
Protective and Osteogenic Effects of Crude Water Extract from Cuscuta japonica Choisy at Gene Expression Level in Human Gingival Cells
Fahsai Kantawong*, Suraiya Sadeeyamoo, Peeraya Wongsit, Montree Tungjai, Phenphichar Wanachantararak, Suruk Udomsom, Jianghua Yang, and Ataya SathirachindaPublished Date : 2022-03-31
DOI : https://doi.org/10.12982/CMUJNS.2022.034
Journal Issues : Number 2, April-June 2022
Abstract This study focused on observing change of gene expression after long-term treatment with high dose of crude water extract from seeds of Cuscuta japonica Choisy (Japanese dodder). The aim of this study was to study the protective and osteogenic effects of dodder seed extract at gene expression level in human gingival cells. In this research, dodder seeds were blended and boiled in water before freeze-drying to preserve as dried powder. Total phenolic content and antioxidant assay were performed. MTT assay was performed with human gingival cells. The concentrations of 100, 250 and 500 µg / mL had no effect on cell viability when cultivated for 48 hours. The extract at the concentration of 250 µg / mL was chosen to treat human gingival cells for many time points to observe the expressions of osteogenic, inflammatory, antioxidant and cancer gene markers by real-time PCR. The results showed that dodder seed water extract could increase the expressions of osteogenic markers; OPN, OCN and Col-I genes. Moreover, dodder seed water extract induced mineralization of human gingival cells cultured in 3D structure. Change of BCL2, CAS3 and LC3 expression indicated involvement in apoptotic process of dodder seed water extract. Significant changes in gene expression of antioxidant markers (GST1, SOD1 and TXNRD1), inflammatory marker (Cox-2) and epithelial cell marker (α-SMA) without change of important cancer genes were observed. These evidences suggested that seeds of Cuscuta japonica Choisy could benefit the application in regenerative medicine and alternative medicine.
Keywords: Cuscuta japonica Choisy, Antioxidant, Osteogenic effects, Gene expression
Funding: The Integrative Associated Medical Sciences Fund 2562 and Associated Medical Sciences Annual Research Fund 2562.
Citation: Kantawong, F., Sadeeyamoo, S., Wongsit, P., Tungjai, M., Wanachantararak, P., Udomsom, S., Yang, J., and Sathirachinda, A. 2022. Protective and osteogenic effects of crude water extract from Cuscuta japonica choisy at gene expression level in human gingival cells. CMU J. Nat. Sci. 21(2): e2022034.
INTRODUCTION
Cuscutae Semen, also known as dodder seed or Tu Si Zi, was the seed of a parasitic vine used in Traditional Chinese Medicine. Phytochemical compounds from edible plants were used to prevent degenerative diseases since ancient time. The seeds of Cuscuta chinensis (Chinese dodder) were widely used as Chinese herbal remedies (Noureen et al., 2019). Dodder seeds contained various phytochemical agents such as flavonoids, phenolic acids, steroids, hydroquinones, volatile oils, lignins, polysaccharides and glycosides (He et al., 2010). In Taiwan, infertile women were treated with dodder seeds (Wu et al., 2018). Most of the previous studies used alcohol and various types of solvents in the extraction processes and tested with cell lines or animal cells. However, the effects of dodder seeds on gene expression in primary cell culture was not yet performed. In this study, we used simple method by blending and boiling dodder seeds in water which was similar to the real-life use. The extract was tested with human gingival cells. Study of Yang et al. indicated that processing of Chinese dodder was able to alter their chemical constituents and affected their bioactivity profiles. Study of Yang et al. also supported that crude products and processed products should prescribe differently in clinic (Yang et al., 2016). Cuscuta chinensis, Cuscuta australis and Cuscuta japonica Choisy were important species of Cuscutae Semen recorded in Chinese Pharmacopoeia (Gao et al., 2017). C. japonica Choisy was also widely used as Traditional Chinese Medicine and it was commercially available in Sichuan, China. However, the study about medicinal properties of C. japonica Choisy was still limited. The study of Moon et al indicated that C. japonica Choisy might improve learning and memory via the enhancement of adult hippocampal neurogenesis by upregulating expression of neurogenic differentiation factor, which was essential for the maturation and differentiation of granule cells in the hippocampus (Moon et al., 2016). The study of Jang et al. indicated that aqueous fraction from C. japonica seeds reduced melanin synthesis and tyrosinase activity in α-MSH-stimulated B16F10 cells (Jang et al., 2012).
This study presented the simple method for crude extraction by blending and boiling C. japonica seeds in water which still provided the active components such as phenolic acids and flavonoids that can be used for prevention of diseases. Most of the previous studies employed solvent extractions which were not common in daily life use.
Previous study presented that water extract from dodder seeds prevented death of osteoblastic cell line (MC3T3-E1) from oxidative stress. Dodder seeds water extract could inhibit reactive oxygen species and malondialdehyde generation and increased activity of superoxide dismutase, glutathione reductase, glutathione S-transferase and glucose-6-phosphate dehydrogenase (Gao et al., 2013). Dodder seeds water extract decreased the cytochrome c releasing from mitochondria and increased anti-apoptotic protein expression but reduced apoptotic protein expression (Gao et al., 2013). The aqueous extract of dodder seeds increased alkaline phosphatase activity, collagen synthesis and bone morphogenetic protein 2 expression in the osteosarcoma cell lines (Yang et al., 2009). Previous studies indicated that kaempferol and hyperoside were active compounds in dodder seeds which possessed osteogenic effect (Yang et al., 2011; Zhang et al., 2014).
Seeds of Cuscuta japonica Choisy were found in the Southern China and were used as substitutes for C. chinensis, which is not readily available (Gao et al., 2017; Oh et al., 2002). No research evidence demonstrated the action of Cuscuta japonica Semen as the osteogenic herbal medicine. The aim of this study was to study the protective and osteogenic effects of Cuscuta japonica seeds at gene expression level in human gingival cells. In the previous study, biomaterials made from gelatin and tri-calcium phosphate combined with Traditional Chinese Medicine were prepared to cultivate rat bone cells. It was found that addition of Cuscuta chinensis extract to the rat bone cell culture clearly promoted proliferation and differentiation of the osteoblasts from their precursor cells. C. chinensis extract reduced amount of tartrate-resistant acid phosphatase (TRAP) which indicated that this herb significantly inhibited osteoclast activities (Yao et al., 2005). In this study, we tried to applied scaffold technology and Traditional Chinese Medicine with human primary cells to prove that dodder seed water extract could enhance bone formation in 3D structure. In this study, we used simple method to prepare 3D-framework filled with gelatin scaffold which was easy for cell culture and treatment (Kantawong et al., 2018). Gelatin scaffold was biocompatible, biodegradable, cost effective and possessed osteogenic (Wang et al., 2016). However, in the real situation, the mechanical properties of gelatin were not matched with bone mechanical properties which lead to the problem with osseointegration when placing the scaffolds to the site of implantation (Wang et al., 2016). In this study, three-dimensional (3D) scaffolds produced with polycaprolactone (PCL) framework filled with gelatin were prepared to solve the problem. PCL was popular in bone tissue engineering because it possessed osteogenic properties and it was not too soft as gelatin (Henkel et al., 2013). The aim of this study is to investigate the osteogenic activity of Cuscuta japonica Semen. In the present study, the researchers will observe that the 3D structure could act as a barrier of Traditional Chinese Medicine treatment or not.
The gingiva is the periodontal tissues which originates from the neural crest. The gingiva consists of connective tissue and epithelium. The gingival tissue is a source of stem cells called gingiva-derived mesenchymal stem cells (GMSCs) (Stefanska et al., 2020). GMSCs present stem-cell properties similar to mesenchymal stem cells (MSCs) which possess multipotent differentiation capacities (Kim et al., 2021). Suitable scaffolds are able to support the bone differentiation ability of GMSCs isolated from gingiva tissues which benefit autologous bone tissue regeneration (Cristaldi et al., 2020; Diomede et al., 2018).
MATERIALS AND METHODS
Preparation of dodder seeds water extracts
Dodder seeds were obtained from local trader in Sichuan (Sichuan Shamgracy Pharmaceutical Co., LTD). Commercial Cuscutae Semen was Cuscuta japonica Choisy as indicated on the package. The voucher specimen of commercial Cuscuta japonica Choisy from Sichuan was previously identified by Prof. Yu-lin Lin (Institute of Medicinal Plant Development (IMPLAD), Chinese Academy of Medical Science and Peking Union Medical College) and deposited at the herbarium of IMPLAD, Chinese Academy of Medical Sciences and Peking Union Medical College. (Gao et al., 2017) Dried dodder seeds amount of 200 grams were blended in a blender for 2 minutes with 3,000 mL of distilled water before boiling for 5 minutes. After that, the boiled solution was cooled down at 4°C overnight. The next day, the solution was filtered through white gauze. The filtrate was aliquoted in lyophilized flasks and frozen at -20°C before lyophilized into powder for 7 days.
Total phenolic content (Folin-ciocalteu assay)
Total phenolic content assay was modified from the previous study (Kantawong et al., 2021a). Dried extract was dissolved in distilled water to the concentrations of 5,000 2,500 1,250 625 and 312.5 µg/mL. Then, 50 µL of each concentration was mixed with 2.5 mL Folin ciocalteau reagent and 2.0 mL Na2CO3 was added.
The reactions were incubated at 45°C in a water bath for 15 minutes and the optical densities were measured at 764 nm. A standard curve was prepared by diluting gallic acid to the concentrations of 10,000, 5,000, 2,500, 1,250, 625 and 312.5 µg/mL and then the same test as described for the dodder seed extract was performed. 2.5 mL of Folin ciocalteau reagent mixed with 2.0 mL Na2CO3 was used as blank. Triplicate controls and tests were prepared for each concentration.
Antioxidant capacity (DPPH assay)
DPPH assay was modified from the previous study (Kantawong et al., 2021a). Dried extract was dissolved in distilled water to the concentrations of 5,000, 2,500, 1,250, 625 and 312.5 µg/mL. After that, 50 µL of each concentration was mixed with 2,950 µL of DPPH reagent (90 µg/mL) and incubated in the dark at room temperature for 15 minutes. A standard curve was prepared by diluting ascorbic acid to the concentrations of 10,000, 5,000, 2,500, 1,250, 625 and 312.5 µg/mL and then performed the same test as described for the dodder extract. Triplicate controls and tests were prepared for each concentration. The absorbances were measured at 515 nm and the optical densities were calculated for % inhibition using the following equation.

