
Seasonal Occurrence of Caddisfly Larval Communities on a Coarse Substrate in the Upper Nan River, Northern Thailand
Pornpimon Buntha and Decha Thapanya*Published Date : 2022-07-11
DOI : https://doi.org/10.12982/CMUJNS.2022.047
Journal Issues : Number 3, July-September 2022
Abstract Caddisfly larvae are commonly found in the Nan River. Larvae were collected monthly from a cobble habitat for a period of two years to study the influence of seasonal changes on larval communities. A Surber sampler was placed in the cobble habitat in the riffle zone at four study sites along the upper Nan River. Field-collected larvae were identified at the family level in the laboratory. Physicochemical factors were measured in terms of water temperature, pH, turbidity, dissolved oxygen (DO) and biochemical oxygen demand (BOD5), as well as for concentrations of ammonia nitrogen (NH3-N), nitrate nitrogen (NO3-N) and orthophosphate (PO43-). Fifteen families of the caddisflies were found. The cool season was associated with the highest degree of density followed by the summer and rainy seasons. Hydropsychidae was the most common caddisfly present throughout the year. Hydroptilidae was common in the summer and cool seasons. Values of water temperature, pH, turbidity, DO, BOD5 and NO3-N among the three seasons were observed to be significantly different. According to the CCA analysis, water temperature, turbidity, BOD5, NO3-N and NH3-N factors had a positive relation to the density of Ecnomidae, Goeridae, Odontoceridae and Polycentropodidae. Remarkably, caddisfies in the families Hydropsychidae, Rhyacophilidae and Stenopsychidae were intolerant to variations in DO and pH values. The results of our study indicate that the physicochemical factors varied in conjunction with the different seasons, while communities and the degree of density of some caddisfly larvae were associated with variations of the physicochemical factors.
Keywords: Trichoptera, Cobble, Environmental factors, Season
Funding: Financial support by a Science Achievement Scholarship of Thailand (SAST) the Biology Department of Chiang Mai University is acknowledged.
Citation: Buntha, P. and Decha Thapanya, D. 2022. Seasonal occurrence of caddisfly larval communities on a coarse substrate in the upper Nan river, Northern Thailand. CMU J. Nat. Sci. 21(3): e2022047.
INTRODUCTION
Creeks, streams and rivers are important running freshwater ecosystems. They provide habitats for both aquatic and terrestrial organisms. Aquatic insects are recognized as the main primary consumers in freshwater ecosystems and play a role as herbivores, carnivores, omnivores and detritivores. They are also a food source for aquatic and terrestrial organisms. For example, caddisflies are preferred by Atlantic salmon, freshwater drums, sturgeons and dippers at both the nestling and adult stages (Ormerod et al., 1987; Ormerod and Tyler, 1991; Nilo et al., 2006; Shubina, 2006; Jacquemin et al., 2014). Importantly, case-building caddisfly can provide refuge for nymphs of mayflies (Nakano et al., 2005).
Caddisflies are classified in the order Trichoptera. To date, fifty-one families of caddisflies have been described (Morse et al., 2019). The egg, larva and pupa stages live in water, whereas the adult stage is known to emerge onto land for the purposes of mating. Caddisflies have three pairs of legs, while the larval body is divided into three parts: the head, thorax and abdomen (McCafferty, 1981; Dudgeon, 1999). They are known to be widely distributed throughout Asia, Africa, Australia, Europe, North America and South America (Zhou et al., 2016). Caddisfly larvae are known to thrive in micro-habitats in both pools and riffles. They are usually found in the deposition area of detritus matters (e.g., fallen leaves and dead wood) and on benthic particles, i.e. sand, pebbles, cobbles, gravel, boulders and rocks. In the Ščavnica stream, the abundance and diversity of the caddisfly larvae on coarse substrates especially those comprised of cobbles were higher than on fine substrates (Urbanič et al., 2005). In the upper Nan River, cobble environments are commonly found in riffle zones. To date, in-depth research on communities of caddisfly larvae that thrive on cobble habitats in the Nan River has not yet been conducted.
The seasons in northern Thailand are recognized as the cool season, the summer and the rainy season. January, February, November and December are the months that make up the cool period, while the months of March, April and May are indicative of summer. Rainy months include June, July, August, September and October (Khedari et al., 2002; Climatological Group, 2015). The characteristics of running water usually vary by changes in the seasons. For example, turbidity and concentrations of nitrogen and phosphorus in the Bakun Reservoir and its downstream river in Sarawak, Malaysia (Tropical zone) during the wet season significantly differ from those of the dry season (Ling et al., 2017). Air temperature and relative humidity are particularly relevant to the life span of adult caddisflies inhabiting Champathong Waterfall, northern Thailand (Prommi et al., 2012). Importantly, seasonal changes are known to affect caddisfly communities. In New Zealand, caddisfly richness on soft to hard-bottomed substrates can vary by season (Stark and Phillips, 2004). An increase in water temperature during the spring and summer has benefited the occurrence of Hydropsyche slossonae and Dolophilodes distinctus larvae in the first-order stream, northern Lower Michigan (Houghton and Shoup, 2014). The diversity and abundance of mayflies, stoneflies and caddisflies were highest during the wet season in the Batu, Teroi and Tupah Rivers, Malaysia (Suhaila et al., 2014). Seasonal occurrence of caddisfly larvae is important for the predators of this species. The amount of food in benthic fish (Salvelinus alpinus) stomachs has been found to be related to seasonal variations associated with the amount of prey available at a given location. This is particularly true with regard to chironomid and caddisfly larvae. Remarkably, the feeding behavior of fish has been enhanced when the population of preying species increases (Kreiling et al., 2021).
In this study, the occurrence of caddisfly larval communities on cobble habitats in the upper Nan River throughout the year was studied in order to identify the relevant seasonal variations in their communities. The density and the number of families were compared during each season. Correlations between larval density and physicochemical factors were also analyzed in order to study the effects of seasonal characteristics related to water in larval communities.
MATERIALS AND METHODS
Study sites
The Nan River is one of four main rivers in northern Thailand. The river originates from the Phi Pan Nam Mountain range. The length of the river is 650 kilometers. The river flows throughout Nan, Uttaradit, Phitsanulok and Phichit Provinces and joins other rivers to become the Chao Phraya River in central Thailand (Jayawardena et al., 1997). Waterfronts of the Nan River in Nan Province are routinely used for the purposes of agricultural and economic development, such as for the growing of paddy, maize and other cash crops. The river also supports the fishing and local tourism industries (Royal Irrigation Department, 2014; Trisurat et al., 2019).
