacillus paracasei subsp. paracasei, two of Latilactobacillus curvatus subsp. curvatus, and one of Lactiplantibacillus plantarum exhibited the antibacterial activities against four standard reference bacteria, and twelve clinical strains of food-poisoning bacteria especially Vibrio parahaemolyticus. The synbiotic culture of L. paracasei 9/5, L. curvatus 87/6 and L. plantarum 89/4 which are cultured with sweet corn, grand red bean, papaya, star fruit, nashi pear, and black ginger exhibited significant inhibitions to V. parahaemolyticus. Bacteriocin which was harvested from the synbiotic culture of L. curvatus 87/6 and nashi pear demonstrated the strongest activity to the tested bacteria. After partial purification, ultrafiltration, and ion exchange column chromatography, this bacteriocin was anionic with 40-80 kDa and pI at 4-5. Its antimicrobial activities significantly decreased at pH > 8.0, with trypsin and pepsin digestions as well as after heating at > 100°C. Analyzed with SDS-PAGE, the suspected bacteriocin band was detected in the increased intensity, lower intensity, and was not detected after harvested from the synbiotic culture, the bacterial culture alone, and only plant powder extract, respectively. It was concluded that the synbiotic culture of L. curvatus with some prebiotics could induce potent antimicrobial bacteriocin production.
Keywords: Lactic acid bacteria, Prebiotics, Synbiotic, Bacteriocin, Vibrio parahaemolyticus
Funding: The authors are thankful for the research funding provided by the Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
Citation: Sookkhee, S., Kumsang, Y., and Boongum, S. 2022. Bacteriocin harvested from the synbiotic culture of selected lactic acid bacteria with various vegetables, cereals, fruits, medicinal and tuber plants: inhibition of Vibrio parahaemolyticus. CMUJ. Nat. Sci. 21(3): e2022050.
INTRODUCTION
Lactic acid bacteria (LAB) are found to reside in the human, animal body as the normal flora (Ahrné et al., 1998) and are widely used as probiotics and possess a beneficial effect on human health. LAB play an important role in the food fermentation of milk, meats, and vegetables, and has been shown to enhance the stability and nutritional value of foods by preventing the growth of pathogenic and spoilage microbes as food contaminating microbes (Ayivi et al., 2020). It is accepted to the “Generally Recognized As Safe or GRAS” (FAO, 2006). Several reports revealed the capability to produce antimicrobial compounds including organic acids for example lactic acid and acetic acid, hydrogen peroxide, and bacteriocins (Sookkhee et al., 2001; Mijač et al., 2006; Tachedjian et al., 2017). Bacteriocins are oligopeptides, proteins or protein complexes possessing antimicrobial activity against both gram-positive and gram-negative bacteria (Yang et al., 2014; Woraprayote et al., 2015). Bacteriocin production can be classified into four classes (McAuliffe et al., 2001; Sookkhee et al., 2017b) including lantibiotics, non-lantibiotics which are low molecular-weight and heat-stable peptides, non-lantibiotics which are large-molecular weight and heat-labile peptides, and complex proteins. Due to the antimicrobial activities, LAB may be beneficial as bioprotective agents to control infections in the intestine (Liévin-Le Moal et al., 2014), vagina (Chee et al., 2020) or oral cavity (Sookkhee et al., 2001). There were several reports mentioned on the antimicrobial activity of LAB bacteriocins against the variety of gastrointestinal pathogens including Listeria monocytogenes (Winkowski et al., 1993), Bacillus cereus (Soria et al., 2014), Clostridium difficile (Lee et al., 2013), and Escherichia coli (Wang et al., 2020). But there are few reports on the bacteriocins affecting the members of Vibrionaceae (Balakrishnan et al., 2014). However, the bacteriocin from such a study was not produced from synbiotic culture. Although some compounds in plants play a role as the natural prebiotics (Wlodarska et al., 2015), there were a few reports relating to the antibacterial activity of bacteriocin produced by the synbiotic culture between LAB and prebiotics or plant nutrients. (Benítez-Chao et al., 2021; Mandal et al., 2009). Therefore, the objectives of the present study were to isolate the antimicrobial LAB recovered from various foods and other fermented products, to determine the selected LAB growth in the synbiotic culture with plant prebiotics, and to assess the inhibitory activity of their bacteriocins against the tested strains.
MATERIAL AND METHODS
Preparation of LAB
Six hundred isolates of LAB which were formerly recovered from the various sources of foods and fermented products in a previous study of our colleagues since 2006-2012. A purified colony of each LAB was isolated on de Mann-Rogosa Sharpe broth (MRS; BactoTM; Bacton Dickinson, Sparks, MD) under 5% CO2 atmosphere at 37°C for 48 hours. before being kept into an aliquot tube containing glycerol as cryoprotectant and stored in the deep freezer managed by Dr. Siriwoot Sookkhee, Department of Microbiology, Faculty of Medicine, Chiang Mai University until use. Numbers and species of these LAB were shown in Table 1.
Identification of LAB
Each isolate has confirmed the species by using API-50 CHL biochemical identification (Pyar et al., 2019) or using VITEK-MS apparatus (Rocca et al., 2019). According to the API-50 CHL kit (bioMerieux Vitek Inc., Hazel Wood, MO), each isolate was cultured on MRS agar under the CO2 atmosphere at 37°C for 48 hours. The colonies were resuspended into MRS Broth to get the heavy suspension and adjusted the optical turbidity as equal to McFarland Standard No. 2 by dropping the bacteria suspension into the novel MRS Broth. Two drops of the adjusted suspension were inoculated into API-50 CHL medium and then covered with a drop of sterilized mineral oil. After 24 and 48 hours of incubation, the results were recorded. According to the biochemical profiles, the species of each isolate was identified by using the identification software (API®50 CHB/E databases; bioMerieux™). Some isolates which were unidentified with Api-50 CHL were also proteomically identified using VITEK MS (bioMérieux™).
For analyzing the proteomic profiles, a single colony of each isolate was picked up and smeared on a well of VITEK MS-DS target slides (bioMérieux™, Marcy l' Etoile, France). The center of each acquisition spot group has a calibrator spot used to detect the normal function of the VITEK MS. E. coli ATCC8739 was applied to the calibrator spot as the control strain. Alpha-4-cyano-4-hydroxycinnamic acid (CHCA) matrix (bioMérieux™, Marcy l' Etoile, France) was then dropped on top of each well to give crystallization. VITEK MS (bioMérieux™, Marcy l' Etoile, France) was run according to the condition as described in the previous report (Lananta et al., 2021). Peptide spectra from each well of the target slide were determined and then processed to interpret the species identification using the Myla® database (bioMérieux™, Marcy l'Etoile, France).
Table 1. LAB species and their numbers of isolatesrecruited in the present study. The species names of LAB were recently classified (Zheng et al, 2020).
|
LAB species |
Numbers of isolates |
|
Bifidobacterium lactis |
6 |
|
Bifidobacterium longum |
12 |
|
Companilactobacillus kimchii (basonym: Lactobacillus kimchii) |
1 |
|
Fructilactobacillus fructivorans (basonym: Lactobacillus fructivorans) |
1 |
|
Lacticaseibacillus casei (basonym: Lactobacillus casei) |
24 |
|
Lacticaseibacillus paracasei subsp. paracasei (basonym: Lactobacillus paracasei subsp. paracasei) |
90 |
|
Lacticaseibacillus rhamnosus (basonym: Lactobacillus rhamnosus) |
27 |
|
Latilactobacillus sakei subsp. sakei (basonym: Lactobacillus sakei subsp. sakei) |
15 |
|
Latilactobacillus curvatus (basonym: Lactobacillus curvatus) |
21 |
|
Lactiplantibacillus plantarum subsp. plantarum (basonym: Lactobacillus plantarum subsp. plantarum) |
90 |
|
Lactobacillus acidophilus |
27 |
|
Lactobacillus crispatus |
27 |
|
Lactobacillus johnsonii |
15 |
|
Lactobacillus delbrueckii subsp. delbrueckii |
33 |
|
Lactobacillus acetotolerans |
4 |
|
Lactobacillus helveticus |
21 |
|
Lactobacillus jensenii |
9 |
|
Lactococcus lactis |
33 |
|
Lentilactobacillus buchneri (basonym: Lactobacillus buchneri) |
5 |
|
Lentilactobacillus kefiri (basonym: Lactobacillus kefiri) |
1 |
|
Levilactobacillus brevis (basonym: Lactobacillus brevis) |
3 |
|
Levilactobacillus cerevisiae |
1 |
|
Limosilactobacillus fermentum (basonym: Lactobacillus fermentum) |
54 |
|
Limosilactobacillus reuteri (basonym: Lactobacillus reuteri) |
39 |
|
Liquorilactobacillus capillatus (basonym: Lactobacillus capillatus) |
3 |
|
Pediococcus acidilactici |
36 |
|
Secundilactobacillus kimchicus (basonym: Lactobacillus kimchicus) |
1 |
|
Secundilactobacillus oryzae (basonym: Lactobacillus oryzae) |
1 |
Bacterial Indicators
Staphylococcus aureus ATCC 25923, Sarcina lutea ATCC 9341, Escherichia coli ATCC 25922, and Bacillus subtilis ATCC 6633 were performed as the tested indicators to screen the primary antimicrobial activity using agar-well diffusion assay (Gonelimali et al., 2018). Six clinical strains of gastroenteritis pathogenic bacteria were recruited to screen the secondary antimicrobial activity using agar-cup diffusion assay including Bacillus cereus CM02, Shigella flexneri CM01, E. coli O157:H7 CM05 and CM06, Vibrio cholerae PT001, and Vibrio parahaemolyticus SR02. Additionally, six environmental strains, PT03, NS02, CP01, KB03, RN01, and PK04 of Vibrio parahaemolyticus which were collected from raw oysters cultivated at the floating cages near the coast were also tested. They were also stored at deep freezer in the Bacteria Unit of the Department of Microbiology, Faculty of Medicine, Chiang Mai University, Thailand. For the preparation of these bacteria, they were separately grown in Tryptic soy broth (TSB; Difco™, Detroit, MI) at 37°C for 24 hours before adjusting to a turbidity equivalent to McFarland No. 0·5.
