
Effects of Carbonate Hydroxyapatite (CHA) on the Development of Heart and Cranium Cartilage of Zebrafish (Danio rerio Hamilton, 1882) Larvae
Sandi Fransisco Pratama, Bambang Retnoaji*, and Ika Dewi AnaPublished Date : 2022-07-11
DOI : https://doi.org/10.12982/CMUJNS.2022.041
Journal Issues : Number 3, July-September 2022
Abstract One of the most commonly utilized materials for dental implants is carbonate hydroxyapatite (CHA). However, its usage must be free of tissue toxicity. Therefore, this study aimed to investigate how the use of CHA as a dental implant material affected the development of zebrafish (Danio rerio) embryos. CHA treatment was administered to 3–3.5 hours to 72 hours post-fertilization (hpf) embryos. Alterations in embryo and larva shape were all studied to determine toxicity. Several abnormalities that might arise during development were also examined by monitoring morphological alterations in embryos and larvae. Furthermore, heart morphology in larvae aged 72 hpf; heart rate in embryos aged 24, 48, and 72 hpf; and cardiac histology structure in larvae aged 30 dpf were all used to study heart development. Alizarin Red and Alcian Blue (ARAB) staining was employed to determine the anatomy of the cranial cartilage in larvae aged 6 dpf. Data were statistical analysis with SPSS ver. 21 and the significance was determined using one-way analysis of variance (ANOVA). The morphological examination results revealed that the embryo and larvae had no morphological abnormalities. Furthermore, the heart was developing normally, according to examinations of morphology, histology, and heart rate. The cranial cartilage had no flaws, the structure was complete, and the length and angle of the cranial cartilage did not change between the control and CHA treatments. Overall, CHA exposure did not affect the development of zebrafish (Danio rerio) embryos, according to the findings.
Keywords: CHA, Cranium, Embryo, Heart, Toxicity, Zebrafish
Funding: The authors are grateful for the research and study funding provided by the LPDP RI Scholarship.
Citation: Pratama, S.F., Retnoaji, B., and Ana, I.D. 2022. Effects of carbonate hydroxyapatite (CHA) on the development of heart and cranium cartilage of zebrafish (Danio rerio Hamilton, 1882) larvae. CMU J. Nat. Sci. 21(3): e2022041.
INTRODUCTION
Carbonate hydroxyapatite (CHA) is a type of ceramic biomaterial that is widely used as a material for bone and dental implants (Donath et al., 1987; Endres et al., 2003; Debelian et al., 2016). CHA, the main inorganic component of bones and teeth (Kumar et al., 2016), possesses bioactive and osteoconductive properties (Liao et al., 2011; Nagai et al., 2015). Such bioactivity related to its osteoconductivity allows the migration of osteoblasts to the surface of the material (Frayssinet et al., 1998) before directly binding to the bone. Due to these properties, CHA has been widely used in biomedical implants and bone and tooth regeneration applications (Ana et al., 2018; Oley et al., 2018).
When used as a dental implant material, CHA is placed in a location that is in direct contact with the periapical tissue; hence, its non-toxicity and biocompatibility are very important considerations (Escobar et al., 2016). The need for materials that are non-toxic and have good biocompatibility requires toxicity testing. Among the methods proposed in the literature, in vivo testing has gained immense relevance because all experimental settings are under controlled laboratory conditions. In particular, this assay allows a scientific explanation of the biocompatibility of dental materials (Pratama, 2021).
Zebrafish is one type of animal model that is widely used as a model animal for toxicity testing. These animals have the same embryonic development as mammals (Zhu et al., 2007; Hill et al., 2011). Given that zebrafish have genetic and physiological similarities with humans (Clemens et al., 2013; Stephens et al., 2016; Jiang et al., 2019), the toxicity of dental implant materials in zebrafish can be easily correlated with the latter. Therefore, the current study aimed to examine the effect of CHA on heart development and the structure of the cranium cartilage of zebrafish larvae.
MATERIAL AND METHODS
Materials
The materials used in this study included zebrafish wild type AB/TL, carbonate hydroxyapatite (synthesized by the Faculty of Dentistry, Universitas Gadjah Mada), egg water media (1,000 mL water, 1.5 mL salt water, and 1–2 drops methylene blue), Bouin’s fixative solution, alcohol (Genera Labora), aquadest (Genera Labora), xylol (Merck, Darmstadt, F.R. Germany), toluol (BDH, Analar), paraffin (Leica), Mayer’s albumin (Sigma), entellan (Merck KgaA, Darmstadt, F.R. Germany), hematoxylin (Himedia), eosin (Merk KgaA, Darmstadt, F.R. Germany), H2O2 3% (Merck KgaA, Darmstadt, F.R. Germany), KOH 2% (Merk KgaA, Darmstadt, F.R. Germany), Gliserin 100% (Merk KgaA, Darmstadt, F.R. Germany), and Alizarin Red and Alcian Blue (ARAB) stain solution.
Zebrafish embryo preparation
Zebrafish wild-type ABTL were kept in an aquarium measuring 20 × 20 × 30 cm. Zebrafish were reared under standard conditions: temperature of 27°C–28°C, light/dark periods of 14/10 hours, and dissolved oxygen 6–8. Embryos were obtained by spawning male and female brooders in a spawning aquarium. Spawning was done with a ratio of two males and one female. The spawning eggs were collected for research.
Zebrafish embryo decorionization
Decorionization was conducted according to the modified Henn and Braunbeck (2011) protocol. Zebrafish embryos were allowed to reach the blastulation stage (high to the oblong stage). This was to ensure that the selected embryos were live ones. The embryos were then transferred to a petri dish. Decorionization was subsequently performed under a surgical microscope using two sharps, pointed tweezers. The chorion was gently clamped with the tip of the tweezers and then torn off to form a larger gap after the chorion gap was formed. The presence of this gap ensured that the embryo was fully exposed to CHA.
Exposure of zebrafish embryo on CHA
The CHA used in this study was synthesized from the Faculty of Dentistry, Universitas Gadjah Mada. First, 100% pure CHA was dissolved in egg water media at different concentrations. The decorated embryos were transferred to well plates containing egg water and CHA at concentrations of 125, 500, and 2,000 g/mL. Each treatment concentration was replicated three times. Each replication consisted of 10 embryos, so that each concentration consisted of 30 embryos. The well plate was exposed to CHA at 14 light and 10 dark photoperiods, 28 °C temperature, and dissolved oxygen 6–8. The exposure was conducted from the dome phase embryo (3.5–4 hpf) to the 72 hpf embryo.
Larvae morphological observations
Morphological observations were performed by observing any morphological changes through stereo microscope observations and by taking photos. Several important parameters were assessed, including edema and blood accumulation, as well as head, tail, and yolk malformation. The characteristics of malformations that can occur during embryonic to larval development are shown in Figure 1. Morphological changes were assessed on zebrafish aged 24, 48, and 72 hpf.
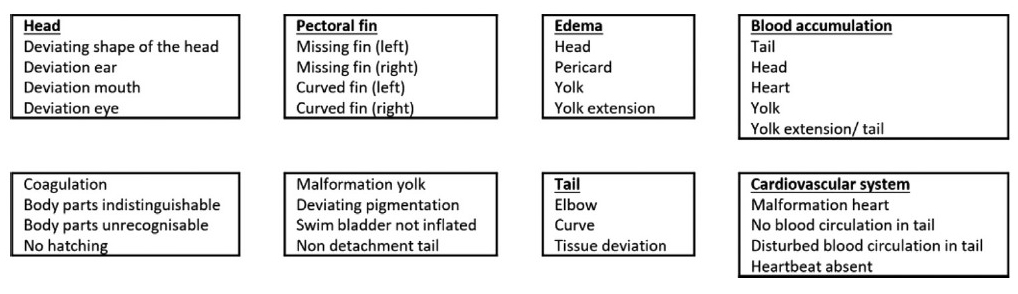
Figure 1. Descriptions of malformations during zebrafish embryonic development (Source: Hoyberghs et al., 2020)
Heart development observation
Heart morphology
Heart morphology observations were conducted on 72 hpf larvae. Every single larva of each treatment was placed in a petri dish and photographed under ventral and lateral views. The parameters that can be observed in cardiac morphology include the position of the atrium and ventricles, the presence of an indentation or barrier in the atrioventricular canal (AVC), and the distance between the sinus venosus (SV) and the bulbous arteriosus (BA). The SV-BA distance was measured using the ImageJ software. The data were statistically analyzed to determine the difference in the mean SV-BA lengths between treatments.