Human gingival cell culture
Human gingival cells (n = 2; passage 3) were seeded into 6-well plate at the density of 40,000 cells/well and incubated in CO2 incubator for 24 hours. In the next day, media was discarded and replaced with complete DMEM containing 250 µg/mL dodder extract. Human gingival cells (passage 3) at the density of 40,000 cells/well cultured in plain complete DMEM were used as control groups. Cultured media was changed every 3 days. Cells were cultured for 3 days, 8 days and 14 days. The experiment was divided into 2 groups: the control group (cells cultured in complete DMEM) and the treated group (cells cultured in DMEM containing 250 µg/mL dodder seed extract). Triplicate controls and tests were prepared for each time point and independent t-test (SPSS 17.0) was used to determine the difference between groups.
Cell viability study (MTT assay)
Human gingival tissues were obtained according to a protocol approved by the Ethics Committee, Faculty of Dentistry, Chiang Mai University (70/2019). Human gingival tissues (n = 2) were harvested during a surgical procedure and preserved in a sterile phosphate buffer saline. Human gingival tissues were cut with a scalpel in a Petri dish containing complete DMEM before culture in an incubator at 37°C with 5% CO2. Complete DMEM was changed every two days until the cells reached 80% confluent. Human gingival cells (passage 3) were seeded in complete DMEM with the density of 20,000 cells/well in the 12-well plates and incubated at 37°C for 24 hours in the CO2 incubator. The next day, media was discarded and replaced with complete DMEM containing dodder extracts dissolved at various concentrations and incubated for 48 hours at 37°C in a CO2 incubator. Human gingival cells (passage 3) cultured in normal complete DMEM was used as controls. When the incubation time was over, media was discarded and replaced with 2 mL of media containing MTT (1 mg/mL) before incubating for 2 hours in CO2 incubator. After the media was discarded, 2 mL of DMSO was added to dissolve the formazan crystals. The absorbance was measured at 570 nm and 630 nm. The optical densities were used to calculate % cell viability using the following equation. The experiments were repeated 3 times and independent t- test (SPSS 17.0) was used to determine the difference between groups.

Real-time PCR
RNA extraction was performed using NucleoSpin® RNA II, according to the protocol provided by the manufacturer (Yin et al., 2020). Total RNA amounts were measured with Nanodrop 2000. The reaction solutions were prepared with 6 µL of RNA, 4 µL of 5x RT Master Mix and 10 µL of DEPC water. The RNA was converted to cDNA in the thermocycler using the following program: 37°C 15 minutes, 50°C for 5 minutes, 98°C for 5 minutes. cDNA was stored at -20°C. NO-RT control was prepared with the same method using 5x RT buffer. Real-time PCR reaction were prepared with 8 µL RNA, 10 µL SYBR Green Mastermix and 2 µL primer (the final concentration of each primer was 1.0 µM) in the total volume of 20 µL. The PCR program was set as following: (1) Pre-incubation at 95°C for 2 minutes. (2) PCR consisted of denature at 95°C, annealing at 60°C and extension at 72°C. Real-time PCR was performed for 40 cycles. Two reference genes; GAPDH and beta-actin were used for normalization. Gene expression was calculated by the relative real-time analysis in LightCylcler 1.5 using the Pfaffl analysis method (Pfaffl, 2001).
The sequences of primers used in this study was shown in Table 1. The experiment was divided into 2 groups: the control (cells cultured in complete DMEM) and the treatment (cells cultured in DMEM containing 250 µg/mL dodder seed extract). Triplicate controls and tests were prepared for each time point and independent t-test (SPSS 17.0) was used to determine the difference between groups. Fold change of gene expressions were presented in the form of mean ± SD when P
Table 1. Primer sequences used in this study.
|
Primer |
Sequence (5'->3') |
References |
Accession number |
|
Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) |
(F) AAATCCCATCACCATCTTCCAGGAGC |
(Prins et al., 2014) |
NM_001357943.2 |
|
(R) CATGGTTCACACCCATGACGAACA |
|||
|
Osteopontin (OPN) |
(F) GGACAGCCAGGACTCCATTG |
(Lv et al., 2013) |
NM_000582.3 |
|
(R) TGTGGGGACAACTGGAGTGAA |
|||
|
Collagen type -I (Col-I)
|
(F) GATGGATTCCAGTTCGAGTATG |
(Yoo et al., 2016) |
NM_000088.4 |
|
(R) GTTTGGGTTGCTTGTCTGTTTG |
|||
|
BCL-2
|
(F) GATGTGATGCCTCTGCGAAG |
(Mollaei et al., 2017) |
NM_000633.3 |
|
(R)CATGCTGATGTCTCTGGAATCT |
|||
|
Caspase-9 (CAS9)
|
(F)ATGGACGAAGCGGATCGGCGGCTCC |
(Krisnamurti et al., 2016) |
NM_001278054.2 |
|
(R) GCACCACTGGGGGTAAGGTTTTCTAG |
|||
|
Cycloxygenase‑2 (COX-2) |
(F)CCCTTGGGTGTCAAAGGTAA |
(Yang and Han, 2014) |
NM_000963.4 |
|
(R) GCCCTCGCTTATGATCTGTC |
|||
|
Glutathione S-transferase 1 (GST1) |
(F) GCCACACTCTCCGTCAA |
(Spivack et al., 2004) |
NM_000853.3 |
|
(R)TGCCAAGAAGAACGACATTCC |
|||
|
Thioredoxin Reductase 1 (TXNRD1) |
(F) GAAGATCTTCCCAAGTCCTATGAC |
(Kahlos et al., 2001) |
NM_182743.3 |
|
(R)ATTTGTTGCCTTAATCCTGTGAGG |
|||
|
Histone acetyltransferase 1 (HAT1) |
(F)CAGATATATAAGGCTGACATGAC |
(McGraw et al., 2003) |
NM_003642.4 |
|
(R) GCTGTAATATCAAGAACTGTAGG |
|||
|
Alpha-smooth muscle actin |
(F)TGACAATGGCTCTGGGCTCTGTAA |
(Talior-Volodarsky et al., 2012) |
NM_001613.4 |
|
(R) TTCGTCACCCACGTAGCTGTCTTT |
|||
|
Transgelin (TAGLN) |
(F) CGCGAAGTGCAGTCCAAAATCG |
(van Tuyn et al., 2005) |
NM_003186.5 |
|
(R) GGGCTGGTTCTTCTTCAATGGGC |
|||
|
α-actin |
(F) AGAAAATCTGGCACCACACC |
(Deng et al., 2013) |
NM_001101.5 |
|
(R) AGAGGCGTACAGGGATAGCA |
|||
|
Osteocalcin (OCN) |
(F)GCAAGTAGCGCCAATCTAGG |
(Riksen et al., 2014) |
NM_001393915.1 |
|
(R) GCTTCACCCTCGAAATGGTA |
|||
|
BAX |
(F)GGTTGTCGCCCTTTTCTA |
(Mollaei et al., 2017) |
NM_001291430.2 |
|
(R) CGGAGGAAGTCCAATGTC |
|||
|
Caspase-3 (CAS3) |
(F)TTCAGAGGGGATCGTTGTAGAAGTC |
(Krisnamurti et al., 2016) |
NM_001354783.2 |
|
(R) CAAGCTTGTCGGCATACTGTTTCAG |
|||
|
LC3
|
(F)GAGAAGCAGCTTCCTGTTCTGG |
(Peng et al., 2015) |
NM_001085481.3 |
|
(R) GTGTCCGTTCACCAACAGGAAG |
|||
|
NF-kB |
(F) ATGGCTTCTATGAGGCTGAG |
(Pekkala et al., 2015) |
NM_001243985.2 |
|
(R) GTTGTTGTTGGTCTGGATGC |
|||
|
Superoxide Dismutase 1 (SOD1) |
(F) GTAATGGACCAGTGAAGGTGTG |
(Rush et al., 2000) |
NM_000454.5 |
|
(R)CAATTACACCACAAGCCAAACG |
|||
|
Histone Deacetylase 3 (HDAC3) |
(F) TATGCAAGGCTTCACCAAGAG |
(McGraw et al., 2003) |
NM_001355039.2 |
|
(R) TCCGTATTTGTGGAAGGACAC |
|||
|
Lysine acetyltransferase 2A (Kat2a) |
(F) CAGAATGTCTTTTCCCACCAG |
(McGraw et al., 2003) |
NM_001376227.1 |
|
(R) GGATTCAGCTCACACTCCATC |
|||
|
Calponin 2 (CNN2) |
(F)CTGCAGAGCGGGGTGGACATTGGC |
(Ueda et al., 2002) |
NM_001303499.2 |
|
(R) GCCGGCCTCCTCCTGGTAGTAAGG |
Gelatin preparation for mineralization assay
3 ml of 12% gelatin solution was mixed with 30 µl of 25% glutaraldehyde and placed into each well of the 6-well plate. The 6-well plate was left for 24 hours at room temperature to allow gelatin cross-linking. The next day, glutaraldehyde remnant was washed out with distilled water 3 times (30 minutes at a time). The cross-linked gelatins were soaked overnight in distilled water at -4°C after that distilled water was discarded and polymerized gelatins were frozen for 48 hours before freeze-drying. Before using in cell culture, dried gelatins were sterilized by immerging in 70% ethanol for 30 minutes before rinsing with sterile distilled water 3 times (30 minutes at a time) then polymerized gelatins could be used for further experiments as shown in Figure 1A. Gelatins were equilibrated in complete DMEM at 37°C overnight before cell seeding.
3D structure fabrication
3D structure was designed by OpenSCAD program which can adjust the percent of chamber size. The in-houses Fused Deposition Modeling (FDM) machine or 3D printer with 0.4 mm of nozzle size was employed for the scaffold fabrication. The scaffolds with various % of chamber size were print by using polycaprolactone (PCL) filament at layer height = 0.2 mm, % Infill =100, and speed of printing = 10 mm/min. 3 ml of 12% gelatin solution was mixed with 30 µl glutaraldehyde and filled into the space of the 3D structures before standing at room temperature for 24 hours to allow gelatin polymerization as shown in Figure 1B. The 3D structures (with polymerized gelatin filler) were placed into 6-well plate (1 piece per well) and sterilized with 70% ethanol before washing with sterilized water 3 times (30 minutes at a time). 3D structures were equilibrated in complete DMEM at 37°C overnight before cell seeding.