Primary screening of antimicrobial activity
The antimicrobial activity was primarily determined by using an agar-well diffusion method against the reference strains of S. aureus, S. lutea, B. subtilis and E. coli. Each overnight culture of tested strain was separately adjusted turbidity as equal to McFarland Standard No. 0.5 before swabbed on Mueller Hinton Agar (MHA; BactoTM Bacton Dickinson) plate and make wells by a cork borer. The cell-free supernatant of each LAB which was cultured in MRS broth under LAB condition for 48 hours-cultivation was harvested by centrifuging with 4,121 g at 4°C for 30 minutes and then filled into the well on the swabbed agar before incubating at 37˚C for 24 hours. The experiments were done in duplicate. Its antimicrobial activities against these tested indicators were evaluated according to the average inhibition zones. Ten percent of total tested LAB, 60 isolates, which gave the strongest activities were selected.
Secondary antimicrobial activity
The antimicrobial activity of these selected isolates against 12 clinical tested bacteria was investigated using agar cup diffusion assay (Bonev et al., 2008) on MHA plates. The preparations of tested indicators had similar the preparation in the agar-well diffusion assay. Cell-free supernatant of LAB was freshly prepared and filled into the cylinder cup which was placed on the swabbed MHA plates and then grown at 37°C for 24 hours. Its antimicrobial activities against these tested strains were evaluated according to the average inhibition zones. The experiments were done in triplicate. Ten percent of them, six isolates, which exhibited the largest inhibition zones were selected for further studies as the selected antimicrobial LAB.
Preparations of Plant Powder Extracts Sixty-two plants which are shown in Table 2 including 16 cereals, 10 vegetables, 20 fruits, 8 medicinal plants, and 8 tuber plants were prepared using a soybean machine or juice extractor, or food mixer. Their powders were made using the freeze-dried technique (CHRIST ALPHA1-4, Memmingen, Germany). Two percent w/v of inulin (Merck™; Merck KGaA, Darmstadt, Germany) was used as the positive control. These plants were organically planted and provided by The Organic Plantation of Royal Project Foundation.
Table 2. Lists of the tested cereals, vegetables, tuber plants, and fruits with Thai, general and scientific names.
|
No. |
Scientific names |
Thai names |
General names |
|
|
Cereals |
|
|
|
|
|
1 |
Arachis hypogaea |
Tua-li-song |
Pea nut |
|
|
2 |
Bruguiera cylindrica |
Ma-led-tua-kao |
Navy bean |
|
|
3 |
Cajanus cajan |
Tua-rae |
Pigeon pea |
|
|
4 |
Chenopodium quinoa |
Qui-noa |
Quinoa |
|
|
5 |
Glycine max |
Tua-lueng |
Soybean |
|
|
6 |
Linum usitatissimum |
Ma-led-li-nin |
Linseed |
|
|
7 |
Oryza sativa |
Ja-muk-kao |
Rice bran |
|
|
8 |
Phaseolus vulgaris |
Tua-daeng |
Red bean |
|
|
9 |
Phaseolus vulgaris |
Tua-kaek |
Green bean |
|
|
10 |
Pisum sativum |
Tow-maew |
Green pea |
|
|
11 |
Sesamum indicum |
Nga-dum |
Black sesame |
|
|
12 |
Sesamum indicum |
Nga-kao |
White sesame |
|
|
13 |
Sorghum bicolor |
Khao-fang |
Sorghum |
|
|
14 |
Vigna angularis |
Tua-a-zu-ki |
Azuki bean |
|
|
15 |
Vigna radiata |
Tua-kaew |
Mung bean |
|
|
16 |
Zea mays |
Kao-pod |
Sweet corn |
|
|
Vegetables |
||||
|
17 |
Brassica oleracea |
Broc-co-li |
Broccoli |
|
|
18 |
Cucumis sativus |
Taeng-kwa-yee-pun |
Japanese cucumber |
|
|
19 |
Cucurbita moschata |
Fag-thong |
Pumpkin |
|
|
20 |
Cucurbita pepo |
Zuc-chi-ni |
Summer squash |
|
|
21 |
Cynara scolymus |
Ar-ti-choke |
Globe artichoke |
|
|
22 |
Hibiscus sabdariffa |
Kra-jeab |
Roselle |
|
|
23 |
Luffa acutangula |
Boab |
Angled loofah |
|
|
24 |
Sechium edule |
Cha-yo-te |
Chayote |
|
|
25 |
Solanum melongena var. serpentinum |
Ma-kue-maung |
Egg plant |
|
|
26 |
Telosma minor |
Kha-jorn |
Cowslip creeper |
|
|
Fruits |
||||
|
27 |
Ki-wi |
Kiwifruit |
||
|
28 |
Ananas comosus |
Sup-pa-rod |
Pineapple |
|
|
29 |
Averrhoa carambola |
Ma-fueng |
Star fruit |
|
|
30 |
Carica papaya |
Ma-la-kor |
Papaya |
|
|
31 |
Citrus hindsii |
Som-kum-quat |
Hong kong kumquat |
|
|
32 |
Citrus maxima |
Som-o-thong-dee |
Pummelo |
|
|
33 |
Cucumis melo var. cantaloupensis |
Can-ta-loupe |
Cantaloupe |
|
|
34 |
Hylocereus undatus |
Kaew-mung-korn |
Dragon fruit |
|
|
35 |
Malus domestica |
Ap-ple |
Gala apple |
|
|
36 |
Manilkara zapota |
La-mood |
Sapodilla |
|
|
37 |
Passiflora edulis |
Saw-wa-rod |
Passion fruit |
|
|
38 |
Persea americana |
A-vo-ca-do |
Avocado |
|
|
39 |
Phyllanthus emblica |
Ma-kham-pom |
Indian gooseberry |
|
|
40 |
Prunus persica |
Tor |
Peach |
|
|
41 |
Prunus salicina |
Luk-nai-daeng |
Japanese plum |
|
|
42 |
Punica granatum |
Tub-tim |
Pomegranate |
|
|
43 |
Pyrus bretscheideri |
Sa-lee-num-pung |
Ya pear |
|
|
44 |
Pyrus pyrifolia |
Sa-lee-hi-mah |
Nashi pear |
|
|
45 |
Vitis labrusca |
A-ngoon-moung |
Purple table grapes |
|
|
46 |
Zizyphus mauritiana |
Pud-sa-num-nom |
Jujube |
|
|
Medicinal plants |
||||
|
47 |
Alpinia galanga |
Kah |
Greater galangal |
|
|
48 |
Andrographis paniculata |
Fah-Ta-Lai-Jon |
Creat |
|
|
49 |
Angelica sinensis |
Tung-kui |
Dong quai |
|
|
50 |
Boesenbergia rotunda |
Kra-chai-khao |
Chinese ginger |
|
|
51 |
Cymbopogon citratus |
Ta-krai |
Lemon grass |
|
|
52 |
Kaempferia galanga |
Proh-hom |
Galanga |
|
|
53 |
Kaempferia parviflora |
Kra-chai-dum |
Black ginger |
|
|
54 |
Ocimum basilicum |
Maeng-luk |
Sweet basil |
|
|
Tuber plants |
|
|
||
|
55 |
Arctium lappa |
Som-jak-ka-pat |
Greater burdock |
|
|
56 |
Beta vulgaris |
Beet-root |
Beetroot |
|
|
57 |
Colocasia esculenta |
Phuek |
Taro |
|
|
58 |
Daucus carota |
Car-rot |
Carrot |
|
|
59 |
Helianthus tuberosus |
Kaen-ta-wan |
Jerusalem artichoke |
|
|
60 |
Ipomoea batatas |
Mun-ted |
Sweet potato |
|
|
61 |
Raphanus sativus |
Ra-dish |
Radish |
|
|
62 |
Raphanus sativus var. longipinnatus |
Hua-chi-tao |
Chinese radish |
|
Inhibitory activity of the powders against six selected antimicrobial LABs
Due to the synbiotic culture, the selected LAB must be not inhibited by the antimicrobial activity of plant powder extracts. In the present study, five percent w/v of each plant powder was resuspended in the sterilized distilled water. The suspensions which exhibited the activity against the tested LAB must be excluded from the synbiotic study. All suspensions were determined the inhibitory activity against each selected LAB isolate under 5% CO2 atmosphere at 37°C for 48 hours using the agar-cup diffusion assay as described above.
Synbiotic cultures
Each selected LAB was separately cultured in the presence (synbiotic culture or LPM) and absence (LAB culture alone or LM) of 5% w/v plant powder extract in the modified MRS broth (volume ratio of L:P:M was 2:3:5) for determining the bacterial growth and antimicrobial activity. Each 5% w/v plant powder suspension in the modified MRS broth (PM) was sterilized by autoclaving at 110°C, 15 pounds for 10 minutes. Each LAB isolate was grown in MRS broth at 37°C for 48 hours under a 5% CO2 atmosphere. The volume of these suspensions was 4°C centrifuged at 4,121 g for 10 minutes. The pellet was resuspended with Phosphate Buffer Solution (PBS) pH 7.2 to make initial amounts of bacteria in LM and LPM by adjusting the optical density as equal to McFarland No.2. The modified MRS broth (M) that being depleted the carbon source was prepared from the formula as follows for using as the LPM synbiotic culture media; 0.2 %w/v proteose peptone, 0.2 %w/v beef extract, 0.5 %w/v yeast extract, 0.2 %w/v tween-80, 0.4 %w/v ammonium citrate, 1 %w/v sodium acetate, 0.02 %w/v magnesium sulfate, 0.01 %w/v manganese sulfate, and 0.4 %w/v dipotassium phosphate. After 48 hours of LPM synbiotic culture, cell-free supernatant was harvested after centrifuging at 4,121 g for 10 minutes. The determination of antimicrobial activity among these systems in each isolate was done as described above. The activity of PM towards each selected LAB was also tested. LPM cultures that exhibited the largest size of inhibition zone against the clinically tested strains were selected as the selected LPM synbiotic cultures for further study.
Statistical analysis
Data were expressed as means ± SD. They were done triplically. Differences were considered statistically significant by paired T-test at α value of P = 0.05.
Antimicrobial compounds produced from the selected LPM synbiotic cultures
The growths of LAB in LPM and LM cultures of each plant powder extract were compared by colony enumeration with spread plate technique on MRS agar after incubated at 37°C for 48 hours under 5% CO2 atmosphere. The pH of their cell-free supernatants was determined. The concentration of hydrogen peroxide in the cell-free supernatant was also determined (Chakravarty et al., 2016) using the Quantitative Peroxide Assay Kit (PeroXOquantTM; PIERCE, Rockford, IL.) according to the protocol of the manufacturer. The absorbance was measured by using a spectrophotometer at 595 nm. The concentration was calculated according to the standard curve.
