
Effect of Pre-And Postharvest Treatments with Salicylic Acid on Physicochemical Properties of Pineapple cv. MD2
Diego Mauricio Cano-Reinoso, Loekas Soesanto1, Kharisun, and Condro Wibowo*Published Date : 2022-07-11
DOI : https://doi.org/10.12982/CMUJNS.2022.039
Journal Issues : Number 3, July-September 2022
Abstract Salicylic acid applied pre-and postharvest can impact positively the fruit quality, although more experiments are necessary to clarify its influence on pineapple. Therefore, this study aimed to evaluate the effect of pre-and postharvest treatments with salicylic acid on physicochemical properties of MD2 pineapple. Treatments were, A (Control: Waxing but without SA application pre-and postharvest), B (SA (2 mM) preharvest (sprayed) + SA (5 mM) postharvest (dipping)), C SA (2 mM) preharvest (sprayed) + SA (7 mM) postharvest (dipping)) and D (SA (2 mM) preharvest (sprayed) + SA (9 mM) postharvest (dipping)). Fruit total soluble solids, total acidity, ascorbic acid, β-carotene, fruit weight, percentage of weight loss, firmness, respiration rate, shell colour and flesh translucency were examined. The ascorbic acid, respiration rate and flesh translucency were determined as the variables more influential in this study. Treatments with postharvest concentrations higher than 5 mM increased the ascorbic acid (˃ 300 mg/kg) and reduced the translucency incidence (˂ 20 %), while with postharvest concentrations between 5-7 mM reduced the respiration rate (˂ 12 mL CO2/kg*h). All the treatments provided ideal values for the rest of the quality variables studied. Finally, treatment C was considered the most beneficial for the fruit, delivering the most elevated ascorbic acid content (385.89 mg/kg), the lowest respiration rate (10.46 mL CO2/kg*h) and translucency incidence (16.67 %).
Keywords: Antioxidants, Ascorbic acid, Enzyme, Respiration rate, Translucency
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Citation: Cano-Reinoso, D.M., Soesanto, L., Kharisun, and Wibowo, C. 2022. Effect of pre-and postharvest treatments with salicylic acid on physicochemical properties of pineapple cv. MD2. CMU J. Nat. Sci. 21(3): e2022039.
INTRODUCTION
Pineapple is a valuable crop in tropical and subtropical areas of the world (Hossain, 2016; Shamsudin et al., 2020). Low acid hybrids are currently the most exported in the industry, presenting new opportunities and challenges for the farmers (Kleemann, 2016; Cano-Reinoso et al., 2021a). MD2 is one of these low acid hybrids with a significant reputation, receiving a price three times higher than other pineapple hybrids (Bin Thalip et al., 2015). MD2 is characterised for its bright-gold colour, sweeter taste, high ascorbic acid (AsA), lower fibre and acidity content, and more uniform size than other cultivars (Bin Thalip et al., 2015).
A constant problem with MD2 is their susceptibility to natural flowering, shell burning and physiological disorders like flesh translucency, representing major threats to its quality (Soteriou et al., 2014; Cano-Reinoso et al., 2021a). Because of these susceptibilities, the implementation of supplementary mineral fertilizations together with exogenous applications of natural plant compounds have become a medium to control and maintain the optimal quality of diverse pineapple hybrids (Lu et al., 2011; Goñi et al., 2017).
Currently there is an increased interest in using natural alternatives employing compounds presented in plants that can be beneficial for their physiology, causing a lower environmental impact and health risks (Ponce et al., 2011; Goñi et al., 2017). Those natural substances have turned into a viable substitute to chemical applications in agricultural industries (Goñi et al., 2017). Salicylic acid (SA) is a natural compound known for its potent regulatory roles in plant metabolism (Hayat et al., 2010; Ali et al., 2017; Goñi et al., 2017). SA as a signal molecule can regulate stress response and plant developmental processes like photosynthesis, stomatal conductance, transpiration, ion uptake and transport, disease resistance, seed germination, crop yield and glycolysis (Asghari and Aghdam, 2010; Ali et al., 2017; Goñi et al., 2017). Besides, this compound has been proved to delay fruit ripening, affecting ethylene biosynthesis and maintaining postharvest quality and shelflife of horticultural products (Goñi et al., 2017). For example, exogenous applications of SA reduced the ethylene production, causing inhibition of the activity of the cell wall and membrane degrading enzymes like polygalacturonase (PG), lipoxygenase, cellulase and pectinemethylesterase; these circumstances leading to a decrease in the fruits softening rate (Asghari and Aghdam, 2010; Goñi et al., 2017). Also, regarding diseases tolerance, SA can cause a positive impact in the induction of systematic acquire resistance in plants (SAR), provoking a higher antioxidant enzyme activity, specially by phloem, favoring fruit metabolism and protection against any attack of bacteria and fungi (De Freitas and Resender Nassur, 2017; Surendran et al., 2018).
In a previous study in pineapple, Lu et al. (2010) investigated the effects of postharvest SA treatments on fruit quality and anti-oxidant metabolism during cold storage. They demonstrated that postharvest treatments with 5.0 mM of SA delayed the occurrence of internal browning, extending its shelflife. On top of that, SA treatments decreased the respiration rate and the activities of peroxidase (POD), polyphenol oxidase (PPO) and phenylalanine ammonia-lyase (PAL) enzymes, compared with the non-SA treated controls. In a similar way than the previous experiment described, Lu et al. (2011) explored the pre-and postharvest SA treatments to alleviate the internal browning maintaining the winter pineapple quality. Their results showed that all SA treatments significantly reduced the internal browning incidence and intensity. Furthermore, SA did not affect total soluble solids content (TSS), total acidity (TA) and total phenolic content.
Nevertheless, few papers have been documented in detail on the effect of this natural compound on pineapple fruit characteristics and metabolic activities, especially in low acid hybrids. Moreover, the available information on pineapple has focused more on SA postharvest employments than pre-harvest. This situation has led to questions about possible SA effects on the fruit when this is only used pre-harvest or mixed with postharvest applications; in addition, the optimal concentration of SA in the solutions necessary to obtain an ideal quality have not been properly discussed. Therefore, based on this previous information, this study aims to evaluate the effect of pre-and postharvest treatments with salicylic acid on physicochemical properties of MD2 pineapple.
MATERIALS AND METHODS
Experiment design and treatments
This experiment was conducted in pineapple plantations of Lampung, Sumatra island of Indonesia. MD2 pineapple cultivar was employed in this research. The experiment was set in two parts: preharvest field treatments and a second part about postharvest treatments. The first part was carried out from May to July of 2020, while the second part from July until Augusts of 2020.
The first part of the experiment consisted of a randomised complete block design, with each treatment having four replications with twenty fruits per replication. Four rows in each block were set with a width and length of 0.4 and 3.75 m, respectively. Pineapple plants were arranged in two lines of ten plants inside the row with a separation of 0.25 m. Besides, the final date of harvest coincided with one week of the commercial market of the fruit; for this reason, the fruit was harvested between 144-147 days after the plant flower induction, when it has been reported MD2 obtain the optimal physical and chemical characteristics (Bin Thalip et al., 2015; Ding and Syazwani, 2016).
Before starting this part of the experiment, the soil after preparing the rows was fertilized with 200 kg/ha Di-ammonium Phosphate, 1000 kg/ha K2SO4 and 200 kg/ha Kieserite crystal. Later, foliar sprayings were done three months after planting employing 700 kg/ha Urea, 700 kg/ha (NH₄)₂SO₄, 1000 kg/ha K2SO4, 170 kg/ha MgSO4, 60 kg/ha FeSO4, 60 kg/ha ZnSO4, in intervals of 30 days. Finally, borax was sprayed in the plants in doses of 30 kg/ha at flower induction time. The physical and mineral composition of the soil is presented in table 1. Furthermore, a weather station (LSI Lastem; equipped with a CR6 data logger from Campbell Scientific; Italy) provided during the experiment length an average of 77.66% of relative humidity (RH), 23.45°C, 9.18 w/m of solar radiation and rainfall of 153.15 mm for this experiment part.