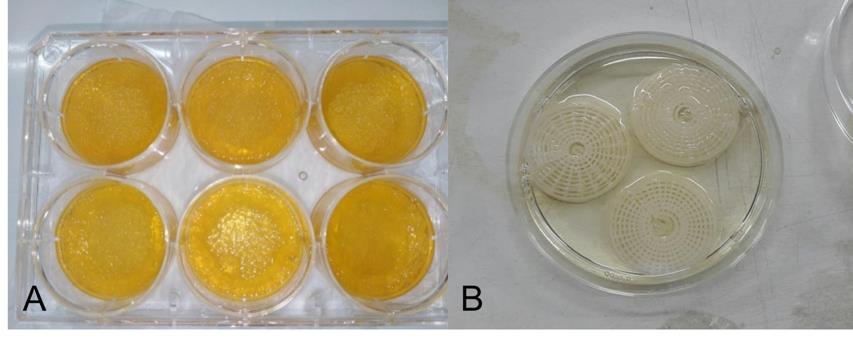
Figure 1. Scaffolds used for mineralization assay. (A) polymerized gelatins in 6-well plate. (B) 3D structures with gelatin filler.
Cell culture for mineralization assay
Human gingival cells (passage 3) 40,000 cells were seeded into each well of 6-well plate with complete DMEM then incubated for 24 hours in CO2 incubator to allow cell attachment. The next day, complete DMEM was discarded and replaced with 5 mL complete DMEM containing dodder seed extract at the concentration of 250 µg/mL. Cell culture was maintained for 14 days. Complete DMEM containing dodder seed extract at the concentration of 250 µg/mL was changed every 5 days.
Cell staining
4% formaldehyde was added onto cell cultures and left for 10 minutes at room temperature. After that, 4% formaldehyde was discarded and replaced with 1% Alizarin red S solution for 10 minutes. Alizarin red S solution was washed out with tap water overnight and cell morphology was observed under the inverted microscope.
Statistical analysis
Triplicate controls and tests were prepared and independent t-test was analyzed using SPSS 17.0 to determine the difference between groups.
DPPH assay and total phenolic content
Dried powder of dodder seed water extract 14.97 g was obtained when 200 grams of dried dodder seeds were used in the extraction and %yield was equal to 7.5%.
The DPPH radical scavenging of dodder seed water extract at the concentrations of 5,000, 2,500, 1,250, 625 and 312.5 µg/mL were shown in Figure 2A. It was found that the extract at the concentration of 5,000 µg/mL showed 13.77% inhibition. The % inhibition of dodder seed extract 5,000 µg was equivalent to ascorbic acid 21.9 µg. When the extract was continuously diluted to various concentration, the % inhibition decreased proportionally as shown in Figure 2A. The extract at the concentrations of 5,000, 2,500, 1,250, 625 and 312.5 µg/mL contained the total phenolic content equivalent to standard gallic acid were shown in Figure 2B. The total phenolic content of dodder seed extract 5,000 µg was equivalent to gallic acid 181.36 µg.
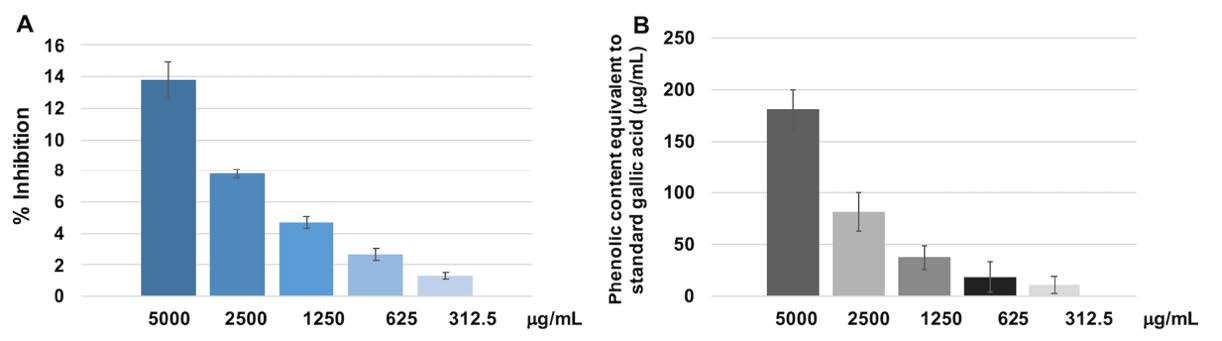
Figure 2. (A) The % inhibition of dodder seed extract at various concentrations. (B) The relationship between the amount of phenolic content in the extract at various concentrations equivalent to gallic acid standard. The DPPH scavenging activity and phenolic content for each concentration were triplicated. Mean and standard deviation were presented for each concentration.
Study on cell viability using MTT assay
Dodder seed extract at the concentrations of 100, 250 and 500 µg/mL were employed to test human gingival cell viability. It was found that the concentrations of 100, 250 and 500 µg/mL had no effect on cell viability when cultivated for 48 hours as shown in Figure 3. The concentration of 250 µg/mL was therefore chosen for further experiments.
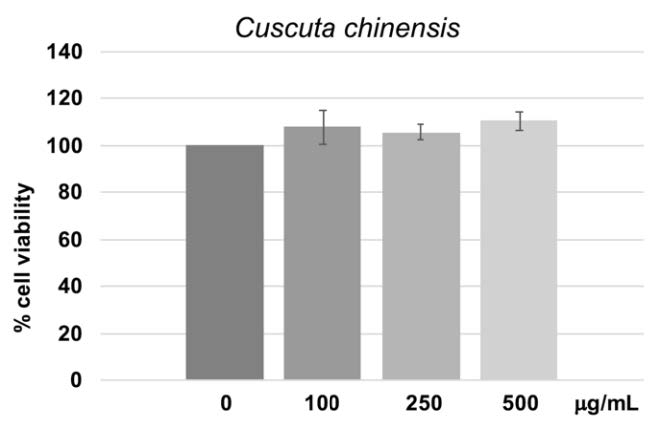
Figure 3. Dodder extract at various concentrations and % cell viability after 48 hours of culture in the extract compared to controls. The MTT assay for each concentration was triplicated. Mean and standard deviation were presented for each concentration.
Antioxidant marker and bone differentiation marker
Antioxidant gene expression in human gingival cells treated with 250 µg/mL for 14 days was shown in Figure 4. GST1 was slightly increased (Figure. 4A). The expression of SOD1 and TXNRD1 increased by 3.8 times (Figure. 4B & 4C).
Osteopontin (OPN) gene was 3 times higher than control (Figure. 4D) while osteocalcin (OCN) gene was found 1.8 times higher than control (Figure. 4E). The expression of the Col-I gene increased by 3.8 times (Figure. 4F).
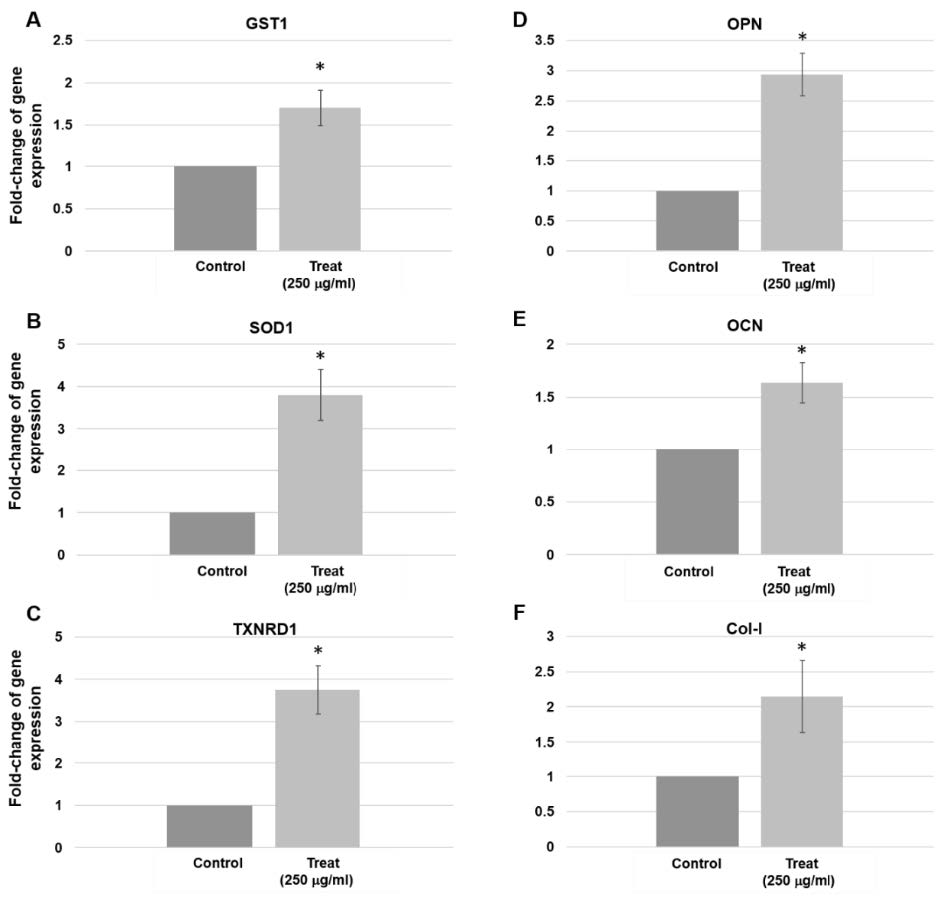
Figure 4. Gene expression in human gingival cells treated with 250 µg/mL for 14 days. (A) GST1 expression increased by 1.7 times (P = 0.028). (B) SOD1 expression increased by 3.8 times (P = 0.015). (C) TXNRD1 gene expression increased by 3.8 times (P = 0.014). (D) OPN expression increased by 3 times (P = 0.011). (E) OCN gene expression increased by 1.8 times (P = 0.028). (F) Col-I gene increased by 3.8 times (P = 0.018). Three pairs of control and test samples were investigated. Mean and standard deviation were presented for each concentration.