The concentration of lactic acid was measured by using Gutmann’s method (Gerez et al., 2006). The tested reagents were freshly prepared with the mixture of reagent buffer (140 µl), lactate solution (100 µl), β-NAD solution (15 µl) (MerckTM; Merck KGaA, Darmstadt, Germany) and deionized water (35 µl) by comparing with the blank reagent which is prepared with the mixture of reagent buffer (140 µl), β-NAD solution (15 µl) and deionized water (35 µl). The absorbance was monitored by using a spectrophotometer at 340 nm (A340nm) and recorded the initial absorbance (Ai) of both the tested and blank experiments. Afterward, their reactions were added 50 µg/ml of L-lactic dehydrogenase (Sigma-Aldrich; St.Louis, MO) immediately and mixed until reactions were complete. The absorbances of reactions were monitored by using a spectrophotometer at 340 nm and recorded the final absorbance (Af) of both the tested and blank experiments. The concentration of lactic acid was calculated by reference to its assay absorbance compared to the standard curve.
The total protein concentration of each LPM synbiotic culture was determined (Lizier et al., 2010) by using Qubit® 2.0 fluorometer and Qubit™ protein assay kit (Molecular Probe™, Eugene, OR) according to the protocol of the manufacturer.
Partial purification of bacteriocins harvested from synbiotics
The cell-free supernatants of selected LPM synbiotic cultures were harvested by centrifuging the fresh culture at 4°C, 4,800 rpm for 30 minutes to eliminate the bacterial cells and insoluble residue of plant powder. Bacteriocins were partially purified from cell-free supernatant using vivaflow-50™ ultrafiltration at the cut-off 10 and 30 kDa (Sartorius Stedem Lab, Stonehouse, UK). Each bacteriocin fraction was then separately purified with anion exchange chromatography (Yi et al., 2016) via AKTA® Explorer apparatus (GE Healthcare Life Science). HitrapTM Capto S, a strong sulfoethyl cation exchanger (Amersham Biosciences AB) was performed as a stationary phase. Buffer A (20 mM Tris-HCl pH8.0) was performed to equilibrate the column and to wash out the unbound substance. The expected proteins were eluted by buffer B (20 mM tris-HCl pH8.0 supplemented with 1.0 M NaCl) in three segmented gradients, including 35%, 55%, and 100% with flow rate 1.0 ml/min, and then separately collected. Ionic salts were removed by using the desalting media column (HiTrapTM Desalting; Amersham Biosciences AB, Uppsala, Sweden). The desalted protein was eluted with the sterilized PBS pH 7.2. The protein concentration of each fraction was then determined by Qubit fluorometric assay with Qubit® 2.0 fluorometer as described above. The antimicrobial activity of the bacteriocin fraction of each selected LPM was also determined. The bacteriocin fraction which exhibited antimicrobial activity was selected as the selected crude bacteriocin.
Characterization of Bacteriocin
Crude bacteriocin activities were characterized by pH, heat and protease sensitivity (Zhang et al., 2018) as follows. For pH sensitivity, crude bacteriocins were each adjusted with 1N HCl or 1N NaOH to pH 1-14 before filling into the cup which was swabbed with V. parahaemolyticus, an important seafood-contaminant bacteria. The plates were incubated at 37°C for 24 hours. The residual activity of each treated bacteriocin against V. parahaemolyticus was determined. For heat sensitivity, crude bacteriocin was separately treated at 60°C, 80°C, 100°C for 30 minutes, and 121°C for 15 minutes. They were then processed as described above. For proteolytic enzyme sensitivity, crude bacteriocins were separately treated with various concentrations of trypsin (MerckTM; Merck KGaA, Darmstadt, Germany) and pepsin (MerckTM; Merck KGaA, Darmstadt, Germany), 0.125, 0.25 and 0.5 mg/ml, at 37ºC for 1 hour. Acetate buffer pH 4.4 was used to control the action of these enzyme. Until 1 hour of actions, pH condition of reaction was increased to pH 6.0 before the enzymes were inactivated with heat at 75ºC for 30 minutes. They were then processed as described above. The experiments were done in duplicate.
One-dimensional polyacrylamide gel electrophoresis
The molecular weight of the selected bacteriocin was investigated (Yamamoto et al., 2003). Twenty µg of crude bacteriocins were denatured with sodium dodecyl sulfate (AMRESCO®; AMRESCO, Solon, OH) and β-mercaptoethanol (BIO-RAD®; BIO-RAD Laboratory, Hercules, CA) before being boiled at 95°C for 10 minutes in a water bath. These denatured proteins were electrophoresed in SDS-PAGE chamber (Mini-PROTEAN® IV cell; BIO-RAD Laboratory) under 80 volts constant current condition for 4% stacking gel and 90 volts for 12% separating gel in running buffer pH 8.3 until the bromophenol tracking dye (MerckTM; Merck KGaA) reached the bottom of the separating gel. The silver staining techniques were performed to visualize the proteins in gels (Halami et al., 2011) according to the protocol of the manufacturer (PlusOneTM; GE Healthcare, Bio-Sciences AB, Uppsala, Sweden).
Two-dimensional polyacrylamide gel electrophoresis
The bacteriocin was desalted four times by using the 2D clean-up reagent kit (EttanTM; Sample Preparation Kits; Amersham Biosciences). Fifty µg of cleaned protein was loaded directly onto the immobilized pH gradient (IPG) strips pH 3-10 for a length of 7 cm (ImmobilineTM; DryStrip; Amersham Biosciences, Uppsala, Sweden) and then put into the rehydration buffer at room temperature for 12 hours before placing on the electrophoresis chamber (Multiphor II Electrophoresis System; Pharmacia Biotech ABTM; Uppsala, Sweden). Both steps of 2-D PAGE were processed as described in a previous report (Sookkhee et al., 2017a).
RESULTS
Screening of antimicrobial isolates
Interestingly, 566 of 600 tested isolates demonstrated antimicrobial activities against four ATCC tested indicators, S. aureus ATCC 25923, S. lutea ATCC 9341, E. coli ATCC 25922, and B. subtilis ATCC 6633. They could inhibit these tested indicators in a variety of activities. However, 60 tested isolates which exhibited the broadest-spectrum activity and largest inhibition zones were selected. Sixty-nine isolates of nine species were not selected namely C. kimchi, F. fructivorans, L. casei, L. johnsonii, L. helveticus, L. kefiri, L. cerevisiae, L. capillatus, and S. kimchicus. Among thirty-five isolates of six species, a strongest isolate of each species namely B. lactis, B. longum, L. acetotolerans, L. jensenii, L. brevis, and S. oryzae was selected. Additionally, results also demonstrated the selection of the antimicrobial isolates from other 13 species including 2 of L. sakei subsp. sakei, 2 of L. buchneri, 3 of P. acidilactici, 3 of L. rhamnosus, 3 of L. curvatus, 4 of L. acidophilus, 4 of L. crispatus, 4 of L. lactis, 4 of L. reuteri, 4 of L. fermentum, 6 of L. delbrueckii subsp. delbrueckii, 7 of L. plantarum subsp. plantarum, and 9 of L. paracasei subsp. paracasei. After the secondary screening, only six selected isolates which possessed the strongest antimicrobial activities against the tested bacteria were selected then called the potent antimicrobial isolates. They were L. rhamnosus 68/7, L. curvatus 87/6, L. fermentum 20/6, L. plantarum subsp. plantarum 89/4, L. paracasei subsp. paracasei 9/5 and 60/13. Therefore, it may be possible that they produced antimicrobial substances especially bacteriocins for inhibiting the tested bacteria. Their activities were also demonstrated in Table 3. The antimicrobial activity of the most potent isolate, L. curvatus 87/6 was demonstrated in Figure 1.
Table 3. Selected antimicrobial LAB isolates after tested by agar-cup diffusion assay.
|
Tested bacteria |
Average diameter of inhibition zone obtained from cell-free supernatant of isolate (mm+SD) |
|||||
|
9/5 |
20/6 |
60/13 |
68/7 |
87/6 |
89/4 |
|
|
S. aureus ATCC 25923 |
24.50 + 0.76 |
25.00 + 0.29 |
24.00 + 1.00 |
25.00 + 0.87 |
25.00 + 0.76 |
26.00 + 0.76 |
|
S. lutea ATCC 9341 |
31.00 + 0.29 |
31.50 + 0.87 |
28.50 + 0.76 |
31.50 + 0.29 |
32.00 + 0.76 |
29.00 + 0.87 |
|
E. coli ATCC 29213 |
21.00 + 0.76 |
20.50 + 0.76 |
20.00 + 1.00 |
19.50 + 0.29 |
25.00 + 0.29 |
19.50 + 0.29 |
|
B. subtilis ATCC 6633 |
29.50 + 0.76 |
24.00 + 0.87 |
26.50 + 1.00 |
23.50 + 0.76 |
29.00 + 0.87 |
29.50 + 0.76 |
|
B. cereus CM02 |
26.50 + 1.00 |
22.50 + 0.76 |
22.50 + 0.29 |
24.80 + 0.87 |
27.50 + 0.76 |
24.50 + 1.00 |
|
S. flexneri CM01 |
25.00 + 0.87 |
23.00 + 0.29 |
24.50 + 0.87 |
22.00 + 1.00 |
25.50 + 1.00 |
27.00 + 0.29 |
|
E. coli O157:H7 CM05 |
27.00 + 0.76 |
23.00 + 0.76 |
23.50 + 0.87 |
22.00 + 0.29 |
27.00 + 0.29 |
25.00 + 0.87 |
|
E. coli O157:H7 CM06 |
27.50 + 0.29 |
25.50 + 0.76 |
27.50 + 0.76 |
28.00 + 0.76 |
27.50 + 0.76 |
24.50 + 0.76 |
|
V. cholerae PT001 |
22.50 + 0.76 |
20.50 + 1.00 |
21.50 + 0.76 |
22.00 + 0.29 |
23.50 + 0.29 |
21.00 + 0.87 |
|
V. parahaemolyticus SR02 |
19.00 + 0.87 |
18.00 + 0.87 |
18.50 + 0.29 |
18.00 + 0.87 |
20.50 + 0.87 |
18.50 + 1.00 |
|
V. parahaemolyticus PT03 |
22.00 + 0.76 |
22.50 + 0.29 |
21.50 + 0.87 |
21.00 + 1.00 |
24.00 + 0.76 |
23.50 + 0.29 |
|
V. parahaemolyticus NS02 |
23.50 + 0.87 |
22.50 + 0.76 |
21.50 + 0.76 |
22.50 + 0.76 |
24.50 + 0.87 |
22.50 + 0.76 |
|
V. parahaemolyticus CP01 |
21.00 + 0.87 |
19.50 + 0.87 |
18.00 + 0.87 |
18.00 + 0.29 |
20.50 + 0.87 |
20.50 + 0.87 |
|
V. parahaemolyticus KB03 |
21.00 + 0.29 |
19.50 + 0.76 |
18.00 + 0.76 |
18.00 + 0.87 |
20.50 + 0.76 |
20.50 + 0.87 |
|
V. parahaemolyticus PK04 |
20.50 + 0.76 |
20.00 + 0.87 |
19.00 + 0.76 |
18.50 + 0.76 |
21.00 + 1.00 |
21.00 + 0.29 |
|
V. parahaemolyticus RN01 |
23.00 + 0.87 |
22.00 + 0.76 |
21.00 + 0.29 |
23.50 + 0.29 |
23.50 + 0.29 |
22.00 + 0.87 |
Note: Bold numbers revealed to the largest inhibition zone of each indicator bacteria
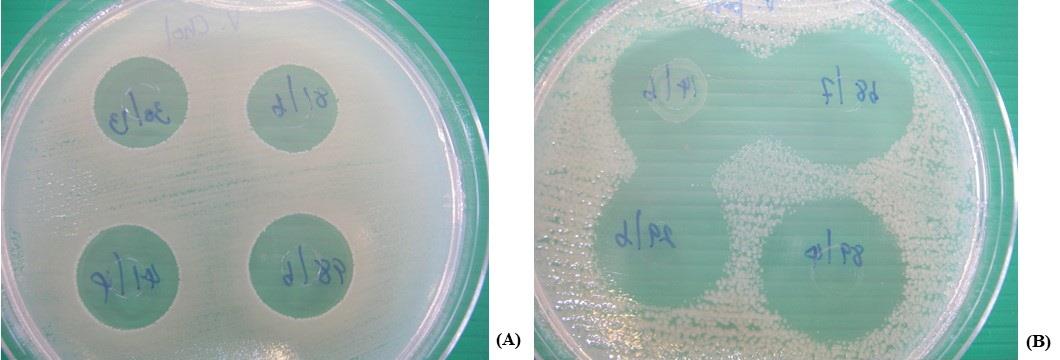
Figure 1. Antimicrobial activity of some LAB against E. coli O157:H7 CM05 (A), and a clinical strain of V. parahaemolyticus NS02 (B) using agar-cup diffusion assay.