Table 1. Physical and mineral characteristics of the soil in the experiment.
|
Texture |
Values |
|
|
Clay (%) |
22.41 |
|
|
Loam (%) |
9.00 |
|
|
Sand (%) |
66.63 |
|
|
Chemical composition |
Values |
|
|
pH (H2O) |
5.11 |
|
|
C (%) |
1.30 |
|
|
N (mg/kg) |
750.00 |
|
|
P (mg/kg) |
10.21 |
|
|
K (mg/kg) |
150.90 |
|
|
Ca (mg/kg) |
210.00 |
|
|
Mg (mg/kg) |
110.20 |
|
|
Na (mg/kg) |
5.21 |
|
In the case of the second part of the experiment, randomly ten fruits per replication were picked at harvest and arranged by their respective treatments inside a cold storage during 40 days, at 8°C and 90% of RH. Four fruits per treatment (one per replication) were selected to be analysed in intervals of eight days. Summarizing, the treatments implemented in this experiment are presented in table 2.
Table 2. The organisation of the cover treatments implemented in the research.
|
Treatment |
Characteristics |
|
A |
Control (Waxing but without SA application pre-and postharvest) |
|
B |
SA (2 mM) preharvest (sprayed) + SA (5 mM) postharvest (dipping) |
|
C |
SA (2 mM) preharvest (sprayed) + SA (7 mM) postharvest (dipping) |
|
D |
SA (2 mM) preharvest (sprayed) + SA (9 mM) postharvest (dipping) |
Note: *SA employed in dipping applications was mixed with: 1% (v/v) ethanol, and 0.01% (v/v) tween 20 (emulsifier).
All the treatments including the control employed fungicide and waxing before cold storage in postharvest. The fungicide product employed was Prochloraz in doses of 2 cc/L; meanwhile, Sta-Fresh 2952 in doses of 74 g/L was used as a waxing. Both, fungicide and waxing, in that order, were implemented in dipping applications for ten seconds, just after the dipping of the fruit in SA, which was done during five minutes. The postharvest SA solutions were dissolved in a water container of 25 L. Concerning the preharvest sprayings with SA, those were performed in six and three weeks before harvest at night, applied on the fruit shell and crown until runoff. For this procedure it was employed a hand-sprayer with a solution having a concentration of 2.0 mM. The sprayings on the shell and crown were done following the information concerning the positive effect on mineral and photo-assimilates mobility by these pineapple plant structures after flower induction of Chen and Paull (2000) and Vásquez-Jiménez and Bartholomew (2018).
The SA solutions applied pre-and postharvest together with the dipping time were selected after evaluating the previous results of Lu et al. (2010) and (2011). They proved that mixed SA applications pre-and postharvest had more beneficial effects than just one implementation; on top of that, they demonstrated that the minimum concentration in a solution to cause a beneficial effect in the pineapple quality should be 2 mM before harvest and 5 mM in postharvest. Also, the optimal dipping time recommended in their experiments was between five and ten minutes.
TSS and TA determinations
The TSS and TA were measured according to the procedure explained in Shamsudin et al. (2020), in each fruit per replication of every treatment arranged. TSS was detected using a hand-held refractometer (MASTER-53 α; Atago; Japan); TA was determined by titration to pH 8.1 with 0.1 M NaOH using phenolphthalein an indicator and expressed as a percentage of citric acid.
AsA and β-carotene determinations
The AsA was calculated using the method reported in Siti Roha et al. (2013), employing a High-Performance Liquid Chromatography (Hitachi; USA) model L-2000 instrument with a Refractive Index detector model L-2490. A juice extracted from the fruit flesh adjacent to the core was used. The samples were obtained from each fruit per replication of every treatment implemented. To develop a curve of AsA level, standard solutions of 200, 400 and 800 mg/L were used. Thereafter, the standard solutions were dissolved with distilled water and filtered through a Millipore 0.45 µm membrane filter. The AsA content was calculated comparing the peak area by a chromatographic procedure. The chromatographic conditions for AsA determination were as follow:
Column: Purospher® STAR NH2 (250 x 4 (mm), 5 µm). Guard column: LiChocart® 4-4 / LiChrospher® 100 NH2, 5 µm. Column temperature: Room temperature (22°C). Mobile phase: Pipette of 0.14 mL H2SO4 (0.0025 M) concentrated at 97% is introduced in a volumetric flask, then add 1000 ml of distilled water. Injection volume: 20 μL. Duration of analysis: 15 min.
In the case of β-carotene, this was examined according to the method described in Owolade et al. (2017). A juice extracted from the adjacent part to the flesh core was used. The samples were obtained from each fruit per replication of every treatment implemented. Therefore, 25 mg of β-carotene were weighed and dissolved in 2.5 mL of chloroform and thereafter diluted to 250 mL, using petroleum ether. After that, concentrations of 2, 10, 20, 30, 40 and 50 mg/L were implemented for the subsequent absorbance observations employing a spectrometer (Spectroquant® Pharo 300; Thomas Scientific; USA) 452 nm with 3 % of acetone using petroleum ether as blank. The β-carotene content was measured using the respective standard curve.
Fruit firmness determination
The firmness of the flesh was obtained based on the methodology reported in (Ding & Syazwani, 2016). Firmness was determined in the centre region of each fruit, adjacent to the core, using one fruit per every replication of each treatment applied. A penetrometer (GY-2 Portable Hardness Tester; Zhejiang Top Cloud-Agri Technology Co., Ltd; China) with a pressure head size of 8 mm diameter; according to its respective characteristic (scale value 0.5-4 kg/cm (x105pa), accuracy: ± 2 mm, Insertion depth of pressure head: 10 mm and Size: 140 mm *60 mm *30 mm), results are expressed in N.
Pineapple shell colour
The shell colour of the fruits was determined similar to the procedure explained in Mandal et al. (2015), in one fruit per replication on each of the arranged treatments using a scale from zero to six based on the following characteristics: Zero (Fruit shell green), one (10% of the shell area starting from the base turned yellow), two (10-20% of the shell area starting from the base turned yellow), three (20-35% of the shell area starting from the base turned yellow), four (35-50% of the shell area starting from the base turned yellow), five (50-75% of the shell area starting from the base turned yellow) and six (˃75% of the shell area starting from the base turned yellow). The final score was reported in every observation as the mean value of the replications in each treatment.
Fruit respiration rate, weight and weight loss(%)
For these measurements, one fruit was chosen and set from the first day of observation until the final day in every replication of the treatment applied. Different to the others, these variables were obtained every five days until 40 days of postharvest storage of the pineapple fruits. To calculate the respiration rate, a similar method described in Fonseca et al. (2002) and Bhande et al. (2008) was employed. First, changes in the CO2 concentrations were detected in a sealed glass container of 31 cm height *24 cm wide with 9 L of capacity. A portable AZ7788A carbon dioxide detector was used for this procedure (CO2 range: 0-5000 mg/kg, 10-95% RH, 0-50°C; AZ Instrument Corp; Taiwan). Before starting the CO2 measuring, in the cold storage at 90 % RH and 8 °C conditions, the fruit weight was calculated according to Shamsudin et al. (2007), using a weighing scale. After that, the fruits and the portable detector were introduced in the container, carefully closed, avoiding the escape or introduction of air. Changes in the CO2 concentrations were calculated during 30 min in every fruit, and after that, the respiration rate was determined using the following equation, similar to Fonseca et al. (2002) and Bhande et al. (2008):
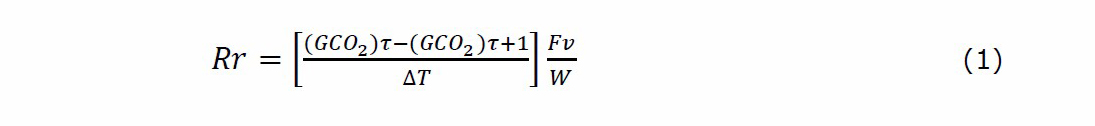
Where Rr is the respiration rate in mL (CO2)/kg*h, (GCO)2 is the gas concentrations for CO2 in mL/L, τ is the storage time in hours, ∆T the time difference between two gas measurements, Fv is the free volume of the respiration chamber in L and W is the weight of the fruit in kg. Free volume was obtained as the total volume of the container minus the volume occupied by its content using a water displacement method as described in Bhande et al. (2008). A picture of the respiration rate method arrangements in this experiment previously described is presented in Figure 1. Furthermore, percentage of weight loss was determined as a percentage of weight loss relative to the initial value taken as 100%, employing the same methodology reported in Hu et al. (2011).

