
Effect of Immersion Time in Simulated Body Fluid on Adhesion Strength of Hydrothermally Treated Hydroxyapatite-Titanium Nitride Films on Polyetheretherketones
Kwanchanok Koonrungsesomboon, Dheerawan Boonyawan, Kullapop Suttiat, and Piriya Yavirach*Published Date : 2022-07-11
DOI : https://doi.org/10.12982/CMUJNS.2022.042
Journal Issues : Number 3, July-September 2022
Abstract This study sought to investigate the effect of immersion time in simulated body fluid (SBF) on the adhesion strength of hydrothermally treated hydroxyapatite-titanium nitride (HA-TiN) films on polyetheretherketone (PEEK) substrates. The HA-TiN films were deposited on PEEK substrates via magnetron sputtering and annealed with hydrothermal treatment. The crystalline phase and element compositions on the deposited films were confirmed by X-ray diffractometry (XRD), and X-ray photoelectron spectrometry (XPS). The samples were then immersed in SBF at 37°C for 7 to 56 days, where the surface characterization and chemical composition of the films were analyzed using scanning electron microscopy (SEM), atomic force microscopy (AFM), and XPS. After the in vitro degradation in SBF, the adhesion strength between HA-TiN films and PEEK substrates were measured by a universal testing machine and further investigated the failure mode using a stereomicroscope and SEM. The results demonstrated the improvement of crystallinity on HA-TiN sputtered films after hydrothermal treatment. After immersion in SBF, the coating surface revealed some nucleation without any detachment and exhibited an increase of surface roughness. The hydroxyapatite and titanium dioxide were revealed on the surface throughout the 56 days, while the Ca/P ratio decreased and remained constant during immersion. The adhesion strength did not significantly differ in all groups. These findings concluded that hydrothermally treated HA-TiN sputtered films on PEEK substrates showed the stability of adhesion strength throughout 56 days in simulated physiological conditions. The dissolution and precipitation during immersion represented the favorable characteristics of the films in the orthopedic or dental application.
Keywords: Adhesion, Degradation, Thin films, Hydroxyapatite, Titanium dioxide
Funding: This work was supported by Faculty of Dentistry, Chiang Mai University, and Graduate School, Chiang Mai University, Chiang Mai, Thailand.
Citation: Koonrungsesomboon, K., Boonyawan, D., Suttiat, K., and Yavirach, P. 2022. Effect of immersion time in simulated body fluid on adhesion strength of hydrothermally treated hydroxyapatite-titanium nitride films on polyetheretherketones. CMU J. Nat. Sci. 21(3): e2022042.
INTRODUCTION
Polyetheretherketone (PEEK) is a thermoplastic polymer that has been used as an alternative material instead of metal for orthopedic and dental applications (Ma and Tang, 2014; Almasi et al., 2016). This material exhibits several favorable properties, including excellent biocompatibility, mechanical durability, chemical resistance, and consistency in sterilization (Kurtz and Devine, 2007; Ma and Tang, 2014; Almasi et al., 2016). The elastic modulus of PEEK (3-4 GPa) is closer to human cortical bone (18 GPa) than the metallic materials like titanium alloy (110 GPa). This property causes less stress concentrated at the bone-implant interface, reducing bone resorption (Kurtz and Devine, 2007; Boonyawan et al., 2016; Ozeki et al., 2017). Additionally, the radiolucency of PEEK allows the postoperative radiographic assessment of bone growth at the surgical site (Hahn et al., 2013). However, this polymer is biologically inert, limiting the interaction with surrounding tissues, and leading to low bone-implant integration (Rabiei and Sandukas, 2013). Thus, improving the bioactivity of PEEK is considered important to achieve effective application within the medical field.
Attaining a bioactive surface and retaining advantageous properties of PEEK, the coating of the surface with bioactive substances is recommended (Rabiei and Sandukas, 2013; Almasi et al., 2016). In particular, hydroxyapatite (HA) which is a biocompatible calcium phosphate-based ceramic has been widely used as a bioactive material (Sinha et al., 2001). Since HA is the primary inorganic component of bone, this material can promote bone ingrowth to the implantation site (Muresan, 2015). Due to the brittleness and low strength of HA (Fiume et al., 2021), titanium nitride (TiN) has been applied to reinforce the mechanical properties for the reason that TiN provides enhanced hardness and resistance to wear and corrosion (Shi et al., 2015; Boonyawan et al., 2016). The HA-TiN composite film was developed to achieve exceptional features from both materials on the surface of PEEK (Boonyawan et al., 2016; Nupangtha and Boonyawan, 2017).
Magnetron sputtering is the effective surface coating method that can deposit various types of material, including metals, alloys, and ceramics (De Groot et al., 1998) onto the polymer substrate such as PEEK (Boonyawan et al., 2016; Nupangtha and Boonyawan, 2017). This technique attains a homogenous thin film with less than 1 micron in thickness (De Groot et al., 1998). Nevertheless, the as-sputtered film provides the amorphous phase of HA, which is rapidly dissolved in the living body. Therefore, the post-deposition annealing by hydrothermal treatment should be performed so as to crystallize the sputtered films (Ozeki et al., 2003). According to the previous study by Buranapanich et al. (2020), the hydrothermal treatment at 100°C for 12 hours was the most favorable condition for improving the HA crystallinity on HA-TiN films. This condition yielded the closest Ca/P ratio to the stoichiometry of HA and did not exceed the glass transition temperature of PEEK (143°C) (Buranapanich et al., 2020). Additionally, the study of Shi et al. (2015) suggested performing the hydrothermal treatment for TiN coating with a temperature below 120°C to preserve the hardness of TiN (Shi et al., 2015).
For the long-term application of coated materials in the living body, the optimal and stable adhesion strength of the film and substrate is one of the significant parameters that should be of concerned, so as to obtain a durable bone-implant integration (Ding et al., 1999; Buranapanich et al., 2020). In order to simulate the living body condition, the in vitro test using simulated body fluid (SBF), which has ion concentrations close to human blood plasma (Kokubo and Takadama, 2006), was performed at different immersion periods before testing the bond strength of the film. The aim of this study was to evaluate the effect of immersion time in SBF on the adhesion strength of hydrothermally treated HA-TiN films on PEEK substrates.
MATERIALS AND METHODS
Sample preparation
Preparation of PEEK substrates
Pure PEEK disks (Ketron PEEK-1000, Quadrant Engineering Plastic Products, USA), with a diameter of 6 mm and thickness of 2 mm, were prepared. All disks were sequentially polished by 400, 800, 1,500, and 2,000-grit silicon carbide grinding paper. The samples were ultrasonically washed with ethanol and acetone to remove any abrasive particle remnants, then was air dried, and stored in a desiccator before the deposition process.
Deposition of HA-TiN films
The HA-TiN films were deposited on PEEK disks by the reactive pulsed DC magnetron sputtering technique. PEEK disks were placed in the stainless-steel sputtering chamber with a dimension of 37×37×37 cm3. A cathodic target was a 99.99% pure Ti disk, with 100 mm diameter, covered with a 75 mm diameter and 3.5 mm thickness HA disk as a co-axis target. The distance from the target to the substrate was fixed at 7 cm. The sputtering chamber was vented to achieve a base pressure below 9×10-6 Torr. In this experiment, 99.99% argon gas and 99.99% nitrogen gas were used as working gases with a constant flow rate of 3.5 sccm and 1.4 sccm, respectively. The sputtering pressure varied in the range of 3×10-2 to 5×10-2 Torr. The discharge voltage was set at 400 V, and the pulse frequency was 50 kHz. The sputtering process was maintained for 240 min.