Identification
The species identification was acceptable at the levels of 80% identity. According to the API®50 CHB/E database, two isolates of L. paracasei subsp. paracasei 9/5 and 60/13 exhibited the % identity at 99.2 and 99.5, respectively. Moreover, % identity of four selected isolates including L. rhamnosus 68/7, L. curvatus 87/6, L. plantarum subsp. plantarum 89/4, and L. fermentum 20/6 were 99.8, 99.9, 99.9, and 99.6, respectively.
Inhibitory activity of plant powder extracts against six selected antimicrobial lab isolates
Among 62 plants recruited in the present study, their powder suspensions were made as described above then the physicochemical properties and antimicrobial activities were determined against these selected antimicrobial LAB isolates. Five plant powder extracts, A. galanga (Greater galangal; No. 47), B. rotunda (Chinese ginger; No. 50), C. citratus (Lemon grass; No. 51), P. emblica (Indian gooseberry; No. 39), and P. granatum (Pomegranate; No. 42) demonstrated the antimicrobial activity to the tested isolates. They were excluded from the further study of the LPM synbiotic cultures.
Antimicrobial activity of the cell-free supernatant of lpm synbiotic cultures
The LPM synbiotic cultures of the selected LAB isolates and plant extracts were evaluated according to the inhibitory activity against V. parahaemolyticus and the growth of LAB isolates. The results of LPM synbiotic cultures were demonstrated in Figure 2-3.
Besides five plant extracts that inhibited the selected antimicrobial isolates, some plant extracts also affected the growth of the selected isolates in LPM cultures after comparison with LM, namely C. quinoa (No.4), L. usitatissimum (No.6), P. sativum (No.10), C. sativus (No.18), A. comosus (No.28), and P. americana (No.38). They were not the prebiotic sources for supporting the growth of LPM. However, results further demonstrated that the high fold change of LPM growths of these selected isolates with some cereals, including P. vulgaris (No.8), S. bicolor (No.13), V. radiata (No.15), and Z. mays (No.16), with some vegetable, S. melongena var. serpentinum (No.25), with some fruits, including A. carambola (No.29), C. papaya (No.30), H. undatus (No.34), M. zapota (No.36), P. pyrifolia (No.44), and V. labrusca (No.45), with tuber plants including A. lappa (No.55), C. esculenta (No.57), H. tuberosus (No.59), and R. sativus var. longipinnatus (No.62). These plant extracts were selected and then the antimicrobial activity was determined against the tested V. parahaemolyticus NS02. It may be suspected that these selected plant extracts contained the prebiotic compounds for helping the growth of selected isolates in LPM after compared with their LM.
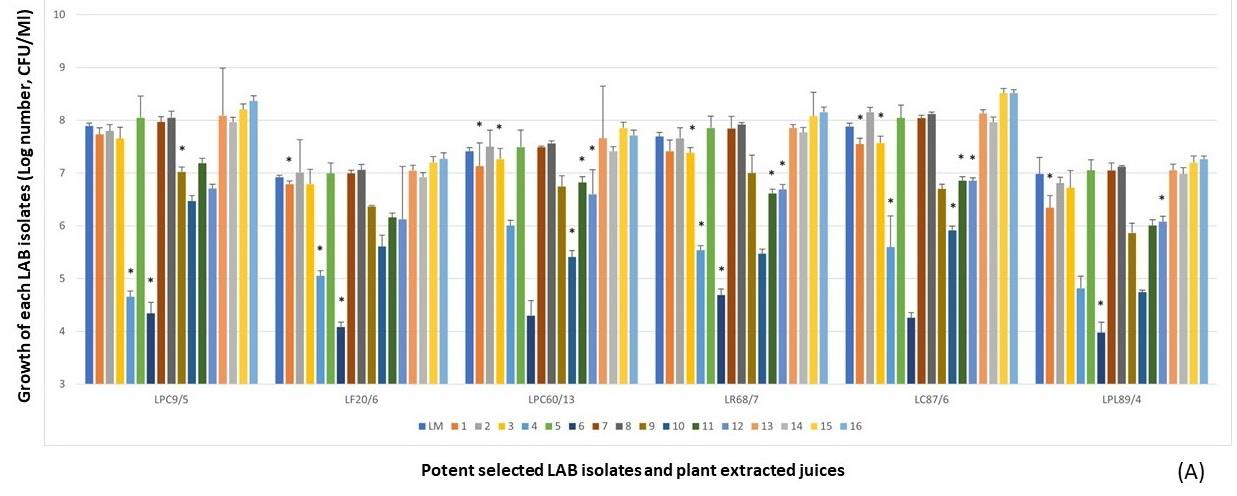
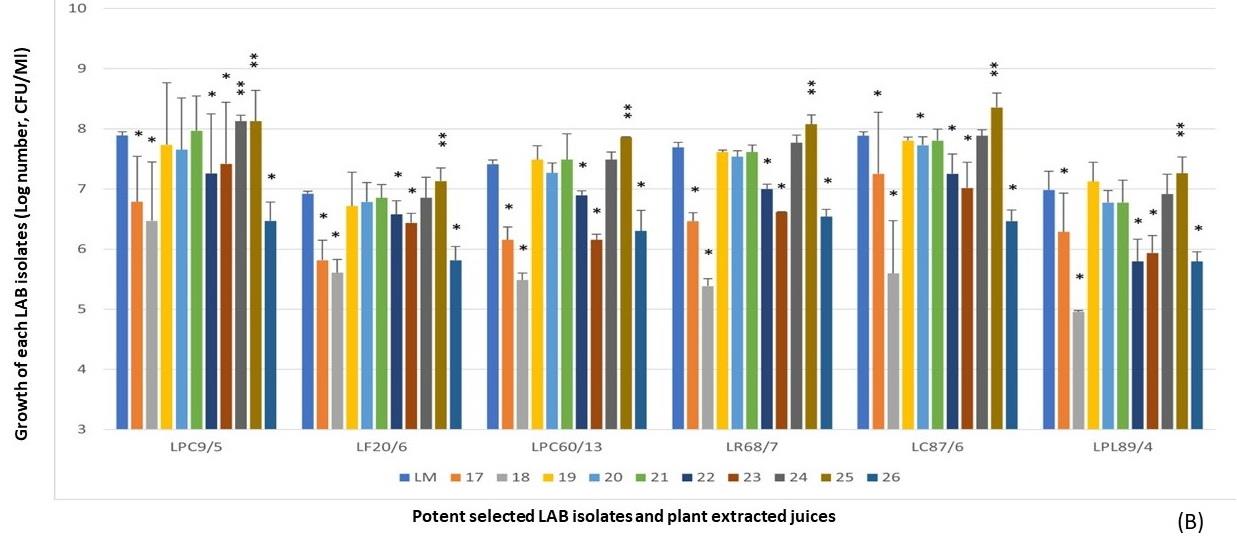
Figure 2. LPM synbiotic growth of each selectedantimicrobial isolate and plant powder extracted from cereals (A) and vegetables (B). Data are shown as the fold change of bacterial growth after compared with LM.