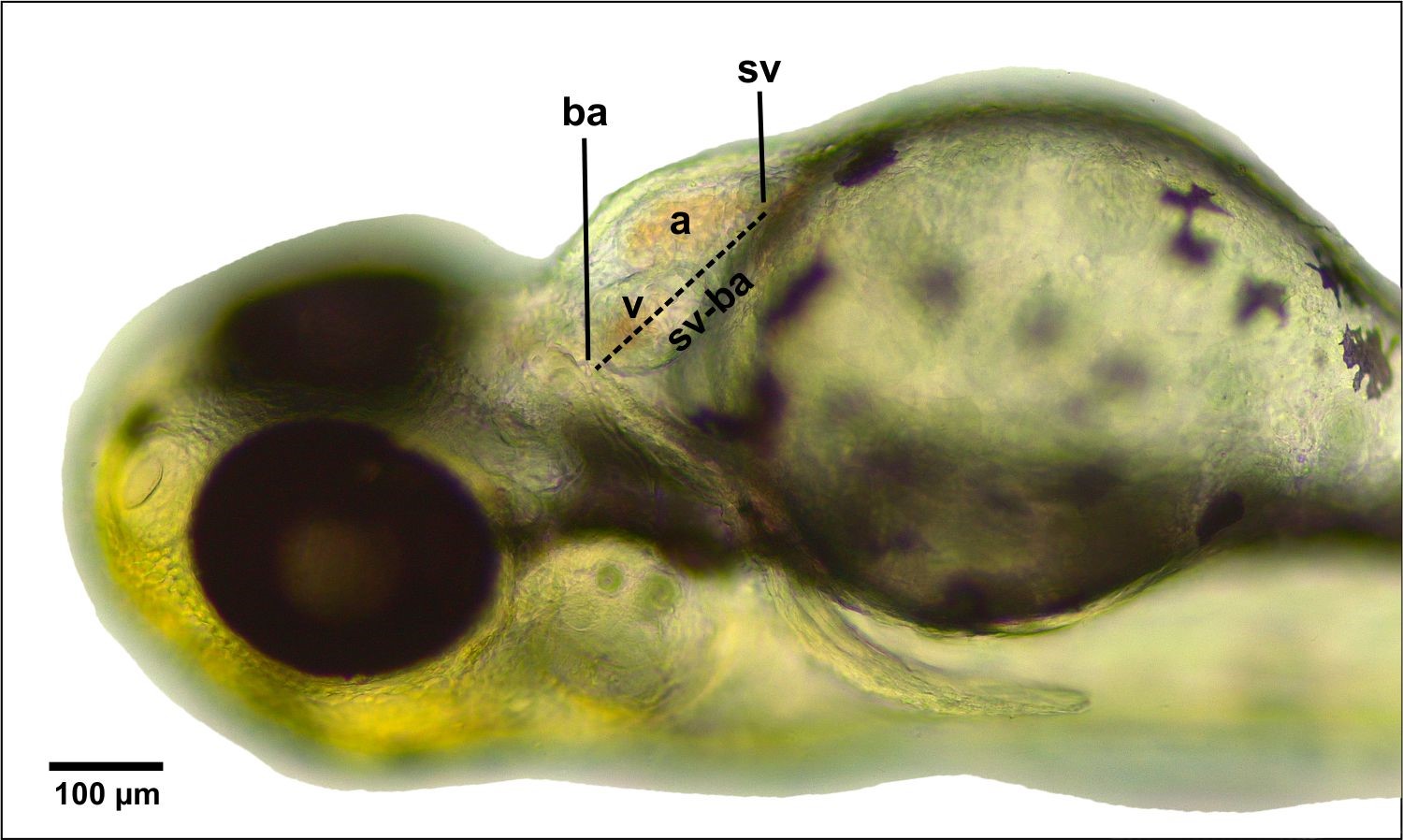
Figure 2. Measurement of sinus venosus and bulbous arteriosus distances. Abbreviations: (A) atrium, (V) ventricle, (BA) bulbous arteriosus, (SV) sinus venosus. Bar scale: 100 µm.
Heart Histology
Zebrafish larvae aged 30 dpf were euthanized for the cardiac histology preparations. Three larvae per replication in each treatment were prepared for the subsequent histological observations. The larvae were fixed using a solution for one night. Then, they were decalcified using nitric acid, washing, and fixation using alcohol (70%, 80%, 90%, 96%, and 100%); cleared using toluol; and embedded on paraffin. The embedded larvae were then cut (5 µm thick with transverse sections) using a rotary microtome. Staining was done by double staining hematoxylin and eosin. Photographs were taken using a Leica ICC50 HD microscope. The captured images were observed for the orientation and position of the ventricular muscle structure and the heart (atria and ventricles).
Observation of Cranial Cartilage Structure
Larvae aged 5 dpf were observed to determine the structure of the cranial cartilages. ARAB stains were used to stain the bones and cartilages of the cranium. Staining followed the protocol of Retnoaji et al. (2014) and was slightly modified. Larvae collection was performed using a 2 mL microtube. Next, the larvae were fixed using 96% alcohol for 2 days and then stained with ARAB (Et-OH 100%, Alcian Blue, Alizarin Red, Acetic Acid, Water) for 3 days. The ARAB solution was discarded, after which the larvae were washed using distilled water until clean. To these, 1 mL of bleach solution (H2O2 3% and KOH 2%) was added, and each sample was allowed to stand for 10 minutes. This bleach solution helped remove the color pigment in the larvae. Once the bleach solution was discarded, 1 mL of 0.05% KOH was added, and the samples were again allowed to stand for 5 minutes. The 0.05% KOH was replaced with a mol solution (20 mL of 20% Glycerin + 1 mL of 1% KOH + 79 mL of distilled water), then allowed to stand for 12–24 hours. Finally, the larvae were transferred to 3% methylcellulose for shooting.
Photographs were taken using a Leica ICC50 microscope, and the captured images were measured using the ImageJ software. Several measurement parameters are shown in Figure 3. Data analysis was performed using ANOVA to determine the significance between treatments (P < 0.05).
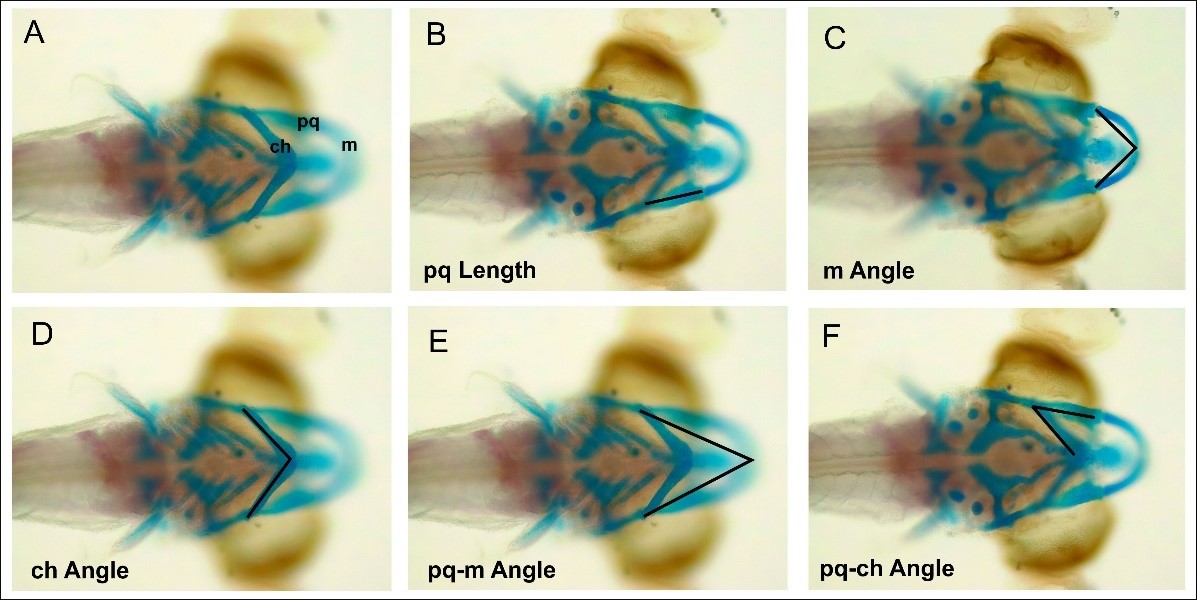
Figure 3. Measurement of the angle and length of the cranium of a zebrafish that has been stained with ARAB staining. (A) Structure of the cranium. (B) pq length. (C). m-length. (D) ch angle. (E) pq-m angle. (F) pq-ch ang
RESULTS
Effects of CHA on Larvae Morphology
Morphological changes are important parameters in assessing the effects of chemical and material toxicity. In this work, several important parameters were assessed, including edema and blood accumulation, as well as head, tail, and yolk malformation. Morphological observations on larvae aged 72 hpf (Figure 4) showed no malformations in larvae.