Cell staining
Alizarin red s staining showed calcium accumulation in dodder seed water extract treated group as shown in Figure 5. Mineralized nodule was slightly observed after 1 week (Figure 5B). Mineralization nodules could be clearly observed in gelatin (Figure 5D) and in 3D structure (Figure 5E & 5F).
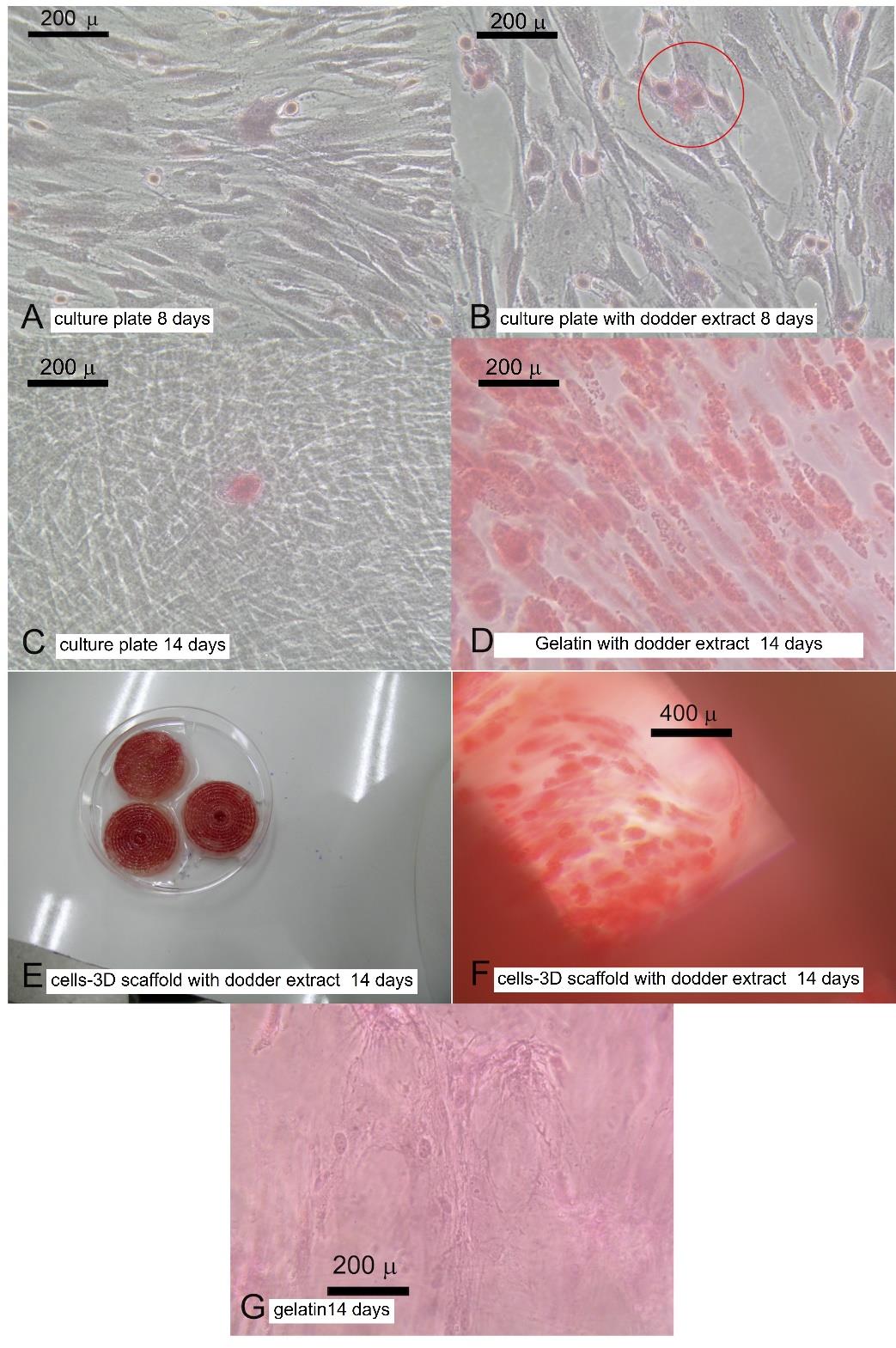
Figure 5. Mineralization. (A) human gingival cells cultured in tissue culture plate and complete DMEM for 8 days. (B) human gingival cells cultured in tissue culture plate and complete DMEM contained dodder seeds water extract for 8 days. (C) human gingival cells cultured in tissue culture plate and complete DMEM for 14 days. (D) human gingival cells cultured in gelatin and complete DMEM contained dodder seeds water extract for 14 days. (E) Cells-3D structures after 14 days treatment in complete DMEM contained dodder seeds water extract. (F) human gingival cells cultured in 3D structures and complete DMEM contained dodder seeds water extract for 14 days. (G) human gingival cells cultured in plain gelatin for 14 days.
Epithelial markers
In addition, epithelial marker was also found up-regulated, dodder seed extract increased the expression of Alpha-smooth muscle actin (α-SMA) in the human gingival cells. The expression of α-SMA increased by 2.5 and 3 times compared to control at 3 days and 14 days, respectively (Figure. 6A & 6B). When confirmed with other biomarkers such as calponin, no obvious changes were found (Figure 6C & 6D).
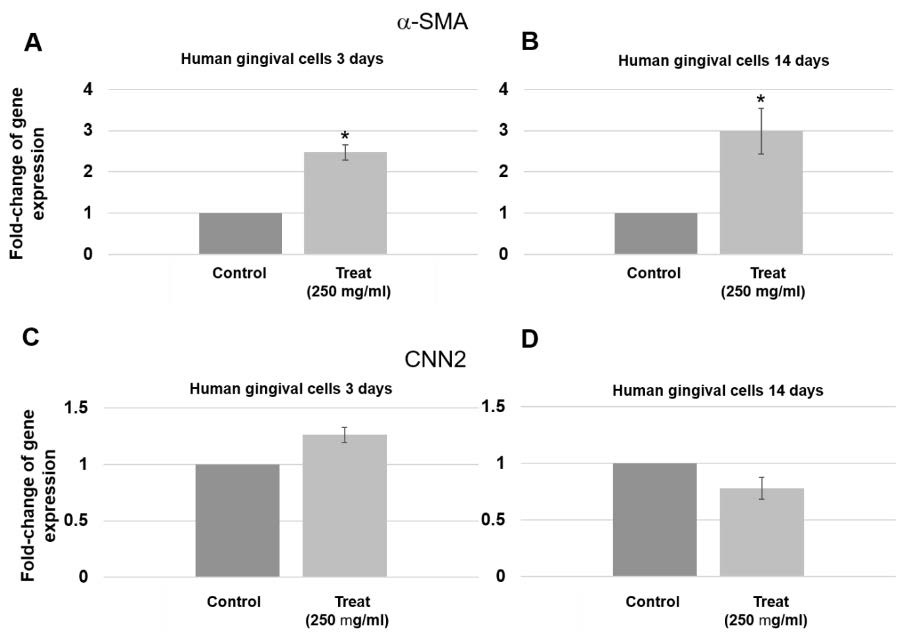
Figure 6. Expression of epithelial markers in human gingival cells treated with 250 µg/mL for 3 days and 14 days. (A & B) α-SMA gene expression increased at 3 days (P = 0.005) and 14 days (P = 0.025). (C & D) Insignificant change in expression of CNN2 gene was found at 3 days and 14 days. Three pairs of control and test samples were investigated. Mean and standard deviation were presented for each concentration.
Apoptosis and autophagy markers
There was no change in expression of BAX gene when cultured in the extract for 3 days and 14 days (Figure 7A & 7F). BCL2 gene expression was not altered at 3 days, but increased more than 3 times when culturing in the extract for 14 days (Figure 7B & 7G). There was no change in expression of CAS3 gene at 3 days but the expression increased more than 2 times when cultured in the extract for 14 days (Figure 7C & 7H). There was no change in expression of CAS9 gene when cultured in the extract for 3 days and 14 days (Figure 7D & 7I). After culturing the human gingival cells in dodder seed extract for 3 days, the expression of the LC3 gene was not much different from the control group. LC3 expression increased more than 4 times when culturing for 14 days (Figure 7E & 7J).
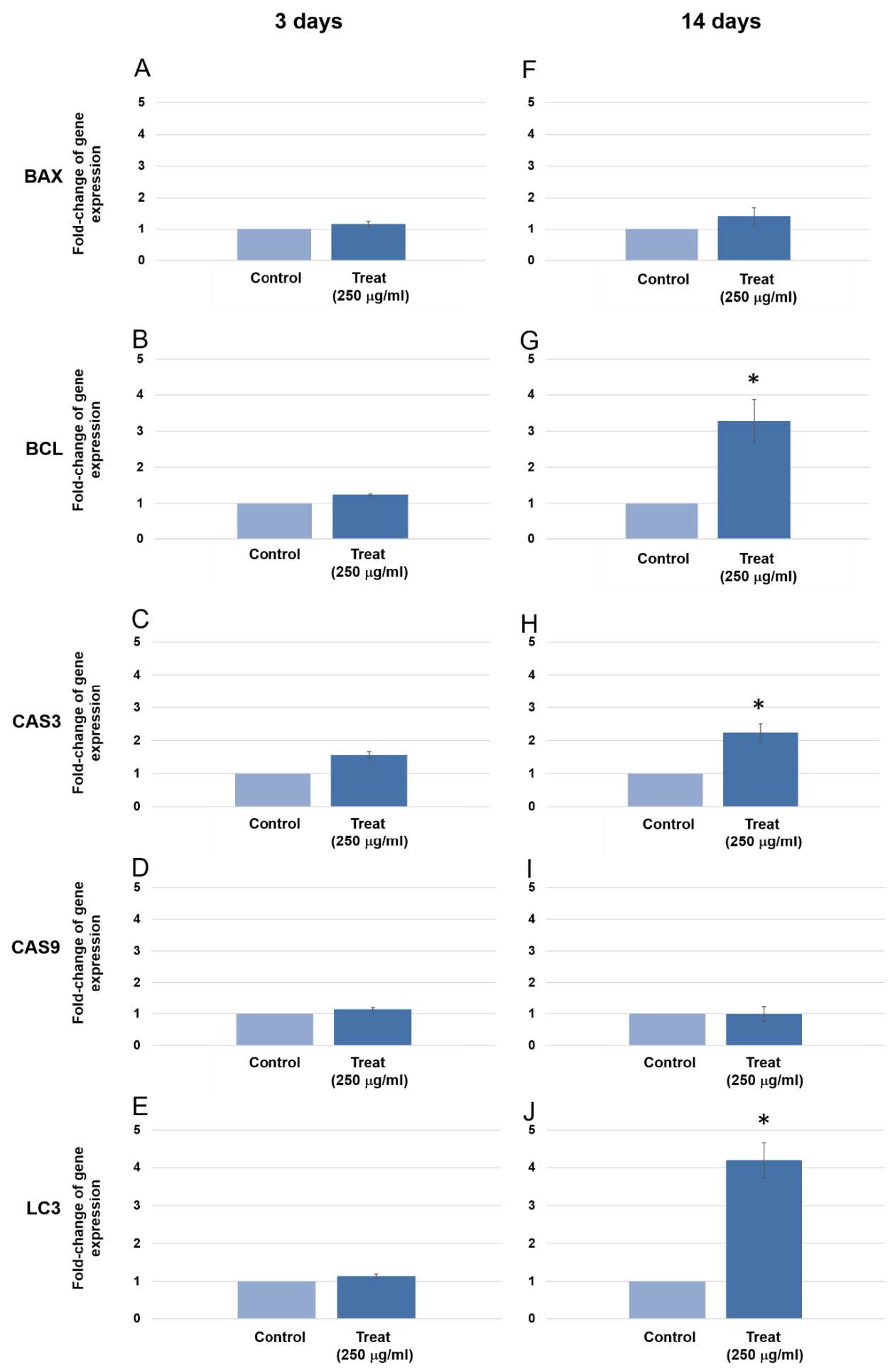
Figure 7. The expression of BAX, BCL2, CAS3, CAS9 and LC3 gene after treated human gingival cells with the extracts for 3 days and 14 days. BCL2, CAS3 and LC3 genes were found significantly upregulated at 14 days culture (P = 0.022, 0.015 and 0.007, respectively) Three pairs of control and test samples were investigated. Mean and standard deviation were presented for each concentration.
