
Effect of Calorie Restriction on Intestinal Inflammation in Mice Treated with a High-Calorie Diet
Nadya Meta Harlinda, Sundari Indah Wiyasihati*, Nila Kurniasari and Kristanti Wanito WigatiPublished Date : 2022-07-11
DOI : https://doi.org/10.12982/CMUJNS.2022.036
Journal Issues : Number 3, July-September 2022
Abstract Inflammatory bowel disease (IBD) is classified into ulcerative colitis and Crohn's disease. It is chronic inflammation of gastrointestinal tract and idiopathic disease. Sugar is proved to be risk factor for IBD. Calorie restriction is a nutrition intervention to reduce energy intake and can reduce chronic desease. This study aims to determine the difference in the effect of a high-calorie diet and calorie restriction on intestinal inflammation. Eight-week-old healthy female Balb/c mice were divided into two groups. The high calorie (HC) group was fed standard diet plus oral gavage of glucose solution every day for 4 weeks. The dose of glucose solution was 0.0325 ml/gBW or 3-5% additional calories each day. The calorie restriction (CR) group had their calorie intake restricted to 50% of standard diet 3 times a week. After 4 weeks of treatment, the mice had their colon removed. The assessment of the intestinal inflammation was carried out using a light microscope, with a scoring system by Erben et al (2014) in HE staining. The mean score of the HC group was 1.22, this was higher than the CR group with a mean score of 1.18 even tough the result revealed that the scores of intestinal inflammation were not significantly different in the two groups (P = 0.827). However, there is no evidence for the underlying mechanism of intestinal inflammation by high-calorie diet and improvement of intestinal inflammation in high-calorie diet via calorie restriction. Thus, further research is needed on other calorie restriction methods in high-calorie diet study.
Keywords: High calorie diet, Calorie restriction, Intestinal inflammation
Funding: The authors are grateful for the research funding provided by the Fundamental Research Program Ministry of Research, Technology and Higher Education, Indonesia.
Citation: Harlinda, N.M., Wiyasihati, S.I., Kurniasari,N. and Wigati, K.W., 2022. Effect of Calorie Restriction on Intestinal Inflammation in Mice Treated with a High-Calorie Diet. CMU J. Nat. Sci. 21(3): e2022036.
INTRODUCTION
Inflammatory bowel disease (IBD) is classified into ulcerative colitis and Crohn's disease. Crohn's disease can affect all parts of the gastrointestinal tract from the mouth to the anus transmurally, whereas ulcerative colitis mainly affects the colonic mucosa. Both of these diseases tend to appear in young adults, but can actually appear at any age (Wehkamp et al., 2016). It is an idiopathic and chronic disease and can result in significant morbidity and mortality, as shown in data from Denmark (Yan, 2012; Jess et al., 2013).
In the Western population, the data shows a stable increase in the incidence of inflammatory bowel disease (IBD) (Gibson, 2009; Shivashankar et al., 2017). In Indonesia, 19 out of 921 patients who underwent a colonoscopy procedure at the Ciptomangunkusumo Hospital were diagnosed with Crohn's disease (Pratama et al., 2011), while at Dr. Saiful Anwar Hospital, Malang, the number of ulcerative colitis patients during 2010-2014 was 176 patients (8.2 %) (Mustika and Triana, 2016).
Environmental, infectious, genetic, immunological and microbiome factors play a role in the pathogenesis of inflammatory bowel disease (Amre et al., 2007). A study aimed at evaluating the role of dietary factors in the etiology of IBD found that sugar and fat were proved to be risk factors, especially for Crohn's disease (Sakamoto et al., 2005). Other studies have found that monosaccharide and disaccharide diets can increase the risk of Crohn's disease (Knight-sepulveda et al., 2015), especially in a westernized diet which is also high in sugar. In rats, it has been found that a diet high in fat or carbohydrates can cause dysbiosis, thus resulting in changes in the barrier function of the host (Li et al., 2015). Intake of food high in fat and carbohydrates will increase the expansion of pathogens and decrease the commensal microbiota in the gastrointestinal tract. Consequently, the host is highly exposed to increased metabolic products such as H2S and decreased exposure to beneficial metabolic products of commensal bacteria such as short chain fatty acids (SCFAs), resulting in an increase in exposure to immunogenic antigens that encourage the production of inflammatory cytokines that can increase inflammation in the gut (Leone et al., 2013).