Figure 1. Experimental set up respiration rate measurement. A) CO2 detector, B) Electricity cable of the detector introduced by the top, C) Pineapple fruit, and D) Sealed container.
Scanning microscope (SEM) analysis and flesh translucency
Using a similar method described in Hu et al. (2012), a SEM analysis was carried out at 40 days of postharvest storage. A small piece of tissue in the middle of the flesh adjacent to the core (5 mm3 × 5 mm3 × 2 mm3) was split with a tweezer. Before scanning, the slices were dehydrated with ethanol solutions and dried at a critical point of liquid CO2 with a desiccator. The samples were mounted onto aluminium specimen stubs using conductive silver glue and sputter-coated with gold. SEM was done with a scanning electron microscope (ZEISS/EVO MA 10; German) equipped with an energy dispersive spectroscopy (EDS) at 15 Kv.
On the other hand, translucency incidence was determined in each of the fruits per replication in every one of the treatments at the moment of each observation. The accounted number of fruits affected from the total examined since harvest until the experiment’s final determined the incidence. The results are expressed in percentage.
Statistical analysis
Statistical analyses were performed using SPSS Version 22.0 software (SPSS Inc.; Chicago, IL, USA). All data were analysed by an analysis of variance of one-way (ANOVA). Mean significant differences at P < 0.05 were determined by Duncan’s multiple range tests and Kruskal-Wallis test (for the shell colour, and translucency data).
RESULTS
Fruit TSS, TA, and TSS/TA ratio
The TSS and TA did not expose significant differences in the results of the treatments after 40 days of cold storage. For the TSS, the average values were around 16 % (Table 3). In the case of the TA content, the values obtained were on average 0.6 % (Table 3). Furthermore, the TSS/TA ratio provided outcomes with some variances, however not sufficient to cause significant differences by the statistical analysis.
AsA and β-carotene level in the fruit
The results of this experiment concerning AsA exposed significant differences. The highest AsA levels after 40 days were obtained in treatment C and D (385.89 and 383.86 mg/kg, respectively), while the lowest one was observed in treatment B (203.75 mg/kg) (Table 3). The AsA content during the postharvest storage of the fruit is displayed in Figure 2. In there it is possible to observe a decreasing trend until 40 days, with a slight increase some days before finishing the observations.
Table 3. Influences of the treatments applied on the physicochemical characteristics of the fruit after 40 days of cold storage.
|
Variables studied |
|||||
|
Treatment |
TSS (%) |
TA (%) |
TSS/TA |
AsA (mg/kg) |
β-carotene (mg/kg) |
|
A |
15.43 ± 0.02a |
0.58 ± 0.20a |
26.59 ± 0.02a |
246.64 ± 0.01c |
0.51 ± 0.01a |
|
B |
15.35 ± 0.01a |
0.53 ± 0.08a |
31.76 ± 0.08a |
203.75 ± 0.02b |
0.40 ± 0.04b |
|
C |
16.03 ± 0.02a |
0.66 ± 0.08a |
26.53 ± 0.07a |
385.89 ± 0.02a |
0.38 ± 0.02b |
|
D |
16.58 ± 0.02a |
0.55 ± 0.04a |
30.65 ± 0.02a |
383.86 ± 0.01a |
0.55 ± 0.01a |
Note: ** Each value represents a mean ± standard error. Mean values in each column followed by the same lower-case letters are not statistically different by Duncan’s multiple range test (P < 0.05).
***A (Control: Waxing but without SA application pre-and postharvest), B (SA (2 mM) preharvest (sprayed) + SA (5 mM) postharvest (dipping)), C (SA (2 mM) preharvest (sprayed) + SA (7 mM) postharvest (dipping)), D (SA (2 mM) preharvest (sprayed) + SA (9 mM) postharvest (dipping).
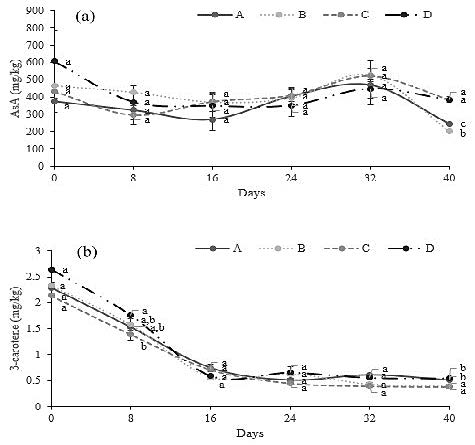
Figure 2. Effects of the SA treatments employed on the ascorbic acid (a) and β-carotene content (b). A (Control: Waxing but without SA application pre-and postharvest), B (SA (2 mM) preharvest (sprayed) + SA (5 mM) postharvest (dipping)), C (SA (2 mM) preharvest (sprayed) + SA (7 mM) postharvest (dipping)), D (SA (2 mM) preharvest (sprayed) + SA (9 mM) postharvest (dipping). Values are the mean four replicates, and vertical bars represent ± standard error. Each data followed by the same lower-case letters are not statistically different by Duncan’s multiple range test.
In the case of the β-carotene, this variable shows significant differences in the results examined. The most reduced values were obtained in treatment C (0.38 mg/kg) and the most elevated in treatment A and D (0.51 and 0.55 mg/kg, respectively) (Table 3). As higher the concentration of SA used, more elevated was the AsA content in the fruits. This circumstance was not observed in β-carotene; moreover, there was not a correlation between the AsA and β-carotene outcomes. Figure 2 shows how the trend during postharvest storage in β-carotene differs from the AsA, decreasing linearly during the first 16 days and thereafter, staying constant without having the variations observed in the AsA trend.
Fruit weight, percentage of weight loss and firmness
The fruit weight, percentage of weight loss and firmness did not demonstrate significant differences in the outcomes after 40 days of storage; nevertheless, it is vital to analyse their average values and trend through the storage life. The fruit weight after 40 days was on average 1,400 g, while the firmness was 4 N (Table 4); furthermore, the percentage of weight loss exposed an increasing trend from the first day of storage until the final day in all the treatments implemented (Figure 3). Also, this figure showed how the firmness had a decreasing trend with some minor variations.
Table 4. Influences of the treatments applied on the physicochemical characteristics of the fruit after 40 days of storage.
|
Variables studied |
||||||
|
Treatment |
Weight (g) |
Weight loss (%) |
Firmness (N) |
R. Rate mL CO2/kg*h |
Shell colour |
Translucency (%) |
|
A |
1,505.00 ± 0.01a |
8.79 ± 0.01a |
4.13 ± 0.03a |
12.39 ± 1.16a |
3.75ab |
25.00a |
|
B |
1,557.25 ± 0.05a |
8.76 ± 0.01a |
4.42 ± 0.03a |
11.43 ± 1.07a |
3.54b |
35.42a |
|
C |
1,457.50 ± 0.04a |
9.18 ± 0.01a |
4.68 ± 0.02a |
10.46 ± 0.57a |
3.71ab |
16.67a |
|
D |
1,399.50 ± 0.07a |
9.39 ± 0.01a |
4.67 ± 0.02a |
13.17 ± 1.32a |
4.29a |
25.00a |
Note: ** Each value represents a mean ± standard error. Mean values in each column followed by the same lower-case letters are not statistically different by Duncan’s multiple range test and Kruskal-Wallis test (for the shell colour and translucency incidence data) (P < 0.05).
***A (Control: Waxing but without SA application pre-and postharvest), B (SA (2 mM) preharvest (sprayed) + SA (5 mM) postharvest (dipping)), C (SA (2 mM) preharvest (sprayed) + SA (7 mM) postharvest (dipping)), D (SA (2 mM) preharvest (sprayed) + SA (9 mM) postharvest (dipping).