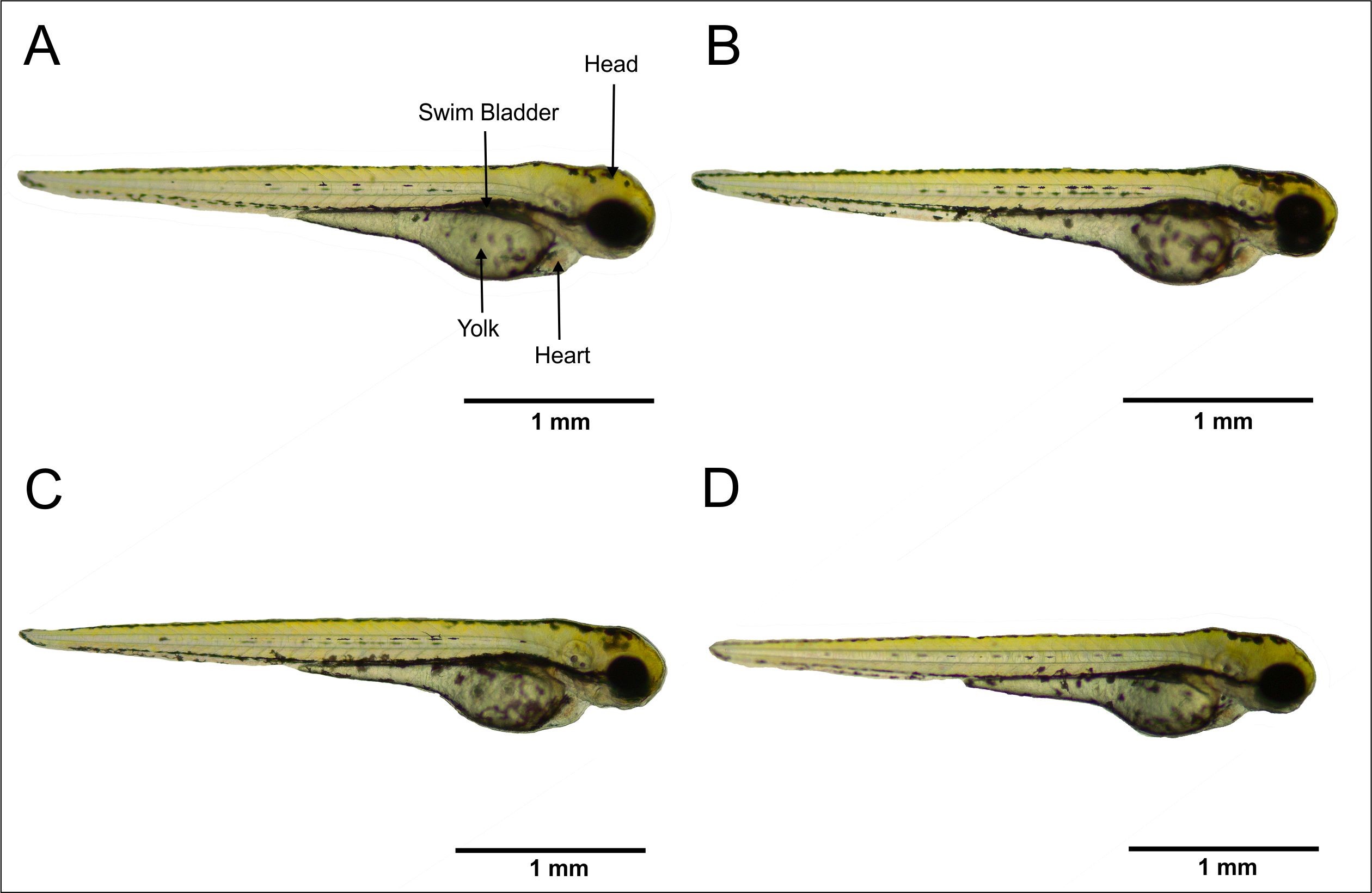
Figure 4. Morphologies of 72 hpf zebrafish larvae. (A) Control. (B) CHA concentration 125 µg/mL. (C) CHA concentration 500 µg/mL. (D) CHA concentration 2,000 µg/mL. Bar scale 1 mm.
Table 1. Malformation assessment in zebrafish morphology.
|
Malformation Parameter |
Treatment |
|
||||||
|
Control |
CHA 125 µg/mL |
CHA 500 µg/mL |
CHA 2000 µg/mL |
|||||
|
Head Malformation |
x |
x |
x |
x |
||||
|
Edema |
x |
x |
x |
x |
|
|||
|
Tail Malformation |
x |
x |
x |
x |
|
|||
|
Blood Coagulation |
x |
x |
x |
x |
|
|||
|
Yolk Malformation |
x |
x |
x |
x |
|
|||
Explanation: The cross (x) indicates that there is no malformation
Table 1 shows several parameters for observing embryonic malformations in zebrafish larvae. Observations on the head involve several parameters of malformations, including head shape, mouth, ear, and eye deviations. Based on the observations, it is known that there is no malformation on the head. Observations related to the possibility of edema were performed using several parameters, including elongation of the yolk and edemas of the head, pericardium, and yolk. The observations revealed that there were no edemas in these parts. Furthermore, there was no malformation in the tail of the zebrafish in both the control and the CHA treatment groups with concentrations of 125, 500, and 2,000 µg/mL.
In addition, blood clotting was used as a parameter for abnormalities during development. These blood clots can be found in several parts, including the head, tail, yolk, and the heart. The results of the observations revealed that there were no blood clots in some parts of the body. Based on the results of morphological observations shown in Table 1, it can be concluded that there were no malformations in zebrafish larvae in both the control and the CHA treatment groups at concentrations of 125, 500, and 2,000 µg/mL.
Effects of CHA on Heart Development
Heart Morphology
Morphological observations were made by observing several malformations that might occur in the heart. Some of the malformations that can be found in the heart include pericardial edema, the orientation of the ventricles and atria, and a clear boundary in the AVC zone. The results of cardiac morphology observations (Figure 5) revealed that there was no pericardial edema, both in the control and in the CHA treatment groups.
In addition to the observation of pericardial edema, another parameter that was examined was the orientation of the ventricles and atria. The observations showed that the control and CHA treatments were located side by side on both sides, forming an S-shaped structure. Moreover, in the control and CHA treatments, there was also a clear boundary between the atria and ventricles. This boundary is known as the atrioventricular canal.