Hydrothermal treatment
The PEEK disks with as-deposited HA-TiN coating were placed in a hydrothermal synthesis autoclave reactor (Suzhou Shenghua Instrument Technology, China) containing 10 ml of deionized water. The hydrothermal treatment was performed at 100°C for 12 hours in a hot air oven.
Coating characterization analysis
The crystallized HA-TiN surface coating was analyzed by an X-ray diffractometer (XRD) (SmartLab, Rigaku, Japan) with a CuKα radiation source that operated at 40 kV. The analysis was performed in the range of 2Ɵ = 10-60° with a step size of 0.01°.
The chemical compositions of the films were confirmed by X-ray photoelectron spectroscopy (XPS) (Axis Ultra X-ray photoelectron spectrometer, Kratos Analytical, UK). The XPS parameters were set according to a previous study (Boonyawan et al., 2016). The monochromatized aluminum Kα X-rays were used for excitation at the anode power of 150 W. The survey spectra were detected with a pass energy of 80 eV at 1 eV intervals. The high-energy resolution spectra were obtained with a pass energy of 20 eV at 0.1 eV intervals. All XPS spectra were referenced by setting the C 1s of C-C and C-H bonds in hydrocarbons at the binding energy of 284.6 eV.
In vitro degradation
The prepared samples were randomly divided into six groups (n=15/group), including control (group 1), immersion in SBF for 7 days (group 2), 14 days (group 3), 21 days (group 4), 28 days (group 5), and 56 days (group 6). Each sample in the experimental groups was placed in a tube containing 9 mL of SBF (Hung et al., 2017) (SBF, Phygene Biotechnology, China), then incubated at 37°C for different durations according to the pre-designed groups.
Surface characterization analysis
The surface morphology of the films was observed under a scanning electron microscope (SEM) (JSM-5910LV, JEOL USA Inc., USA) with an accelerating voltage of 15 kV and a magnification 5,000X. The surface roughness of the films was measured by an atomic force microscope (AFM) (Auto-Probe CP, Park Scientific Instruments, USA) in non-contact mode. The SEM analysis was evaluated from 3 samples per group, and the surface roughness values were determined from 12 samples in each group.
Chemical composition analysis
The chemical composition and Ca/P ratio of the films following the in vitro degradation test were analyzed by X-ray photoelectron spectroscopy (XPS) (Axis Ultra X-ray photoelectron spectrometer, Kratos Analytical, UK) with similar XPS parameters as revealed above.
Adhesion strength test
The adhesion strength between HA-TiN film and PEEK substrate in each group was measured using a universal testing machine (Instron 5566 universal testing machine, Instron, USA). The testing rods were adhered to the top of the HA-TiN film and the sandblasted base of the PEEK substrate by using a cyanoacrylate adhesive (Model repair II blue, Dentsply, USA) (Figure 1). The excess adhesive covering the border of a sample was removed. The axial load at a constant crosshead velocity of 1.0 mm/min was applied until separation was reached. The maximum force (N) and cross-sectional area (mm2) were recorded for each sample. Twelve samples of each group were tested. The adhesion strength was calculated using the following equation.
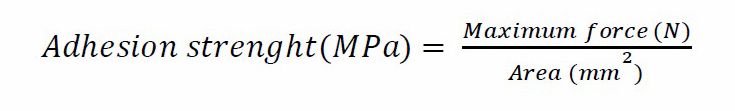
The mode of failure was observed using a stereomicroscope (Model SZ2-ILST, Olympus Corporation, Japan) and a scanning electron microscope (SEM) (JSM-5910LV, JEOL USA Inc., USA) with an accelerating voltage of 15 kV and a magnification 100X and 5,000X.
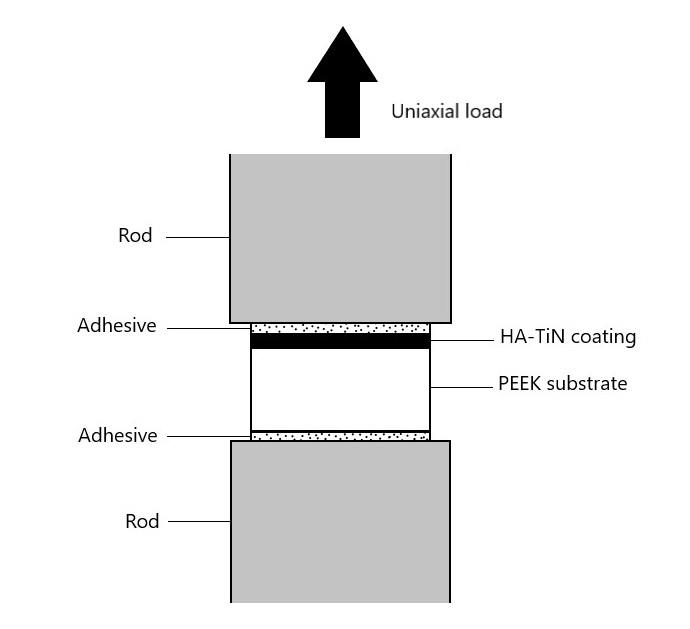
Figure 1. Schematic illustration for the evaluation of adhesion strength.
RESULTS
Coating characterization
The crystalline phase of the coatings was evaluated by XRD prior to the in vitro degradation test. The XRD analysis of the as-deposited and hydrothermally treated HA-TiN films on PEEK substrates are presented in Figure 2. The intensities of HA (002), HA (211), HA (300), TiO2 (200), and TiO2 (111) peaks were found in the as-deposited film. No TiN peak was observed. After hydrothermal treatment, the intensity of HA (002), HA (211), and HA (300) peaks increased in comparison with the untreated sample, as classified in Figure 2. These XRD patterns demonstrated the presence of hydroxyapatite (HA) and titanium dioxide (TiO2) on the film after the sputtering process and the crystallinity improvement in the coating after hydrothermal treatment.
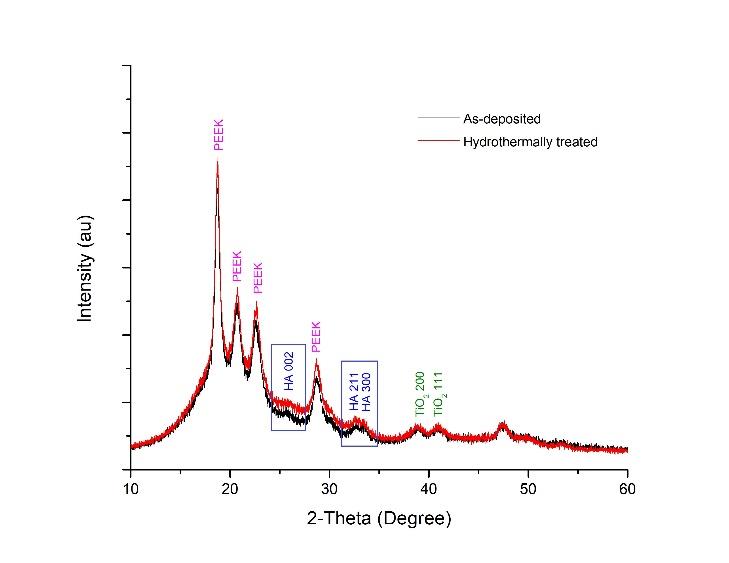
Figure 2. XRD patterns of the as-deposited and hydrothermally treated HA-TiN films on PEEK substrates demonstrate the intensities of HA (002), HA (211), HA (300), TiO2 (200), and TiO2 (111) peaks. The hydrothermally treated film shows higher intensities of HA peaks than the as-deposited film.