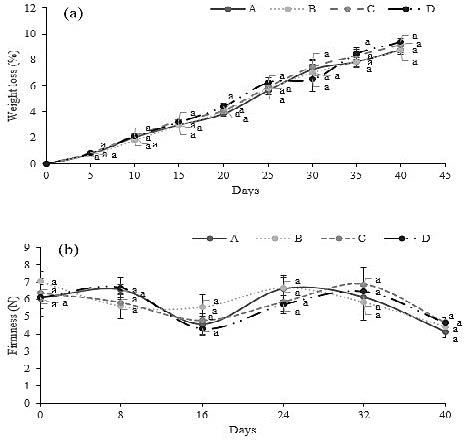
Figure 3. Effects of the SA treatments employed on the percentage of weight loss (a) and flesh firmness level (b). A (Control: Waxing but without SA application pre-and postharvest), B (SA (2 mM) preharvest (sprayed) + SA (5 mM) postharvest (dipping)), C (SA (2 mM) preharvest (sprayed) + SA (7 mM) postharvest (dipping)), D (SA (2 mM) preharvest (sprayed) + SA (9 mM) postharvest (dipping). Values are the mean four replicates, and vertical bars represent ± standard error. Each data followed by the same lower-case letters are not statistically different by Duncan’s multiple range test.
Fruit respiration rate
The fruit respiration rate did not significantly differ after 40 days of storage; however, the previous studies outcomes suggested that in this variable is essential to analyse its storage trend. The trend can deliver an idea about the impact of any treatment implemented in the fruit metabolic process. The trend of the respiration rate value exposed that starting from five days after harvest, treatment C always delivered the lowest values while D and A provided the highest results (Figure 4). This circumstance infers that treatment C helps the fruit to reduce its metabolic senescent process while D and A have a negative effect on that.
Shell colour and flesh translucency
The result of this experiment demonstrated significant differences for the shell colour, having its highest score in treatment D (4.29) and the lowest one in B (3.54) (Table 4); although B had similar significant differences with A and C. Regarding translucency, this variable did not expose significant differences; however, the analysis of its mean values could be helpful to understand the impact of SA in the fruit physiology. The highest value was obtained in treatment B (35.42 %), while the lowest in C (16.67 %) (Table 4). Figure 5 exposed the shell and flesh colour physical appearance during storage. In this figure it is possible to observe some shell colour changes during postharvest storage; also, more pronounce symptoms of flesh translucency, especially in treatment B, which is reflected in the incidence outcomes obtained for this treatment (Table 4).
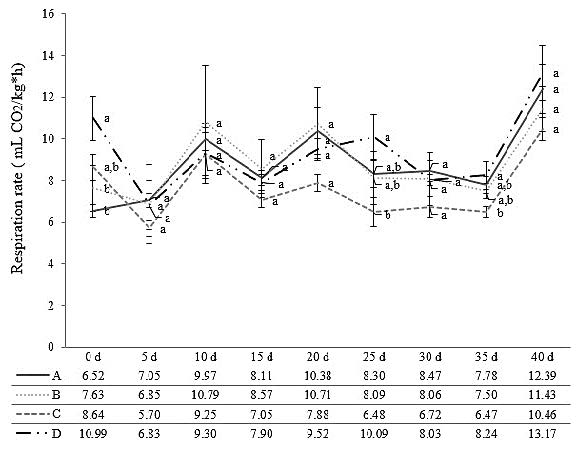
Figure 4. Effects of the SA treatments employed on the fruit respiration rate during the experiment, with the average value in each observation time. A (Control: Waxing but without SA application pre-and postharvest), B (SA (2 mM) preharvest (sprayed) + SA (5 mM) postharvest (dipping)), C (SA (2 mM) preharvest (sprayed) + SA (7 mM) postharvest (dipping)), D (SA (2 mM) preharvest (sprayed) + SA (9 mM) postharvest (dipping). Values are the mean four replicates, and vertical bars represent ± standard error. Each data followed by the same lower-case letters are not statistically different by Duncan’s multiple range test.
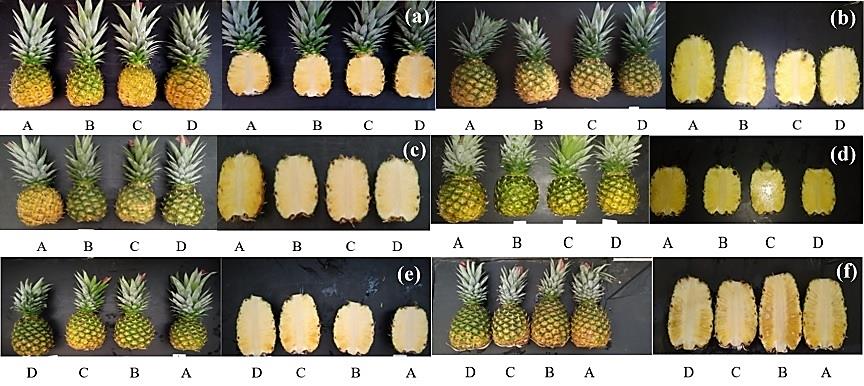
Figure 5. Shell and flesh colour condition since harvest until the final storage day of the experiment (a: 0 days, b: 8 days, c: 16 days, d: 24 days, e: 32 days, and f: 40 days). A (Control: Waxing but without SA application pre-and postharvest), B (SA (2 mM) preharvest (sprayed) + SA (5 mM) postharvest (dipping)), C (SA (2 mM) preharvest (sprayed) + SA (7 mM) postharvest (dipping)), D (SA (2 mM) preharvest (sprayed) + SA (9 mM) postharvest (dipping).
SEM analysis
SEM examinations exposed that the cell walls of the fruits in every treatment had in their primary layer symptoms of integrity and consistency, characterised by cell arrows with more significant thickness; however, there were observed some cells with arrows not attached to the vascular bundles, attributed to an unhealthy cell wall (Figure 6).
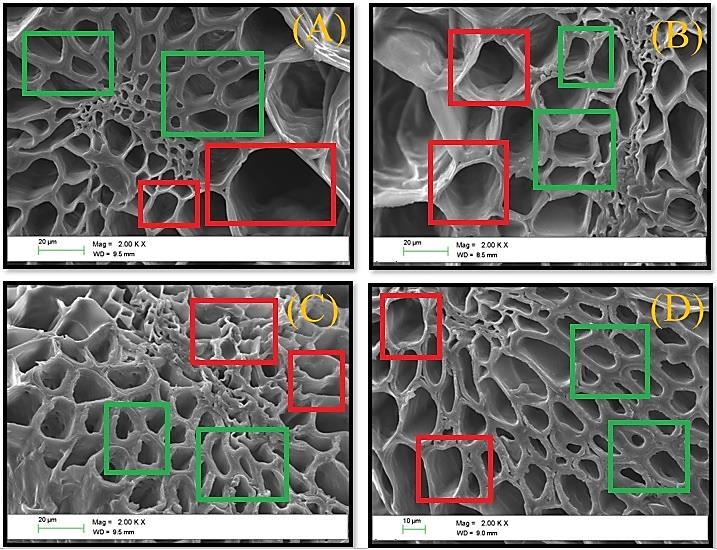
Figure 6. Effects of the SA treatments implemented on the cell walls of the fruits after 40 days of cold storage detected by SEM (20 and 10 µm size, 2000x of magnification). A (Control: Waxing but without SA application pre-and postharvest), B (SA (2 mM) preharvest (sprayed) + SA (5 mM) postharvest (dipping)), C (SA (2 mM) preharvest (sprayed) + SA (7 mM) postharvest (dipping)), D (SA (2 mM) preharvest (sprayed) + SA (9 mM) postharvest (dipping). The smaller thickness and undulated arrows of the cell wall (red square) and more significant thickness and non-undulated arrows (green square) were analysed. Each data followed by the same lower-case letters are not statistically different by Duncan’s multiple range test.