Cancer genes
When 250 µg/mL were used to treat the human gingival cells for 8 days, the expression of 6 cancer genes (PRKACB, STAT5B, ADORA2B, LPAR1, LPAR3, LPAR4) were not rendered (Figure 8). None of these genes showed significant alteration of gene expression.
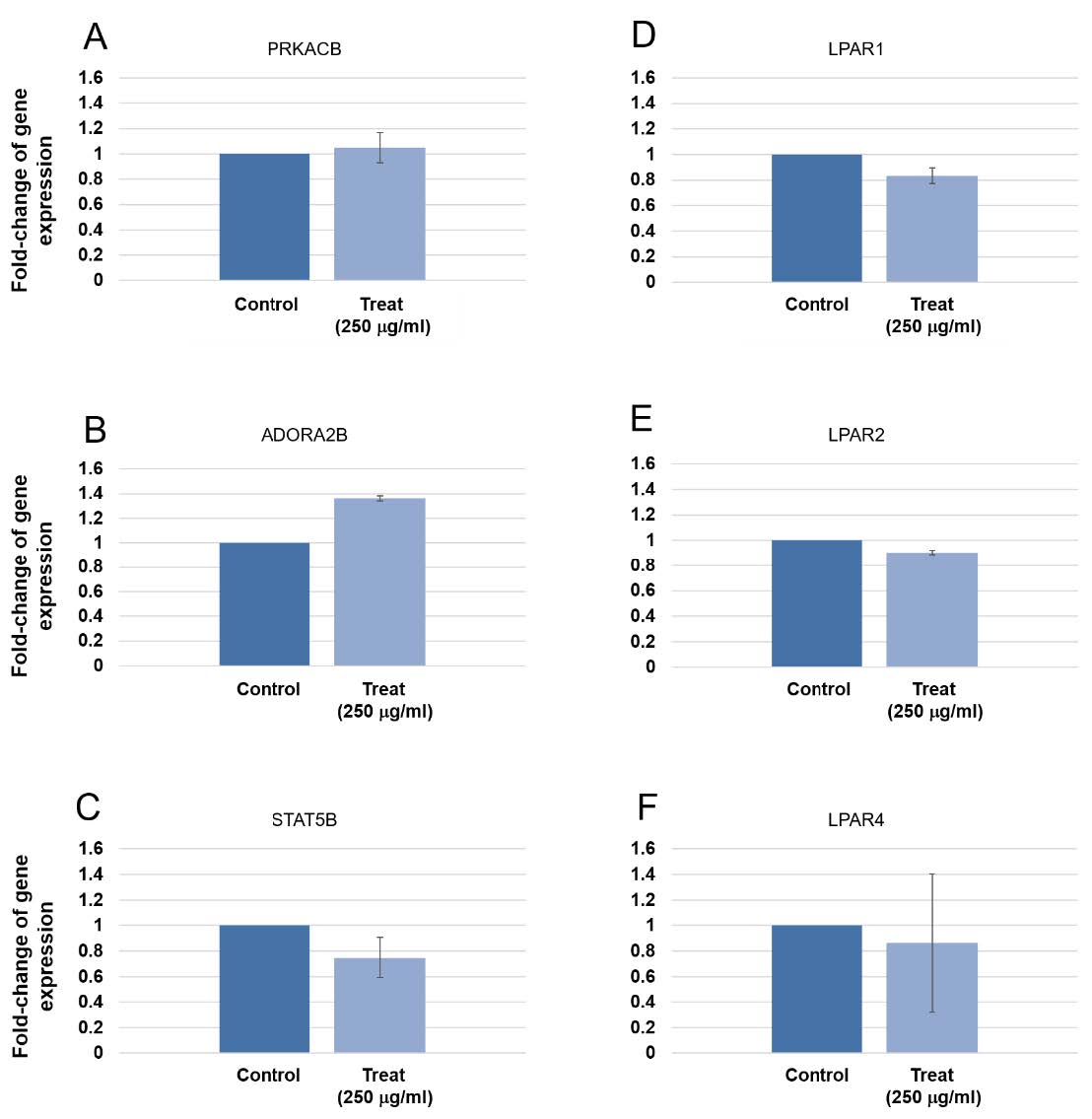
Figure 8. Expression of tumorigenic gene in human gingival cells at 8 days treatment. Three pairs of control and test samples were investigated. Mean and standard deviation were presented for each concentration. The expression of PRKACB, STAT5B, ADORA2B, LPAR1, LPAR3, LPAR4 were not rendered.
Other gene markers
Epigenetic gene markers and inflammatory gene markers were tested to observe other possible effects that could happen after long-term treatment with 250 µg/ml Cuacuta japonica water extract (Figure 9). The purpose was to investigate molecular mechanisms associated with inflammation-promoted tumorigenesis. Abnormal epigenetic changes including DNA methylation, histone modification and chromatin remodeling occur during the transformation of chronic inflammation.
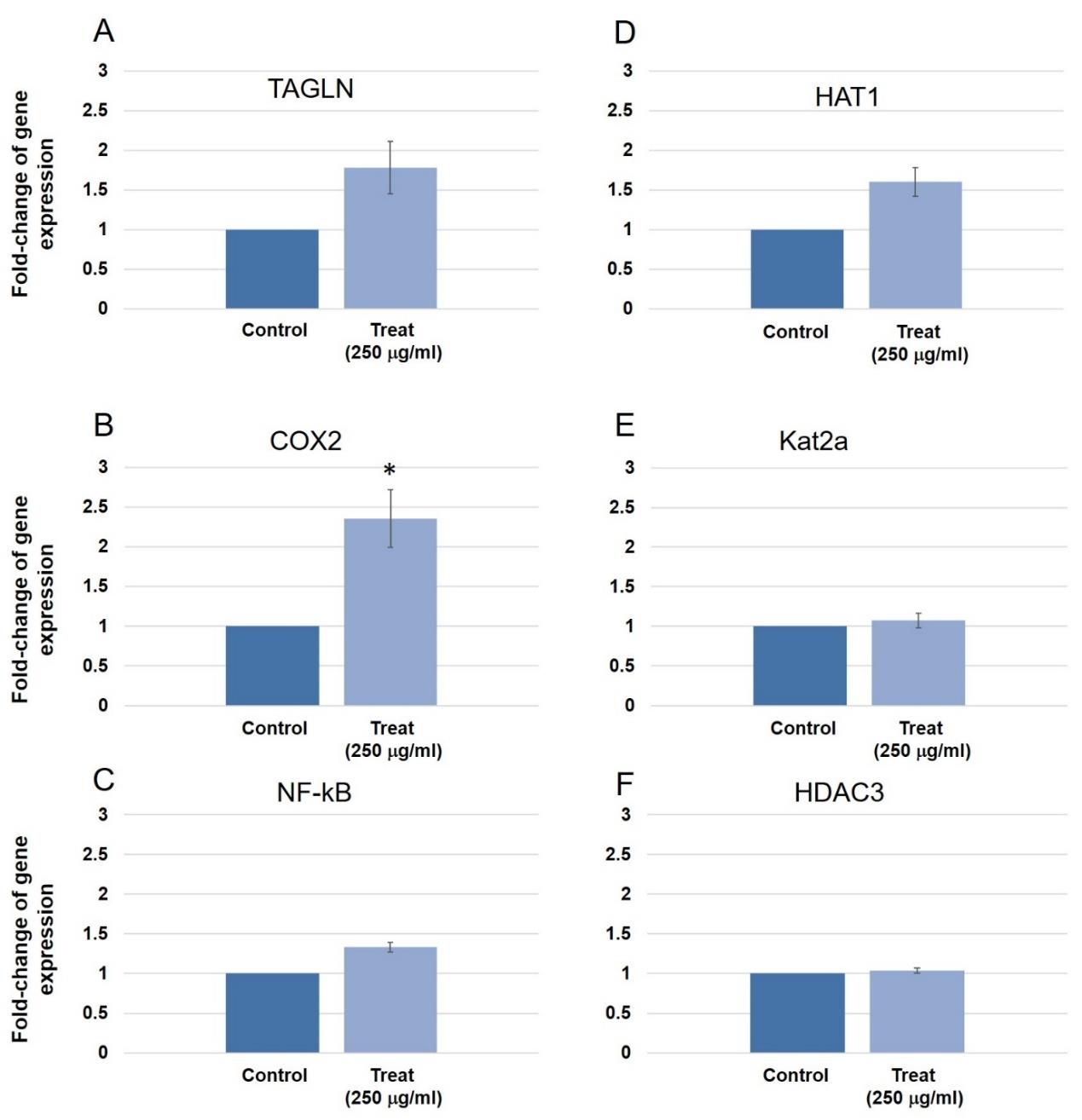
Figure 9. Other gene expression in human gingival cells. (A) The expression of the Runx2 gene in dodder seed extract for 3 days showed no significant change compared to control. (B) TAGLN gene expression in triplicate experiments increased 1.7 times compared to the control group when cultured for 3 days. (C) Cox-2 gene expression in triplicate experiments at 3 days culture increased 2.4 times compared to control group (p = 0.023). (D) NF-kB gene expression in triplicate experiments showed no difference compared to the control group at 3 days culture. (E) ALP gene expression in triplicate experiments found no difference compared to the control group at 14 days culture. (F) HAT1 gene expression in triplicate experiments found increased 1.6 times compared to the control group at 3 days culture. (G) Kat2a gene expression in triplicate experiments found no difference in expression compared to the control group at 3 days. (H) HDAC3 gene expression in triplicate experiments found no differences compared to the control group at 3 days culture. Three pairs of control and test samples were investigated. Mean and standard deviation were presented for each concentration.