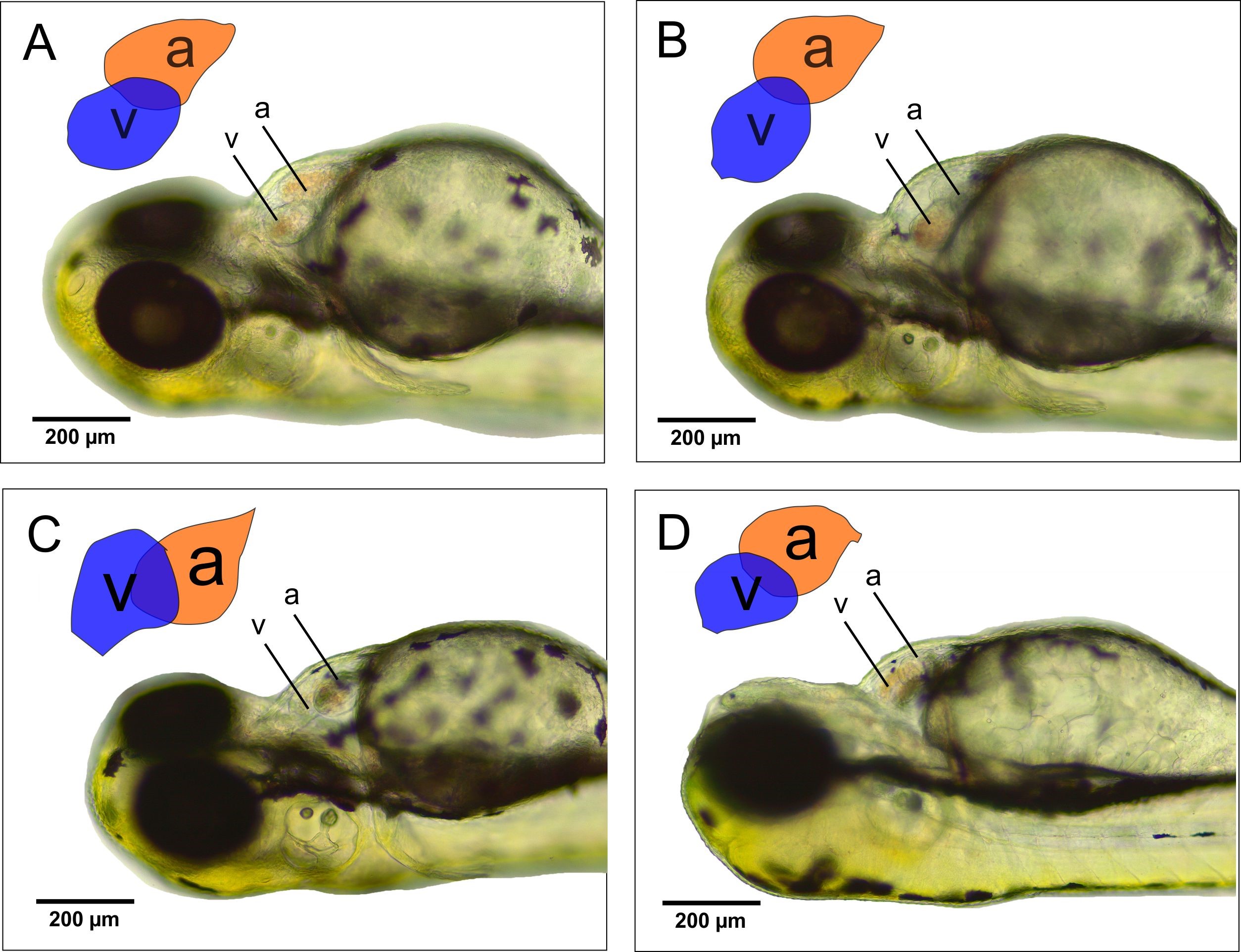
Figure 5. Effects of CHA on zebrafish heart morphology. Abbreviations: (a) atrium, (v) ventricle. Bar scale 200 µm.
One of the parameters to measure the effects of CHA toxicity on zebrafish heart is to measure the distance between the blood inlet in the SV and the outlet in the BA. Based on the measurement results using the ImageJ software (Figure 5), the SV-BA distance in the control treatment is 192.2 µm, and the concentrations of CHA treatment were 125 g/mL; 196.2 µm, 500 g/mL; 182.9 µm and 2,000 g/mL; and 180.8 µm. Statistical analysis using ANOVA (P < 0.05) revealed no significant difference between the control treatment and the CHA treatment with concentrations of 125, 500, and 2,000 µg/mL.
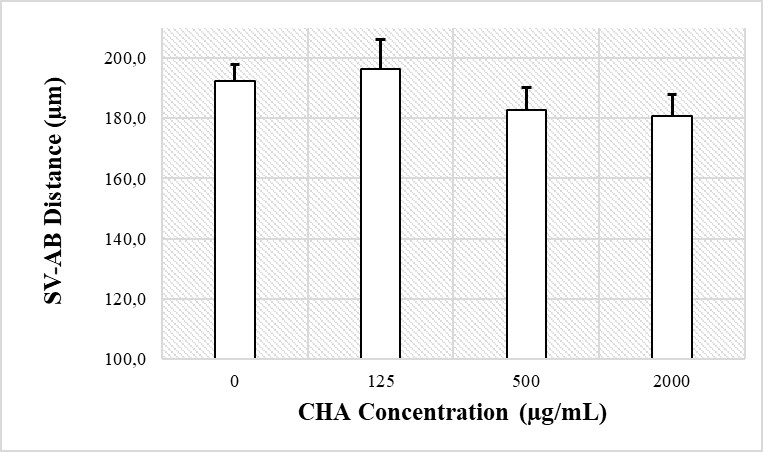
Figure 6. Graph of the average SV-BA distance of 72 hpf zebrafish larvae in the control and CHA treatment groups at concentrations of 125, 500, and 2,000 µg/mL. The results of the ANOVA statistical test (P < 0.05) showed no significant difference between the two groups.
Heartbeat Rate
Observations were made at several periods of zebrafish embryo development, including 24, 48, and 72 hpf (Figure 7). The results of the observation of the embryonic heart rate at 24 hpf revealed that the embryonic heart rate in the control treatment was 123 beats/minute. Meanwhile, the embryonic heart rate in the CHA treatment with concentrations of 125 µg/mL were 114 beats/min, 500 µg/mL; 111 beats/min, and 2,000 µg/mL; and 106 beats/minute. Statistical tests using ANOVA (P < 0.05) revealed no significant difference in heart rate in the control and CHA treatments with concentrations of 125, 500, and 2,000 µg/mL.
Furthermore, heart rate observations were performed on 48 hpf age zebrafish. The results showed that the average number of heartbeats in the control treatment was 150 beats/minute. Meanwhile, the embryonic heart rate in the CHA treatment with a concentration of 125 µg/mL were 147 beats/min, 500 µg/mL; 155 beats/min, 2,000 µg/mL; and 151 beats/minute. The results of statistical testing using ANOVA (P < 0.05) showed that there was no significant difference between the control treatment and the CHA treatment.
Observations of the number of heartbeats were also conducted on 72 hpf zebrafish. The results of the observations of the heart rate of larvae aged 72 hpf showed that the average heart rate of larvae in the control treatment was 187 beats/minute. Meanwhile, the heart rate of zebrafish larvae in CHA treatment with several concentrations, including concentrations of 125 µg/mL were 178 beats/min, 500 µg/mL; 176 beats/min, and 2,000 µg/mL; and 176 beats/minute. Statistical testing using ANOVA (P < 0.05) showed that no significant difference between the control and the CHA treatments with concentrations of 125, 500, and 2,000 µg/mL.
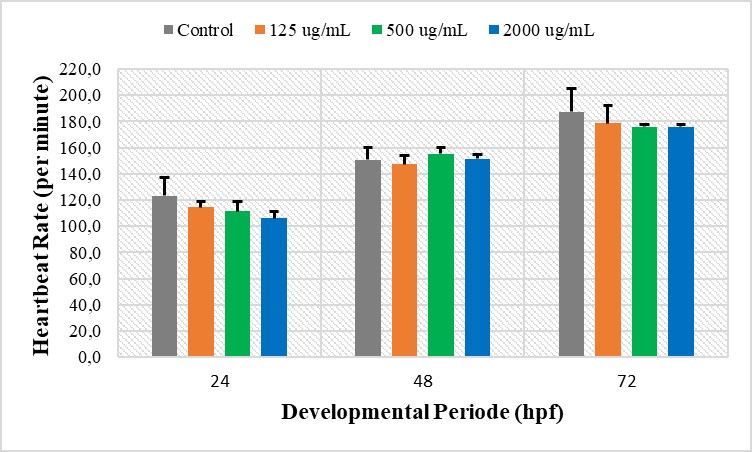
Figure 7. Heart rate graphs at different periods of zebrafish development, including 24, 48, and 72 hpf. The results of the ANOVA statistical test (P < 0.05) showed no significant difference between the control and CHA treatment groups.
Heart Histology
During the early stages of embryonic development, exposure to certain chemicals can cause a variety of structural malformations of the heart. These malformations can include abnormalities in the orientation and position of the heart, as well as the heart muscle. In this study, the orientation and position of the heart (atrium and ventricles) and the histological structure of the ventricles were observed.
In the transverse section of zebrafish larvae (Figure 8), we can see the structure of the internal organs of zebrafish, including the spinal cord (sc), notochord (nc), operculum (o), esophagus (e), ventricle (v), and atrium (a). The results revealed the locations of the heart in the ventral part of the esophagus. The heart was also found to be symmetrically located in the dorsal middle. Thus, there was no abnormality in the looping process during cardiac morphogenesis. In an abnormal heart, the position of the heart tends to be asymmetrical.