Calorie restriction is a nutrition intervention to reduce energy intake. In a long-term observational study of calorie restriction, it was found that calorie restriction can reduce body weight and the risk of chronic disease in people who are not obese (Zou et al., 2019). Calorie restriction plays a role in creating a balance and diversity of the gut microbiota ecosystem (Wang et al., 2018), preventing excess energy from causing systemic inflammation that occurs when adipose tissue expands to accommodate extra nutrient storage (Ye and Keller, 2010).
However, research on the effects of calorie restriction in humans is still in its early stages (Holloszy and Fontana, 2007) and the mechanisms regarding the effects of high-calorie diets are still poorly understood and the role of experimental animals is needed to help overcome the lack of knowledge (Herawati et al., 2016). Because previous studies regarding the effect of a high-calorie diet and calorie restriction on intestinal inflammation still cannot explain the mechanism, the researchers intend to examine the effect of calorie restriction on intestinal inflammation in mice treated with a high-calorie diet by evaluating the inflammation score, which consists of inflammatory cell infiltration, epithelial changes and changes in the architecture of the intestinal mucosa on microscopic examination with HE staining.
MATERIALS AND METHODS
Approval for this research has been obtained from Health Research Ethics Committee, Faculty of Medicine, Universitas Airlangga (No. 11/EC/KEPK/FKUA/2021).
Animals
This study is an experimental study with post-test-only control group design. Experimental animals used in this study were female mice (Mus musculus) BALBc strain from Pusvetma (Pusat Veteriner Farma), Surabaya, Indonesia. Mice are routinely selected as experimental animals for research on intestinal inflammation because mammals have the same basic immunological mechanisms in both intracellular and extracellular signaling pathways. Mice also have advantages over other experimental animals in that they are inexpensive, require care that is not difficult, and represent the same gut microbiota as humans (Uhlig and Powrie, 2009). The inclusion criteria for the experimental animals were being 8 weeks of age, weighing 20-25 grams, and being in good health. Meanwhile, the exclusion criteria for the experimental animals were weight loss more than 10% during the acclimatization period and illness during the study. The care and treatment of the experimental animals during the study were based on the 3R principle (Replacement, Reduction, and Refinement). All experimental animals included in the inclusion criteria were acclimatized by being placed in a treatment room in the form of experimental animal cages with the size of 20 x 20 x 25 cm, at the Faculty of Veterinary Medicine, Universitas Airlangga, and were given food and equal treatment for 7 days. Mice used in this study were divided into two groups: high calorie group (HC) and calorie restriction group (CR). Each group consisted of twelve mice with sampling being done using simple random sampling, specifically probability sampling technique. Experimental animals were given food from comfeed broiler II, and the glucose solution for additional calorie used in this experiment was from Otsu-D40.
High calorie group treatment
Mice were given a high calorie diet consisting normal amount of food ad libitum per day added with oral gavage of glucose solution with D40 in the amount of
3-5% or 0.0325 ml/gBB (Herawati et al., 2020) every day for 4 weeks. The determination of the oral gavage dose was carried out individually for each mouse by weighing the weight of the mice every week.
Restriction group treatment
Mice were given ad libitum calorie restriction of 50% of the normal amount (Pósa et al., 2015) or 5 grams of feed/day intermittently on Tuesday, Thursday and Saturday. They were also still given D40 glucose fluid by oral gavage every day for 4 weeks to create a high-calorie diet. Based on previous research, intermittent administration of 50% ad libitum restriction 3 times a week in female mice with a high-calorie diet can reduce the expansion of fat tissue to store excess nutrients, known to decrease visceral fat weight (Lutfi et al., 2021).
Preparations
Experimental animals were euthanized in the 4th week, dissected, and the intestines were taken, cleaned, and fixed with 10% formalin in a sample bottle. Tissue processing was carried out at the Department of Anatomical Pathology, Faculty of Medicine, Universitas Airlangga. Colonic tissue was processed to make paraffin blocks and then cut (1-2 micrometers thick) transversely for Haematoxylin-Eosin (HE) staining.
Histopatological scoring
Assessment of the intestinal inflammation utilized the scoring in a study conducted by Erben et al., (2014) titled “A guide to histomorphological evaluation of intestinal inflammation in mouse models”. The intestinal inflammation was assessed specifically in the colon. The higher the value, the more severe the inflammation is. The assessment was carried out on three aspects, namely inflammatory cell infiltration, epithelial changes, and changes in the architecture of the intestinal mucosa with a magnification of 100x and 400x using a light microscope (Table 1).