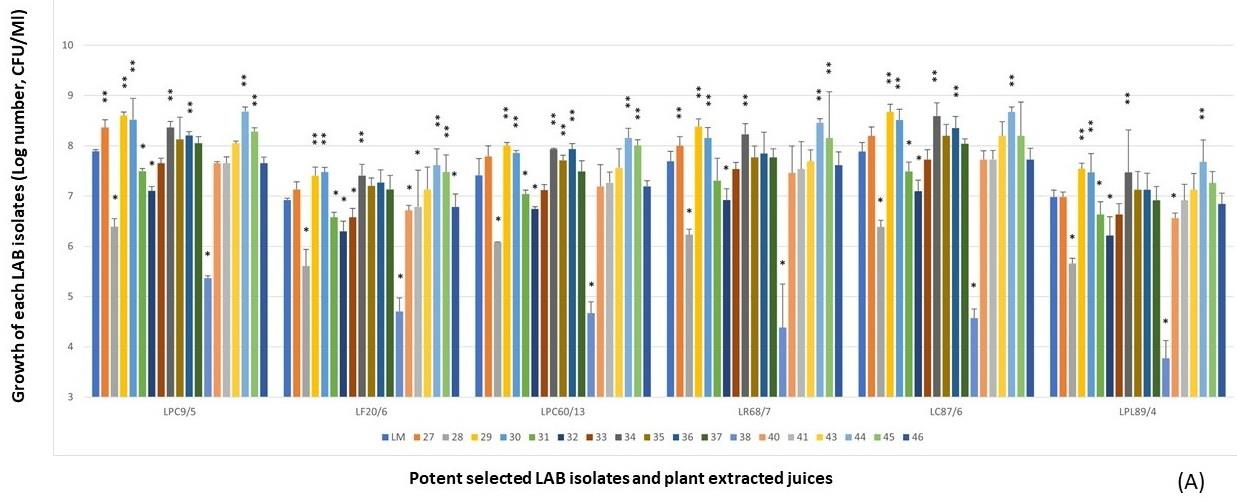
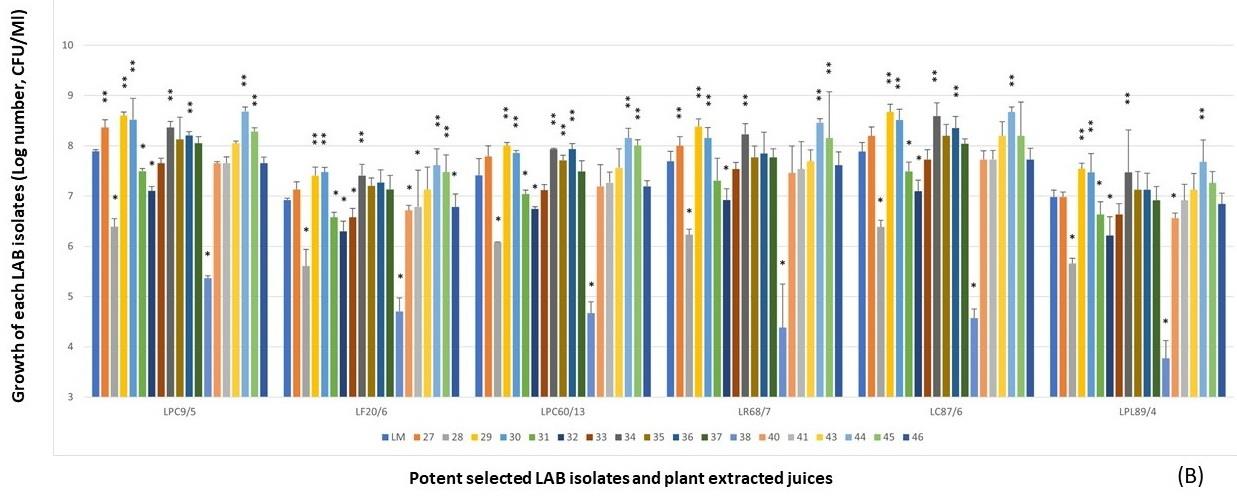
Figure 3. LPM synbiotic growth of each selected antimicrobial isolate and plant powder extracted from fruits (A), medicinal plants and tuber plants (B). Data are shown as the fold change of bacterial growth after compared with LM.
Due to the prebiotic effect, these tested plants were the important sources that synergically affected the antimicrobial activity of these selected antimicrobial LAB isolates as shown in Figure 4, including Z. mays (No.16), A. carambola (No.29), M. zapota (No.36), P. pyrifolia (No.44), V. labrusca (No.45), and H. tuberosus (No.59). The potent activity was demonstrated in the LPM cultures of these plant extracts after being compared with their inhibitions of LM cultures.
From the results of each selected LAB isolate, it was found that the larger inhibition zones were demonstrated in the LPM synbiotic culture after being compared with only bacterial cultures alone, LM. It was noted that these plant extracts could act as natural prebiotics for supporting more growth and antimicrobial activity of such isolates. Among six LPM cultures of these isolates, the cultures added with the powder extracts of P. pyrifolia (No.44), H. tuberosus (No.59), and Z. mays (No.16) were shown the strong antimicrobial activity after determining the average inhibition zone towards four tested strains of V. parahaemolyticus (Figure 5). As well as L. curvatus 87/6, the LPM synbiotic cultures of L. paracasei 9/5, and L. plantarum 89/4 with P. pyrifolia or Nashi pear (No.44) also exhibited the highest activity in the same manners. P. pyrifolia or Nashi pear (No.44) was the only selected prebiotic-rich fruit for supporting the growth and antimicrobial activity of this LAB isolate. Besides the prebiotic source of fruit, Z. mays or sweet corn (No.16), only one selected cereal, showed the potent antimicrobial activity in the LPM synbiotic culture of this isolate. Lastly, H. tuberosus or Jerusalem artichoke (No.59) which is well-known as a high rich inulin source was also selected as for its potent antimicrobial activity.
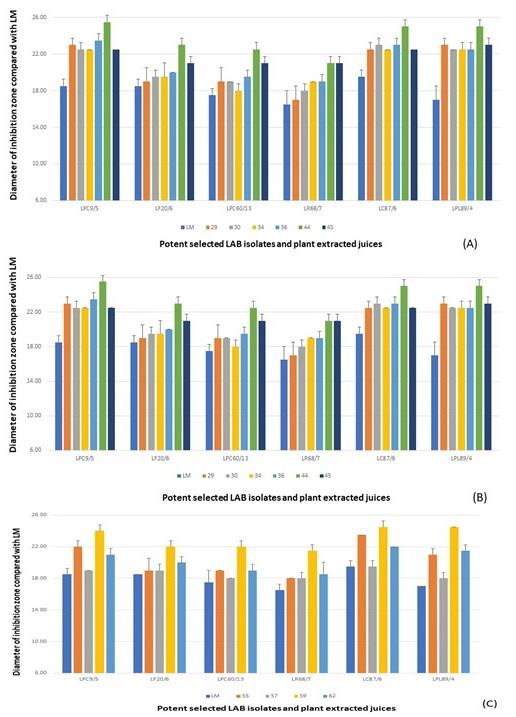
Figure 4. Antimicrobial activity of LPM synbiotic cultures of each selected antimicrobial isolate and plant powder extracted from cereals and vegetables (A), fruits (B), and medicinal plants and tuber plants (C) against V. parahaemolyticus NS02. Data are shown as the diameter of the inhibition zone after being compared with LM.
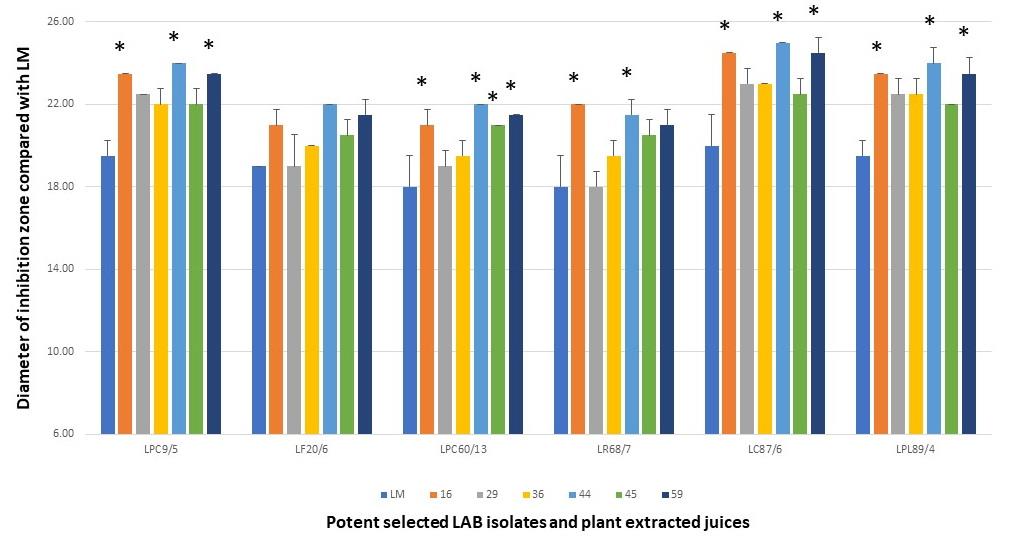
Figure 5. Antimicrobial activity of LPM synbiotic cultures of each selected antimicrobial isolate and selected plant powder extracts against four tested V. parahaemolyticus. Data are shown as the diameter of the inhibition zone after being compared with LM.
Characterization of active substances harvested from selected synbiotics
The lactic acid, hydrogen peroxide, total protein concentration, and pH of cell-free supernatant of the selected LPM cultures were investigated. As described above that the prebiotic effect of plants could result in the increasing growth of LAB isolates. However, the changes in pH were not affected to the antimicrobial activity of tested LPM cultures. It may be assumed that the enhancing growth and antimicrobial activity were not affected by the pH of synbiotic cultures. Based on LAB, lactic acid could be detected in a culture of these LAB isolates. The significant increase of acid was demonstrated in the LPM cultures of L. paracasei 9/5, and L. plantarum 89/4 with the selected phytonutrient, Z. mays (No.16) and H. tuberosus (No.59) while the lactic acid productions of L. curvatus 87/6 in the LPM cultures with Z. mays (No.16), P. pyrifolia (No.44), and H. tuberosus (No.59) were not significantly different as shown in Figure 6. Results demonstrated that the increasing concentrations of hydrogen peroxide and lactic acid could be observed in the LPM cultures of L. paracasei 9/5, L. curvatus 87/6 and L. plantarum 89/4 in LPM cultures. The detectable concentrations of such LPM cultures were less than the MICs of hydrogen peroxide and lactic acid standard. It may result in a synergistic effect to enhance the antimicrobial activity (Figure 6). Total protein concentrations of the LPM culture of L. curvatus 87/6 and the selected plant extracts statistically differed from its LM culture (Figure 6). After comparing the active substances among the culture of L. curvatus 87/6 with their extracts, the highest protein concentration was in the LPM cultures of L. curvatus 87/6 with Z. mays (No.16), P. pyrifolia (No.44), and H. tuberosus (No.59) in the same manner that differed from its LM cultures. The major interesting agents were focused on the antimicrobial proteins and/or peptides (called bacteriocins) that could be detected in these LPM synbiotic cultures.