The validation of the chemical composition on the deposited film after hydrothermal treatment was performed by using XPS. Figure 3 shows the survey scan spectrum, which exhibits the element compositions on the coating. The results express the peaks represented Ca 2p, P 2p, O 1s, and Ti 2p. Small amounts of Fe 2p and Cr 2p were found as contamination from the stainless-steel HA target holder.
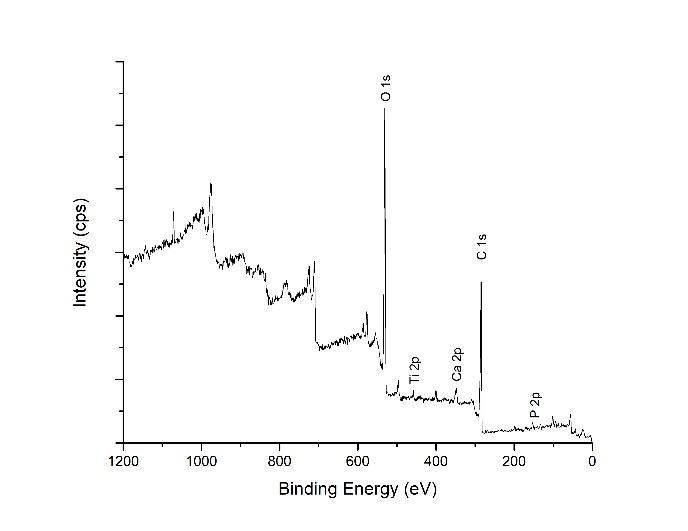
Figure 3. XPS survey scan spectrum of hydrothermally treated HA-TiN film on PEEK substrate shows the elements on the film, including P 2p, Ca 2p, Ti 2p, and O 1s at the binding energies of 133.0 eV, 347.0 eV, 458.0 eV, and 531.0 eV, respectively.
The high-energy resolution spectra of Ca 2p, P 2p, O 1s, and Ti 2p in the film are explained in Figure 4. The Ca 2p spectrum (Figure 4a) was fitted with two peaks, including Ca 2p3/2 and Ca 2p1/2, at the binding energies of 347.7 eV and 351.2 eV, respectively. This finding characterized the calcium bonding in hydroxyapatite. Figure 4b presents the deconvolution of the P2p spectrum. The P 2p3/2 peak, at the binding energy of 133.1 eV, and the P 2p1/2 peak, at the binding energy of 134.1 eV, indicated the phosphate phase of hydroxyapatite in the film. The peaks of O 1s (Figure 4c), positioned at 530.2 eV, 531.5 eV, and 532.8 eV, exhibited the oxygen bonds in the deposited film. These findings included oxide bond from TiO2, hydroxide and phosphate groups in the hydroxyapatite structure, and -OH bond of adsorbed water, consecutively. Figure 4d reveals two peaks of Ti 2p, which centered at 458.4 eV and 464.1 eV, attributed to Ti 2p3/2 and Ti 2p1/2 of TiO2. The TiN was not detected from the XPS scan. The XPS analyses, as mentioned above, confirmed the structure of hydroxyapatite and titanium dioxide in the coating prior to SBF immersion.
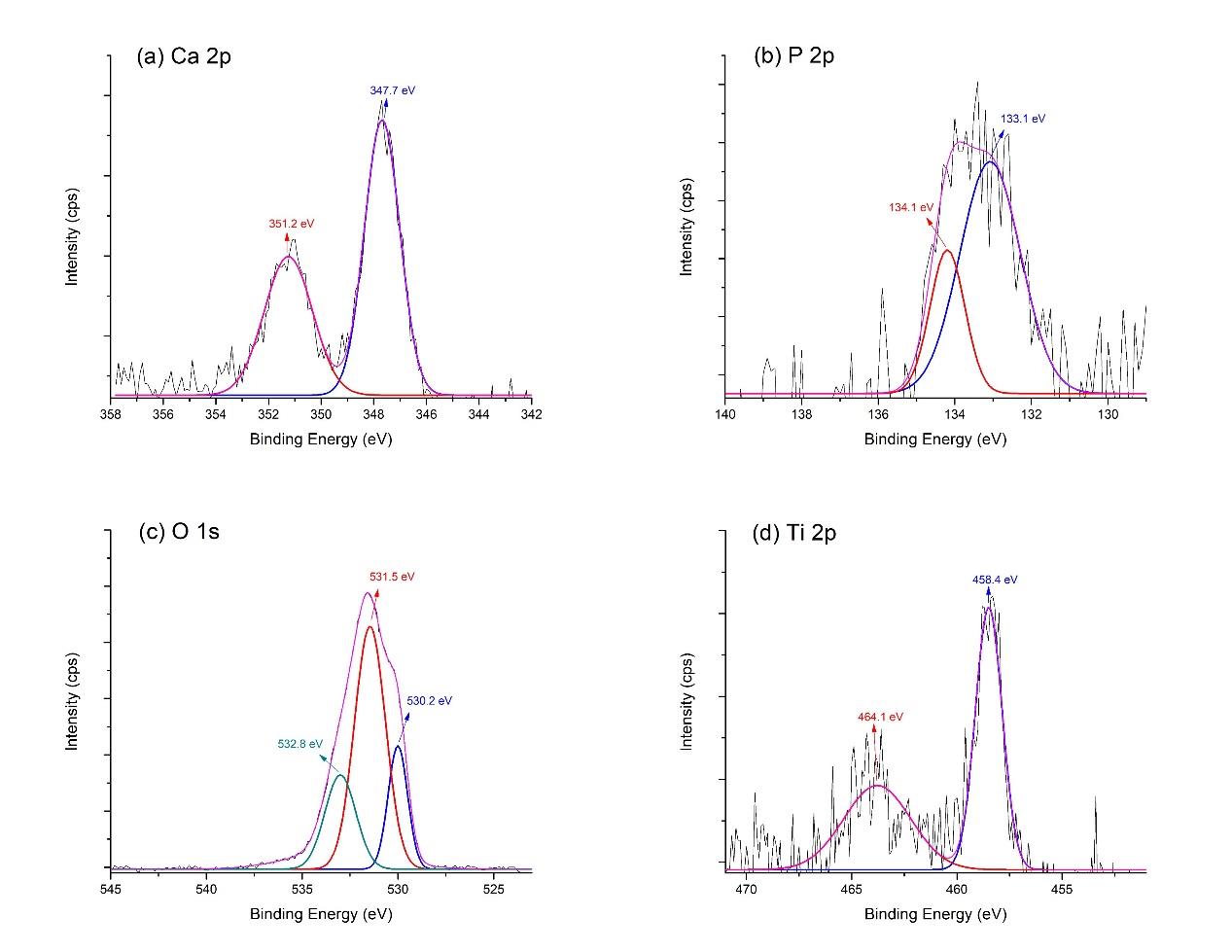
Figure 4. XPS high-energy resolution spectra of hydrothermally treated HA-TiN films on PEEK substrates, showing (a) Ca 2p, (b) P 2p, (c) O 1s, and (d) Ti 2p peaks and respective fitting peaks.