Table 1. Scoring for inflammation from “A guide to histomorphological evaluation of intestinal inflammation in mouse models”.
|
Inflammatory Cell Infiltrate |
Epithelial Changes |
Mucosal Architecture |
Score |
|
|
Severity |
Extent |
|||
|
Minimal |
Mucosa |
Minimal hyperplasia |
|
1 |
|
Mild |
Mucosa, sometimes Submucosa |
Mild hyperplasia, mild goblet cell loss ± cryptitis ± erosions |
|
2 |
|
Moderate |
Mucosa, Submucosa |
Moderate hyperplasia, moderate goblet cell loss, cryptitis ± crypt abscesses |
Ulcerations |
3 |
|
Marked |
Mucosa, Submucosa, often Transmural |
Marked hyperplasia, marked goblet cell loss, multiple crypt abcesses |
Extended ulcerations ± pseudopolyps |
4 |
Statistic analysis
Descriptive test was conducted on the obtained data to see the distribution of the data. Then, normality test was performed and comparison test was carried out with Mann-Whitney test using SPSS version 25.0 software. The difference of data is significant if the significance value (sig.) is less than 0.05 (P < 0.05).
RESULTS
Score for intestinal inflammation in each group
The distribution of the number of samples for each intestinal inflammation score in both groups after 4 weeks of treatment, there were ten experimental animals in the HC group and eleven in the CR group that were assessed for the score of inflammation. The difference in the total count of each group was due to the exclusion of some experimental animals before euthanasia. The number of experimental animals excluded in the HC group was three, and one was excluded in the CR group. In the HC group, two preparations had the score of 2 for the inflammation, seven preparations had the score of 1 and no preparations had the score of 3 or 4. In the CR group, two preparations had the score of 2 for the inflammation, nine preparations had the score of 1 and no preparations had the score of 3 or 4. The higher the score, the more severe the intestinal inflammation is.
Figure 1 shows the intestinal inflammation with a score of 1. Inflammatory cell infiltration was minimal, characterized by fewer and scattered inflammatory cells, and only found in the mucosa. There were no changes in the epithelium and architecture of the intestinal mucosa detected using 40x magnification on a light microscope. Whereas, Figure 2 shows the intestinal inflammation with a score of 2. Inflammatory cell infiltration occurred mildly, characterized by inflammatory cells in greater numbers and found in clusters. There were no changes in the epithelium and architecture of the intestinal mucosa detected using 40x magnification on a light microscope.
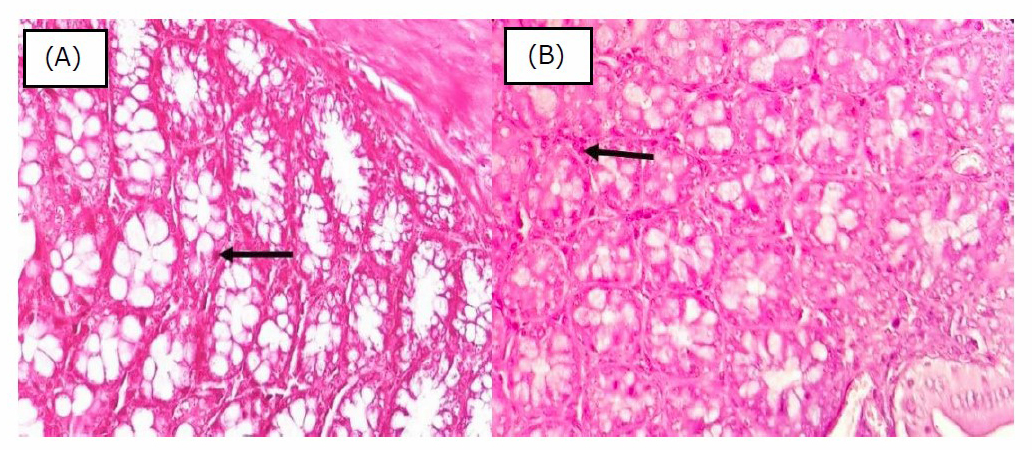
Figure 1. Histopathology of colon mucosa with score 1. Black arrow showed scattered lymphocytes in the lamina propria (obj. 40x, H&E). [A] High Calorie Diet Group, [B] Restriction Calorie Group.