DISCUSSION
The main substances found in dodder seed water extract were polysaccharides, phenolic acids and flavonoids (Kantawong et al., 2021b). The previous study by Kantawong et al. showed the crude extract of C. japonica contained l-arabinose, pinoresinol, 2-pentylfuran, chlorogenic acid, p-coumaric acid, quercetin, rutin, hyperoside, isorhamnetin, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid and 4,5-dicaffeoylquinic acid.
After treating the human gingival cells in dodder seed extract for 14 days, the expressions of bone differentiation genes were detected. Dodder seed water extract induced bone differentiation because the expression of bone differentiation markers (OPN, OCN and collagen type I) increased significantly. OPN, OCN and collagen type I were extracellular matrix proteins responsible for mineral accumulation (Addison et al., 2015; Foster et al., 2018). Recent study reported that OPN plays a crucial role during the formation of the collagen fibrils (Depalle et al., 2020) which correlated with the increased expression of collagen type I. The result from mineralization assay was correlated with the result of gene expression. However, some of our previous studies indicated that the osteogenesis also depended on the quality of human gingival cells because some batches of human gingival cells did not show strong positive bone mineralization even if the OPN and OCN expressions were up-regulated (data not shown).
Pinoresinol and quercetin induced osteoblast differentiation and mineralization by regulating BMP-2/Runx2 signaling because it had similar effects to estradiol (E2) (Jiang et al., 2019; Pang et al., 2018). Chlorogenic acid can promote the osteogenic differentiation of hDPSCs by regulating Wnt signaling (Hu et al., 2021). Rutin affected the osteogenic differentiation and proliferation of PDLSCs via PI3K/AKT/mTOR signal pathway (Zhao et al., 2020). Hyperoside was a plant flavonoid which contained osteogenic effects (Zhang et al., 2014). These evidences suggested the osteogenic effects of dodder seed water extract.
Gelatin provided a biomimetic peptide that promoted cell adhesion and supported tissue regeneration. However, gelatin possessed some disadvantages. Gelatin had poor mechanical properties, lack thermal stability, and presented relatively shorter degradation rate (Bello et al., 2020). Printed framework and a gelatin filler for bone regeneration presented gradient degradation performance to satisfy the uniform adhesion and proliferation of cells in the scaffold at the early stage of implantation, as well as providing space for the subsequent regeneration of bone tissue (Dou et al., 2021). Over swelling and thermal instability made gelatin presented characteristics similar to those of soft tissues which lead to low cell retention and uneven cell distribution as shown in Figure 5G. The printed framework prevented gelatin from over swelling and collapsing. The use of 3D bioprinting technology is shown to have a great potential to fabricate gelatin-based scaffolds for application in the field of bone tissue engineering. The 3D printed scaffolds showed uniform cell distribution and superior bone nodule formation compared with normal gelatin as seen by the alizarin red staining (Figure 5E&5F).
The phenolic acids found in dodder seed extract were chlorogenic acid, caffeic acid, p-coumaric acid, 3, 4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid and 4,5-dicaffeoylquinic acid. In the previous studies, phenolic acids showed important roles in oxidative stress prevention and metabolism. 3, 5-Dicaffeoylquinic acid was a potent α-glucosidase inhibitor which contributed to the antihyperglycemic action of the compound (Simeonova et al., 2019). The beneficial effects of 3,5-dicaffeoylquinic acid support the traditional use of Cuscuta spp. for the treatment of diabetes (Noureen et al., 2019). Oh et al. indicated that C. japonica contained 3,5-Di-O-caffeoylquinic acid, methyl 3, 5-Di-O-caffeoylquinate, 3,4-Di-O-caffeoylquinic acid, and methyl 3,4-Di-O-caffeoylquinate which might be responsible for the antihypertensive action (Oh et al., 2002).
In the present study, dodder seed extract affected the expression of antioxidant enzyme SOD1 or copper-zinc superoxide dismutase (Cu/Zn SOD1). This enzyme located in the cytoplasm, transforming superoxide radical into oxygen and hydrogen peroxide (Mondola et al., 2016). Another enzyme that found to increase expression was thioredoxin reductase 1 (TXNRD1). TXNRD1 changed the oxidized form of thioredoxin back into a reduced form which could accelerate reduction of disulfide bond in many proteins to enable their function (Emelyanov and Fyodorov, 2016). GST1, a microsomal glutathione transferase (Schaffert, 2011), showed slightly increased.
Increased expression of α-SMA might be the advantage for this study because α-SMA was identified as a biomarker of early osteoprogenitor cells in the periosteum (Matthews et al., 2020). Slightly increase of transgelin (TAGLN) was also observed at 3 days (Figure 9A). Transgelin was expressed in vascular and visceral smooth muscle, and it was an early marker of smooth muscle differentiation (Matsui et al., 2018). Vascularization was important for diffusion of nutrients and regulatory molecules inside the 3D scaffold (Freeman et al., 2019). However, upregulation of the α-SMA could be used as a marker of the epithelial to mesenchymal transition (EMT) in various types of cancers (Lazarevic et al., 2020). Thus, the investigation of various cancer-related gene expression was also performed in this study (Figure 8). It was found that treatment with 250 µg/mL dodder seed water extract longer than 1 week did not significantly affect the expression of 6 cancer genes (PRKACB, STAT5B, ADORA2B, LPAR1, LPAR3, LPAR4). Moreover, dodder seed water extract did not affect the expression of 3 epigenetic genes (HDAC3, HAT1, Kat2a) at 3 days culture (Figure 9D, 9E, 9F).
Quercetin, rutin, hyperoside and isorhamnetin were flavonoids found in dodder seed extract. These substances prevented cancer and oxidative stress. Pinoresinol was a phytoestrogen with antitumor activity, inhibiting breast cancer cells (López-Biedma et al., 2016). 4,5-Dicaffeoylquinic acid maintained prostate cancer cell line in the S-phase (Lodise et al., 2019). These evidences were concordant with the result of cancer gene expression.
The expression of cancer related genes in human gingival cells was observed at 8 days culture. cAMP-dependent catalytic β (PRKACB) proteins involved in many cancers was decreased in non-small cell lung cancer, causing the cancer cells to multiply. Upregulation of PRKACB expression could prevent the progression of non-small cell lung cancer (Chen et al., 2013). In addition, the PRKACB fusions gene was found to be involved in cholangiocarcinoma (Mertens et al., 2018). Signal transducer and activator of transcription (STAT) was a transcription factor known to be involved in cancer progression. Increased expression of STAT5B promoted cell proliferation and resistance in mantle cell lymphoma by activating Akt signaling pathway (Zhang et al., 2019). The expression of STAT5A, STAT5B and STAT6 was used as a prognosis in hepatocellular carcinoma (Dong et al., 2019). STAT5A or STAT5B stimulated T-cell lymphoma development (Maurer et al., 2019). Adenosine A2b receptor (ADORA2B) was associated with oral squamous cell carcinoma, (Kasama et al., 2015) breast cancer and colorectal cancer (Ma et al., 2010).
In addition, ADORA2B controlled cellular proliferation via HIF-1α activation (Lan et al., 2018). Lysophosphatidic acids (LPA) regulated cell processes, including cell cycle progression, viability and wound healing (Llona-Minguez et al., 2015). Lysophosphatidic acid receptors (LPAR) showed increased expression in hepatocellular carcinoma (Mazzocca et al., 2015). In our study, none of these genes showed significant alteration of gene expression.
Various proinflammatory signaling pathways including the NF-κB, IL-6/STAT3, cyclooxygenase-2 (COX-2)/PGE2, and IL-23/Th17 participate in the transformation of inflammation into cancer. Proinflammatory signaling pathways induce the production of inflammatory mediators, upregulate the expression of antiapoptotic genes, stimulate cell proliferation and angiogenesis, and thereby contribute to tumorigenesis (Yang et al., 2019).
BAX gene (Bcl-2 Associated X-protein) was a pro-apoptotic gene (Naseri et al., 2015; Zhu et al., 2015) while BCL2 was responsible for anti-apoptosis (Opferman and Kothari, 2018). CAS3 or caspase3 was an enzyme involved in the apoptosis process and its main function was to drive the process of apoptosis.
The upregulation of CAS3 also played other roles in cell functions such as the development into other cells (Nakajima and Kuranaga, 2017; Wang and Luo, 2014). CAS3 played important roles in and autophagy (Wu et al., 2014). Upregulation of BCL2 and CAS3 without change of BAX and CAS9 might imply that dodder seed water extract prevented intrinsic but not extrinsic apoptosis (Voss and Strasser, 2020). The LC3 was a protein involved in the formation of autophagosome fusion with the lysosome. Dodder seed extract might cause autophagy due to the upregulation of LC3. Previously, dodder extract had been reported to increase LC3 expression which caused autophagy in the Jurkat cells (Al-Daghri et al., 2012). However, the increase in LC3 could be found in developing cells such as cell differentiation (Hale et al., 2013). Dodder seed extract induced a noticeable effect on the expression of the COX-2 gene, a prostaglandin inflammatory mediator (Figure 9B).