In vitro degradation
Surface characterization of the films
After the in vitro degradation test, the surface characterization of the films was evaluated. The SEM images (Figure 5) depict the surface morphology of the HA-TiN films on PEEK substrates before and after immersion in SBF. The sputtered film after hydrothermal treatment (Figure 5a) exhibited a uniform and dense coating layer over the entire surface, without any apparent porosity. Some microcracks were found due to stress-relieving while the material was cooling to room temperature (Buranapanich et al., 2020). After seven days of immersion, some nucleation partially precipitated on the surface (Figure 5b). The nucleation formed into the more noticeable agglomeration after being immersed for 14 days (Figure 5c) and 21 days (Figure 5d). On day 28, a less compact structure was observed (Figure 5e). This conversion of surface morphology resembled the 56 days immersion group (Figure 5f). No detachment of the coatings was recognized in all groups at all observing periods.
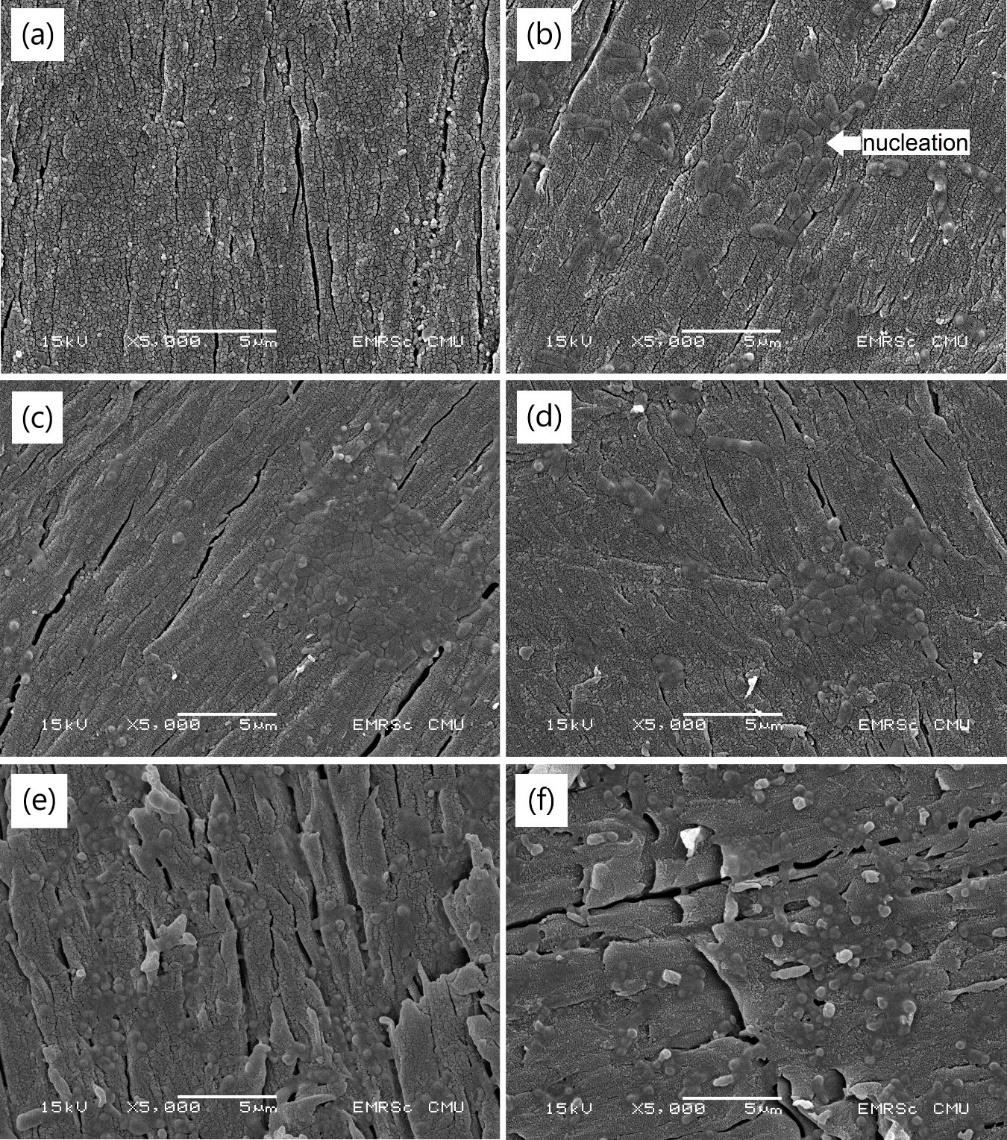
Figure 5. SEM images of HA-TiN films on PEEK substrates (a) before immersion, (b) after immersion in SBF for 7 days, (c) 14 days, (d) 21 days, (e) 28 days, and (f) 56 days. After immersion in SBF, the nucleation and agglomeration were found without detachment of the films throughout 56 days.
The average surface roughness of each group, measured by AFM, is summarized in Table 1. The value increased after the samples were immersed for 14 days and ranged between 67.74 to 117.45 nm. These surface roughness values were significantly higher than the initial value (P <0.05). The three-dimensional images of typical surface topography in each group obtained from AFM are presented in Figure 6.
Table 1. The surface roughness of HA-TiN films on PEEK substrates before and after different immersion times in SBF.
|
Immersion time (Days) |
Surface roughness (nm) |
|
Before immersion 7 14 |
49.80 ± 13.82 a |
|
46.10 ± 12.21 a |
|
|
67.74 ± 15.17 b |
|
|
21 28 56 |
117.45 ± 31.48 d 72.00 ± 15.71 b 93.80 ± 16.75 c |
Note: The values indicated with different letters were statistically significant differences from each other (P <0.05).