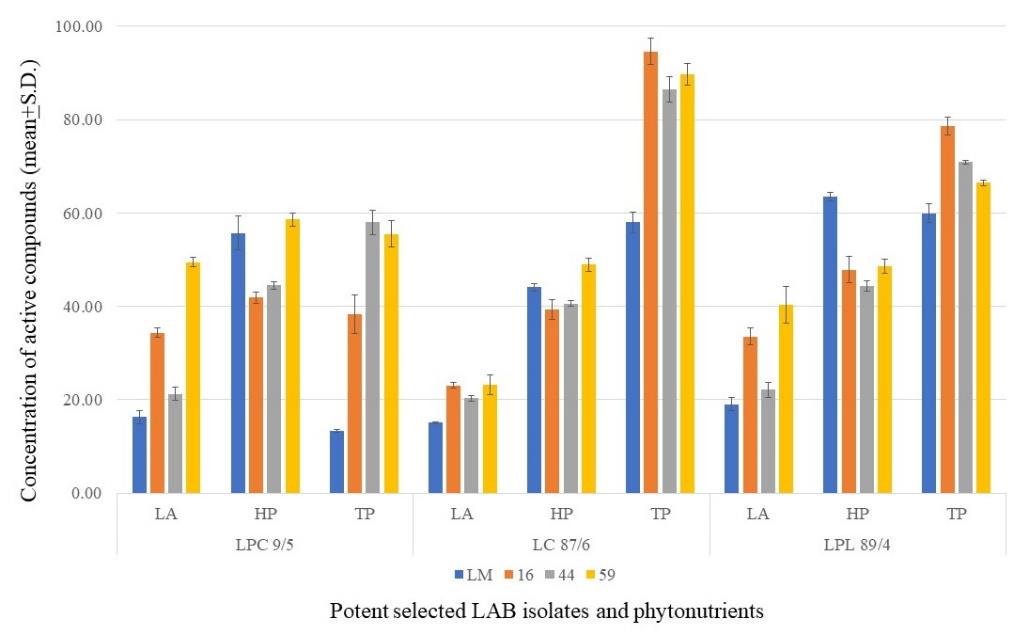
Figure 6. Concentration of Active Compounds. LA, Lactic acid (µg/ml); HP, Hydrogen peroxide (µg/ml); TP, Total proteins (mg/ml). Data showed with average and standard deviation.
Characterization
The antimicrobial activities against V. parahaemolyticus of the treated supernatant harvested from the LPM culture of L. curvatus 87/6 with P. pyrifolia (No.44) demonstrated acidic pH and gradually reduced to pH >8. The activities were also reduced at a higher temperature than 100°C. For the proteolytic enzyme sensitivity, the activities were inhibited after digesting with 0.5 mg/ml of trypsin and pepsin. It may suggest that this crude bacteriocin was acidic, heat-labile protein and also belonged to class III bacteriocin. From these results, it may be possible that the activity was due to the proteinaceous compounds, especially the antimicrobial protein called bacteriocin.
One-dimensional polyacrylamide gel electrophoresis
The protein extract from the LPM synbiotic culture of L. curvatus 87/6 with P. pyrifolia (No.44) could be detected in the observable levels by using the silver stain assay. The different results exhibited the detectable protein bands in LPM, LM and PM lane on the gel of SDS-PAGE were shown in Figure 7. This figure shows three different patterns of protein bands. Firstly, the protein bands could be detected both in LPM and LM (blue arrows). These bands were suspected as the original producing proteins of L. curvatus 87/6. Secondly, a protein band could be detected both in LPM and PM (yellow arrow). These bands were suspected as the original producing proteins of P. pyrifolia (No.44). Lastly, the protein bands could be detected only in LPM (red arrows). These bands were suspected as the additional proteins of L. curvatus 87/6 that were produced after being cultured with plant extracts.
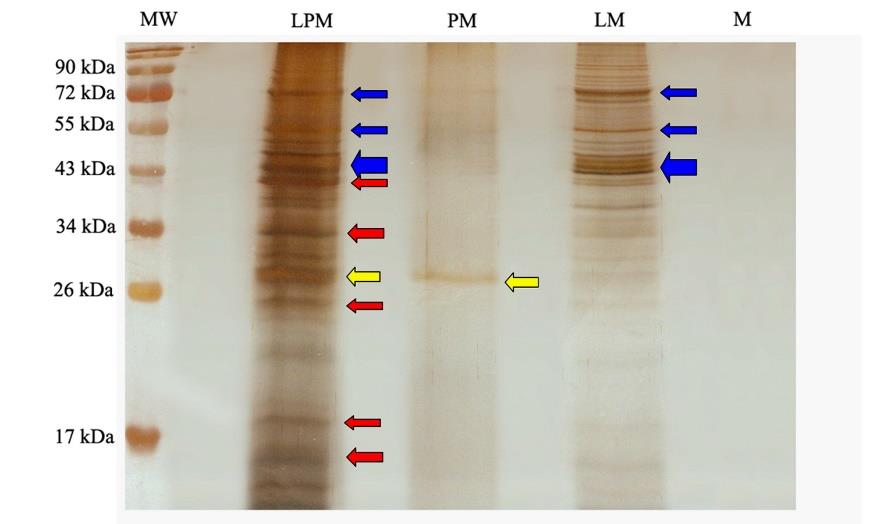
Figure 7. Protein patterns of crude bacteriocin harvested from the culture of L. curvatus 87/6 with P. pyrifolia (No.44) (LPM), P. pyrifolia (No.44) alone (PM), and L. curvatus 87/6 alone (LM). MW, molecular weight marker (Page RulerTM; Fermentas INC). M, only modified MRS broth without C- and N- sources of nutrients.
Two-dimensional polyacrylamide gel electrophoresis
The protein patterns in 2D-PAGE of crude bacteriocin from the cell-free supernatant of LPM synbiotic culture of L. curvatus 87/6 and P. pyrifolia (No.44) were shown in Figure 8. Their molecular weights were demonstrated in a range between 15-100 kDa. Their charges were in both cationic and anionic zones. Interestingly, the suspected protein from P. pyrifolia (No.44), the yellow oval, exhibits pI in a range of 9-10. It may be suggested that the protein contained in this plant extract showed a different pI from the other proteins produced from L. curvatus 87/6. From 2D-PAGE, the high molecular weight of original proteins produced from L. curvatus 87/6 exhibited their pI in a range of 4-7 as shown in blue ovals. While the molecular weight of additional proteins produced from L. curvatus 87/6 which being synbiotic cultured with P. pyrifolia (No.44) was lower with pI between 3-7 as shown in red ovals (Figure 8).
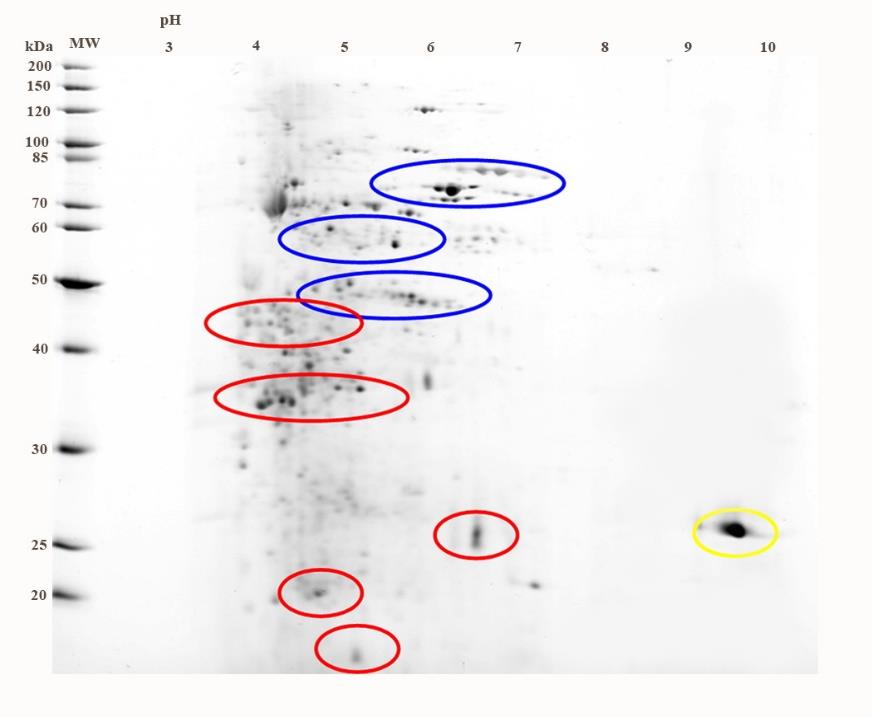
Figure 8. Protein pattern of L. curvatus 87/6 synbiotic cultured with P. pyrifolia (No.44).
DISCUSSION
LAB are widely used in both food and medical industries because of their ability to ferment sugar to lactic acid and produce other antimicrobial substances such as hydrogen peroxide and bacteriocins. As LAB are considered as Gram-positive bacteria, its membrane can tolerate in various kinds of environments and high oxidative stress. According to the previously mentioned characteristics, almost all LABs play a role as probiotics, especially Lactobacillus and Bifidobacterium (Klaenhammer et al., 2005). The antimicrobial activities of LAB against the oral (Sookkhee et al., 2001), intestinal (Campana et al., 2017), and vaginal pathogenic bacteria (Happel et al., 2017) have been demonstrated. Nevertheless, few studies have reported the activity of LAB towards Vibrio paraheamolyticus, an important seafood contaminant, and the causative agent of gastroenteritis from seafood. The present study was to explore the numbers of LAB isolates which able to inhibit the growth of V. parahaemolyticus for the further benefit to decrease and/or eliminate the seafood contamination and risk of gastroenteritis. Results of the present study demonstrated almost LAB could inhibit the tested intestinal pathogens. In that meaning, it can be firmly stated that selected LAB isolates could be found from various sources especially foods. Based on this classical classification, antimicrobial LAB isolates were identified to be L. paracasei, L. plantarum, and L. curvatus. It may be noted that foods are also an important source of selected antimicrobial LABs which play a role as probiotics.
Presently, molecular genetic techniques should be carried out to identify the bacterial species, investigate the pathogenic and/or virulent factors, and study the resistance mechanisms (Kwon et al., 2004). Due to the lack of species-specific DNA primer used in the PCR technique for all LAB species, we chose the other methods which could identify the species of the selected LAB. API 50CHL biochemical identification has been recommended in the studies of LAB (Pyar et al., 2019). From the results of antimicrobial screening of LAB, three LAB isolates that possessed the most potent activity were identified as L. paracasei subsp. paracasei, (L. curvatus and L. plantarum. These species were also reported as the potent bacteriocin producer in other studies (Bendjeddou et al., 2012; Barbosa et al., 2015; Marques et al., 2017; Todorov et al., 2017).