DISCUSSION
TSS, TA, and TSS/TA ratio in the fruit
The typical value of TSS for a low acid hybrid like MD2 should be as minimal as 12% (Bartholomew and Sanewski, 2018; Cano-Reinoso et al., 2021b); the outcomes of this experiment reflected that finding. Lu et al. (2011) demonstrated that mix of SA application pre-and postharvest on pineapple did not affect negatively the TSS and also did not cause a significant increase during postharvest storage. Similar results were obtained by Sangprayoon et al. (2019); together with other SA experiments in mangosteen (Mustafa et al., 2018a), dragon fruit (Mustafa et al., 2018b) and carambola fruit (Mustafa et al., 2014). However, in a study done on pineapple fruit reported by Mandal et al. (2014), postharvest dipping applications of SA increased the TSS content after 15 days of storage. The primordial sugars in pineapple are sucrose, glucose and fructose, and TSS is mainly a determination of the sucrose content in the fruit (Chen and Paull, 2017; Paull and Chen, 2018). These findings suggest that the SA treatments implemented could not cause a representative superior accumulation of non-reducing sugars like sucrose during storage compared with the control. Besides, based on preliminary observations, the same phenomenon described could have happened during the fruit ripening, as it was determined in the harvest outcomes, where there were no significant differences in the results between treatments, ranging around 15% all of them. Furthermore, the higher content of TSS during postharvest reported in some experiments could be related to the concentration of SA employed and the application time; nevertheless, further studies should be done on this aspect.
Regarding the TA, ideal values in MD2 should be around 0.4-0.7% (Saradhuldhat and Paull, 2007; Paull and Chen, 2018). The results obtained in this research were in that recommended range suggesting no harmful impact of the treatments used on TA. Despite pre-and postharvest SA applications in fruits like mango (Hong et al., 2014) and orange (Aminifard et al., 2013) reported an increase in the TA content during postharvest, results in pineapple have not displayed any increase in this variable during storage conditions (Lu et al., 2011; Mandal et al., 2015; Sangprayoon et al., 2019). In pineapple, TA represents a determination of citric acid, which accounts for around 65-70% of the total acid content of the fruit, followed by malic acid, having 18-30% of this combined percentage (Chen and Paull, 2017; Paull and Chen, 2018). Seemly the SA treatments implemented did not cause a representative increase in the organic acids content of the fruit after 40 days of storage, essentially the citric acid; therefore, the lack of significant differences exposed compared with the control. Also, according to the preliminary examinations carried out, the same circumstances could have occurred during the fruit development prior to harvest, because no significant differences between treatments were detected in the harvest results, having mean values around 0.4%.
On the other hand, the TSS/TA ratio is considered an important indicator to determine fruit flavour and quality. In low acid hybrids like MD2, its value range between 15-20, although some studies have proved that it could be higher than 20 with values close to 30 (Chen et al., 2009; Ding and Syazwani, 2016). Champa et al. (2014) and Mustafa et al. (2018b) demonstrated that pre-harvest SA sprays in grapes and dragon fruit, respectively, suppressed the TSS and TA degrading during postharvest, maintaining optimal values until the final storage day. Several studies concerning pineapple quality and shelflife during postharvest have been associated with a reduction of the TSS and TA content through the initial days of fruit’s cold storage, if this was not harvested with an ideal quality, which also cause a decrease in its final TSS/TA value. Similar to the previous study described, the results of this experiment suggested that pre-and postharvest SA use can mitigate the reduction of the TSS, TA and with that the TSS/TA to undesirable values, which is in agreement also with the results of Lu et al. (2011) and Mandal et al. (2015); moreover, the research outcomes also evidence that the fruits were harvested with an optimal value of TSS and TA, as the control still provided outstanding outcomes after 40 days of postharvest handling.
Pineapple AsA and β-carotene content
AsA is considered a soluble vitamin essential to protect the cell from oxidative stress due to its scavenger properties (Akram et al., 2017; Noichinda et al., 2017). For low acid hybrids, an optimal AsA level should be between 300-600 mg/kg (Lu et al., 2014; Paull and Chen, 2018). The outcomes observed in the treatments with the most superior content (C and D), were inside the optimal range suggested. In previous studies was reported that pre-and postharvest SA spraying in similar concentrations with the treatment C (2 mM pre-and 7 mM postharvest), got to reduce the AsA degrading, obtaining the most superior level after 30 days of storage (Lu et al., 2011; Mandal et al., 2015). Hu et al. (2012), in an experiment on pineapple waxing treatments, explained that typically the decline in the AsA content is associated with the rise of reactive oxygen species (ROS), like superoxide (O2-), hydrogen peroxide (H2O2), and the hydroxyl radical (OH).
SA has proved to synthesize protective compounds and enzymes that function as antioxidants, which can act as scavenger agents protecting against ROS (Mustafa et al., 2015; Goñi et al., 2017). A continuous ROS production can enhance the signalling pathway of hormones like SA, provoking a rise of the AsA (Mustafa et al., 2015; Akram et al., 2017). For example, Lu et al. (2010) proved that SA in postharvest solutions with a concentration of 5 mM could encourage the AsA production together with the catalyzation of peroxidase enzymes in pineapple. These previous facts suggest that treatments C and D were the ones that more lessen the AsA degrading by increasing the antioxidant enzyme activities, and protective compounds as phenols, derivate from a superior sensing of the SA signalling pathway. Besides, as evidenced by the mean values outcomes, the SA signalling could have been superior in treatment D at harvest; although downregulated during postharvest, decreasing some of the positive antioxidant properties previously described, causing similar outcomes with treatment C after 40 days of storage.
Furthermore, the pigment responsible for the yellow colour of the flesh in pineapple is the β-carotene (Vásquez-Jiménez and Bartholomew, 2018; Steingass et al., 2020). This pigment belongs to the plants carotenoids, which are known for their antioxidant properties, eliminating singlets of ROS (Fanciullino et al., 2014; Noichinda et al., 2017). The outcomes of these experiments inferred that the no use of SA or high concentrations of this (˃ 7 mM) are more beneficial for the β-carotene content in the fruit than intermedial concentrations, like in treatment A and D. Although there are no sufficient studies on the SA impact on β-carotene in pineapple, the available information documented, like in mandarin (Baswal et al., 2020) and papaya (Supapvanich and Promyou, 2017), indicates that a rising of the carotenoids in those fruits was associated with lower concentrations of SA during postharvest treatments. Based on this finding, there may be an effect coming from the low and high concentrations of SA on the formation and degrading of the β-carotene affecting its biosynthesising via the mevalonate pathway (Noichinda et al., 2017). Besides, as exposed by the mean values outcomes at harvest, the mevalonate pathway could have been more active in fruits affected by treatment D; nevertheless, this pathway could have been downregulated during storage, causing a lower value in this treatment after 40 days.
More research should be done in this aspect to know better the impact of SA in pineapple pigments. Furthermore, these results are concomitant with the information of Kongsuwan et al. (2009) and Paull and Chen (2018), where the AsA was determined as the main antioxidant scavenger of pineapple fruit and did not provide a strong correlation with the β-carotene content. In this experiment, not all the treatments having the most elevated AsA had a high β-carotene level in the fruits.
Fruit weight, percentage of weight loss and firmness in the fruit
The fruit weight average after 40 days was between the normal range recommended for optimal quality in MD2. The weight range of MD2 typically is between 1,300-2,500 g, depending on the consumer preferences (Bin Thalip et al., 2015; Cano-Reinoso et al., 2021b). Furthermore, concerning the percentage of weight loss, previous studies reporting pre-and postharvest applications with SA in pineapple caused a reduction of the percentage of weight loss; nevertheless, those experiments were arranged until 20 days of storage (Lu et al., 2010; Mandal et al., 2015). Comparing the results of those studies at 20 days with the average values of this experiment at the same date (4%) (Figure 3), it is possible to infer that the treatments implemented got to reduce the percentage of weight loss. However, because there were no significant differences compared with the control, those beneficial effects of the treatments stated could be linked to the waxing application used. Hu et al. (2012) got to reduce the percentage of weight loss in pineapple employing Sta-Fresh 2952 in doses of 60 g/L; because in this experiment the doses were 74 g/L for all the treatments, it could be suggested that this higher dose caused that the reduction of the percentage of weight loss was more significant than former studies. Fruit weight loss is essentially associated with respiration and water content evaporation through the shell of pineapple. Waxing act as a barrier, restricting water transfer and delaying dehydration (Hernández-Muñoz et al., 2008; Hu et al., 2012). Seemly the waxing in this experiment caused more influences than SA concerning this variable, delaying the pineapple dehydration and water evaporation.