Four study sites were situated along the length of the upper Nan River, Nan Province (Figure 1). Study sites NR1 and NR3 were located in conservation areas that are managed by local community regulations. Macroalgae are usually found along the river from the cool season to the summer. Leaf packs and plant remains from forest and riparian areas were commonly found in the cobble habitats of the river. Agricultural activities were found on the riverbank at three of the study sites, NR2, NR3 and NR4 (Table 1).
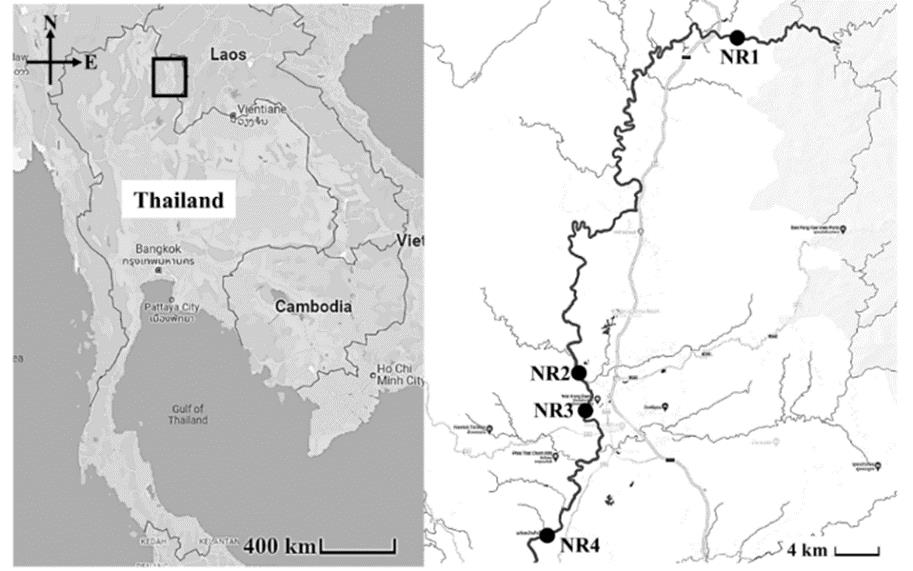
Figure 1. Location of four sampling sites in the upper Nan River, northern Thailand (NR1, NR2, NR3 and NR4).
Table 1. Descriptions of four study sites located in the upper Nan River.
|
Study site |
Location |
Altitude (meter) |
Waterfront areas |
Sedimentations and vegetation in the cobble habitat in the 3 seasons |
|||
|
Latitude(˚N) |
Longitude(˚E) |
Cool |
Summer |
Rainy |
|||
|
NR1 |
19°31'00.2” |
100°55'57.0” |
371 |
Forest |
Macroalgae, detritus |
Macroalgae, detritus |
detritus |
|
NR2 |
19°19'00.4” |
100°50'43.2” |
259 |
Agriculture |
|||
|
NR3 |
19°18'16.4” |
100°50'55.1” |
256 |
Agriculture |
|||
|
NR4 |
19°13'31.7” |
100°49'30.0” |
252 |
Forest, agriculture |
|||
Collecting samples
Cobbles in riffle zones are one of habitats for caddisfly larvae in the running water sources (Urbanič et al., 2005). In this study, cobble habitats located in riffle zones with 10 – 50 cm of water depth were selected for the collection of samples at all sampling sites throughout the three seasons. Caddisfly larvae were collected with the use of a 25 x 25 cm2 Surber sample. Twenty sampling times for each study site in the 4th week of each month were employed. Collecting the larvae began in January of 2017 and was terminated in December of 2018 (24 months). Eighty percent ethyl alcohol solution was used to preserve these caddisfly larvae for laboratory identification.
Physicochemical factors
Three replicates of eight water parameters were measured monthly in the Nan River at each study site. The physicochemical factors included water temperature, pH, turbidity, dissolved oxygen (DO), biochemical oxygen demand (BOD5), ammonia nitrogen (NH3-N), nitrate nitrogen (NO3-N) and orthophosphate (PO43-). A thermometer was directly dipped in the water at each field site in order to measure water temperature (°C). The multi-parameter analyzer CONSORT Model C533 with a pH probe was employed to determine pH values. The azide modification method was used to determine DO and BOD5 (mg/L) values. The absorptometric, nesslerization, cadmium reduction and ascorbic acid methods in conjunction with a HACH model DR/2000 spectrophotometer were used to measure water turbidity (FTU) and concentrations of NH3 –N, NO3-N and PO43- (mg/L).
Data analysis
Caddisfly larval specimens were identified at the family level under a stereomicroscope in the AIRU laboratory, Biology Department of Chiang Mai University. The textbook entitled “Tropical Asian Streams” by Dudgeon (1999) and a published research paper entitled “Descriptions of the pupae and larvae of Stenopsyche siamensis Martynov, 1931 (Trichoptera: Stenopsychidae) with notes on larval biology” by Ismail et al. (1996) were referenced in this study.
A normality test employing the Shapiro-Wilk method was used to test the distribution patterns in the density data (individuals/m2) of caddisfly larvae at the family level and the data related to the physicochemical factors of each season. The distribution pattern of the caddisfly density data was identified as non-normal. The data for the relevant physicochemical factors, with the exception of pH and NO3-N, were indicative of non-normal distribution. A one-way ANOVA (for normal distribution data) and the Kruskal-Wallis (for non-normal distribution data) were employed to identify any statistical differentiations in the physicochemical factors and the density data of caddisfly larvae throughout the three seasons. Canonical correspondence analysis (CCA) is a multivariate statistic method that is used to explain the relationships between biological assemblages and environmental factors (Ter Braak and Verdonschot, 1995). CCA was used to analyze the relationship between caddisfly communities at the family level and the relevant physicochemical factors. Total statistical analyses were conducted using the Past program, version 4.03.
RESULTS
Caddisfly larval communities and their degrees of density in the upper Nan River
The total number of families and the density of caddisfly larvae tended to increase during the early cool season (November) and exhibited the highest degree of fluctuation in the middle of the cool season (December and January) followed by the summer and rainy season. Both the number of families and the degree of larval density began to decrease in the late cool season. This decreasing trend continued through to September in the rainy season, while the number of families and the larval density began to increase in the late rainy season (October). A rapid increase in caddisfly larval individuals and the number of families occurred from November to January (Figure 2).