The antimicrobial substances are not only produced by probiotics or lactic acid bacteria. Their productions are also dependent on cultural composition. In the case of synbiotics, prebiotics contained in the tested plants could also affect their production. Prebiotics are non-digestible food ingredients that beneficially affected the host by selectively stimulating the growth, antimicrobial activity, or both of several bacterial species already resident in the colon (Chen et al., 2016). Fructans and inulin are obtained from the natural sources including vegetables, cereals, tuber plants and fruits. Jerusalem artichoke, Chicory, Dahlia tubers, garlic, and Asparagus root were reported as rich sources of fructans or inulin which have been well-known as the important prebiotic compounds (Roberfroid, 2005; Stiverson et al., 2014; Yadav et al., 2014). Oligosaccharide raffinose is also an ingredient in soybeans (Espinosa-Martos et al., 2006). Guarner et al. reported that the increases in normal flora metabolism, colon inflammatory prevention, and the growth of lactobacillus are caused by the effects of inulin and oligofructose (Guarner, 2005).
Various vegetables, cereals, fruits, medicinal and tuber plants are interesting sources of a prebiotic compound including oligosaccharides and proteins that promote the growth and the antimicrobial activity of LABs. The present study showed the valuable nutrients contained in the tested vegetables, fruits, cereals, medicinal and tuber plants. These nutrients exhibited the prebiotic effects to promote the growth of selected LAB isolates in the synbiotic cultures and enhance the large production and release of antimicrobial compounds to inhibit the tested enteropathogenic bacteria, especially V. parahaemolyticus. Therefore, the prebiotic substances contained in the selected synbiotics of the present study were selected for further investigation.
According to the results, prebiotics of some plants especially cereals and fruits play a role as the enhancer of the growth and antimicrobial activity of synbiotic cultures. A study by Likotrafiti et al (Likotrafiti et al., 2013) reported that growth activation and enhancement of the antimicrobial activity of Lactobacillus could be observed in the synbiotic culture of Limosilactobacillus fermentum against E. coli. The study of Alander et al (2001) reported that the volunteers who take synbiotics, prebiotics and probiotics in yogurt, for two weeks showed an increase of LAB in the gastrointestinal tract more than the other group who only took prebiotics or probiotics (Alander et al., 2001).
Bacteriocins which are produced from lactobacillus could destroy the cell membrane of bacteria, inhibit replication, translation, transcription and interfere with the activity of bacterial enzymes (And et al., 2003). The increase of bacteriocin production in synbiotic culture after comparison with the selected LAB culture alone was determined as the research questions in our present study. The prebiotic effect from various phytonutrients, and the enhancement of antimicrobial activity due to the hyperproduction of bacteriocins in some synbiotic cultures were mentioned. The results demonstrated that LAB isolates cultured in mMRS broth,modified MRS broth which depleted carbon source, produced the different extracted proteins from their synbiotics cultures according to SDS-PAGE analysis. In Figure 7, five different proteins (red arrows) were demonstrated and suspected to be the bacteriocin. However, these proteins will be further purified to identify the active bacteriocin that inhibits V. parahaemolyticus. It may be explained that these isolates were propagated in mMRS broth which depleted the C- sources of nutrients as the high stress condition to multiply. But the prebiotics contained in these plants could promote the bacterial growth. Then bacteria secreted the antimicrobial compounds, bacteriocins, as the secondary metabolites. Their synbiotic cultures were performed as the stress condition for increasing the production and yields of bacteriocins. Considerably, these selected LAB isolates could produce the larger amounts of bacteriocins than other tested LAB isolates.
Generally, Lactobacillus can produce the large amounts of bacteriocins, the proteinaceous compounds. In the present study, crude proteins included bacteriocins were separated by SDS-PAGE on 12% SDS-polyacrymide gel and then visualized by silver staining (Contreras et al., 1997). In the present study, the synbiotics produced a low concentration of protein which could not be detected by the sensitivity of Coomassie Brilliant Blue R250 staining, therefore, the silver staining, a high sensitive visualization of protein, was carried out in the present study.
However, these isolates produced the low concentration of bacteriocin which harvested from the synbiotic culture but the stronger antimicrobial activity of proteins was observed. Especially to the synbiotic culture of L. curvatus 84/7 and nashi pear, the harvested crude proteins possessed the highest antimicrobial activity could be demonstrated. The charges and molecular weights of proteins extracted from the above synbiotic culture were characterized in 2D-PAGE analysis. These results were important for the decision of techniques for protein purification. These proteins (in red ovals) were expected to be a bacteriocin produced from synbiotic culture and should be further purified by size exclusion chromatography, and ion exchange chromatography column chromatography, characterized the bacteriocin activity and identified by using the mass spectrometry.
From the results, the molecular weight of the purified bacteriocins were characterized by SDS-PAGE and 2D-PAGE and found that their sizes were at a range of 20-50 kDa belong to class III bacteriocins. The example of class III bacteriocins was helveticin J, 37 kDa, produced by Lactobacillus helveticus 481 (Joerger et al., 1990). Atanassova’s results in 2003 reported that the 43 kDa anionic bacteriocin of L. paracasei subsp. paracasei strain M3 was efficient to inhibit Helicobacter pylori and Candida albicans and could be partially purified by anion exchange column, reverse phase chromatography and HPLC, respectively (Atanassova et al., 2003). Similar to Atanassova’s report in 2003, the stronger antimicrobial activity of our bacteriocin was demonstrated in the anionic bacteriocin which visualized by 2D-PAGE. These partial purified bacteriocins should by further purified and analyzed in the proteomic level.
CONCLUSION
It was concluded that the LPM synbiotic cultures between the selected antimicrobial LAB isolates and nashi pear could produce the antimicrobial bacteriocin which possessed the strongest antimicrobial activity against V. parahaemolyticus and other gastroenteritic bacteria. It may be suggested that these suspected anionic bacteriocins harvested from the LPM synbiotic culture of each LAB species with nashi pear will be carried out to further characterize, purify and analyze the proteomic level.
ACKNOWLEDGMENTS
This work was financial supported by the Faculty of Medicine, Chiang Mai University. We would like to express our gratitude to Assist. Prof. Sumalee Pruksakorn, Department of Medicine, Faculty of Medicine, Chiang Mai University, for her invaluable guidance. We wish to thank the technicians at the Division of Central Laboratory, Maharaj Nakorn Chiang Mai Hospital, and at the Department of Microbiology, Faculty of Medicine, Chiang Mai University, Thailand who provide us laboratories.
AUTHOR CONTRIBUTIONS
Siriwoot Sookkhee provided the conception and designed the experiment, consulted the research proposal and submitted the research funding, provided the bacterial isolates and techniques, did the experiments and wrote the manuscript. Yothin Kumsang wrote the research proposal, did the experiments and wrote the manuscript. Sathian Boongum did the experiments and wrote the manuscript. All authors approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Ahrné, S., Nobaek, S., Jeppsson, B., Adlerberth, I., Wold, A. E., and Molin, G. 1998. The normal Lactobacillus flora of healthy human rectal and oral mucosa. Journal of Applied Microbiology. 85: 88-94.
Alander, M., Mättö, J., Kneifel, W., Johansson, M., Kögler, B., Crittenden, R., et al. 2001. Effect of galacto-oligosaccharide supplementation on human faecal microflora and on survival and persistence of Bifidobacterium lactis Bb-12 in the gastrointestinal tract. International Dairy Journal. 11: 817-825.
And, H. C., and Hoover, D. G. 2003. Bacteriocins and their food applications. Comprehensive Reviews in Food Science and Food Safety. 2: 82-100.
Atanassova, M., Choiset, Y., Dalgalarrondo, M., Chobert, J. M., Dousset, X., Ivanova, I., et al. 2003. Isolation and partial biochemical characterization of a proteinaceous anti-bacteria and anti-yeast compound produced by Lactobacillus paracasei subsp. paracasei strain M3. International Journal of Food Microbiology. 87: 63-73.
Ayivi, R. D., Gyawali, R., Krastanov, A., Aljaloud, S. O., Worku, M., Tahergorabi, R., et al. 2020. Lactic Acid Bacteria: Food Safety and Human Health Applications. Dairy, 1:202-232.
Balakrishnan, B., Ranishree, J. K., Thadikamala, S., and Panchatcharam, P. 2014. Purification, characterization and production optimization of a vibriocin produced by mangrove associated Vibrio parahaemolyticus. Asian Pacific Journal of Tropical Biomedicine. 4: 253-261.
Barbosa, M. S., Todorov, S. D., Jurkiewicz, C. H., and Franco, B. D. G. M. 2015. Bacteriocin production by Lactobacillus curvatus MBSa2 entrapped in calcium alginate during ripening of salami for control of Listeria monocytogenes. Food Control, 47: 147-153.
Bendjeddou, K., Fons, M., Strocker, P., and Sadoun, D. 2012. Characterization and purification of a bacteriocin from Lactobacillus paracasei subsp. paracasei BMK2005, an intestinal isolate active against multidrug-resistant pathogens. World Journal of Microbiology and Biotechnology. 28: 1543-1552.
Benítez-Chao, D. F., León-Buitimea, A., Lerma-Escalera, J. A., and Morones-Ramírez, J. R. 2021. Bacteriocins: An overview of antimicrobial, toxicity, and biosafety assessment by in vivo models. Frontiers in Microbiology. 12: 630695.
Bonev, B., Hooper, J., and Parisot, J. 2008. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. Journal of Antimicrobial Chemotherapy. 61: 1295-1301.
Campana, R., van Hemert, S., and Baffone, W. -2017-. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathogens. 9: 12.
Chakravarty, D., Banerjee, M., Bihani, S. C., and Ballal, A. 2016. A salt-inducible Mn-catalase (KatB) protects Cyanobacterium from oxidative stress. Plant Physiology. 170: 761-773.
Chee, W. J. Y., Chew, S. Y., and Than, L. T. L. 2020. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microbial Cell Factories. 19: 203-203.
Chen, Y. L., Liao, F. H., Lin, S. H., and Chien, Y. W. 2016. A prebiotic formula improves the gastrointestinal bacterial flora in toddlers. Gastroenterology Research and Practice. 2016: 3504282.