On the other hand, optimal firmness values in the fruit flesh in MD2 pineapple have been ranged between 4-7 N (Ding and Syazwani, 2016). The values obtained in this research were inside that ranged. Despite the lack of information regarding the impact of SA in pineapple fruit firmness, the results obtained in other fruits, like sweet orange (Ahmad et al., 2013) and cherry (Valero et al., 2011), demonstrated that SA delayed firmness losses during storage employing pre-and postharvest treatments. Usually, during postharvest, the firmness of the flesh tends to decrease as long as the storage time increase, having some variations due to metabolic changes occurring in the cell wall (De Freitas and Resender Nassur, 2017; Wang et al., 2017;).
The situation previously exposed was observed in the decreasing trend of the firmness (Figure 3). The degrading of the cell wall is considered an essential symptom related to firmness reduction and fruit softening (Hu et al., 2012; Gao et al., 2020). This phenomenon is connected with degrading enzymes activities and an increase of ROS production, causing a membrane lipid peroxidation together with the breakdown of the Ca2+ ions crossed-bridged in the cell wall (Hu et al., 2012; De Freitas and Resender Nassur, 2017). The reason behind why despite at 40 days of storage still the fruit have a firmness value attributed to optimal conditions at harvest can be related to the waxing treatment employed, similar to the percentage of weight losses case. Hu et al. (2011), in their trials testing several doses of Sta-Fresh in pineapple, got to mitigate the reduction of the firmness by increasing the waxing doses. Therefore, in this experiment, the waxing doses used were necessary to maintain this variable in a preferable standard to be consumed.
Pineapple respiration rate
The fruit respiration rate did not show significant differences; although, the analyses of its trend is an important aspect to consider, because of the information that this provides regarding metabolic process. The lowest trend was observed in treatment C and the more superior was detected in A and D. Like explained for the AsA and the β-carotene, it appears that high concentrations of SA (˃ 7 mM), and the no use of this, cause a negative impact on the quality of the fruit (treatment A and D). These results are similar to the ones obtained by Lu et al. (2010) and Mandal et al. (2015), where elevated postharvest concentrations of 5mM of SA provided the lowest respiration rate between treatments used, showing a similar trend with this experiment.
The respiration rate measurements give an indicator of the tissue metabolic activity and quality of the horticultural products (Saltveit, 2016). SA has been found to alter the alternative oxidase (AOX) activity, an enzyme essential in regulating the oxidation of ubiquinol/ubiquinone pool and the reduction of oxygen to water (Goñi et al., 2017). When there is no adequate ATP synthesis in the mitochondria, together with the alteration of AOX, ROS in the mitochondria are affected, triggering the fruit’s resistance mechanism (SAR) (Goñi et al., 2017). These mechanisms are associated with a more superior production of phenolic compounds, carotenoids and AsA. Besides, SA concentrations around 5 mM in pineapple were linked to higher activity of antioxidant enzymes like catalyze (CAT), superoxidase dismutase (SOD), and ascorbic peroxidase (APX) (Lu et al., 2011; Mandal et al., 2015). Seemly, low SA concentrations (˂ 5 mM) or higher (˃ 7 mM) applied pre-and postharvest, which influences the AOX activity and mitochondrial ROS level, do not cause same intensity than the employment of concentrations between 5 to 7 mM. Therefore, the phenol compounds and antioxidant substances are not activated in pineapple, causing more free radical accumulation and subsequent rise in the fruit metabolic activities and respiration rate. Further studies should be done in this matter as there is no enough information on the way of action of SA in pineapple resistance mechanism under different concentrations in solutions applied.
Shell colour and flesh translucency in the fruit
Pineapple clones like MD2 in optimal quality typically display a bright green-yellow colour (Nadzirah et al., 2013; Ding and Syazwani, 2016). This bright green-yellow colour usually is related to a superior carotenoid content and antioxidant capacity in the shell (Nadzirah et al., 2013; Ding and Syazwani, 2016). Despite the lack of information regarding SA effects on pineapple shell colour either with pre-or postharvest applications, the available information can help understand its influences. In Peaches (Tareen et al., 2012), SA applied after harvest maintained the skin colour of the fruit without noticeable chlorophyll degrading during storage conditions with concentrations of 2 mM; also, in kiwi fruit (Zhang et al., 2003), SA solutions with a concentration of 1 mM used after harvest reduced the biosynthesis of ethylene, retarding the climacteric rise in ethylene production. These results suggested that concentrations of SA higher than 7 mM (like in treatment D) can encourage shell chlorophyll degrading by enhancing the biosynthesis of ethylene during postharvest. Nevertheless, it is suggested that further experiments should be done to study the impact of SA in pineapple shell colour and metabolic-related process.
On the other hand, translucency is a physiological disorder affecting pineapple characterised by water soaking symptoms (Paull and Chen, 2015, 2018). Field temperature, fruit sugar accumulation and low calcium levels have been determined as the critical factors related to this physiological disorder (Paull and Chen, 2018; Cano-Reinoso et al., 2022). Typically, symptoms of translucency appear weeks close to harvest and do not tend to increase if the fruit is handled with an adequate temperature and relative humidity during postharvest (Chen and Paull, 2017; Paull and Chen, 2018). Therefore, translucency incidence can be detected in any stage during storage life of the fruit, while its severity impact can be controlled with an ideal manipulation. The fruit samples employed to elaborate Figure 5 were selected randomly; based on that, the physical characteristics observed may not reflect some of the outcomes obtained in this research. This situation may explain the variability of the shell colour characteristics, essentially after 24 days of storage; in addition, the lack of translucency identification trough the flesh condition represented until 32 days postharvest. For example, in this experiment symptoms of this physiological disorder were noticed in all the treatments since harvest until the final storage day.
On top of that, lately the calcium content has been regarded as the most essential aspect to control translucency incidence (Paull and Chen, 2018; Cano-Reinoso et al., 2022). Mandal et al. (2015) in their experiment employing SA after harvest with a concentration of 5 mM reduced the translucency in pineapple after more than 15 days of storage, although it was not clarified the SA impact on the calcium metabolism and enzyme related activities. Vallarino and Osorio (2012) explained that when there is a change in the cell wall polysaccharide matrix, the cell senses and activates the SA signalling pathway and subsequent SAR. In addition, Es-sbihi et al. (2020) explained that one of the mechanisms related to SAR caused by SA under stress conditions is a higher calcium Ca2+ assimilation into the cell wall and subsequent rise of its content in the plant.
Therefore, exogenous applications of SA could encourage a fast activation of the SAR in the fruit causing a more Ca2+ ions associated with the cell wall matrix. Despite the lack of significant differences, analyzing the mean results of the experiment and the previous information exposed it is possible to infer that concentrations lower than 5 mM or higher than 7 mM could reduce the fruit response to activate the SA pathway. Meanwhile, a concentration of 7 mM only (treatment C) could be the most optimal to encourage SA signalling. On top of that, it is important to mention that some symptoms of internal browning (IB) were observed in all the treatments after 32 days of storage, more pronounced in treatment A. Concerning this situation, in previous trials before the beginning of this research it was detected that IB appeared typically between 20-24 days postharvest, in fruits without any postharvest treatment application, and earlier than the time observed in this experiment. This fact remarks the potential of employing SA to control IB to extend the shelflie of pineapple, as reported by previous authors (Lu et al., 2011; Sangprayoon et al., 2019). Finally, further studies are suggested on SA concentrations in solutions implemented and its influences on calcium content and metabolism in pineapple.
SEM observations
In pineapple, hemicelluloses represent the essential component of the primary cell wall layer (41.8%), followed by cellulose (33.6%) and pectin (21.2%) (Jarvis, 2011; Ding and Syazwani, 2016). As inferred previously, ROS together with cell wall degrading enzyme are agents essential to consider concerning cell wall healthiness. For example, Pectin methyl esterase (PME) or Polygalacturonase (PG) are degrading enzymes that breaks down glycosidic links between units of desterified galacturonic acids, causing a cell wall breakdown (De Freitas and Resender Nassur, 2017). By this SEM analysis was complicated to determine an exact difference between the treatments employed at the cellular level; also, ROS or degrading enzyme influences causing an unhealthy cell wall. Therefore, an examination that permits in more detail the observation of the whole cell wall layers is needed, a transmission electron microscope (TEM) can be recommended.