Fifteen families of caddisfly larvae were identified. The highest number of families (15 families) were found in the cool season, followed by the summer (12 families) and the rainy season (8 families). Ecnomidae, Hydropsychidae, Leptoceridae, Odontoceridae, Philopotamidae, Polycentropodidae and Psychomyiidae larvae were found throughout the year. Six families, namely Goeridae, Leptoceridae, Odontoceridae, Philopotamidae, Polycentropodidae and Psychomyiidae, were collected at all study sites during the cool season and the summer. Hydropsychidae and Hydroptilidae larvae made up the dominant group during the cool season through to the summer, while Hydropsychidae larvae made up the dominant group throughout the three seasons (Table 2).
In the cool season, the degrees of density of Glossosomatidae, Hydropsychidae, Hydroptilidae, Leptoceridae, Philopotamidae and Psychomyiidae larvae were significantly higher than in the summer and rainy season (P < 0.05). In the summer, the density of Ecnomidae was higher than in the cool and rainy seasons. Conversely, the density of these families was lowest during the rainy season (Table 2).
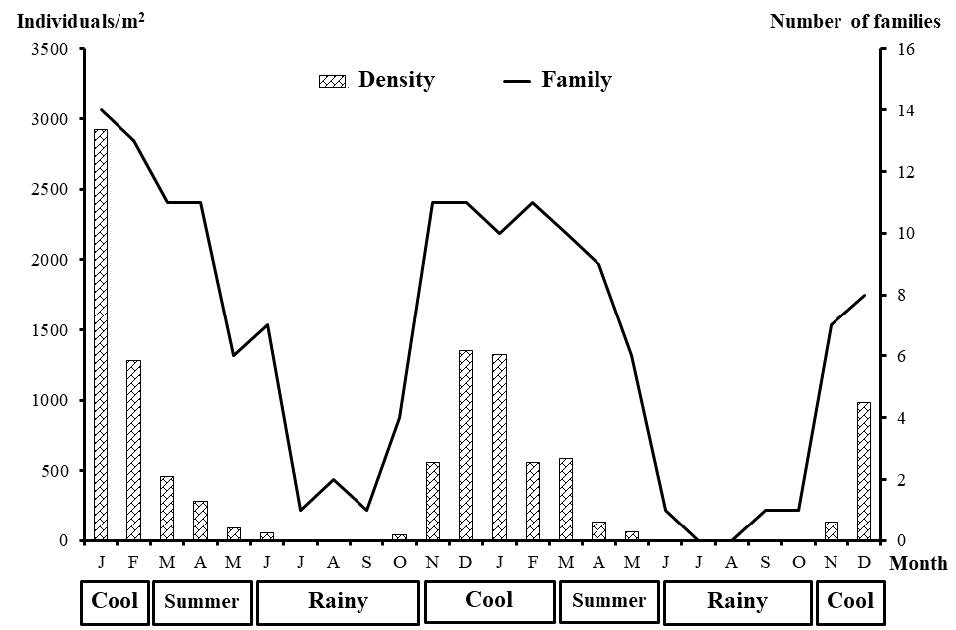
Figure 2. Total number of families and degree of density (individuals/m2) of caddisfly larvae in each month from January, 2017 to December, 2018.
Table 2. Number of families and degree of density (individuals/m2) of caddisfly larvae at four study sites in the cool, summer and rainy seasons are shown in the table below. The Kruskal-Wallis test highlights differences in the degrees of density throughout the three seasons. Different letters (a, b, and c) indicate significant differences at P < 0.05.
|
Family |
Cool |
Summer |
Rainy |
Total study sites |
||||||||||||
|
NR1 |
NR2 |
NR3 |
NR4 |
NR1 |
NR2 |
NR3 |
NR4 |
NR1 |
NR2 |
NR3 |
NR4 |
Cool |
Summer |
Rainy |
||
|
BCC |
0 |
0* |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0a |
0a |
0a |
|
|
CLM |
0* |
0* |
0 |
0* |
0* |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0a |
0ab |
0b |
|
|
ECN |
1 ± 2 |
0 ± 1 |
0 ± 1 |
0 |
17 ± 20 |
1 ± 2 |
1 ± 3 |
2 ± 3 |
0 |
0* |
0* |
0 |
0 ± 1b |
5 ± 11a |
0b |
|
|
GLS |
3 ± 4 |
1 ± 2 |
0* |
0 ± 1 |
1 ± 3 |
0 ± 1 |
0 |
0 |
0 |
0 |
0 |
0 |
1 ± 2a |
0 ± 1b |
0c |
|
|
GRD |
1 ± 2 |
0 ± 1 |
1 ± 4 |
1 ± 1 |
1 ± 2 |
1 ± 1 |
1±3 |
0* |
0 |
0 |
0 |
0 |
1 ± 2a |
1 ± 1a |
0b |
|
|
HDP |
873 ± 1034 |
367 ± 283 |
598 ± 569 |
774 ± 770 |
326 ± 183 |
65 ± 81 |
84 ± 154 |
51 ± 57 |
10 ± 23 |
12 ± 27 |
10 ± 30 |
8 ± 20 |
653 ± 606a |
131 ± 153b |
10 ± 20c |
|
|
HDT |
312 ± 442 |
631 ± 658 |
271 ± 340 |
305 ± 357 |
10 ± 10 |
173 ± 227 |
68 ± 154 |
116 ± 121 |
0 |
0 |
0 |
0 |
380 ± 401a |
92 ± 134b |
0c |
|
|
LTC |
22 ± 19 |
27 ± 22 |
9 ± 7 |
28 ± 32 |
11 ± 11 |
19 ± 22 |
3 ± 4 |
10 ± 10 |
0 ± 1 |
0 |
0 |
0 |
22 ± 19a |
11 ± 12b |
0c |
|
|
LPD |
0 |
1 ± 2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 ± 1a |
0a |
0a |
|
|
ODT |
9 ± 8 |
9 ± 7 |
4±5 |
2 ± 2 |
8 ± 8 |
5 ± 5 |
13 ± 23 |
6 ± 5 |
0 |
0* |
0* |
0 |
6 ± 6a |
8 ± 11a |
0b |
|
|
PLP |
79 ± 70 |
31 ± 59 |
61±77 |
3 ± 3 |
11 ± 23 |
15 ± 32 |
19 ± 42 |
0 ± 1 |
0* |
0 |
0 |
0 |
43 ± 56a |
11 ± 24b |
0c |
|
|
PLC |
10 ± 6 |
9 ± 8 |
4 ± 8 |
2 ± 3 |
2 ± 3 |
4 ± 8 |
3 ± 3 |
2 ± 3 |
0 ± 1 |
1 ± 2 |
0 |
0* |
6 ± 6a |
3 ± 4a |
0 ± 1b |
|
|
PCM |
21 ± 30 |
9 ± 8 |
23 ± 42 |
21 ± 23 |
5 ± 7 |
7 ± 8 |
2 ± 5 |
4 ± 7 |
0 |
0 ± 1 |
0* |
0 |
18 ± 24a |
5 ± 6b |
0 ± 1c |
|
|
RYC |
0* |
0 |
0* |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0a |
0a |
0a |
|
|
STN |
29 ± 21 |
0 |
0 |
0* |
7±11 |
0 |
0 |
0 |
0 |
0* |
0 |
0 |
7 ± 15a |
2 ± 5ab |
0b |
|
|
Number of Families |
13 |
13 |
11 |
11 |
12 |
10 |
9 |
9 |
4 |
6 |
4 |
2 |
15a |
12a |
8b |
|
Note: BCC = Brachycentridae; CLM = Calamoceratidae; ECN = Ecnomidae; GLS = Glossosomatidae; GRD = Goeridae; HDP = Hydropsychidae; HDT = Hydroptilidae; LTC = Leptoceridae; LPD = Lepidostomatidae; ODT = Odontoceridae; PLP = Philopotamidae; PLC = Polycentropodidae; PCM = Psychomyiidae; RYC = Rhyacophilidae; STN = Stenopsychidae. An asterisk (*) indicates that the density was less than 1 individual/m2.