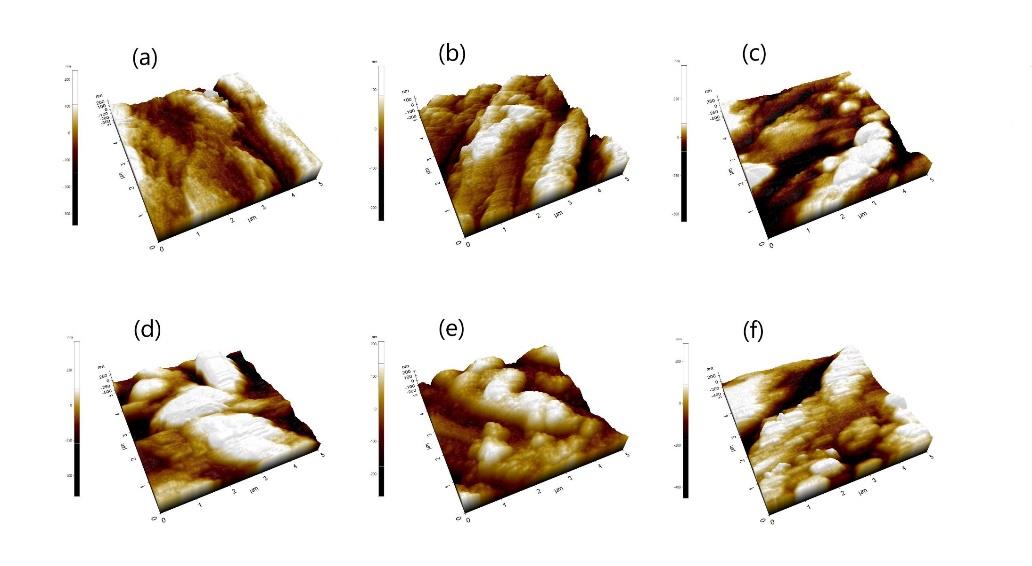
Figure 6. 3D images of surface topography derived from AFM, showing HA-TiN films on PEEK substrates (a) before immersion, (b) after immersion in SBF for 7 days, (c) 14 days, (d) 21 days, (e) 28 days, and (f) 56 days. The roughness of the surfaces from AFM images corresponds to the AFM data and SEM images.
Chemical composition of the films
The chemical composition of the HA-TiN film was analyzed by XPS following the in vitro degradation test. The survey scan spectra revealed the element compositions, including Ca 2p, P 2p, O 1s, and Ti 2p, exhibited on the coatings after different immersion times, as presented in Figure 7. Additionally, the XPS spectra of all elements in every group are shown in Figure 8. The binding energies representing hydroxyapatite and titanium dioxide were still presented on the surfaces after immersion in SBF for up to 56 days. As the films were immersed for longer durations, the O 1s spectrum demonstrated a slight shoulder shift toward the higher binding energy without shifting O 1s core-level binding energies (Figure 8c). This outcome revealed the increase of peak area of the high-binding-energy oxygen, indicating that more O atoms have formed the -OH bond of adsorbed water. In addition, the binding energy values of Ca 2p, P 2p, O 1s, and Ti 2p still characterized their normal oxidation states in every periodic assessment, as shown in Figure 8a, 8b, 8c, and 8d, respectively. These results confirmed that the compositions of the deposited films, namely hydroxyapatite and titanium dioxide, were exhibited on the surfaces of PEEK substrates throughout 56 days when immersed in SBF.
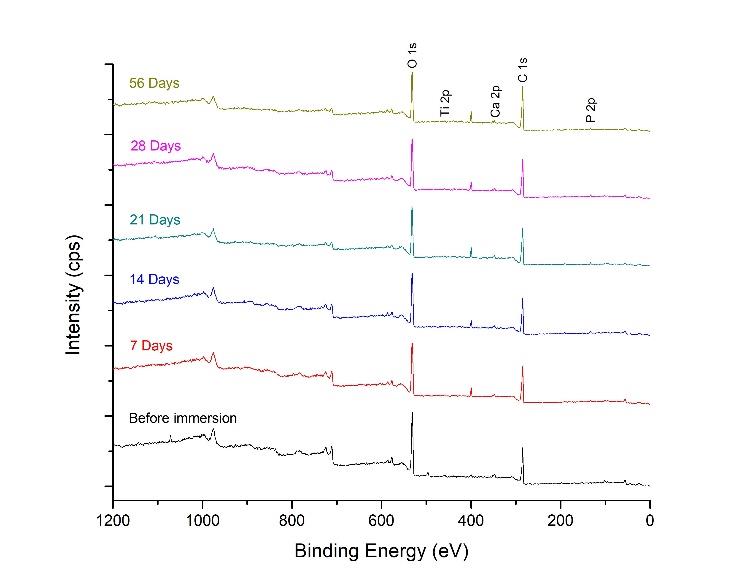
Figure 7. XPS survey scan spectra of HA-TiN films on PEEK substrates before and after immersion in SBF for 7, 14, 21, 28, and 56 days show the elements on the films, including P 2p, Ca 2p, Ti 2p, and O 1s at the binding energies of 133.0 eV, 347.0 eV, 458.0 eV, and 531.0 eV, respectively.
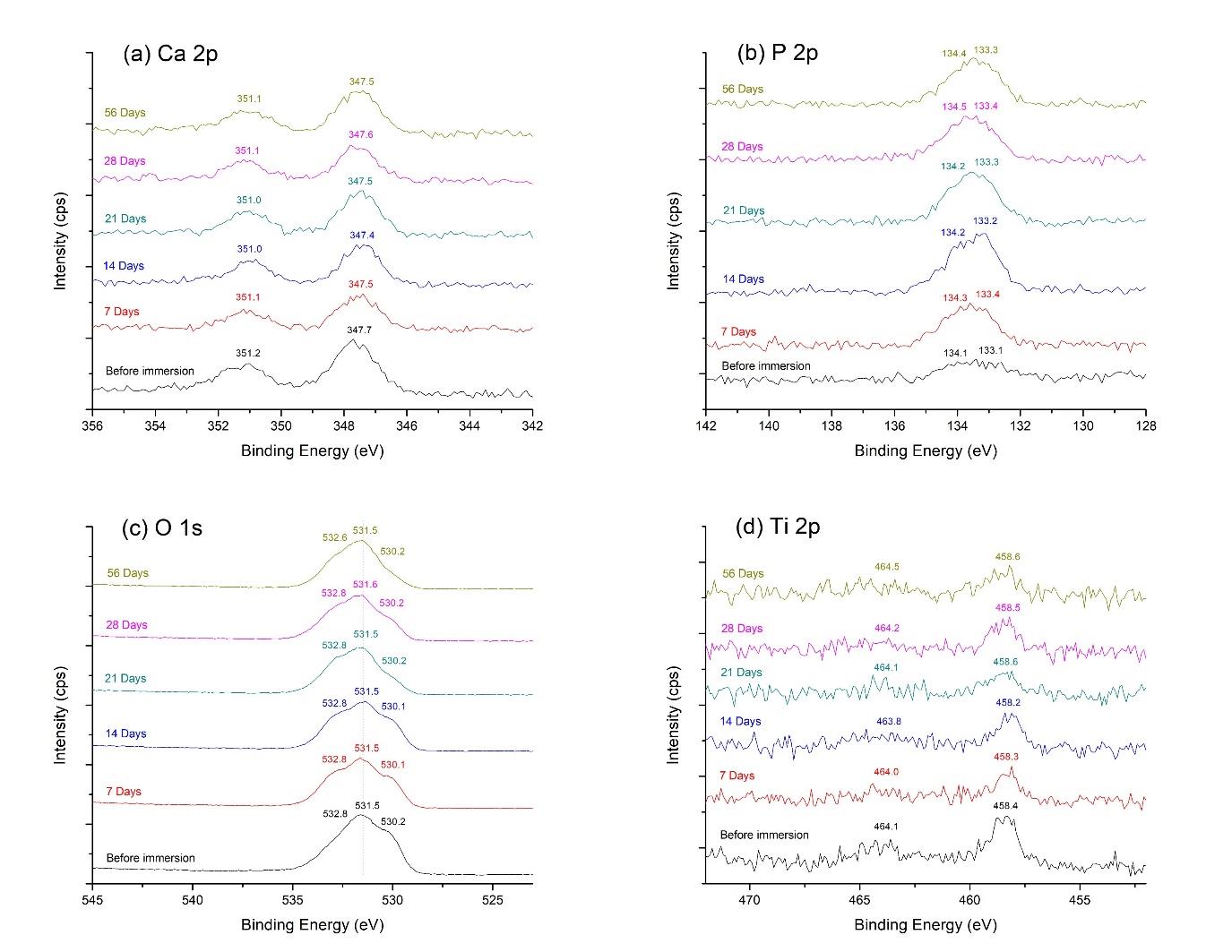
Figure 8. XPS (a) Ca 2p, (b) P 2p, (c) O 1s, and (d) Ti 2p spectra of HA-TiN films on PEEK substrates before and after immersion in SBF for 7, 14, 21, 28, and 56 days. The binding energy values in all groups characterize the bonding of hydroxyapatite and titanium dioxide in the deposited films.