Histologically, the CHA treatments with concentrations of 125 µg/mL (Figure 8B), 500 µg/mL (Figure 8C), and 2000 µg/mL (Figure 8D) all showed normal orientations and positions similar to the control treatment (Figure 8A). The differences in the size of the atria and ventricles in each treatment were caused by the size of the fish and the serial differences at the time of cutting using a microtome.
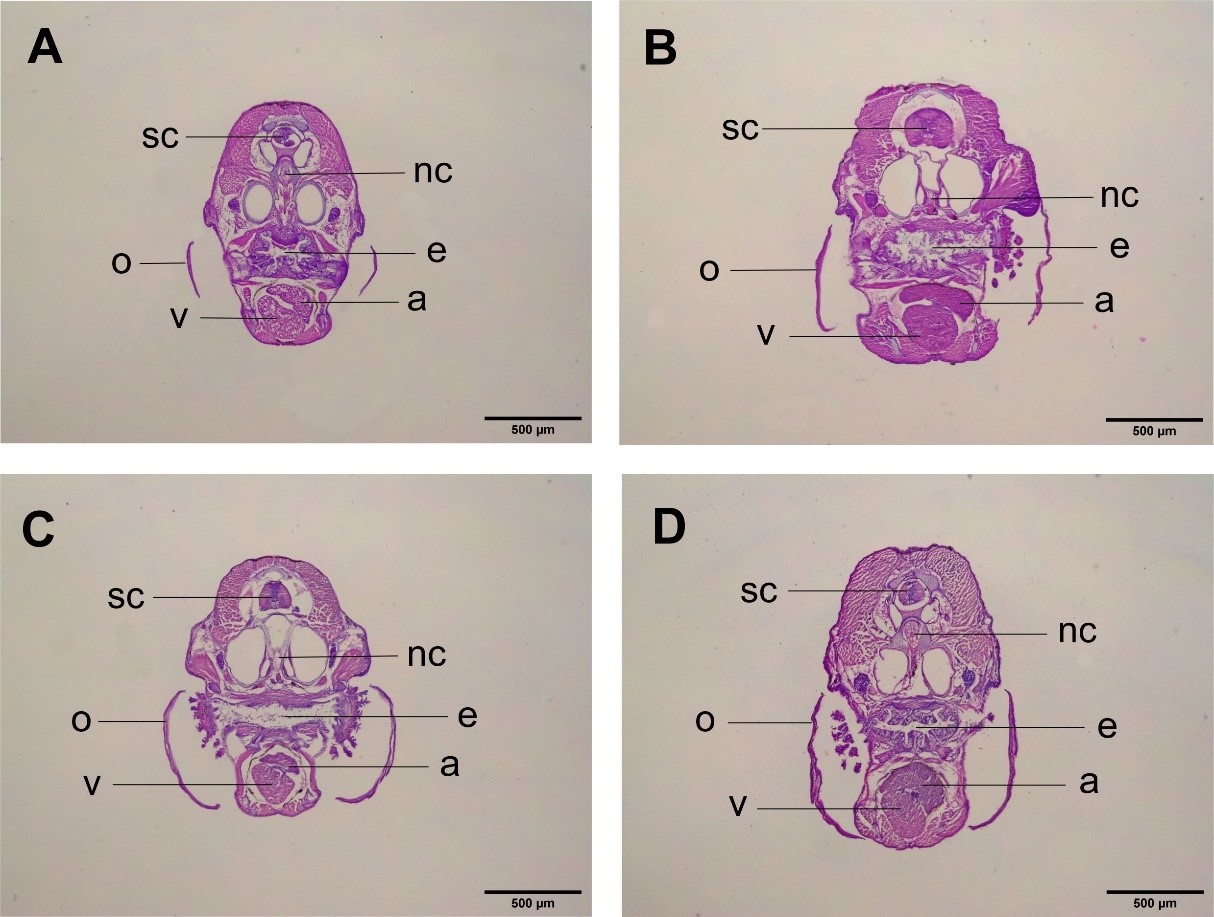
Figure 8. Histological structure of 30 dpf zebrafish organs with transverse sections. (A) Control. (B) CHA concentration of 125 µg/mL. (C) CHA concentration of 500 µg/mL. (D) CHA concentration of 2000 µg/mL. (sc) spinal cord. (nc) noto cord. (o) operculum. (e) esophagus. (v) ventricles. (a) atrium. 4× magnification. Bar scale 500 µm.
The histological structure of the ventricle through a transverse section (Figure 9) showed the structure of the zebrafish ventricle. The myocardium consisted of a compact myocardium (cm) on the outside and a trabecula myocardium (tm) on the inside. Between the trabecula myocardium, we found the trabecula (t), which was filled by blood cells normally pumped by the ventricles. The pumping of blood by the heart muscle is rapid and occurs continuously. This is supported by the characteristics of cardiac muscle, including striated muscle fibers (Figure 9, box). The results also showed intercalations between fibers (Figure 9, arrows) and the nucleus contained in each cell.
The results of the observation of the histological structure of the ventricles in the control treatment (Figure 9A) showed that the ventricles had a compact myocardium structure consisting of a compact external part and thickly arranged trabecula myocardium in the internal part. This is a normal structure of the myocardium of zebrafish. Furthermore, observations of the muscle structure showed that the muscle fibers were connected.
Observations on the ventricles of zebrafish treated with CHA (Figures 9A, B, and C) showed the same structure as the control treatment: the myocardium on the outside was compactly arranged and the trabecula myocardium was thickly arranged on the inside. In addition, the intercalation of the muscle fibers showed that the muscle fibers were connected.
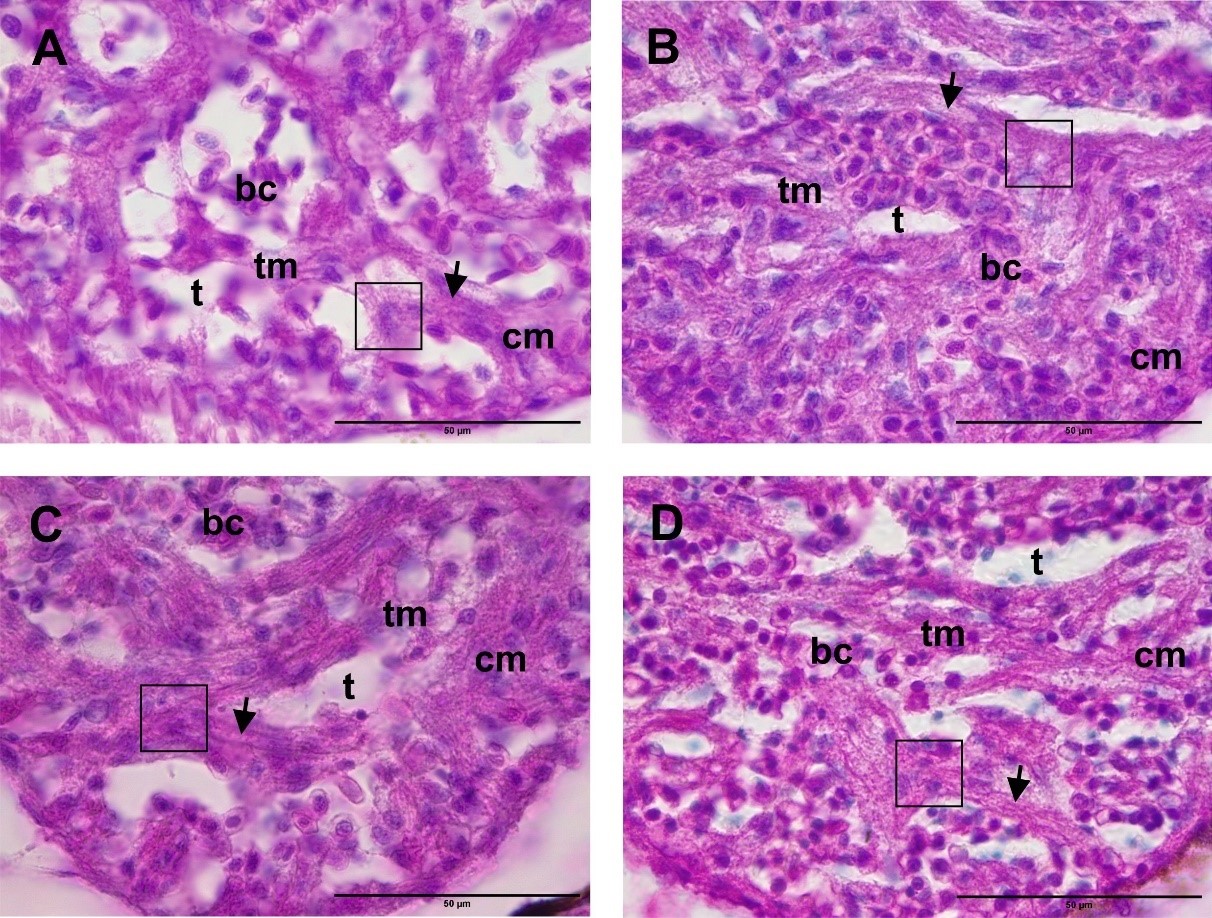
Figure 9. Histological structure of the heart (ventricles) with a transverse section. (A) Control. (B) CHA concentration of 125 µg/mL. (C) CHA concentration of 500 µg/mL. (D) CHA concentration of 2,000 µg/mL. (cm) compact myocardium. (tm) trabecula myocardium. (t) trabeculae. (bc) blood cells. (arrows) intercalation of muscle fibers. (box) muscle fibers. 100× magnification. Bar scale: 50 µm.