Physicochemical factors and their relationship to communities of caddisfly larvae at the family level
Water temperature values were the lowest in the cool season (18.3 – 29.0°C) and highest in the summer (25.0 – 32.0°C). Turbidity values were highest in the rainy season (13 – 1553 FTU), followed by the summer (4 – 821 FTU) and the cool season (0 – 29 FTU). Dissolved oxygen (DO) and pH values in the cool season were significantly higher than in the other seasons (P< 0.05). The biochemical oxygen demand (BOD5) values in the summer and the rainy season were significantly higher than in the cool season, whereas the BOD5 values in the rainy season and the summer were not different. Concentrations of NH3-N and PO43- among the three seasons were not found to be different, while NO3-N values in the rainy season were higher than in the summer (Table 3).
The relationship between communities of caddisfly larvae at the family level and the physicochemical factors was analyzed by employing canonical correspondence analysis or CCA (Figure 3). The CCA axis 1 and axis 2 indicated 48.8% and 35.1% of the variances within the family, which were in line with the physicochemical factors. Axis 1 was associated with water temperature, turbidity, BOD5, NO3-N and NH3-N. Families with high positive scores on axis 1 included Ecnomidae, Goeridae, Odontoceridae and Polycentropodidae. Families with high negative scores on axis 1 included Brachycentridae, Glossosomatidae, Hydroptilidae, Lepidostomatidae, Philopotamidae, Rhyacophilidae and Stenopsychidae.
Axis 2 was associated with DO and the pH of the water. Families with high positive scores on axis 2 included Brachycentridae, Glossosomatidae, Goeridae, Hydroptilidae, Leptoceridae, Lepidostomatidae, Odontoceridae and Polycentropodidae, while Hydropsychidae, Rhyacophilidae and Stenopsychidae were associated with high negative scores on axis 2 (Figure 3).
Table 3. Differences in physicochemical factors among the cool, summer and rainy season from 2017 to 2018. Different letters (a, b, and c) indicate significant differences at P < 0.05.
|
Physicochemical factors |
Cool Season |
Summer |
Rainy Season |
|
Water temperature (°C) |
22.8 ± 2.5c |
27.8 ± 1.7a |
25.6 ± 1.5b |
|
pH |
8..19 ± 0.38a |
7.95 ± 0.35b |
7.63 ± 0.27c |
|
Turbidity (FTU) |
8 ± 6c |
100 ± 188b |
155 ± 254a |
|
DO (mg/L) |
9.0 ± 1.0a |
8.2 ± 1.1b |
8.2 ± 0.6b |
|
BOD5 (mg/L) |
0.58 ± 0.54b |
1.06 ± 0.92a |
1.41 ± 1.48a |
|
NH3 –N (mg/L) |
0.14 ± 0.10a |
0.56 ± 0.80a |
0.26 ± 0.21a |
|
NO3-N (mg/L) |
1.6 ± 0.3ab |
1.5 ± 0.3b |
1.7 ± 0.4a |
|
PO43- (mg/L) |
0.22 ± 0.14a |
0.20 ± 0.07a |
0.19 ± 0.07a |
Note: DO = Dissolved Oxygen; BOD5 = Biochemical Oxygen Demand; NH3-N = Ammonia nitrogen; NO3-N = Nitrate nitrogen; PO43- = Orthophosphate
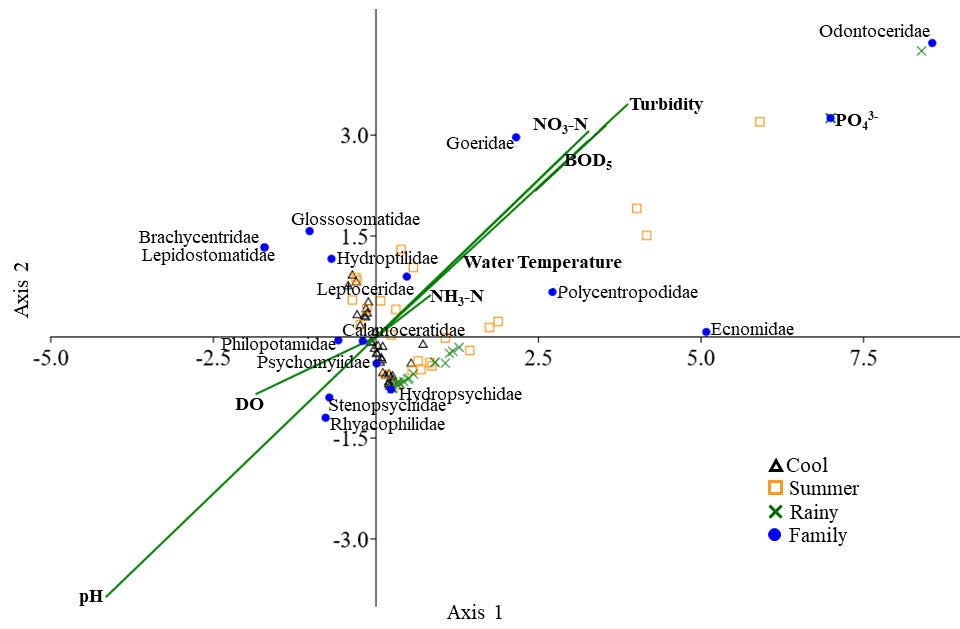
Figure 3. The Canonical correspondence analysis (CCA) diagram shows the relation between communities of caddisfly larvae at the family level and the relevant physicochemical factors in the Nan River. Eigenvalue axis 1 = 0.07 and eigenvalue axis 2 = 0.05. The caddisfly groups are represented by solid circles, while triangles represent the cool season; squares represent the summer; cross marks represent the rainy season.