In addition, the atomic concentration percentages of elements in each group were examined from XPS analyses, as summarized in Table 2. The Ca/P ratio of the films before immersion in SBF was 1.96 ± 0.95. After immersion in SBF, the Ca/P ratio of the films decreased from the initial value and ranged from 0.61 to 1.09, which showed no statistically significant difference among these groups (P >0.05).
Table 2. The Ca/P ratio of HA-TiN films on PEEK substrates before and after different immersion times in SBF.
|
Immersion time (Days) |
Ca/P ratio |
|
Before immersion 7 14 |
1.96 ± 0.95 b |
|
0.94 ± 0.40 a |
|
|
0.77 ± 0.03 a |
|
|
21 28 56 |
0.91 ± 0.27 a 0.61 ± 0.08 a 1.09 ± 0.13 a |
Note: The values indicated with different letters were statistically significant differences from each other (P<0.05).
Adhesion strength
The adhesion strength of the HA-TiN films to the PEEK substrates was evaluated by a universal testing machine after being immersed in the SBF at different periods. Prior to SBF immersion, the adhesion strength was 13.01 ± 2.60 MPa. After being immersed in SBF for 7 to 56 days, the adhesion strength ranged from 11.40 to 15.10 MPa, as outlined in Table 3. Nonetheless, these values showed no statistically significant difference among all groups (P >0.05).
Table 3. The adhesion strength of HA-TiN films on PEEK substrates before and after different immersion times in SBF.
|
Immersion time (Days) |
Adhesion strength (MPa) |
|
|
Before immersion 7 14 |
13.01 ± 2.60 |
|
|
14.46 ± 3.02 |
||
|
13.06 ± 3.58 |
||
|
21 28 56 |
15.10 ± 2.49 12.98 ± 3.43 11.40 ± 2.96 |
|
Note: The results showed no statistically significant difference between each group (p>0.05).
Figure 9 shows the image from a stereomicroscope and the SEM micrographs of the bonding failures after the adhesion strength test. The bonding failures were the mixed mode in which the cohesive failure occupied most parts of all the samples.
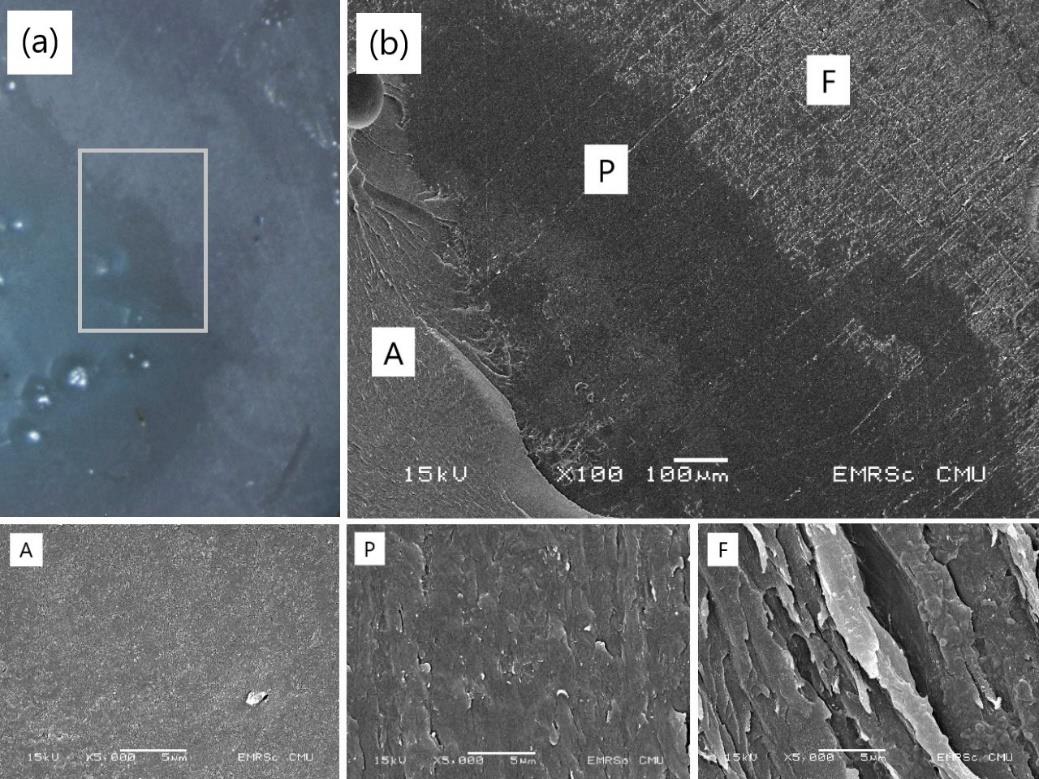
Figure 9. The failure modes of HA-TiN film on PEEK substrate after the adhesion strength test observing under (a) a stereomicroscope and (b) a scanning electron microscope. The letters A, P, and F indicate adhesive, PEEK surface, and film.
DISCUSSION
In this study, the reactive pulsed DC magnetron sputtering technique was successfully utilized to modify the surface of PEEK. After the post-deposition hydrothermal annealing process, the XRD result validated HA and TiO2 as well as the phase change of HA, indicating the improvement of the crystallinity in the coating. However, these HA diffraction peaks were broad, expressed in the low-intensity spectrum. It was possible that the treated film was partially crystalline with some amorphous phase, or composed of fine nanocrystalline HA grains which were not detectable in XRD (Rabiei and Sandukas, 2013). In addition, the presence of element compositions in the coating was confirmed by XPS before performing the in vitro degradation test. After hydrothermal treatment, the XPS spectra demonstrated HA and TiO2 in the deposited films, correlating with the XRD result, and no TiN was detected. The absence of TiN may be clarified as the formation of TiO2 from the oxidation process of titanium during the hydrothermal treatment (Buranapanich et al., 2020). Furthermore, the study by Boonyawan et al. (2016) explained that this finding occurred due to the lower enthalpy of the formation of TiO2 (-944 kJ/mol) than that of TiN (-388 kJ/mol) (Boonyawan et al., 2016). This principle indicated that TiO2 could be easily formed compared to TiN (Sattha et al., 2019).