Contreras, B. G., De Vuyst, L., Devreese, B., Busanyova, K., Raymaeckers, J., Bosman, F., et al. 1997. Isolation, purification, and amino acid sequence of lactobin A, one of the two bacteriocins produced by Lactobacillus amylovorus LMG P-13139. Applied and Environmental Microbiology. 63: 13-20.
Espinosa-Martos, I., and Rupérez, P. 2006. Soybean oligosaccharides. Potential as new ingredients in functional food. Nutricion Hospitalaria. 21: 92-96.
FAO. 2006. Probiotics in food : health and nutritional properties and guidelines for evaluation : Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria, Cordoba, Argentina, 1-4 October 2001 [and] Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London, Ontario, Canada, 30 April -1 May 2002. Rome [Italy]: Food and Agriculture Organization of the United Nations, World Health Organization.
Gerez, C. L., Rollán, G. C., and de Valdez, G. F. 2006. Gluten breakdown by lactobacilli and pediococci strains isolated from sourdough. Letters in Applied Microbiology. 42: 459-464.
Gonelimali, F. D., Lin, J., Miao, W., Xuan, J., Charles, F., Chen, M., et al. 2018. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Frontiers in Microbiology. 9: 1639.
Guarner, F. 2005. Inulin and oligofructose: impact on intestinal diseases and disorders. British Journal of Nutrition, 93 Suppl 1: S61-65.
Halami, P. M., Badarinath, V., Manjulata Devi, S., and Vijayendra, S. V. N. 2011. Partial characterization of heat-stable, antilisterial and cell lytic bacteriocin of Pediococcus pentosaceus CFR SIII isolated from a vegetable source. Annals of Microbiology. 61: 323-330.
Happel, A. U., Jaumdally, S. Z., Pidwell, T., Cornelius, T., Jaspan, H. B., Froissart, R., et al. 2017. Probiotics for vaginal health in South Africa: what is on retailers' shelves? BMC Women's Health. 17: 7.
Joerger, M. C., and Klaenhammer, T. R. 1990. Cloning, expression, and nucleotide sequence of the Lactobacillus helveticus 481 gene encoding the bacteriocin helveticin J. Journal of Bacteriology. 172: 6339-6347.
Klaenhammer, T. R., Barrangou, R., Buck, B. L., Azcarate-Peril, M. A., and Altermann, E. 2005. Genomic features of lactic acid bacteria effecting bioprocessing and health. FEMS Microbiology Reviews. 29: 393-409.
Kwon, H. S., Yang, E. H., Yeon, S. W., Kang, B. H., and Kim, T. Y. 2004. Rapid identification of probiotic Lactobacillus species by multiplex PCR using species-specific primers based on the region extending from 16S rRNA through 23S rRNA. FEMS Microbiology Letters. 239: 267-275.
Lananta, S., Siriratanagool, P., Sommanawan, N., Lerttrakarnnon, P., Boonchuay, S., Jirawattanapong, S., et al. 2021. Different responses of ESBL indicative peptide spectra to various β- lactam exposures among community acquired urinary tract infected Escherichia coli by using the MALDI-TOF Technique. Chiang Mai University Journal of Natural Sciences. 20: e2021095.
Lee, J.-S., Chung, M.-J., and Seo, J.-G. 2013. In vitro evaluation of antimicrobial activity of lactic acid bacteria against Clostridium difficile. Toxicological Research. 29: 99-106.
Liévin-Le Moal, V., and Servin, A. L. 2014. Anti-infective activities of Lactobacillus strains in the human intestinal microbiota: from probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clinical Microbiology Reviews. 27: 167-199.
Likotrafiti, E., Tuohy, K. M., Gibson, G. R., and Rastall, R. A. 2013. Development of antimicrobial synbiotics using potentially-probiotic faecal isolates of Lactobacillus fermentum and Bifidobacterium longum. Anaerobe, 20: 5-13.
Lizier, M., Sarra, P. G., Cauda, R., and Lucchini, F. 2010. Comparison of expression vectors in Lactobacillus reuteri strains. FEMS Microbiology Letters. 308: 8-15.
Mandal, V., Sen, S. K., and Mandal, N. C. 2009. Effect of prebiotics on bacteriocin production and cholesterol lowering activity of Pediococcus acidilactici LAB 5. World Journal of Microbiology and Biotechnology. 25: 1837-1847.
Marques, J. L., Funck, G. D., Dannenberg, G. D. S., Cruxen, C., Halal, S., Dias, A. R. G., et al. 2017. Bacteriocin-like substances of Lactobacillus curvatus P99: characterization and application in biodegradable films for control of Listeria monocytogenes in cheese. Food Microbiology. 63: 159-163.
McAuliffe, O., Ross, R. P., and Hill, C. 2001. Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiology Reviews. 25: 285-308.
Mijač, V. D., Đukić, S. V., Opavski, N. Z., Đukić, M. K., and Ranin, L. T. 2006. Hydrogen peroxide producing lactobacilli in women with vaginal infections. European Journal of Obstetrics & Gynecology and Reproductive Biology. 129: 69-76.
Pyar, H., and Kok, P. 2019. Confirmation of the identity of Lactobacillus species using carbohydrate fermentation test (API 50 CHL) identification system. Journal of Applied Sciences. 19: 797-802.
Roberfroid, M. B. 2005. Introducing inulin-type fructans. British Journal of Nutrition. 93(S1): S13-S25.
Rocca, M. F., Barrios, R., Zintgraff, J., Martínez, C., Irazu, L., Vay, C., et al. 2019. Utility of platforms Viteks MS and Microflex LT for the identification of complex clinical isolates that require molecular methods for their taxonomic classification. PLoS One. 14: e0218077.
Sookkhee, S., Chulasiri, M., and Prachyabrued, W. 2001. Lactic acid bacteria from healthy oral cavity of Thai volunteers: inhibition of oral pathogens. Journal of Applied Microbiology. 90: 172-179.
Sookkhee, S., Khantawa, B., and Srihinkong, W. 2017a. Proteomic analysis of DNA starvation/stationary phase protection proteins from extended spectrum β - lactamase producing Escherichia coli. Chiang Mai UniversityJournal of Natural Sciences. 16: 215–230.
Sookkhee, S., and Sakonwasun, C. 2017b. Lantibiotics : synthesis and antimicrobial activity. Chiang Mai Medical Journal. 56: 231-241.
Soria, M. C., and Audisio, M. C. 2014. Inhibition of Bacillus cereus strains by antimicrobial metabolites from Lactobacillus johnsonii CRL1647 and Enterococcus faecium SM21. Probiotics and Antimicrobial Proteins. 6: 208-216.
Stiverson, J., Williams, T., Chen, J., Adams, S., Hustead, D., Price, P., et al. 2014. Prebiotic oligosaccharides: comparative evaluation using in vitro cultures of infants' fecal microbiomes. Applied and Environmental Microbiology. 80: 7388-7397.
Tachedjian, G., Aldunate, M., Bradshaw, C. S., and Cone, R. A. 2017. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Research in Microbiology. 168: 782-792.
Todorov, S. D., Holzapfel, W., and Nero, L. A. 2017. In vitro evaluation of beneficial properties of bacteriocinogenic Lactobacillus plantarum ST8Sh. Probiotics Antimicrobial Proteins. 9: 194-203.
Wang, G., Tang, H., Zhang, Y., Xiao, X., Xia, Y., and Ai, L. 2020. The intervention effects of Lactobacillus casei LC2W on Escherichia coli O157:H7 -induced mouse colitis. Food Science and Human Wellness. 9: 289-294.
Winkowski, K., Crandall, A. D., and Montville, T. J. 1993. Inhibition of Listeria monocytogenes by Lactobacillus bavaricus MN in beef systems at refrigeration temperatures. Applied and Environmental Microbiology. 59: 2552-2557.
Wlodarska, M., Willing, B. P., Bravo, D. M., and Finlay, B. B. 2015. Phytonutrient diet supplementation promotes beneficial Clostridia species and intestinal mucus secretion resulting in protection against enteric infection. Scientific Reports. 5: 9253.
Woraprayote, W., Pumpuang, L., Tosukhowong, A., Roytrakul, S., Perez, R. H., Zendo, T., et al. 2015. Two putatively novel bacteriocins active against Gram-negative food borne pathogens produced by Weissella hellenica BCC 7293. Food Control, 55: 176-184.
Yadav, S., Gite, S., Lanjekar, V., Nilegaonkar, S., and Agte, V. 2014. In vitro screening of indigenous plant materials for prebiotic potential. International Journal of Current Microbiology and Applied Sciences, 3:137-150.
Yamamoto, Y., Togawa, Y., Shimosaka, M., and Okazaki, M. 2003. Purification and characterization of a novel bacteriocin produced by Enterococcus faecalis strain RJ-11. Applied and Environmental Microbiology. 69: 5746-5753.
Yang, S.-C., Lin, C.-H., Sung, C. T., and Fang, J.-Y. 2014. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Frontiers in Microbiology. 5: 241.
Yi, L., Dang, Y., Wu, J., Zhang, L., Liu, X., Liu, B., et al. 2016. Purification and characterization of a novel bacteriocin produced by Lactobacillus crustorum MN047 isolated from koumiss from Xinjiang, China. Journal of Dairy Science. 99: 7002-7015.
Zhang, J., Yang, Y., Yang, H., Bu, Y., Yi, H., Zhang, L., et al. 2018. Purification and partial characterization of bacteriocin Lac-B23, a novel bacteriocin production by Lactobacillus plantarum J23, isolated from Chinese traditional fermented milk. Frontiers in Microbiology. 9: 2165.
Zheng, J., Wittouck, S., Salvetti, E., Franz, C., Harris, H. M. B., Mattarelli, P., et al. 2020. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. International Journal of Systematic and Evolutionary Microbiology. 70: 2782-2858.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand.
https://cmuj.cmu.ac.th
Siriwoot Sookkhee1,*, Yothin Kumsang1, and Sathian Boongum2
1 Department of Microbiology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
2 Department of Biotechnology, Faculty of Agro-industry, Chiang Mai University, Chiang Mai, Thailand.
Corresponding author: Siriwoot Sookkhee, E-mail: siriwoot.s@cmu.ac.th
Total Article Views
Editor: Sirasit Srinuanpan,
Chiang Mai University, Thailand
Article history:
Received: March 1, 2022;
Revised: June 29, 2022;
Accepted: July 1, 2022;
Published online: July 7, 2022