DISCUSSION
Caddisfly larval communities and their degrees of density in the upper Nan River
Caddisfly larvae in this study were found to be present in the highest degrees of density in the cool season and the summer. The numbers of families in the cool season and the summer were higher than in the rainy season. Degrees of density of Ecnomidae, Glossosomatidae, Hydropsychidae, Hydroptilidae, Leptoceridae, Philopotamidae and Psychomyiidae larvae were different among the three seasons, for which the highest degree of density of these larvae, with the exception of the family Ecnomidae, were observed during the cool season. Notably, Ecnomidae revealed the highest degree of density in the summer. The results indicate that changes in the seasons affected the number of families and degrees of density of these larvae. The community structures of the caddisfly also displayed a tendency to exhibit differences among the three seasons according to CCA analysis (Figure 3).
The total number of individuals and species of adult caddisflies from the Champathong Waterfall located in northern Thailand were highest in the early summer of March, 2009 (Prommi et al., 2012). The greatest number of adult caddisflies from the upper Nan River was also observed in March of 2017 (Buddhanurak, 2021). The development stages from larva to pupa and the emergence of adults in the upper Nan River occurred from the late cool season until the summer. Adult caddisflies came back to the Nan River for the purpose of spawning during the late rainy season. Noticeably, the larval stage repeatedly recovered from the late rainy season until the early cool season.
Net-spinning caddisflies in the family Hydropsychidae were the dominant and common groups in the cobble habitats of the upper Nan River throughout the year during all of the seasons. Degrees of density of Hydropsychidae and Hydroptilidae in the cobble habitats were highest in the cool season through to the summer. Other families, namely Ecnomidae, Goeridae, Leptoceridae, Odontoceridae, Philopotamidae, Polycentropodidae and Psychomyiidae, were also present in the cobble habitats of all study sites during both seasons. Urbanič et al. (2005) reported that the highest degree of abundance of the species of caddisfly larvae in the Ščavnica stream occurred in cobble habitats. This would indicate that the cobble substrate was a suitable habitat for the larvae. However, degrees of density of Brachycentridae, Calamoceratidae, Lepidostomatidae and Rhyacophilidae in the cobble habitats of the upper Nan River were very low at all study sites throughout the year. Gunn (1985) explained that Brachycentrus subnubilus (Brachycentridae) larvae lived on gravel with macrophytes in the River Frome, Dorset. Prommi and Payakka (2015) found that greater numbers of Calamoceratidae and Lepidostomatidae larvae lived in man-made concrete, gravel and woody debris substrates than in the cobble habitats of the Mae Tao and Mae Ku Watershed, northern Thailand. Rhyacophilidae larvae were closely associated with rock substrates in southern Ontario streams (Martin, 1985). Therefore, the cobble habitat of the Nan River may be not a suitable microhabitat for these larvae.
Physicochemical factors in the Nan River
Variations in water temperature were introduced by changes in air temperature (Allan and Castillo, 2007; Yang and Peterson, 2017). The lowest range of water temperature in the Nan River was recorded in the cool season, whereas the highest range was recorded in the summer. The results indicate that the change of season can influence variations in water temperature. The rapid increase in water turbidity occurred in the late summer to the rainy season, in which the water color in the Nan River changed from clear to brick-orange. Because heavy rain cannot rapidly infiltrate the soil, surface runoff typically brings soil matter into water channels. Turbidity values were then increased by the dissolution of these sediments into the water (Lloyd et al., 1987). In the water sampling process from January, 2017 to December, 2018, rainfall was found to primary occur in the late summer through the rainy season. In the rainy season, rainfall did not always occur every day. Consequently, a fluctuation of turbidity was observed in both seasons.
The optimum range of pH for living aquatic animals was 6.5 – 9.0 (Boyd, 2015). Although pH values in the Nan River among the three seasons were different, the pH values in all of the seasons were optimum for the aquatic animals inhabiting the river. DO concentrations were highest in the cool season, while water temperatures were lowest in the cool season. Decreases in water temperature can support oxygen solubility in water (Allan and Castillo, 2007). The amount of oxygen for microorganisms was measured in terms of the biochemical oxygen demand (BOD5). Rainfall and runoff can introduce the microorganisms that are present on soil particles and in litter into water sources (Boyd, 2015). Thus, the BOD5 values increased in the late summer through to the rainy season.
Nutrients from the soil can be brought into natural water sources by rain (Sharma et al., 2013). In the rainy season, concentrations of NO3-N in the upper Nan River were slightly higher than in the other seasons. However, concentrations of NH3 –N and PO43- were not the highest during the rainy season. This was probably due to the increase in water content in the river that occurred during the rainy season, which would have diluted the concentrations of these nutrients.
Relationship between communities of caddisfly larvae at the family level and relevant physicochemical factors
According to the CCA analysis on axis 1, caddisfly larvae included Ecnomidae, Goeridae, Odontoceridae and Polycentropodidae, all of which had high positive scores for water temperature, turbidity, BOD5, NO3-N and NH3-N. This indicated that an increase in the degree of density in these families was associated with an increase in these environmental factors. Brachycentridae, Glossosomatidae and Lepidostomatidae larvae were associated with high negative scores in relation to these environmental factors. Consequently, decreases in water temperature, turbidity, BOD5, NO3-N and NH3-N were associated with a decrease in the degree of density for the three families.
An increase in water temperature within the range of 9 – 12°C was considered an optimum condition for Glossosomatidae larvae in the forested streams of Hokkaido, northern Japan (Kishi et al., 2005). However, water temperature could have a negative correlation with caddisfly larvae in both tropical (northern, Thailand) and temperate zones, as has been observed in Japan, the Netherlands and Turkey (Kishi et al., 2005; Van den Brink et al., 2013; Prommi and Thamsenanupap, 2015; Küçükbasmaci and Findik, 2020; Thamsenanupap et al., 2021). An increase in the range of water temperature could stimulate oxygen consumption and the metabolic rates for Hydropsyche larvae (Hydropsychidae), which would then affect the larval biomass (Bales and Badcock, 1986; Roux et al., 1992). This would indicate that different families of caddisfly larvae exhibited different responses to variations in water temperature.