The exposure of the HA-TiN films on PEEK substrates to simulated physiological conditions after different immersion times lead to the change of the surface characterization and the decrease of Ca/P ratio from the initial value. The reduction of the Ca/P ratio resulted from the release of calcium content from the hydroxyapatite films (Durham III and Rabiei, 2016). The dissolution might occur from the amorphous HA that existed on the coating, as revealed in the XRD result, which tends to dissolve faster than the crystalline HA (Ozeki et al., 2006). Since the SBF contained calcium and phosphate ions from the dissolved coating and SBF itself, these elements could deposit back onto the calcium phosphate coating. The dissolution and precipitation of elements were confirmed by the reduction and elevation of the Ca/P ratio on the films during the 7 to 56 days in SBF. This finding correlated with a previous study, which found the degradation of the hydroxyapatite films and the precipitation of the calcium and phosphorus ions during the 28 days of SBF immersion (Hung et al., 2017). Furthermore, the observation from SEM images showed nucleation on the surface, of which the morphological appearance was similar to the growth of calcium phosphate content on the HA-coated surface in a previous study (Xu et al., 2005). This outcome correlated with the rougher outermost coating surface from AFM examination, confirming the chemical dissolution and precipitation on the coating surface during immersion in SBF.
According to the XPS results which showed the existence of the coating as well as the chemical dissolution and precipitation at the surface during 56-day immersion in SBF, these findings were expected to be advantageous for bone-implant integration in a living body. After implant placement, the implant surface can influence the osteogenesis around the implantation site (Liu et al., 2020). The HA dissolution by releasing calcium and phosphate ions from the coating surface affect the cellular response, especially osteoblast and osteoclast, and influenced the new bone formation at the implant surface (Goharian, 2019; Su et al., 2019). As the woven bone was formed on the implant surface in 5 to 7 days (Wang et al., 2016) and continued over a further 1 to 2 weeks (Liu et al., 2020), the chemical dissolution of the coating during this time could support the bone integration and cell growth. Meanwhile, the chemical deposition of calcium and phosphate resulted in the existence of the HA on the coating until the bone-implant gap was fused with mature lamellar bone after 8 to 12 weeks (Wang et al., 2016).
The investigation of coating adhesion after in vitro degradation demonstrated that the increase of immersion time in SBF had no significant effect on the adhesion strength between HA-TiN films and PEEK substrates. The consistency of coating adhesion throughout 56 days in SBF corresponded to the SEM images, which showed no detachment of the films from the substrates after immersion. Additionally, the adhesion strength obtained from this study was close to the determined value of the standard ISO 13779-2 (≥ 15 MPa). These values were much higher than those fabricated from the plasma spraying method in previous reports (2.8 MPa (Ha et al., 1994) and 7.5 MPa (Beauvais and Decaux, 2007)), in which plasma spraying was used as the commercial implant surface coating process (Goharian, 2019). The more consistent adherence of hydrothermally treated-sputtered film to PEEK substrate than plasma-sprayed film might be due to the more uniform, less porous, and much thinner coating compared with the plasma-sprayed film. These properties provide better fracture resistance, resulting in favorable adhesion at the coating-substrate interface (Rabiei and Sandukas, 2013; Remache et al., 2019). Also, the coating in this study exhibited few microcracks and showed no delamination after immersion. Since the cracks cause the deterioration of the coating adhesion by allowing the solution to enter the coating and coating-substrate interface, it leads to the chemical dissolution in the coating and at the interface (Zhang et al., 2008). Thus, the dense coatings with few cracks derived from this study provided favorable integrity that led to the stable adhesion strength of the coating throughout the 56 days in the simulated physiological environment. In addition, the chemical dissolution and deposition as mentioned above may well occur at the outermost region of the coating, according to the change of Ca/P, surface roughness, and surface morphology, without the alteration of adhesion strength.
The durable coating adhesion for up to 56 days in this in vitro assessment may perhaps benefit the bone formation process after implant placement in orthopedic or dental applications. As the space between the implant and host bone is filled with mature lamellar bone in 8 to 12 weeks after implant placement (Wang et al., 2016), the presence of the coatings on substrates throughout this period may continually induce the activity of the osteoblast cells and the formation of bone nodules (Xu et al., 2005). Hence, this could provide enough time for bone ingrowth at the implantation site and complete osseointegration with the surrounding tissue.
Regarding the coating failure modes after the adhesion strength test, the mixed failure was observed in all groups. This included the adhesion failure that existed between the coating and substrate, the cohesive failure occurred in the adhesives, and the cohesive failure within the coatings. The adhesion failure between the coating and the substrate occurred in a small part of the total area, and the cohesive failure within the coatings occupied most parts of all samples. This finding revealed that the exact adhesion strength of the coatings might be higher than those values mentioned above (Zhang et al., 2008; Hahn et al., 2013). The minority of adhesion failure that occurred in this study indicates the strong bond between the hydrothermally treated HA-TiN sputtered films and the PEEK substrates, which were not degraded by increased immersion time in a simulated environment of a living body.
CONCLUSION
In conclusion, the investigation of the adhesion strength and the characteristic of the HA-TiN films on PEEK substrates in vitro was performed in this research. As the sputtered HA-TiN films were successfully coated onto the PEEK substrates via the magnetron sputtering technique, the post-deposition hydrothermal annealing process was carried out to enhance the crystallinity of the deposited films. After immersion in SBF for 7, 14, 21, 28, and 56 days to simulate the biodegradation process in living tissues, the adhesion strengths of the films to the substrates were examined and showed no statistically significant difference compared to the non-immersed HA-TiN coating. These findings suggest that the HA-TiN coated film made by DC magnetron sputtering provided integrity throughout the 56-day in vitro biodegradation test without affecting the adhesion strength between the coating material and PEEK substrate. In this study, the dissolution and deposition of the elements from the environment on the surfaces of coating material, as demonstrated by the alteration of Ca/P ratios, SEM images, and surface roughness values, was confirmed. These desirable characteristics would be beneficial for supporting the process of bone formation when employed in the orthopedic or dental field. Additionally, the in vivo and clinical studies ought to be performed for further investigations.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the Dental Material Science Research Center, Faculty of Dentistry, Chiang Mai University, and the Plasma and Beam Physics Research Facility, Department of Physics and Materials Science, Faculty of Science, Chiang Mai University for their kind support in equipment. Our thanks also go to Vanasanan Buranapanich and Chanchai Umongno for their assistance in HA-TiN films preparation; and Dr. Thanapat Sastraruji for his assistance in statistical analysis.
AUTHOR CONTRIBUTIONS
Kwanchanok Koonrungsesomboon designed and conducted the experiments, performed the data analysis, and wrote the manuscript. Dheerawan Boonyawan and Piriya Yavirach designed the experiments, assisted in performing the data analysis, and wrote the manuscript. Kullapop Suttiat designed the experiments and wrote the manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
Almasi, D., Iqbal, N., Sadeghi, M., Sudin, I., Abdul Kadir, M.R., and Kamarul, T. 2016. Preparation methods for improving PEEK's bioactivity for orthopedic and dental application: a review. International Journal of Biomaterials. 2016: 8202653.