CONCLUSION
The pre-and postharvest applications of SA affected the physicochemical properties of MD2 pineapple after 40 days of storage. The AsA, respiration rate, and translucency incidence were determined as the variables more influenced by the treatments implemented. The treatment C (SA (2 mM) preharvest (sprayed) + SA (7 mM) postharvest (dipping)) was considered as the more beneficial for the pineapple quality, delivering the most elevated AsA content, the lowest respiration rate and translucency incidence. Further research is suggested concerning the influences of SA concentrations and application moment on TSS and TA. Also, more studies on SA impacts on pineapple plant pigments, mechanisms of resistance against ROS are recommended, especially those affecting the cell wall.
ACKNOWLEDGEMENTS
The authors would like to express their sincere thanks to PT Great Giant Pineapple and their research department in Lampung Indonesia for their logistical support during the research period.
AUTHOR CONTRIBUTIONS
Diego Mauricio Cano-Reinoso designed and conducted all the experiments, performed the statistical analysis, data visualization and wrote the manuscript. Loekas Soesanto, Kharisun and Condro Wibowo help to the visualization, supervision, methodology, writing-reviewing and edition of the manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Ahmad, S., Singh, Z., Khan, A.S., and Iqbal, Z. 2013. Pre-harvest application of salicylic acid maintain the rind textural properties and reduce fruit rot and chilling injury of sweet orange during cold storage. Pakistan Journal of Agricultural Science. 50: 559-569.
Akram, N.A., Shafiq, F., and Ashraf, M. 2017. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Frontiers in Plant Science. 8: 1-5.
Ali, A., Yeoh, W.K., Forney, C., and Siddiqui, M.W. 2018. Advances in postharvest technologies to extend the storage life of minimally processed fruits and vegetables. Critical Reviews in Food Science and Nutrition. 58: 2632-2649.
Aminifard, M.H., Mohammadi, S., and Fatemi, H. 2013. Inhibition of green mould in blood orange (Citrus sinensis var. Moro) with salicylic acid treatment. Archives of Phytopathology and Plant Protection. 46: 695-703.
Asghari, M. and Aghdam, M.S. 2010. Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends in Food Science and Technology. 21(10): 502-509.
Bartholomew, D.P. and Sanewski, G.M. 2018. Inflorescence and fruit development and yield. In G.M. Sanewski, D.P. Bartholomew, and R.E. Paull (Eds.). The pineapple: botany, production and uses (pp. 223-268). CABI Publishing, London, UK.
Baswal, A.K., Dhaliwal, H.S., Singh, Z., Mahajan, B.V.C., and Gill, K.S. 2020. Postharvest Biology and Technology Postharvest application of methyl jasmonate, 1-methylcyclopropene and salicylic acid extends the cold storage life and maintain the quality of ‘Kinnow’ mandarin (Citrus nobilis L. X C. deliciosa L.) fruit. Postharvest Biology and Technology. 161: 111064.
Bhande, S.D., Ravindra, M.R., and Goswami, T.K. 2008. Respiration rate of banana fruit under aerobic conditions at different storage temperatures. Journal of Food Engineering. 87: 116-123.
Bin Thalip, A.A., Tong P.S., and Casey Ng. 2015. The MD2 “Super Sweet” pineapple (Ananas comosus). Utar Agriculture Science Journal. 1: 14-17.
Cano-Reinoso, D.M., Soesanto, L., Kharisun, and Wibowo, C. 2021a. Review : Fruit collapse and heart rot disease in pineapple : Pathogen characterization, ultrastructure infections of plant and cell mechanism resistance. Biodiversitas. 22: 2477-2488.
Cano-reinoso, D.M., Soesanto, L., Kharisun, and Wibowo, C. 2021b. Effect of pre-harvest fruit covers and calcium fertilization on pineapple thermotolerance and flesh translucency. Emirates Journal of Food and Agriculture 33: 834-845.
Cano-Reinoso, D.M., Kharisun, K., Soesanto, and Wibowo, C. 2022. Effect of calcium and silicon fertilization after flowering on pineapple mineral status and flesh translucency. Plant Physiology Reports. 27: 96-108.
Champa, W.A.H., Gill, M.I.S., Mahajan, B.V.C., and Arora, N.K. 2014. Preharvest salicylic acid treatments to improve quality and postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless. Journal of Food Science and Technology. 52: 3607-3616.
Chen, C.C. and Paull, R.E. 2000. Changes in sugar contents and activities of sugar metabolizing enzymes in pinepple fruit flesh during development. Acta Horticulturae. 529: 191-195.
Chen, N.J. and Paull, R.E. 2017. Production and postharvest handling of low acid hybrid pineapple. Acta Horticulturae. 1166: 25-34.
Chen, N.J., Paull, R.E., Chen, C.C., and Saradhuldhat, P. 2009. Pineapple production for quality and postharvest handling. Acta Horticulturae. 822: 253-260.
De Freitas, S.T. and Resender Nassur, R.C.M. 2017. Calcium treatments. In S. Pareek (Ed.). Novel postharvest treatments of fresh produce (pp. 52-68). CRC Press, Boca Raton, USA.
Ding, P. and Syazwani, S. 2016. Physicochemical quality, antioxidant compounds and activity of MD-2 pineapple fruit at five ripening stages. International Food Research Journal. 23: 549-555.
Es-sbihi, F.Z., Hazzoumi, Z., Benhima, R., and Amrani Joutei, K. 2020. Effects of salicylic acid on growth, mineral nutrition, glandular hairs distribution and essential oil composition in Salvia officinalis L. grown under copper stress. Environmental Sustainability. 3: 199-208.
Fanciullino, A.L., Bidel, L.P R., and Urban, L. 2014. Carotenoid responses to environmental stimuli: Integrating redox and carbon controls into a fruit model. Plant, Cell and Environment. 37: 273-289.
Fonseca, S.C., Oliveira, F.A.R., and Brecht, J.K. 2002. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: A review. Journal of Food Engineering. 52: 99-119.
Gao, J., Zhang, Y., Li, Z., and Liu, M. 2020. Role of ethylene response factors (ERFs) in fruit ripening. Food Quality and Safety. 4: 15-20.
Goñi, M.G., Quirós-Sauceda, A.E., Velderrain-Rodríguez, G.R., Ovando-Martínez, M., Roura, S.I., González-Aguilar, G.A., and Pareek, S. 2017. Salicylic acid treatments. In S. Pareek (Ed.). Novel postharvest treatments of fresh produce (pp. 119-148). CRC Press, Boca Raton, USA.
Hayat, Q., Hayat, S., Irfan, M., and Ahmad, A. 2010. Effect of exogenous salicylic acid under changing environment: A review. Environmental and Experimental Botany. 68: 14-25.
Hernández-Muñoz, P., Almenar, E., Valle, V. Del, Velez, D., and Gavara, R. 2008. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria × ananassa) quality during refrigerated storage. Food Chemistry. 110(2): 428-435.
Hong, K., Gong, D., Xu, H., Wang, S., Jia, Z., Chen, J., and Zhang, L. 2014. Effects of salicylic acid and nitric oxide pretreatment on the expression of genes involved in the ethylene signalling pathway and the quality of postharvest mango fruit. New Zealand Journal of Crop and Horticultural Science. 42: 205-216.
Hossain, M.F. 2016. World pineapple production: An overview. African Journal of Food, Agriculture, Nutrition and Development. 16: 11443-11456.
Hu, H., Lin, X., Chen, D., and Chen, W. 2011. Effect of wax treatment on the quality and postharvest physiology of pineapple fruits. Acta Horticulturae. 10: 7592-7603.
Hu, Huigang, Li, X., Dong, C., and Chen, W. 2012. Effects of wax treatment on the physiology and cellular structure of harvested pineapple during cold storage. Journal of Agricultural and Food Chemistry. 60: 6613-6619.
Jarvis, M.C. 2011. Plant cell walls: Supramolecular assemblies. Food Hydrocolloids. 25: 57-262.
Kleemann, L. 2016. Organic Pineapple Farming in Ghana - A Good Choice for Smallholders? The Journal of Developing Areas. 50: 109-130.
Kongsuwan, A., Suthiluk, P., Theppakorn, T., Srilaong, V., and Setha, S. 2009. Bioactive compounds and antioxidant capacities of phulae and nanglae pineapple. Asian Journal of Food and Agro-Industry. Special Issue: 44-50.