In the Nan River, caddisfly larvae within the families Ecnomidae, Goeridae, Odontoceridae and Polycentropodidae were tolerant to variations in turbidity. In a previous study, other caddisfly larvae, within the family Hydropsychidae were also found to be tolerant to variations in turbidity. Hydropsychidae are net-spinning caddisflies and they tend to clear sediment from the nets of their retreats. Cutting and removing net behaviors were observed when a significant amount of sediment settled upon the nets (Runde and Hellenthal, 2000). However, high turbidity levels could disturb some caddisfly larvae and other invertebrate communities (Wantzen, 2006). Episodic sediments in the water reduced the number of Ceratopsyche and Hydropsyche larvae but did not reduce their growth rates (Strand and Merritt, 1997). Hydroptilidae and Leptoceridae larvae in Spanish Mediterranean streams escaped from the conditions associated with high suspended solids (Bonada et al., 2004). Living in turbid water for a long period of time is likely dangerous for the bodies of these larvae. This would indicate that some caddisfly larvae were more tolerant to turbid water, whereas some caddisfly larvae tend to move out of high turbidity areas.
The CCA diagram on axis 2 indicates that Brachycentridae, Glossosomatidae, Goeridae, Hydroptilidae, Leptoceridae, Lepidostomatidae, Odontoceridae and Polycentropodidae were tolerant to variations in DO and pH in the Nan River, while Hydropsychidae, Rhyacophilidae and Stenopsychidae were intolerant to variations of DO and pH values. The dissolved oxygen concentration is important for respiration of the caddisfly larvae. Under low dissolved oxygen conditions, faster respiration movement behavior was observed in Limnophilus larvae (Limnephilidae), which then allowed them to increase oxygen consumption (Fox and Sidney, 1952). The duration of the egg hatching period and the development of larva to the adult stage of Limnephilidae was slower when they lived in habitats that were associated with less than 4.6 mg/L dissolved oxygen. Conversely, higher than 8.0 mg/L of DO concentrations will promote the percent survival and the successful emergence of Limnephilidae and Hydropsychidae larvae (Nebeker, 1972; Nebeker et al., 1996). The upper Nan River is a permanent running water source that can support the solubility of oxygen in the water. The water depth at all sampling sites in the Nan River was not determined to be too deep (10 – 50 cm). Thus, the oxygen level in the Nan River during both daytime and nighttime was sufficient for caddisfly larvae. Concentrations of DO in the Nan River throughout the year were within the range of 6.8 – 11.5 mg/L. This was determined to be a suitable range for the respiration of the caddisfly larvae inhabiting the river (Boyd, 2015).
Aquatic insects are typically disturbed when they live in water with low pH values. Consequently, a pH value lower than 5.9 can reduce the survival rate and the successful emergence of the caddisfly larvae genera Hydropsyche and Brachycentrus (Branchycentridae) (Bell, 1971). The optimum range of pH for living aquatic animals was 6.5 – 9.0 (Boyd, 2015). In the Nan River, pH values throughout the two years of 2017 and 2018 were within the range of 7.00 – 8.81, which was determined to be the optimum range for aquatic life in the river. Although the DO concentrations and pH values in the river were at sufficient levels for the survival of the larvae, caddisflies in three families, namely Hydropsychidae, Rhyacophilidae and Stenopsychidae, were intolerant to variations in DO and pH values. This would indicate that these larvae exhibited a high degree of sensitivity to increases and decreases in the DO and pH values.
In the Nan River, macroalgae in the genera Cladophora, Microspora, Nostochopsis Rhizoclonium and Spirogyra thrived from the cool season to the summer (Peerapornpisal et al., 2005; Thiamdao, 2011; Kunpradid and Tagun, 2016). The macroalgae are beneficial to some caddisfly larvae. Micro-caddisflies and net-spinning caddisfly larval assemblages can live in cobble habitats with Cladophora algae (Dudley et al., 1986; Hart, 1992; Holomuzki and Van Loan, 2002). Hydropsyche and Polamyia (Hydropsychidae) larvae were determined to be omnivores as they were observed to consume fresh filaments of Cladophora, diatoms, woody debris and insect remains (Williams and Hynes, 1973; Prommi, 2016; Buntha et al., 2020). Hydroptila consimilis (Hydroptilidae) larvae were determined to be herbivores as they consume cell fluids of fresh Cladophora algae and diatoms (Minckley,1963; Resh and Houp, 1986; Feminella and Resh, 1991; Keiper and Foote, 1999). Diatoms on the macroalgal filaments were also a food source for Philopotamidae larvae. (Williams and Hynes, 1973). This would indicate that the environmental factors associated with both seasons provided food sources for the caddisfly larvae. Therefore, many caddisfly larvae were found in the river during the period from the cool season to the summer.
CONCLUSION
Fifteen families of caddisfly larvae were found in cobble habitats in the riffle zones of the upper Nan River throughout the period from 2017 to 2018. Communities of the larvae in seven families, namely Ecnomidae, Hydropsychidae, Leptoceridae, Odontoceridae, Philopotamidae, Polycentropodidae and Psychomyiidae were found throughout the three seasons. Hydropsychidae, Hydroptilidae, Leptoceridae, Philopotamidae and Psychomyiidae larvae were a common group that inhabited cobble habitats. Variations in the physicochemical factors occurred as a result of seasonal changes. The physicochemical variations had both negative and positive effects on caddisfly larval communities. Ecnomidae, Goeridae, Odontoceridae and Polycentropodidae exhibited a positive relationship with water temperature, turbidity, BOD5, NO3-N and NH3-N. Hydropsychidae, Rhyacophilidae and Stenopsychidae larvae were determined to be intolerant to decreases in DO and pH values of the water. Interactions of the physicochemical and biological factors in the cobble habitats during the cool season to the summer created a suitable set of conditions for the livelihood of some caddisfly larvae, particularly Hydropsychidae and Hydroptilidae.
ACKNOWLEDGEMENTS
Financial support was obtained through a Science Achievement Scholarship of Thailand (SAST) and the Biology Department of Chiang Mai University. This funding is gratefully acknowledged. The authors express their appreciation to Mr. Russell Kirk Hollis for his kind assistance in the English correction of this manuscript.
AUTHOR CONTRIBUTIONS
Pornpimon Buntha designed and conducted the sample collecting, data collecting, statistical analysis, wrote and improved the manuscript. Decha Thapanya assisted in the research design, statistical analysis guideline, reading and improvement of the manuscript.
REFERENCES
Allan, J.D., and Castillo, M.M. 2007. Stream ecology: Structure and function of running waters (2nd ed.). Springer, Netherlands.