Effects of CHA on the Cartilage Cranium Structure
Cartilage Cranium Structure
In this study, larvae aged 6 dpf were stained with ARAB, producing red (alizarin red) in true bone (osteum) and blue (alcian blue) in the cartilage (cartilage). The results of this study (Figure 10) showed that in both the control treatment and CHA treatments with CHA concentrations of 125, 500, and 2,000 µg/mL all cartilage structures were stained and complete. Based on observations from the ventral side, several structures comprising the cranium can be observed, including the palatoquadrate (pq), Meckel’s cartilage (m), ceratohyal (ch), and ceratobranchial (cb). The results of visual observations also revealed that there were no defects, such as bending of the cartilage parts.
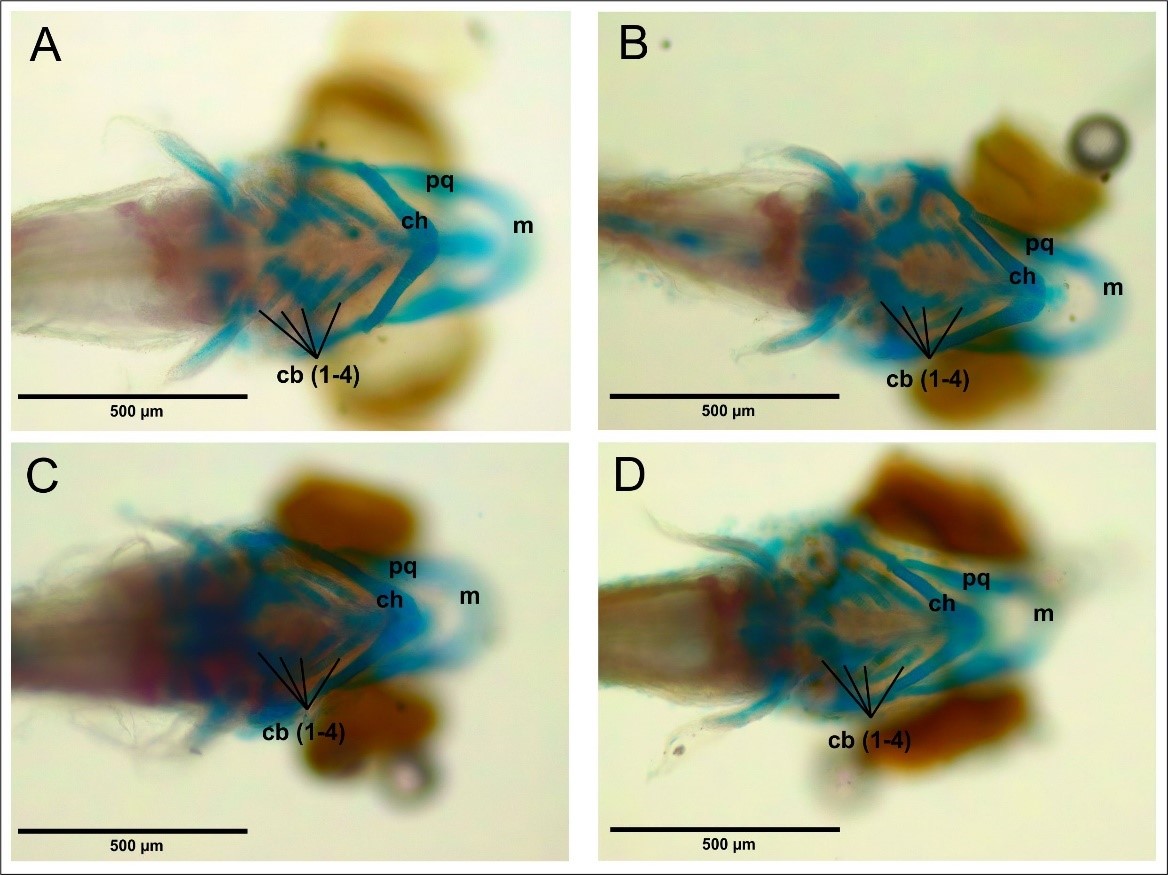
Figure 10. Cranial cartilage structure of 6 dpf zebrafish larvae can be seen ventrally. (A) Control. (B) CHA concentration 125 µg/mL. (C) CHA concentration 500 µg/mL. (D) CHA concentration 2000 µg/mL. (pq) palatoquadrate. (m) Meckel’s cartilage. (ch) ceratohyal. (cb) ceratobranchial. 40x magnification. Bar scale 500 µm.
Cartilage Cranium Assessment
Apart from morphological observations of the cranium, the length and angle of the cartilage components that make up the cranium were also observed. Some of these observations include the palatoquadrate length (pq), the Meckel’s cartilage angle (m), the ceratohyal angle (ch), the ceratohyal angle (ch), the palatoquadrate and Meckel’s cartilage angles (pq-m), and the palatoquadrate and ceratohyal angles (pq-ch) (Figure 11).
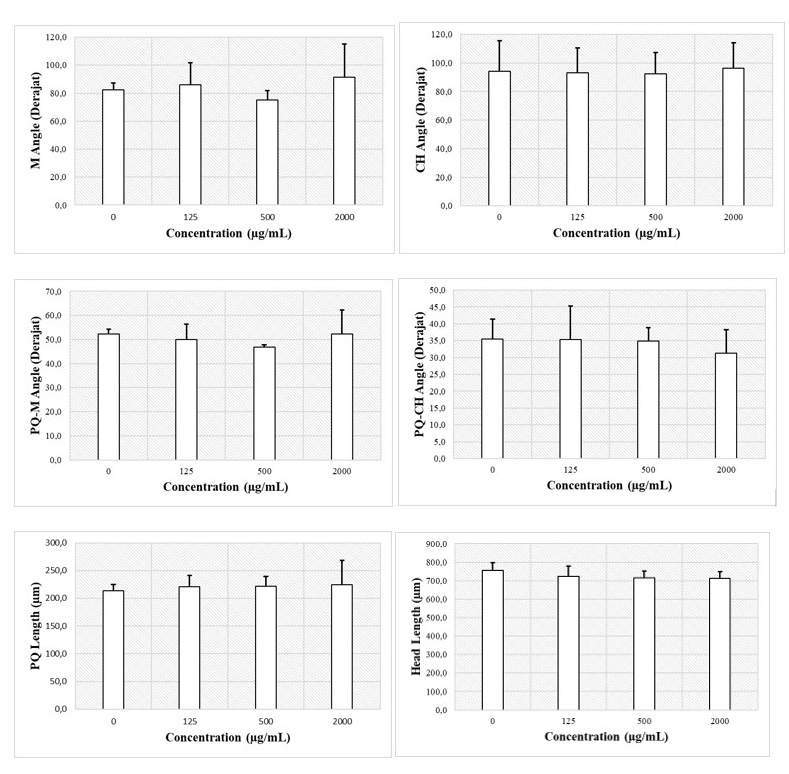
Figure 11. Assessment of the structure of the cranium cartilage by measuring the angles and lengths of each cranium cartilage.