Beauvais, S., and Decaux, O. 2007. Plasma sprayed biocompatible coatings on PEEK implants. Thermal Spray 2007: Global Coating Solutions. 371-377.
Boonyawan, D., Waruriya, P., and Suttiat, K. 2016. Characterization of titanium nitride–hydroxyapatite on PEEK for dental implants by co-axis target magnetron sputtering. Surface and Coatings Technolgy. 306: 164-170.
Buranapanich, V., Boonyawan, D., Sutiat, K., and Yavirach, P. 2020. Effect of temperature and treatment time of hydrothermal treatment on crystallization of titanium nitride-hydroxyapatite films coated on polyetheretherketone. p.080001. AIP Conference Proceedings. AIP Publishing LLC.
De Groot, K., Wolke, J.G.C., and Jansen, J.A. 1998. Calcium phosphate coatings for medical implants. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine. 212: 137-147.
Ding, S.-J., Ju, C.-P., and Lin, J.-H.C. 1999. Immersion behavior of RF magnetron-assisted sputtered hydroxyapatite/titanium coatings in simulated body fluid. Journal of Biomedical Materials Research. 47: 551-563.
Durham III, J.W. and Rabiei, A. 2016. Deposition, heat treatment and characterization of two layer bioactive coatings on cylindrical PEEK. Surface and Coatings Technology. 301: 106-113.
Fiume, E., Magnaterra, G., Rahdar, A., Verné, E., and Baino, F. 2021. Hydroxyapatite for biomedical applications: a short overview. Ceramics. 4: 542-563.
Goharian, A. 2019. Osseoconductive bioactive surface layering for enhancement of osseointegration. p.119-140. In: Goharian, A. (ed) Osseointegration of Orthopaedic Implants. Academic Press, Cambridge.
Ha, S.-W., Mayer, J., Koch, B., and Wintermantel, E. 1994. Plasma-sprayed hydroxylapatite coating on carbon fibre reinforced thermoplastic composite materials. Journal of Materials Science: Materials in Medicine. 5: 481-484.
Hahn, B.-D., Park, D.-S., Choi, J.-J., Ryu, J., Yoon, W.-H., Choi, J.-H., Kim, J.-W., Ahn, C.-W., Kim, H.J., Yoon, B.-H. et al. 2013. Osteoconductive hydroxyapatite coated PEEK for spinal fusion surgery. Applied Surface Science. 283: 6-11.
Hung, K.-Y., Lai, H.-C., and Feng, H.-P. 2017. Characteristics of RF-sputtered thin films of calcium phosphate on titanium dental implants. Coatings. 7: 126.
Kokubo, T., and Takadama, H. 2006. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 27: 2907-2915.
Kurtz, S.M., and Devine, J.N. 2007. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 28: 4845-4869.
Liu, Y., Rath, B., Tingart, M., and Eschweiler, J. 2020. Role of implants surface modification in osseointegration: a systematic review. Journal of Biomedical Materials Research Part A. 108: 470-484.
Ma, R., and Tang, T. 2014. Current strategies to improve the bioactivity of PEEK. International Journal of Molecular Sciences. 15: 5426-5445.
Muresan, L.M. 2015. Corrosion protective coatings for Ti and Ti alloys used for biomedical implants. p.585-602. In: Tiwari, A., Rawlins, J., and Hihara, L.H. (ed) Intelligent Coatings for Corrosion Control. Butterworth-Heinemann, Boston.
Nupangtha, W. and Boonyawan, D. 2017. Fabrication and physical properties of titanium nitride/hydroxyapatite composites on polyether ether ketone by RF magnetron sputtering technique. Journal of Physics: Conference Series. IOP Publishing. 901: 012131
Ozeki, K., Aoki, H., and Fukui, Y. 2006. Dissolution behavior and in vitro evaluation of sputtered hydroxyapatite films subject to a low temperature hydrothermal treatment. Jounal of Biomedical Materials Research Part A. 76: 605-613.
Ozeki, K., Masuzawa, T., and Aoki, H. 2017. Fabrication of hydroxyapatite thin films on polyetheretherketone substrates using a sputtering technique. Materials Science and Engineering C: Materials for Biological Applications. 72: 576-582.
Ozeki, K., Mishima, A., Yuhta, T., Fukui, Y., and Aoki, H. 2003. Bone bonding strength of sputtered hydroxyapatite films subjected to a low temperature hydrothermal treatment. Bio-medical Materials and Engineering. 13: 451-463.
Rabiei, A., and Sandukas, S. 2013. Processing and evaluation of bioactive coatings on polymeric implants. Journal of Biomedical Materials Research Part A. 101: 2621-2629.
Remache, D., Balcaen, Y., Demnati, I., Grossin, D., Alexis, J., and Bertrand, G. 2019. Delamination study of hydroxyapatite coatings for bone orthopedic implants. 24e me Congre s Français de Mécanique. Brest, 26-30 Aug 2019.
Sattha, C., Sakdanuphab, R., Kedkaew, C., and Sakulkalavek, A. 2019. Modelling of titanium oxynitride films for decorative coating by using response surface methodology. Journal of Physics: Conference Series. IOP Publishing. 1259: 012019
Shi, X., Xu, L., Munar, M.L., and Ishikawa, K. 2015. Hydrothermal treatment for TiN as abrasion resistant dental implant coating and its fibroblast response. Materials Science and Engineering C: Materials for Biological Applications. 49: 1-6.
Sinha, A., Ingle, A., Munim, K.R., Vaidya, S.N., Sharma, B.P., and Bhisey, A.N. 2001. Development of calcium phosphate based bioceramics. Bulletin of Materials Science. 24: 653-657.
Su, Y., Cockerill, I., Zheng, Y., Tang, L., Qin, Y.-X., and Zhu, D. 2019. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioactive Materials. 4: 196-206.
Wang, Y., Zhang, Y., and Miron, R.J. 2016. Health, maintenance, and recovery of soft tissues around implants. Clinical Implant Dentistry and Related Research. 18: 618-634.
Xu, S., Long, J., Sim, L., Diong, C.H., and Ostrikov, K. 2005. RF plasma sputtering deposition of hydroxyapatite bioceramics: synthesis, performance, and biocompatibility. Plasma Processes and Polymers. 2: 373-390.
Zhang, S., Wang, Y.S., Zeng, X.T., Khor, K.A., Weng, W., and Sun, D.E. 2008. Evaluation of adhesion strength and toughness of fluoridated hydroxyapatite coatings. Thin Solid Films. 516: 5162-5167.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Kwanchanok Koonrungsesomboon 1, Dheerawan Boonyawan 2, Kullapop Suttiat 1, and Piriya Yavirach 1, *
1 Department of Prosthodontics, Faculty of Dentistry, Chiang Mai University, Chiang Mai 50200, Thailand
2 Plasma and Beam Physics Research Facility, Department of Physics and Materials Science, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
Corresponding author: Piriya Yavirach, E-mail: piriyavdx-18@hotmail.com
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: February 20, 2022;
Revised: April 23, 2022;
Accepted: April 25, 2022;
Published online: May 12, 2022