Bales, M.T., and Badcock, R.M. 1986-Respiration rates and distribution of caddis larvae in relation to acclimation to temperature. p.179–183. In: Bournaud, M., and Tachet, H. (eds) Proceedings of the Fifth International Symposium on Trichoptera. DR W. Junk Publishers, 21 - 26 July 1986. Lyon, France.
Bell, H.L. 1971. Effect of low pH on the survival and emergence of aquatic insects. Water Research. 5: 313–319.
Bonada, N., Zamora-Muñoz, C., Rierdevall, M., and Prat, N. 2004. Ecological profiles of caddisfly larvae in Mediterranean streams: Implications for bioassessment methods. Environmental Pollution. 132: 509–521.
Boyd, C.E. 2015. Water quality: An introduction (2nd ed.). Springer, Switzerland.
Buddhanurak, T. 2021. Diversity, distribution and occurrence of adult caddisflies along the Nan River in Nan Province and their relationship to the presence of macroalgae. Ph.D. Dissertation. Chiang Mai University, Chiang Mai, Thailand.
Buntha, P., Thapanya, D., and Traichaiyaporn, S. 2020. Food source for hydropsychid larvae during an algae bloom in Nan River, Nan Province, Thailand (Trichoptera: Hydropsychidae). Zoosymposia. 18: 9–16.
Climatological Group. 2015. The climate of Thailand. Meteorological Development Bureau, Meteorological Department, Thailand. Available from: https://www.tmd.go.th/en/archive/thailand_climate.pdf
Dudgeon, D. 1999. Tropical Asian streams: Zoobenthos, ecology and conservation. Hong Kong University Press, London.
Dudley, T.L., Cooper, S.D., and Hemphill, N. 1986. Effects of macroalgae on a stream invertebrate community. The North American Benthological Society. 5: 93–106.
Feminella, J.W., and Resh, V.H. 1991. Herbivorous caddisflies, macroalgae, and epilithic microalgae: dynamic interactions in a stream grazing system. Oecologia. 87: 247–256.
Fox, H.M., and Sidney, J. 1952. The influence of dissolved oxygen on the respiratory movements of caddis larvae. Journal of Experimental Biology. 30:235–237.
Gunn, R.J.M. 1985. The biology of Brachycentrus subnubilis Curtis (Trichoptera) in the River Frome, Dorset. Hydrobiologia. 120: 133–140.
Hart, D.D. 1992. Community organization in streams: The importance of species interactions, physical factors, and chance. Oecologia. 91: 220–228.
Holomuzki, J.R., and Van Loan, A.S. 2002. Effects of structural habitat on drift distance and benthic settlement of the caddisfly, Ceratopsyche sparna. Hydrobiologia. 477: 139–147.
Houghton, D.C., and Shoup, L. 2014. Seasonal changes in the critical thermal maxima of four species of aquatic insects (Ephemeroptera, Trichoptera). Physiological Ecology. 43: 1059–1066.
Ismail, A.R., Edington, J.M., and Green, P.C. 1996. Descriptions of the pupae and larvae of Stenopsyche siamensis Martynov, 1931 (Trichoptera: Stenopsychidae) with notes on larval biology. Aquatic Insects. 18: 241–252.
Jacquemin, S.J., Pyron, M., Allen, M., and Etchison, L. 2014. Wabash River freshwater drum Aplodinotus grunniens diet: Effect of body size, sex, and river gradient. Journal of Fish and Wildlife Management. 5: 133–140.
Jayawardena, A.W., Takeuchi, K., and Machbub, B. 1997. Catalogue of rivers for Southeast Asia and Pacific-Volume 2. A UNESCO-IHP Publication, Hong Kong.
Keiper, J.B., and Foote, B.A. 1999. Biology and immature stages of two species of Hydroptila Dalman (Trichoptera: Hydroptilidae) which consume Cladophora (Chlorophyta). Proceeding of the Entomological Society of Washington. 101: 514–521.
Khedari, J., Sangprajak, A., and Hirunlabh, J. 2002. Thailand climatic zones. Renewable Energy. 25: 267–280.
Kishi, D., Murakami, M., Nakano, S., and Maekawa, K. 2005. Water temperature determines strength of top-down control in a stream food web. Freshwater Biology. 50: 1315–1322.
Kreiling, A., O’Gorman, E.J., Pálsson, S., Benhaïm D., Leblanc, C.A., Ólafsson, J.S., and Kristjánsson, B.K. 2021. Seasonal variation in the invertebrate community and diet of a top fish predator in a thermally stable spring. Hydrobiologia. 848: 531–545.
Küçükbasmaci, İ., and Findik, Ö. 2020. The seasonal and spatial distribution of Trichoptera larvae in the Araç Creek (Kastamonu, Karabük, Turkey). Journal of Forestry Faculty. 20: 1–10.
Kunpradid, T., and Tagun, R. 2016-The influence of environmental factors on the distribution on macroalgae, Kai, Cladophora spp., Microspora spp. and Rhizoclonium spp. at Nan River, Tha Wang Pha District, Nan Province. p.419–427. In: Rajabhat University National and International Research and Academic Conference (RUNIRAC IV), 22–24 November 2016. Buriram, Thailand.
Ling, T., Gerunsin, N., Soo, C., Nyanti, L., Sim, S., and Grinang, J. 2017. Seasonal change and spatial variation in water quality of a large young tropical reservoir and its downstream river. Journal of Chemistry. 2017: 1–16.
Lloyd, D.S., Koenings, J.P., and Laperriere, J.D. 1987. Effects of turbidity in fresh waters of Alaska. North American Journal of Fisheries Management 7: 18–33.
Martin, I.D. 1985. Microhabitat selection and life cycle patterns of two Rhyacophila species (Trichoptera: Rhyacophilidae) in southern Ontario streams. Freshwater Biology. 15: 1–14.
McCafferty, W.P. 1981. Aquatic entomology: The fishermen’s and ecologists’ illustrated: Guide to insects and their relatives. Jones and Bartlett Publishers, London.
Minckley, W.L. 1963. The ecology of a spring stream Doe Run, Meade County, Kentucky. Wildlife Society. 11: 3–124.
Morse, J.C., Frandsen, P.B., Grag, W., and Thomas, J.A. 2019. Review diversity and ecosystem services of Trichoptera. Insects. 10: 125.
Nakano, D., Yamamoto, M., and Okino, T. 2005. Ecosystem engineering by larvae of net-spinning stream caddisflies creates a habitat on the upper surface of stones for mayfly nymphs with a low resistance to flows. Freshwater Biology. 50: 1492–1498.