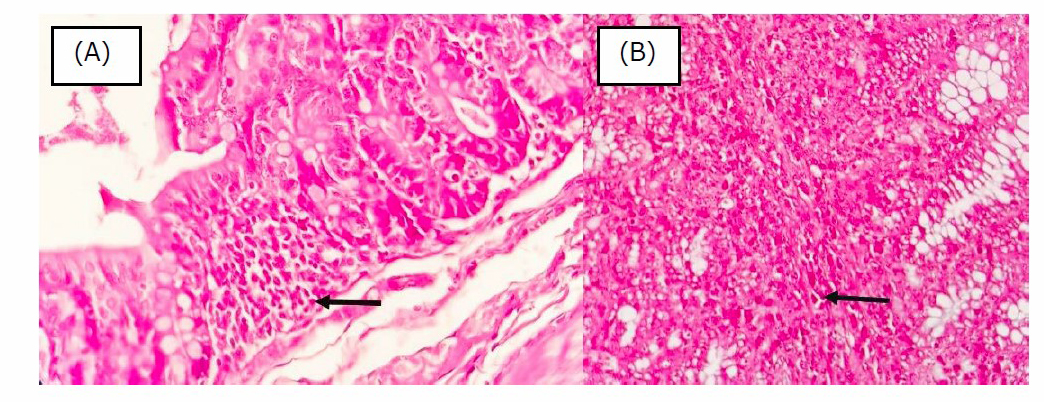
Figure 2. Histopathology of colon mucosa with score 2. Black arrow showed scattered lymphocytes in the lamina propria (obj. 40x, H&E). [A] High Calorie Diet Group, [B] Restriction Calorie Group.
The difference in the score of intestinal inflammation in the two groups
First, we conducted a descriptive analysis to evaluate the minimum, maximum, mean, and standard deviation of the score of intestinal inflammation in mice (Mus musculus) of BALB/c strain. The minimum score were 1 for both groups and the maximum score were 2 for both groups. The mean score for the HC group was 1.22; and the mean score of the CR group was 1.18. Second, we performed a normality test using Shapiro-Wilk test, and it was obtained that the p-value of the two groups was < 0.05, so it can be concluded that the data on the score of intestinal inflammation of mice were not normally distributed. Because the data were not normally distributed, the Mann-Whitney test was carried out, with the results being showed in Table 2. Based on the test results, it was obtained that the P-value was 0.827 or > 0.05. Thus, H0 was accepted, which means there was no difference in the score of intestinal inflammation of mice in the two study groups.
DISCUSSION
The data from this study showed that there was no difference in the score of intestinal inflammation of mice in the two groups. This could be due to several factors. The first factor is a short duration of treatment of high-calorie diet with simple carbohydrates in the form of glucose. A study conducted on mice to examine the effect of short-term high sugar intake on the gut found that there was no difference in the histopathological analysis of the colon in the control group compared to the high sugar intake group after 7 days of treatment and also there was no induction of cell death based on Western blot analysis (Khan et al., 2020). Research by Do et al., (2018) showed an increase in the expression of inflammatory cytokines such as TNFα and IL-1β in the colon that occurred after 12 weeks of high-glucose diet treatment, but did not see inflammation histologically. Another study proved that an increase in Lcn-2 in the feces of mice with a high-fat/ high-sugar diet only occurred at week 5 to week 18, indicating that this diet creates a specific inflammatory environment in the gut, thereby increasing susceptibility to chronic inflammatory bowel disease (Agus et al., 2016). Thus, it is possible that experimental animals given a high-calorie diet in this study have experienced physiological changes as indicated by an increase in inflammatory mediators, but there has not been an anatomically significant change caused by the inflammation.
Another factor that can influence the results of the study is dietary pattern which is not the only factor in the occurrence of intestinal inflammation. Diet is an environmental factor that is one of the various factors that influence the occurrence of intestinal inflammation, such as in IBD, where there is still no clear cause for this inflammation (Perše and Cerar, 2012; Ananthakrishnan et al., 2017). The inflammation involves a complex interaction between genetics, environmental factors including smoking, diet, drugs, geography, social stress, and psychological elements, microbial factors, and immune responses (Loftus, 2004; Zhang and Li, 2014). It is not easy to refute the popular belief that IBD arises from a highly complex interaction between genetic and environmental elements, a disordered immune response and changes in the microbiome, so that not only one of these factors may cause the disease (Zhang and Li, 2014). This may be one of the effects of the absence of significant inflammatory differences in the high-calorie diet group on histological examination because there are many other contributing factors besides dietary patterns.