CHA exposure to zebrafish embryos is known to not affect changes in the cranial morphology of zebrafish larvae. The results of the study showed that the M angles of embryos treated with CHA at concentrations of 125, 200, and 2,000 µg/mL did not differ significantly compared to the control treatment (Figure 11A, P < 0.05). Furthermore, the ceratohyal angles did not differ significantly between CHA treatments of 125, 500, and 2,000 µg/mL and the control treatments (Figure 11B, P < 0.05). The angle formed between the palatoquadrate and Meckel’s did not differ significantly between CHA treatments of 125, 500, and 2,000 µg/mL with control treatment (Figure 11C, P < 0.05). The angle formed between the palatoquadrate and ceratohyal did not differ significantly between CHA treatments of 125, 500, and 2000 µg/mL with control treatments (Figure 11D, P < 0.05). The palatoquadrate length did not differ significantly between treatments at CHA concentrations of 125, 500, and 2,000 µg/mL and the control treatment (Figure 10E, P < 0.05). The head lengths of control treatment and CHA treatments of 125, 500, and 2,000 µg/mL also did not differ significantly (Figure 11E, P < 0.05). In general, CHA treatment had no significant effect (P < 0.05) on the length and size of the cranial cartilage angle of zebrafish larvae.
DISCUSSION
This study provides an overview of the effects of CHA exposure on zebrafish embryo development. Several parameters were observed, including larval morphology, heart development, and cranial cartilage structure. The results of the morphological observations on larvae (Figure 4) showed that the larvae developed normally in both the control and CHA treatments at concentrations of 125, 500, and 2,000 µg/mL. Several indicators of malformations, such as edema and blood clots, as well as head, tail, and yolk malformations (Table 1), showed no abnormalities in larval development. These results indicate that the CHA treatment did not cause malformations in the early development of zebrafish larvae.
Apart from larval morphology, cardiac development is also a parameter that can describe the toxic effects of materials on the development of zebrafish embryos. The heart is the first organ formed in the development of these embryos. Thus, exposure to toxic chemicals early in development can cause malformations in cardiac morphology. In this study, cardiac abnormalities were observed by observing the morphology and histology of the heart.
The results of heart morphology observations (Figure 5) revealed that the control and CHA treatments were located side by side on both sides, forming an S-shaped structure. This result is in line with research conducted by Antkiewicz et al. (2005), who concluded that in a normal heart, the ventricles and atria will lie on both sides and indicste an S-shaped orientation. In comparison, in a heart exposed to toxicants, the ventricles and atria will form a straight line.
In addition, in the control and CHA treatments, there is also a clear boundary-known as the AVC-between the atrium and ventricles. In their study, Sarmah and Marrs (2016) stated that there is boundary in the AVC region inside the heart of a normally developing 50 hpf zebrafish (control). This boundary is the depression between the atria and ventricles. The existence of a clear boundary in the AVC zone indicates that zebrafish larvae developed normally, both in the control and CHA treatments.
Another parameter that can be used to measure the effect of CHA toxicity on the zebrafish heart is the distance between the blood inlet in the SV and the outlet in the BA (Figure 6). The SV-BA distance assessment was performed to determine the effect of CHA treatment on the heart looping of zebrafish embryos. Looping is a crucial step during the early stages of heart formation. At this stage, the heart tube that was originally straight turned into a curved tube. This is the basic pattern of a mature heart (Ramasubramanian et al., 2008). The distance between the SV-BA essentially presents a cardiac loop index, which can provide an overview of the malformations of the heart (Antkiewicz et al., 2005). In the current study, the results of the SV-BA distance measurement (Figure 6) showed no significant difference between the control treatment, on the one hand, and the CHA treatments with concentrations of 125, 500, and 2,000 µg/mL, on the other hand. Therefore, these results indicated that CHA exposure did not affect cardiac development in zebrafish larvae.
It has been reported that heart function is closely related to heart rate and blood flow (Männer et al., 2010). Abnormalities in the heart muscles will cause a decreased heart rate and low blood flow, which can lead to heart failure. Zhu et al. (2007) stated that the heart rate ranges from 140 to 160 beats/minute in a normal developing 48 hpf zebrafish embryo.
The results of this study also indicate that the number of heartbeats in embryos aged 48 hpf ranged from 140 to 160. In addition, there was an increase in the average number of heartbeats over time (24, 48, and 72 hpf). These results indicated normal heart rates of zebrafish embryos in both the control and CHA treatments.
Apart from the general position of the heart, the positions of the atria and ventricles can also describe abnormalities during the loop of the heart. In an abnormal heart, the atrial and ventricular positions usually form a straight line, while the normal one will be side by side to form an S. The transverse section (Figure 8) showed the atria (Figure 8a) and ventricles (Figure 8b) were located side by side. Furthermore, the ventricles were found to be located ventral to the atria. This orientations and positions are in line with the findings of Menke et al. (2011). Through a longitudinal section, Menke explained that the ventricles were located ventral to the anterior of the atrium.
Histologically, the CHA treatments with concentrations of 125 µg/mL (Figure 8B), 500 µg/mL (Figure 8C), and 2,000 µg/mL (Figure 8D) all showed normal orientations and positions, which were the same as the control treatment (Figure 8A). As mentioned previously, the differences in the size of the atria and ventricles in each treatment were caused by the size of the fish and the serial differences at the time of cutting using a microtome. Differences in serial cuts were also evidenced by different perspectives of other organs. Thus, the difference in the size of the atria and ventricles cannot be attributed to the treatment given. These results provide support to our observations of cardiac morphology, which indicated no abnormalities in the orientation and position of the heart.
The results of the observation of the histological structure of the ventricles in the control treatment (Figure 9A) showed that the ventricles had a myocardium structure that was compact on the outside and had thickly arranged trabecula myocardium on the inside. This represents a normal structure of zebrafish myocardium. Observations of the muscle structure also showed that the muscle fibers were connected. Furthermore, observations on the ventricles of zebrafish treated with CHA (Figures 9A, B, C) showed the same structure as the control treatment. The myocardium on the outside was compactly arranged and the trabecula myocardium was thickly arranged on the inside. In addition, the intercalation of the muscle fibers showed that the muscle fibers were connected.
Cranial malformations are an important parameter in assessing teratological effects on zebrafish development. In zebrafish, the process of cartilage formation starts at the age of 2 dpf embryo, and this cartilage structure further forms at 3 dpf (Kimmel et al., 1998). CHA, the main inorganic component of bone, is widely used as an implant material and bone regeneration application because of its bioactive, biodegradable, and osteoconductive properties. As a material, CHA is biocompatible, non-toxic, non-inflammatory, and immunogenic, and can form chemical bonds with the surrounding environment (Spence et al., 2008). CHA contains about 3%–5% carbonate ions with substitutions in the hydroxyapatite lattice and is a major component of bone. This composition allows CHA implants to increase bone formation (Patel et al., 2002)
In the current study, the structure of the cranial cartilage was assessed to determine the effect of CHA exposure. Several important parameters were also evaluated, including the length and angle of the cartilage in the cranium, the length of the cranium, and the completeness of the cartilage within the cranium. The results of statistical tests revealed that no differences between the control treatment and the CHA treatment on some of these observation parameters. Furthermore, exposure to CHA in zebrafish embryos did not result in malformations of the cranial cartilage. However, in contrast to Patel et al. (2002), who reported that CHA exposure can increase bone formation, the results of our study indicated that CHA exposure did not increase the growth of cartilage in the cranium.
The results of this study are in line with the research conducted by Oley et al. (2018), who conducted an in vivo test to investigate the effects of exposure to CHA and platelet-rich plasma (PRP) on bone regeneration in the rat cranium. They found that CHA exposure did not affect bone regeneration. Meanwhile, CHA combined with PRP can effectively increase bone regeneration. These findings indicate that CHA is not significantly able to increase bone growth. Instead, increased bone growth occurs when there is a combination of growth factors.
Meanwhile, bone growth and regeneration are related to four important properties in bone regeneration. First, the osteoconductive matrix acts as a scaffold or framework in which bone growth occurs. Second, osteoconductive factors stimulate bone growth, including growth factors, such as bone morphogenetic protein and transforming growth factor-β (TGF-β). Third, osteogenic cells include osteoblasts, primitive mesenchymal cells, and osteocytes, which guarantee structural integrity (Oley et al., 2018).
CHA provides an important osteoconductive matrix as a scaffold in bone regeneration. However, it does not have osteoconductive factors that can stimulate bone growth. In addition, there are no osteogenic cells in CHA, which is why cartilage growth in zebrafish cranium exposed to CHA does not show significant differences with the control treatment in our study.