Nebeker, A.V. 1972. Effect of low oxygen concentration on survival and emergence of aquatic insects. Transactions of the American Fisheries Society. 101: 675–679.
Nebeker, A.V., Onjukka, S.T., Stevens, D.G., and Chapman, G.A. 1996. Effect of low dissolved oxygen on aquatic life stages of the caddisfly Clistoronia magnifica (Limnepthilidae). Archives of Environmental Contamination and Toxicology. 31: 453–458.
Nilo, P., Tremblay, S., Bolon, A., Dodson, J., Dumont, P., and Fortin, R. 2006. Feeding ecology of juvenile lake sturgeon in the St. Lawrence River system. American Fisheries Society. 135: 1044–1055.
Ormerod, S.J., and Tyler, S.J. 1991. Exploitation of prey by a river bird, the dipper Cinclus cinclus (L.), along acidic and circumneutral streams in upland Wales. Freshwater Biology. 25: 105–116.
Ormerod, S.J., Efteland, S., and Gabrielsen, L.E. 1987. The diet of breeding dippers Cinclus cinclus and their nestling in southwestern Norway. Holarctic Ecology. 10: 201–205.
Peerapornpisal, Y., Pongsirikul, I., and Kanjanapothi, D. 2005. Potential of freshwater macroalgae as food and medicine. Thailand Research Fund (TRF), Bangkok, Thailand.
Prommi, T. 2016. Descriptions of larvae of four species of Hydropsyche (Hydropsychidae: Trichoptera) from Thailand. Zootaxa. 4158: 577–591.
Prommi, T., and Payakka, A. 2015. Aquatic insect biodiversity and water quality parameters of streams in northern Thailand. Sains Malaysiana. 44: 707–717.
Prommi, T., and Thamsenanupap, P. 2015. Diversity and structure of Trichoptera communities and water quality variables in streams, northern Thailand. International Scholarly and Scientific Research and Innovation. 9: 1065–1072.
Prommi, T., Seetapan, K., and Thamsenanupap, P. 2012. Diversity and seasonality of caddisflies (Insecta: Trichoptera) at Champathong waterfall, northern Thailand. Suan Dusit University Research Journal. 5: 125–137.
Resh, V.H., and Houp, R.E. 1986. Life history of the caddisfly Dibusa angata and its association with the red alga Lemanea australis. Journal of the North American Benthological Society. 5: 28–40.
Roux, C., Tachet, H., Bournaud, M., and Cellot, B. 1992. Stream continuum and metabolic rate in the larvae of five species of Hydropsyche (Trichoptera). Ecography. 15: 70–76.
Royal Irrigation Department, 2014. Thailand hydrological yearbook: Water year 2014 volume 57. Office of Water Management and Hydrology, Bangkok.
Runde, J.M., and Hellenthal, R.A. 2000. Effects of suspended particles on net-tending behaviors for Hydropsyche sparna (Trichoptera: Hydropsychidae) and related species. Entomological Society of America. 93: 678–683.
Sharma, S., Dubey, S., Chaurasia, R., and Dave, V. 2013. Macro-invertebrate community diversity in relation to water quality status of Kunda River (M.P.), India. Discovery. 3: 40–46.
Shubina, V.N. 2006. Caddis flies (Trichoptera) in the benthos and food of fish from streams of the Pechora-Ilych State Biosphere Reserve, the northern Urals. Russian Journal of Ecology. 37: 352–358.
Stark, J., and Phillips, N. 2004. Seasonal variability in the macroinvertebrate community index: Are seasonal correction factors required? New Zealand Journal of Marine and Freshwater Research. 43: 867– 882.
Strand, R.M., and Merritt, R.W. 1997. Effects of episodic sedimentation on the net-spinning caddisflies Hydropsyche betteni and Ceratopsyche sparna (Trichoptera: Hydropsychidae). Environmental Pollution. 98: 129–134.
Suhaila, A.H., Che Salmah, M.R., and Huda, N. 2014. Seasonal abundance and diversity of aquatic insects in rivers in Gunung Jerai Forest Reserve, Malaysia. Sains Malaysiana. 43: 667–674.
Ter Braak, C.J.F., and Verdonschot, P.F.M. 1995. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquatic Sciences. 57: 255–289.
Thamsenanupap, P., Seetapan, K. and Prommi, T. 2021. Caddisflies (Trichoptera, Insecta) as bioindicator of water quality assessment in a small stream in northern Thailand. Sains Malaysiana. 50: 655–665.
Thiamdao, S. 2011. Diversity of edible freshwater macroalgae in Thailand during 2007-2008. Ph.D. Dissertation. Chiang Mai University, Chiang Mai, Thailand.
Trisurat, Y., Shirakawa, H., and Johnston, J.M. 2019. Land-use/land-cover change from socio-economic drivers and their impact on biodiversity in Nan Province, Thailand. Sustainability. 11: 649.
Urbanič, G., Toman, M.J., and Krušnik, C. 2005. Microhabitat type selection of caddisfly larvae (Insecta: Trichoptera) in a shallow lowland stream. Hydrobiologia. 541: 1–12.
Van den Brink, F.W.B., Van der Velde, G., and Wijnhoven, S. 2013. Seasonal change in caddis larvae assemblages in river-floodplain habitats along a hydrological connectivity gradient. Hydrobiologia. 716: 75–85.
Wantzen, K.M. 2006. Physical pollution: Effects of gully erosion on benthic invertebrates in a tropical clear-water stream. Aquatic Conservation: Marine and Freshwater Ecosystems. 16: 733–749.
Williams, N.E., and Hynes, H.B. 1973. Microdistribution and feeding of the net-spinning caddisflies (Trichoptera) of a Canadian stream. Oikos. 24: 73–84.
Yang, D., and Peterson, A. 2017. River water temperature in relation to local air temperature in the Mackenzie and Yukon basins. Arctic. 70: 47–58.
Zhou, X., Frandsen, P.B., Holzenthal, R.W., Beet, C.R., Bennett, K.R., Blahnik, R.J., Bonada, N., Cartwright, D., Chuluunbat, S., Cocks, G.V., et al. 2016. The Trichoptera barcode initiative: A strategy for generating a species-level Tree of Life. Philosophical Transactions of The Royal Society B Biological Science, The Royal Society Publishing. 371: 1–11.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand.
https://cmuj.cmu.ac.th
Pornpimon Buntha and Decha Thapanya*
Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
Corresponding author: Decha Thapanya, E-mail: thapanya2@hotmail.com
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: March 21, 2022;
Revised: June 15, 2022;
Accepted: June 16, 2022;
Published online: June 22, 2022