CONCLUSION
Our study has revealed that although calorie restriction in this study cannot give favorable changes in the intestinal inflammation induced by high calorie diet, there is a pattern that caloric restriction tends to have better histopathology changes. However, there is no evidence underlying mechanism of intestinal inflammation by high calorie diet and improvement of intestinal inflammation in high calorie diet via calorie restriction. Thus, further research is needed on other calorie restriction methods in high calorie diet study.
ACKNOWLEDGEMENTS
The authors would like to thank the Faculty of Veterinary, Universitas Airlangga and Anatomical Pathology Laboratorium of Faculty of Medicine, Universitas Airlangga for providing the facilities support.
AUTHOR CONTRIBUTIONS
Nadya Meta Harlinda performed the experiment and analyzed data under the supervision of Sundari Indah Wiyasihati dan Nila Kurniasari. Kristanti Wanito Wigati and Sundari Indah Wiyasihati designed the concept and interpreted the data. Nadya Meta and Sundari Indah Wiyasihati wrote and carried out the proofreading, and finalized the manuscript for publication. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Amre, D., D'Souza, S., Morgan, K., Seidman, G., Lambrette, P., Grimard, G., Israel, D., Mack, D., Ghadirian, P., Deslandres, C., et al. 2007. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for crohn's disease in children. The American Journal of Gastroenterology. 102: 2016-2025.
Agus, A., Denizot, J., Thévenot, J., Martinez-Medina, M., Massier, S., Sauvanet, P., … Barnich, N. 2016. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Scientific Reports. 6: 1–14.
Ananthakrishnan Ashwin N., Xavier, R.J., and Podolsky, D.K. 2017. Epidemology and Pathogenesis. p.3-11. In Inflammatory Bowel Diseases: a clinician’s guide. John Wiley & Sons, Chichester.
Do, M.H., Lee, E., Oh, M.J., Kim, Y., and Park, H.Y. 2018. High-glucose or-fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients. 10: 1–14.
Erben, U., Loddenkemper, C., Doerfel, K., Spieckermann, S., Haller, D., Heimesaat, M. M., … Kühl, A.A. 2014. A guide to histomorphological evaluation of intestinal inflammation in mouse models. International Journal of Clinical and Experimental Pathology. 7: 4557–4576.
Gibson, P.R. 2009. Overview of inflammatory bowel disease in Australia in the last 50 years. Journal of Gastroenterology and Hepatology. 24: 63–68.
Herawati, L., Sari, G.M., and Irawan, R. 2020. High glycemic index diet decreases insulin secretion without altering Akt and Pdx1 expression on pancreatic beta cells in mice. Chiang Mai University Journal of Natural Sciences. 19: 366–378.
Herawati, L., Wigati, K.W., Wibowo, W., and JM, H. 2016. Survival rate animal subject to interval and continuous addition of high calorie diet. p.42-48. In: Proceeding, The Role of Physiology to Increase the Quality of Life Through One Health Concept: Health Care for Human, Animals, Plants and Environment. Yogyakarta.
Holloszy, J.O. and Fontana, L. 2007. Caloric restriction in humans. Experimental Gerontology. 42: 709–712.
Jess, T., Frisch, M., and Simonsen, J. 2013. Trends in overall and cause-specific mortality among patients with inflammatory bowel disease from 1982 to 2010. Clinical Gastroenterology and Hepatology. 11: 43–48.
Khan, S., Waliullah, S., Godfrey, V., Khan, M.A.W., Ramachandran, R.A., Cantarel, B.L., and Zaki, H. 2020. Dietary simple sugars alter microbial ecology in the gut and promote colitis in mice. Science Translational Medicine. 12: 1-15.
Knight-sepulveda, K., Kais, S., Santaolalla, R., and Abreu, M.T. 2015. Diet and inflammatory bowel disease. Gastroenterology & Hepatology. 11: 511–520.