CONCLUSION
Dodder seed water extract contained phenolic compounds and possessed antioxidant activity. Dodder seed water extract 250 µg/mL could be used to accelerate bone differentiation in complex tissue engineering constructs which could benefit time-course transplantation. Dodder seed water extract increased antioxidant gene expression without effect on cancer-related and epigenetic genes. Dodder seed water extract prevented intrinsic apoptosis. These findings provided the future benefit for the use of C. japonica as supplementation for prevention of bone loss instead of using C. chinensis.
AUTHOR CONTRIBUTIONS
Fahsai Kantawong designed and conducted all of the experiments and wrote the manuscript. Suraiya Sadeeyamoo, Peeraya Wongsit, Montree Tungjai, Phenphichar Wanachantararak, Suruk Udomsom4 assisted in conducting the experiments. Montree Tungjai performed the data visualization and wrote the manuscript. Jianghua Yang provided C. Japonica, Ataya Sathirachinda searched for the information about C. Japonica. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflicting interests.
REFERENCES
Addison, W.N., Nelea, V., Chicatun, F., Chien, Y.C., Tran-Khanh, N., Buschmann, M.D., Nazhat, S.N., Kaartinen, M.T., Vali, H., Tecklenburg, M.M., et al. 2015. Extracellular matrix mineralization in murine MC3T3-E1 osteoblast cultures: an ultrastructural, compositional and comparative analysis with mouse bone. Bone. 71: 244-256.
Al-Daghri, N., Alokail, M., Alkharfy, K., Mohammed, A., Abd-Alrahman, S., Yakout, S., Amer, O., and Krishnaswamy, S. 2012. Fenugreek extract as an inducer of cellular death via autophagy in human T lymphoma Jurkat cells. Bmc Complementary and Alternative Medicine. 12: ARTN 202.
Bello, A.B., Kim, D., Park, H., and Lee, S.H. 2020. Engineering and functionalization of gelatin biomaterials: from cell culture to medical applications. Tissue Engineering Part B: Reviews. 26: 164-180.
Chen, Y., Gao, Y., Tian, Y., and Tian, D.L. 2013. PRKACB is downregulated in non-small cell lung cancer and exogenous PRKACB inhibits proliferation and invasion of LTEP-A2 cells. Oncology Letters. 5: 1803-1808.
Cristaldi, M., Mauceri, R., Campisi, G., Pizzo, G., Alessandro, R., Tomasello, L., Pitrone, M., Pizzolanti, G., and Giordano, C. 2020. Growth and osteogenic differentiation of discarded gingiva-derived mesenchymal stem cells on a commercial scaffold. Front. Cell and Developmental Biology. 8.
Deng, C., Li, Y., Liang, S., Cui, K., Salz, T., Yang, H., Tang, Z., Gallagher, P.G., Qiu, Y., Roeder, R., et al. 2013. USF1 and hSET1A mediated epigenetic modifications regulate lineage differentiation and HoxB4 transcription. PLOS Genetics. 9: e1003524.
Depalle, B., McGilvery, C.M., Nobakhti, S., Aldegaither, N., Shefelbine, S.J., and Porter, A.E. 2021. Osteopontin regulates type I collagen fibril formation in bone tissue. Acta Biomaterialia. 120: 194-202.
Diomede, F., Gugliandolo, A., Cardelli, P., Merciaro, I., Ettorre, V., Traini, T., Bedini, R., Scionti, D., Bramanti, A., Nanci, A., et al. 2018. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Research & Therapy. 9.
Dong, Z., Chen, Y., Yang, C., Zhang, M., Chen, A., Yang, J., and Huang, Y. 2019. STAT gene family mRNA expression and prognostic value in hepatocellular carcinoma. Onco Targets and Therapy. 12: 7175-7191.
Dou, Y., Huang, J., Xia, X., Wei, J., Zou, Q., Zuo, Y., Li, J., and Li, Y. 2021. A hierarchical scaffold with a highly pore-interconnective 3D printed PLGA/n-HA framework and an extracellular matrix like gelatin network filler for bone regeneration. Journal of Materials Chemistry B. 9: 4488-4501.
Emelyanov, A.V. and Fyodorov, D.V. 2016. Thioredoxin-dependent disulfide bond reduction is required for protamine eviction from sperm chromatin. Genes & Development. 30: 2651-2656.
Foster, B.L., Ao, M., Salmon, C.R., Chavez, M.B., Kolli, T.N., Tran, A.B., Chu, E.Y., Kantovitz, K.R., Yadav, M., Narisawa, S., et al. 2018. Osteopontin regulates dentin and alveolar bone development and mineralization. Bone. 107: 196-207.
Freeman, F.E., Browe, D.C., Nulty, J., Von Euw, S., Grayson, W.L., and Kelly, D.J. 2019. Biofabrication of multiscale bone extracellular matrix scaffolds for bone tissue engineering. Eur Cell Mater. 38: 168-187.
Gao, J.M., Li, R., Zhang, L., Jia, L.L., Ying, X.X., Dou, D.Q., Li, J.C., and Li, H.B. 2013. Cuscuta chinensis seeds water extraction protecting murine osteoblastic MC3T3-E1 cells against tertiary butyl hydroperoxide induced injury. Journal Ethnopharmacology. 148: 587-595.
Gao, Z., Wang, L., Wang, X., Liu, Y., and Han, J. 2017. Authenticity Survey of Cuscutae Semen on Markets Using DNA Barcoding. Chinese Herbal Medicines. 9: 218-225.
Hale, A., Ledbetter, D., Gawriluk, T., and Rucker, E. 2013. Autophagy Regulation and role in development. Autophagy. 9: 951-972.
He, X.H., Yang, W.Z., Meng, A.H., He, W.N., Guo, D.A., and Ye, M. 2010. Two new lignan glycosides from the seeds of Cuscuta chinensis. Journal of Asian Natural Products Research. 12: 934-939.
Henkel, J., Woodruff, M.A., Epari, D.R., Steck, R., Glatt, V., Dickinson, I.C., Choong, P.F., Schuetz, M.A., and Hutmacher, D.W. 2013. Bone regeneration based on tissue engineering conceptions - a 21st century perspective. Bone Research. 1: 216-248.
Hu, X., Wang, L., He, Y., Wei, M., Yan, H., and Zhu, H. 2021. Chlorogenic Acid Promotes Osteogenic Differentiation of Human Dental Pulp Stem Cells Through Wnt Signaling. Stem Cells and Development. 30: 641-650.
Jang, J.Y., Kim, H.N., Kim, Y.R., Choi, Y.H., Kim, B.W., Shin, H.K., and Choi, B.T. 2012. Aqueous fraction from Cuscuta japonica seed suppresses melanin synthesis through inhibition of the p38 mitogen-activated protein kinase signaling pathway in B16F10 cells. Journal of Ethnopharmacology. 141: 338-344.
Jiang, X., Chen, W., Shen, F., Xiao, W., Guo, H., Su, H., Xiu, J., and Sun, W. 2019. Pinoresinol promotes MC3T3-E1 cell proliferation and differentiation via the cyclic AMP/protein kinase A signaling pathway. Molecular Medicine Reports. 20: 2143-2150.
Kahlos, K., Soini, Y., Säily, M., Koistinen, P., Kakko, S., Pääkkö, P., Holmgren, P., and Kinnula, V.L. 2001. Up-regulation of thioredoxin and thioredoxin reductase in human malignant pleural mesothelioma. International Journal of Cancer. 95: 198-204.
Kantawong, F., Saisuwan, C., Soeratanapant, P., Wanachantararak, P., Nan, J., Wu, J., and Chang, Y. 2021a. Gynura divaricata Water Extract Presented the Possibility to Enhance Neuronal Regeneration. Evidence-Based Complementary and Alternative Medicine. 2021.
Kantawong, F., Saksiriwisitkul, C., Riyapa, C., Limpakdee, S., Wanachantararak, P., and Kuboki, T. 2018. Reprogramming of mouse fibroblasts into neural lineage cells using biomaterials. Bioimpacts. 8: 129-138.
Kantawong, F., Wongsit, P., Yang, J., Wanachantararak, P., Nan, J., and Wu, J. 2021b. Expression of preadipocyte genes in apical papilla cells after treatment with crude water extract of Cuscuta japonica Choisy. Journal of Associated Medical Sciences. 54: 125-134.
Kasama, H., Sakamoto, Y., Kasamatsu, A., Okamoto, A., Koyama, T., Minakawa, Y., Ogawara, K., Yokoe, H., Shiiba, M., Tanzawa, H., et al. 2015. Adenosine A2b receptor promotes progression of human oral cancer. BMC Cancer. 15: 563.
Kim, D., Lee, A., Xu, Q., Zhang, Q., and Le, A. 2021. Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine - A Comprehensive Review. Frontiers in Immunology. 12.
Krisnamurti, D.G., Louisa, M., Anggraeni, E., and Wanandi, S.I. 2016. Drug Efflux Transporters Are Overexpressed in Short-Term Tamoxifen-Induced MCF7 Breast Cancer Cells. Advances in Pharmacological Sciences. 2016: 6702424.
Lan, J., Lu, H., Samanta, D., Salman, S., Lu, Y., and Semenza, G.L. 2018. Hypoxia-inducible factor 1-dependent expression of adenosine receptor 2B promotes breast cancer stem cell enrichment. Proceedings of the National Academy of Sciences of the United States of America. 115: E9640-E9648.
Lazarevic, M., Milosevic, M., Jelovac, D., Milenkovic, S., Tepavcevic, Z., Baldan, F., Suboticki, T., Toljic, B., Trisic, D., Dragovic, M., et al. 2020. Marked epithelial to mesenchymal transition in surgical margins of oral cancer-an. Oncology Letters. 19: 3743-3750.
Llona-Minguez, S., Ghassemian, A., and Helleday, T. 2015. Lysophosphatidic acid receptor (LPAR) modulators: The current pharmacological toolbox. Progress in Lipid Research. 58: 51-75.
Lodise, O., Patil, K., Karshenboym, I., Prombo, S., Chukwueke, C., and Pai, S.B., 2019. Inhibition of prostate cancer cells by 4,5-dicaffeoylquinic acid through cell cycle arrest. Prostate Cancer. 2019: 4520645.