CONCLUSION
This study was conducted to investigate the effects of exposure to CHA on the development of zebrafish embryos. Based on the findings, we can conclude that exposure to CHA at concentrations of 125, 500, and 2000 µg/mL did not affect the morphology and histological structure of the heart, the morphology of zebrafish larvae, and the structure of the cranium cartilages of larvae aged 6 dpf.
AUTHOR CONTRIBUTIONS
Sandi Francisco Pratama designed and conducted the experiments, performed statistical analysis, visualized data, and drafted the paper. Bambang Retnoaji and Ika Dewi Ana assisted in designing the experiment, visualizing the data, and drafting the paper. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Ana, I.D., Satria, G.A.P., Dewi, A.H., and Ardhani, R. 2018. Bioceramics for clinical application in regenerative dentistry. Novel Biomaterials for Regenerative Medicine, 1077: 309–316.
Antkiewicz, D.S., Burns, C.G., Carney, S.A., Peterson, R.E., and Heideman, W. 2005. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicological Sciences, 84: 368–377.
Clemens, D.M., Németh-Cahalan, K.L., Trinh, L., Zhang, T., Schilling, T.F., and Hall, J.E. 2013. In vivo analysis of aquaporin 0 function in zebrafish: Permeability regulation is required for lens transparency. Investigative Ophthalmology and Visual Science, 54: 5136–5143.
Debelian, G. and Trope, M. 2016. The use of premixed bioceramic materials in endodontics. Giornale Italiano di Endodonzia, 30: 70–80.
Donath, K., Rohrer, M.D., and Beck-Mannagetta, J. 1987. A histologic evaluation of a mandibular cross section one year after augmentation with hydroxyapatite particles. Oral Surgery, Oral Medicine, and Oral Pathology, 63: 651–655.
Endres, M., Hutmacher, D.W., Salgado, A.J., Kaps, C., Ringe, J., Reis, R.L., Sittinger, M., Brandwood, A., and Schantz, J.T. 2003. Osteogenic induction of human bone marrow-derived mesenchymal progenitor cells in novel synthetic polymer–hydrogel matrices. Tissue Engineering, 9: 689–702.
Escobar-García, D.M., Aguirre-López, E., Méndez-González, V., and Pozos-Guillén, A. 2016. Cytotoxicity and initial biocompatibility of endodontic biomaterials (MTA and Biodentine TM) used as root-end filling materials. BioMed Research International, 2016, 7926961.
Frayssinet, P., Fages, J., Bonel, G., and Rouquet, N. 1998. Biotechnology, material sciences and bone repair. European Journal of Orthopaedic Surgery and Traumatology, 8: 17–25.
Henn, K. and Braunbeck, T. 2011. Dechorionation as a tool to improve the fish embryo toxicity test (FET) with the zebrafish (Danio rerio). Comparative Biochemistry and Physiology - C Toxicology and Pharmacology, 153: 91–98.
Hill, A., Jones, M., Dodd, A., and Diekmann, H. 2011. A review of developmental toxicity screening using zebrafish larvae. Toxicology Letters, 205, S115.
Hoyberghs, J., Bars, C., Pype, C., Foubert, K., Ayuso Hernando, M., Ginneken Van, C., Ball, J., and Cruchten Van, S. 2020. Refinement of the zebrafish embryo developmental toxicity assay. MethodsX, 7, 101087.
Jiang, J., Lv, L., Wu, S., An, X., Wang, F., Liu, X., and Zhao, X. 2019. Developmental toxicity of kresoxim-methyl during zebra fish (Danio rerio) larval development. Chemosphere, 219: 517–525.
Kimmel, C.B., Miller, C.T., Kruze, G., Ullmann, B., Bremiller, R.A., Larison, K.D., and Snyder, H.C. 1998. The shaping of pharyngeal cartilages during early development of the zebrafish. Developmental Biology, 203: 245–263.
Kumar, G.S., Girija, E.K., Venkatesh, M., Karunakaran, G., Kolesnikov, E., and Kuznetsov, D. 2016. One step method to synthesize flower-like hydroxyapatite architecture using mussel shell bio-waste as a calcium source. Ceramics International, 43.
Liao, H., Qi, R., Shen, M., Cao, X., Guo, R., Zhang, Y., and Shi, X. 2011. Improved cellular response on multiwalled carbon nanotube-incorporated electrospun polyvinyl alcohol / chitosan nanofibrous scaffolds. Colloids and Surfaces B: Biointerfaces, 84: 528–535.
Männer, J., Wessel, A., and Yelbuz, T.M. 2010. How does the tubular embryonic heart work? Looking for the physical mechanism generating unidirectional blood flow in the valveless embryonic heart tube. Developmental Dynamics, 239: 1035–1046.
Menke, A.L., Spitsbergen, J.M., Wolterbeek, A.P.M., and Woutersen, R.A. 2011. Normal anatomy and histology of the adult zebrafish. Toxicologic Pathology, 39: 759–775.
Nagai, H., Kobayashi-Fujioka, M., Fujisawa, K., Ohe, G., Takamaru, N., Hara, K., Uchida, D., Tamatani, T., Ishikawa, K., and Miyamoto, Y. 2015. Effects of low crystalline carbonate apatite on proliferation and osteoblastic differentiation of human bone marrow cells. Journal of Materials Science: Materials in Medicine, 26: 99.
Oley, M.C., Islam, A.A., Hatta, M., Hardjo, M., Nirmalasari, L., Rendy, L., Ana, I.D., and Bachtiar, I. 2018. Effects of platelet-rich plasma and carbonated hydroxyapatite combination on cranial defect bone regeneration: An animal study. Wound Medicine, 21(March): 12–15.
Patel, N., Gibson, I.R., Hing, K.A., Best, S.M., Damien, E., Revell, P.A., and Bonfield, W. 2002. The in vivo response of phase pure hydroxyapatite and carbonate substituted hydroxyapatite granules of varying size ranges. Key Engineering Materials, 218–220: 383–386.
Pratama, S.F. 2021. Effect of Dental Implant Carbonate Hydroxyapatite (CHA) on the Embryonic Development of Zebrafish (Danio rerio (Hamilton, 1822)), Master Thesis, Universitas Gadjah Mada, Indonesia
Ramasubramanian, A., Nerurkar, N.L., Achtien, K.H., Filas, B.A., Voronov, D.A., and Taber, L.A. 2008. On modeling morphogenesis of the looping heart following mechanical perturbations. Journal of Biomechanical Engineering, 130: 061018.
Retnoaji, B., Akiyama, R., Matta, T., Bessho, Y., and Matsui, T. 2014. Retinoic acid controls proper head-to-trunk linkage in zebrafish by regulating an anteroposterior somitogenetic rate difference. Development, 141: 158–165.
Sarmah, S. and Marrs, J.A. 2016. Zebrafish as a vertebrate model system to evaluate effects of environmental toxicants on cardiac development and function. International Journal of Molecular Sciences, 17: 2123
Spence, G., Phillips, S., Campion, C., Brooks, R., and Rushton, N. 2008. Bone formation in a carbonate-substituted hydroxyapatite implant is inhibited by zoledronate: The importance of bioresorption to osteoconduction. Journal of Bone and Joint Surgery, British Volume, 90: 1635–1640.
Stephens, W.Z., Burns, A.R., Stagaman, K., Wong, S., Rawls, J.., Guillemin, K., and Bohannan, B.J.M. 2016. The composition of the zebrafish intestinal microbial community varies across development. The ISME Journal, 10: 644–654.
Zhu, X., Zhu, L., Li, Y., Duan, Z., Chen, W., and Alvarez, P.J. 2007. Short communication developmental toxicity in Zebrafish (Danio Rerio) embryos after exposure to manufactured nanomaterials : Buckminsterfullerene aggregates (nC 60) and Fullerol. Environmental Toxicology and Chemistry, 26: 976–979.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Sandi Fransisco Pratama1, Bambang Retnoaji1,*, and Ika Dewi Ana2
1 Faculty of Biology, Universitas Gadjah Mada, Sleman 55281, Indonesia
2 Faculty of Dentistry, Universitas Gadjah Mada, Sleman 55281, Indonesia
Corresponding author: Bambang Retnoaji, E-mail: bambang.retnoaji@ugm.ac.id
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: December 4, 2021;
Revised: April 4, 2022;
Accepted: April 21, 2022;
Published online: April 28, 2022