Leone, V., Chang, E.B., and Devkota, S. 2013. Diet, microbes , and host genetics : the perfect storm in inflammatory bowel diseases. Journal of Gastroenterology. 48: 315–321.
Li, J., Butcher, J., Mack, D., and Stintzi, A. 2015. Functional impacts of the intestinal microbiome in the pathogenesis of infl ammatory bowel disease. Inflammatory Bowel Diseases. 21: 139–153.
Loftus, E.V. 2004. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 126: 1504–1517.
Lutfi, A.R., Herawati, L., and Sari, G.M. 2021. Calorie restriction and moderate-intensity continuous exercise decrease free fatty acid levels and visceral fat weight on high calorie diet female mice. Indian Journal of Forensic Medicine & Toxicology, 15: 3665–3677.
Mustika, S., and Triana, N. 2016. The prevalence, profile, and risk factor of patients with ulcerative colitis at Dr. Saiful Anwar Malang General Hospital. The Indonesian Journal of Gastroenterology, Hepatology and Digestive Endoscopy, 17: 16–20.
Perše, M. and Cerar, A. 2012. Dextran sodium sulphate colitis mouse model: Traps and tricks. Journal of Biomedicine and Biotechnology. 2012: 1–13.
Pósa, A., Szabó, R., Kupai, K., Csonka, A., Szalai, Z., Veszelka, M., and Varga, C. 2015. Exercise training and calorie restriction influence the metabolic parameters in ovariectomized female rats. Oxidative Medicine and Cellular Longevity. 2015: 1–8.
Pratama, N., Abdullah, M., Kansera, D.D., Estherina, J., Yaruntradhani, R., Hardi, F., … Abineno, P. 2011. Prevalence of crohn’s disease in endoscopic unit Cipto Mangunkusumo Hospital. The Indonesian Journal of Gastroenterology, Hepatology, and Digestive Endoscopy. 12: 85–88.
Sakamoto, N., Kono, S., Wakai, K., Fukuda, Y., Satomi, M., Shimoyama, T., and Tanaka, H. 2005. Dietary risk factors for inflammatory bowel disease a multicenter case‐control study in Japan. Inflammatory Bowel Diseases. 11: 154–163.
Shivashankar, R., Tremaine, W.J., Hamsen, W.S., and Jr, E.V.L. 2017. Colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol. 15: 857–863.
Uhlig, H.H. and Powrie, F. 2009. Mouse models of intestinal inflammation as tools to understand the pathogenesis of inflammatory bowel disease. Europian Journal of Immunology. 39: 2021–2026.
Wang, S., Huang, M., You, X., Zhao, J., Chen, L., Wang, L., and Luo, Y. 2018. Gut microbiota mediates the anti- obesity effect of calorie restriction in mice. Scientific Report. 8: 1–14.
Wehkamp, J., Götz, M., Herrlinger, K., Steurer, W., and Stange, E. F. 2016. Inflammatory Bowel Disease: Crohn’s disease and ulcerative colitis. Deutsches Arzteblatt International. 113: 72–82.
Yan, Y. 2012. Pathogenesis of Inflammatory Bowel Diseases. p.111-134. In Dr. Sami Karoui [ed] Inflammatory Bowel Disease - Advances in Pathogenesis and Management. InTech, Europe.
Ye, J. and Keller, J.N. 2010. Regulation of energy metabolism by inflammation : A feedback response in obesity and calorie restriction. Aging. 2: 361–368.
Zhang, Y.Z., and Li, Y.Y. 2014. Inflammatory bowel disease: Pathogenesis. World Journal of Gastroenterology. 20: 91–99.
Zou, H., Wang, D., Ren, H., Chen, P., Fang, C., Shi, Z., and Zhong, H. 2019. Calorie restriction intervention induces enterotype-associated BMI loss in nonobese individuals. BioRxiv.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Nadya Meta Harlinda1, Sundari Indah Wiyasihati2, *, Nila Kurniasari3 and Kristanti Wanito Wigati4
1 Medical Program, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
2, 4 Department of Physiology, Universitas Airlangga, Surabaya, Indonesia
3 Department of Anatomical Pathology, Universitas Airlangga, Surabaya, Indonesia
Corresponding author: Sundari Indah Wiyasihati, E-mail: sundari-i-w@fk.unair.ac.id
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: December 19, 2021;
Revised: March 12, 2022;
Accepted: March 14, 2022;
Published online: April 1, 2022