Lu, X.H., Sun, D.Q., Mo, Y.W., Xi, J.G., and Sun, G.M. 2010. Effects of post-harvest salicylic acid treatment on fruit quality and anti-oxidant metabolism in pineapple during cold storage. Journal of Horticultural Science and Biotechnology. 85: 454-458.
Lu, X., Sun, D., Li, Y., Shi, W., and Sun, G. 2011. Pre- and post-harvest salicylic acid treatments alleviate internal browning and maintain quality of winter pineapple fruit. Scientia Horticulturae. 130: 97-101.
Lu, Xin Hua, Sun, D.Q., Wu, Q.S., Liu, S.H., and Sun, G.M. 2014. Physico-chemical properties, antioxidant activity and mineral contents of pineapple genotypes grown in China. Molecules. 19: 8518-8532.
Mandal, D., Lalremruata, Hazarika, T.K., and Nautiyal, B.P. 2015. Effect of post-harvest treatments on quality and shelf life of pineapple (Ananas comosus [L.] Merr. ‘Giant Kew’) fruits at ambient storage condition. International Journal of Bio-Resource and Stress Management. 6: 490.
Mustafa, M.A., Ali, A., Seymour, G., and Tucker, G. 2014. The role of the ubiquitous phenolic compound 'salicylic acid' in chilling tolerance of carambola. Acta Horticulturae. 1079: 679-683.
Mustafa, M.A., Ali, A., Seymour, G., & Tucker, G. 2018a. Delayed pericarp hardening of cold stored mangosteen (Garcinia mangostana L.) upon pre-treatment with the stress hormones methyl jasmonate and salicylic acid. Scientia Horticulturae. 230: 107-116.
Mustafa, M.A., Ali, A., Seymour, G., and Tucker, G. 2018b. Treatment of dragonfruit (Hylocereus polyrhizus) with salicylic acid and methyl jasmonate improves postharvest physico-chemical properties and antioxidant activity during cold storage. Scientia Horticulturae. 231: 89-96.
Nadzirah, K.Z., Zainal, S., Noriham, A., Siti Roha, A.M., and Nadya, H. 2013. Physico- chemical properties of pineapple variety N36 harvested and stored at different maturity stages. International Food Research Journal. 20: 225-231.
Noichinda, S., Bodhipadma, K., and Wongs-Aree, C. 2017. Antioxidant Potential and Their changes during postharvest handling of tropical fruits. In S. Pareek (Ed.). Novel postharvest treatments of fresh produce (pp. 633-662). CRC Press, Boca Raton, USA.
Owolade, S.O., Akinrinola, A.O., Popoola, F.O., Aderibigbe, O.R., Ademoyegun, O.T., and Olabode, I.A. 2017. Study on physico-chemical properties, antioxidant activity and shelf stability of carrot (Daucus carota) and pineapple (Ananas comosus) juice blend. International Food Research Journal. 24: 534-540.
Paull, R.E. and Chen, C.C. 2018. Postharvest Physiology, Handling and Storage of Pineapple. In G.M. Sanewski, D.P. Bartholomew, and R.E. Paull (Eds.). The pineapple: botany, production and uses (pp. 295-323). CABI Publishing, London, UK.
Paull, R.E. and Chen, N.J. 2015. Pineapple translucency and chilling injury in new low-acid hybrids. Acta Horticulturae. 1088: 61-66.
Ponce, A., Roura, S.I., and Moreira, M. del R. 2011. Essential oils as biopreservatives: different methods for the technological application in lettuce leaves. Journal of Food Science. 76: 34-40.
Saltveit, M.E. 2016. Respiratory metabolism. Postharvest ripening physiology of crops (pp. 139-156). In S. Pareek (Ed.).CRC Press, Boca Raton, USA.
Sangprayoon, P., Supapvanich, S., Youryon, P., Wongs‐Aree, C., and Boonyaritthongchai, P. 2019. Efficiency of salicylic acid or methyl jasmonate immersions on internal browning alleviation and physicochemical quality of Queen pineapple cv.“Sawi” fruit during cold storage. Journal of Food Biochemistry. 43(12): e13059.
Saradhuldhat, P. and Paull, R.E. 2007. Pineapple organic acid metabolism and accumulation during fruit development. Scientia Horticulturae. 112: 297-303.
Shamsudin, R., Daud, W.R.W., Takriff, M.S., and Hassan, O. 2007. Physicochemical properties of the Josapine variety of pineapple fruit. International Journal of Food Engineering. 3: 1-12.
Shamsudin, R., Zulkifli, N.A., and Kamarul Zaman, A.A. 2020. Quality attributes of fresh pineapple-mango juice blend during storage. International Food Research Journal. 27: 141-149.
Siti Roha, A.M., Zainal, S., Noriham, A., and Nadzirah, K.Z. 2013. Determination of sugar content in pineapple waste variety N36. International Food Research Journal. 20: 1941-1943.
Soteriou, G.A., Kyriacou, M.C., Siomos, A.S., and Gerasopoulos, D. 2014. Evolution of watermelon fruit physicochemical and phytochemical composition during ripening as affected by grafting. Food Chemistry. 165: 282-289.
Steingass, C.B., Vollmer, K., Lux, P.E., Dell, C., Carle, R., and Schweiggert, R.M. 2020. HPLC-DAD-APCI-MSn analysis of the genuine carotenoid pattern of pineapple (Ananas comosus [L.] Merr.) infructescence. Food Research International. 127: 108709.
Supapvanich, S. and Promyou, S. 2017. Hot water incorporated with salicylic acid dips maintaining physicochemical quality of ‘Holland’ papaya fruit stored at room temperature. Emirates Journal of Food and Agriculture. 29: 18-24.
Surendran, A., Siddiqui, Y., Manickam, S., and Ali, A. 2018. Role of benzoic and salicylic acids in the immunization of oil palm seedlings-challenged by Ganoderma boninense. Industrial Crops and Products. 122: 358-365.
Tareen, M.J., Abbasi, N.A., and Hafiz, A. I. 2012. Postharvest application of salicylic acid enhanced antioxidant enzyme activity and maintained quality of peach cv. ‘Flordaking’ fruit during storage. Scientia Horticulturae. 142: 21-228.
Valero, D., Díaz-mula, H.M., Zapata, P.J., Castillo, S., Martínez-romero, D., and Serrano, M. 2011. Postharvest Treatments with Salicylic Acid, Acetylsalicylic Acid or Oxalic Acid Delayed Ripening and Enhanced Bioactive Compounds and Antioxidant Capacity in Sweet Cherry. Journal of Agricultural and Food Chemistry. 59: 5483-5489.
Vallarino, J.G. and Osorio, S. 2012. Signaling role of oligogalacturonides derived during cell wall degradation. Plant Signaling & Behavior. 7: 1-3.
Vásquez-Jiménez, J.H. and Bartholomew, D.P. 2018. Plant nutrition. In G.M. Sanewski, D.P. Bartholomew, and R.E. Paull (Eds.). The pineapple: botany, production and uses (pp. 175-202). CABI Publishing, London, UK.
Wang, M., Gao, L., Dong, S., Sun, Y., Shen, Q., and Guo, S. 2017. Role of silicon on plant-pathogen interactions. Frontiers in Plant Science. 8: 1-14.
Zhang, Y., Chen, K., Zhang, S., and Ferguson, I. 2003. The role of salicylic acid in postharvest ripening of kiwifruit. Postharvest Biology and Technology. 28: 67-74.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
Diego Mauricio Cano-Reinoso1, Loekas Soesanto1, Kharisun1, and Condro Wibowo2,*
1 Department of Agrotechnology, Faculty of Agriculture, Jenderal Soedirman University, Purwokerto, J1 dr. Suparno, Karangwangkal 53123, Indonesia
2 Department of Food Science and Technology, Faculty of Agriculture, Jenderal Soedirman University, Purwokerto J1 dr. Suparno, Karangwangkal 53123, Indonesia
Corresponding author: Condro Wibowo, E-mail: condro.wibowo@unsoed.ac.id
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: January, 16, 2022;
Revised: April 5, 2022;
Accepted: April 7, 2022;
Published online: April 19, 2022