Lv, J., Shao, Q., Wang, H., Shi, H., Wang, T., Gao, W., Song, B., Zheng, G., Kong, B., and Qu, X. 2013. Effects and mechanisms of curcumin and basil polysaccharide on the invasion of SKOV3 cells and dendritic cells. Molecular Medicine Reports. 8: 1580-1586.
López-Biedma, A., Sánchez-Quesada, C., Beltrán, G., Delgado-Rodríguez, M., and Gaforio, J.J. 2016. Phytoestrogen (+)-pinoresinol exerts antitumor activity in breast cancer cells with different oestrogen receptor statuses. BMC Complementary and Alternative Medicine. 16: 350.
Ma, D.F., Kondo, T., Nakazawa, T., Niu, D.F., Mochizuki, K., Kawasaki, T., Yamane, T., and Katoh, R. 2010. Hypoxia-inducible adenosine A2B receptor modulates proliferation of colon carcinoma cells. Human Pathology. 41: 1550-1557.
Matsui, T.S., Ishikawa, A., and Deguchi, S. 2018. Transgelin-1 (SM22α) interacts with actin stress fibers and podosomes in smooth muscle cells without using its actin binding site. Biochemical and Biophysical Research Communications. 505: 879-884.
Matthews, B.G., Wee, N.K.Y., Widjaja, V.N., Price, J.S., Kalajzic, I., and Windahl, S.H. 2020. αSMA osteoprogenitor cells contribute to the increase in osteoblast numbers in response to mechanical loading. Calcified Tissue International. 106: 208-217.
Maurer, B., Nivarthi, H., Wingelhofer, B., Pham, H.T.T., Schlederer, M., Suske, T., Grausenburger, R., Schiefer, A.I., Prchal-Murphy, M., Chen, D., et al. 2020. High activation of STAT5A drives peripheral T-cell lymphoma and leukemia. Haematologica. 105: 435-447.
Mazzocca, A., Dituri, F., De Santis, F., Filannino, A., Lopane, C., Betz, R.C., Li, Y.Y., Mukaida, N., Winter, P., Tortorella, C., et al. 2015. Lysophosphatidic acid receptor LPAR6 supports the tumorigenicity of hepatocellular carcinoma. Cancer Research. 75: 532-543.
McGraw, S., Robert, C., Massicotte, L., and Sirard, M.A. 2003. Quantification of histone acetyltransferase and histone deacetylase transcripts during early bovine embryo development. Biology of Reproduction. 68: 383-389.
Mertens, J.C., Rizvi, S., and Gores, G.J. 2018. Targeting cholangiocarcinoma. Biochimica et Biophysica Acta - Molecular Basis of Disease. 1864(4 Pt B): 1454-1460.
Mollaei, H., Safaralizadeh, R., Babaei, E., Abedini, M.R., and Hoshyar, R. 2017. The anti-proliferative and apoptotic effects of crocin on chemosensitive and chemoresistant cervical cancer cells. Biomed Pharmacother. 94: 307-316.
Mondola, P., Damiano, S., Sasso, A., and Santillo, M. 2016. The Cu, Zn Superoxide Dismutase: Not Only a Dismutase Enzyme. Frontiers in Physiology. 7: 594.
Moon, M., Jeong, H.U., Choi, J.G., Jeon, S.G., Song, E.J., Hong, S.P., and Oh, M.S. 2016. Memory-enhancing effects of Cuscuta japonica Choisy via enhancement of adult hippocampal neurogenesis in mice. Behavioural Brain Research. 311: 173-182.
Nakajima, Y.I. and Kuranaga, E. 2017. Caspase-dependent non-apoptotic processes in development. Cell Death & Differentiation. 24: 1422-1430.
Naseri, M.H., Mahdavi, M., Davoodi, J., Tackallou, S.H., Goudarzvand, M., and Neishabouri, S.H. 2015. Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell International. 15: 55.
Noureen, S., Noreen, S., Ghumman, S.A., Batool, F., and Bukhari, S.N.A. 2019. The genus. Iranian Journal of Basic Medical Sciences. 22: 1225-1252.
Oh, H., Kang, D.G., Lee, S., and Lee, H.S. 2002. Angiotensin converting enzyme inhibitors from Cuscuta japonica Choisy. Journal of Ethnopharmacology. 83: 105-108.
Opferman, J. and Kothari, A. 2018. Anti-apoptotic BCL-2 family members in development. Cell Death and Differentiation. 25: 37-45.
Pang, X., Cong, Y., Bao, N., Li, Y., and Zhao, J. 2018. Quercetin Stimulates Bone Marrow Mesenchymal Stem Cell Differentiation through an Estrogen Receptor-Mediated Pathway. Biomed Research International. 2018.
Pekkala, S., Munukka, E., Kong, L., Pöllänen, E., Autio, R., Roos, C., Wiklund, P., Fischer-Posovszky, P., Wabitsch, M., Alen, M., Huovinen, P., et al. 2015. Toll-like receptor 5 in obesity: the role of gut microbiota and adipose tissue inflammation. Obesity (Silver Spring). 23: 581-590.
Peng, W., DU, T., Zhang, Z., DU, F., Jin, J., and Gong, A. 2015. Knockdown of autophagy-related gene LC3 enhances the sensitivity of HepG. Experimental and Therapeutic Medicine. 9: 1271-1276.
Pfaffl, M.W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 29: e45.
Prins, H.J., Braat, A.K., Gawlitta, D., Dhert, W.J., Egan, D.A., Tijssen-Slump, E., Yuan, H., Coffer, P.J., Rozemuller, H., and Martens, A.C. 2014. In vitro induction of alkaline phosphatase levels predicts in vivo bone forming capacity of human bone marrow stromal cells. Stem Cell Research. 12: 428-440.
Riksen, E.A., Landin, M.A., Reppe, S., Nakamura, Y., Lyngstadaas, S.P., and Reseland, J.E. 2014. Enamel matrix derivative promote primary human pulp cell differentiation and mineralization. International Journal of Molecular Sciences. 15: 7731-7749.
Rush, J.W., Laughlin, M.H., Woodman, C.R., Price, E.M. 2000. SOD-1 expression in pig coronary arterioles is increased by exercise training. American Journal of Physiology-Heart and Circulatory Physiology. 279: H2068-2076.
Schaffert, C.S. 2011. Role of MGST1 in reactive intermediate-induced injury. World Journal of Gastroenterology. 17: 2552-2557.
Simeonova, R., Vitcheva, V., Zheleva-Dimitrova, D., Balabanova, V., Savov, I., Yagi, S., Dimitrova, B., Voynikov, Y., and Gevrenova, R. 2019. Trans-3,5-dicaffeoylquinic acid from Geigeria alata Benth. & Hook.f. ex Oliv. & Hiern with beneficial effects on experimental diabetes in animal model of essential hypertension. Food and Chemical Toxicology. 132: 110678.
Spivack, S.D., Hurteau, G.J., Jain, R., Kumar, S.V., Aldous, K.M., Gierthy, J.F., and Kaminsky, L.S. 2004. Gene-environment interaction signatures by quantitative mRNA profiling in exfoliated buccal mucosal cells. Cancer Research. 64: 6805-6813.
Stefanska, K., Mehr, K., Wieczorkiewicz, M., Kulus, M., Volponi, A., Shibli, J., Mozdziak, P., Skowronski, M., Antosik, P., Jaskowski, J., et al. 2020. Stemness potency of human gingival cells-application in anticancer therapies and clinical trials. Cells. 9.
Talior-Volodarsky, I., Connelly, K.A., Arora, P.D., Gullberg, D., and McCulloch, C.A. 2012. α11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy. Cardiovascular Research. 96: 265-275.
Ueda, T., Araki, N., Mano, M., Myoui, A., Joyama, S., Ishiguro, S., Yamamura, H., Takahashi, K., Kudawara, I. and Yoshikawa, H. 2002. Frequent expression of smooth muscle markers in malignant fibrous histiocytoma of bone. Journal of Clinical Pathology. 55: 853-858.
van Tuyn, J., Knaän-Shanzer, S., van de Watering, M.J., de Graaf, M., van der Laarse, A., Schalij, M.J., van der Wall, E.E., de Vries, A.A., and Atsma, D.E. 2005. Activation of cardiac and smooth muscle-specific genes in primary human cells after forced expression of human myocardin. Cardiovascular Research. 67: 245-255.
Voss, A.K. and Strasser, A. 2020. The essentials of developmental apoptosis. F1000Res 9.
Wang, J., Wang, L., Zhou, Z., Lai, H., Xu, P., Liao, L., Wei, J. 2016. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers (Basel). 8: 115.
Wang, J.Y. and Luo, Z.G. 2014. Non-apoptotic role of caspase-3 in synapse refinement. Neuroscience Bulletin. 30: 667-670.
Wu, H., Che, X., Zheng, Q., Wu, A., Pan, K., Shao, A., Wu, Q., Zhang, J., and Hong, Y. 2014. Caspases: a molecular switch node in the crosstalk between autophagy and apoptosis. International Journal of Biological Sciences. 10: 1072-1083.
Wu, H.W., Feng, Y.H., Wang, D.Y., Qiu, W.Y., Yu, Q.Y., Yang, L.L., Liang, C., Luo, S.P., and Gao, J. 2018. Effect of Total Flavones from Cuscuta Chinensis on Anti-Abortion via the MAPK Signaling Pathway. Evidence-Based Complementary and Alternative Medicine. 2018: 6356190.
Yang, H.M., Shin, H.K., Kang, Y.H., and Kim, J.K. 2009. Cuscuta chinensis extract promotes osteoblast differentiation and mineralization in human osteoblast-like MG-63 cells. Journal of Medicinal Food. 12: 85-92.
Yang, L., Chen, Q., Wang, F., Zhang, G. 2011. Antiosteoporotic compounds from seeds of Cuscuta chinensis. Journal of Ethnopharmacology. 135: 553-560.
Yang, S. and Han, H. 2014. Effect of cycloxygenase-2 silencing on the malignant biological behavior of MCF-7 breast cancer cells. Oncology Letters 8: 1628-1634.
Yang, S., Xu, H., Zhao, B., Li, S., Li, T., Xu, X., Zhang, T., Lin, R., Li, J., and Li, X. 2016. The difference of chemical components and biological activities of the crude products and the salt-processed product from semen Cuscutae. Evidence-Based Complementary and Alternative Medicine. 2016: 8656740.
Yang, Z., Dang, Y., and Ji, G. 2019. Role of epigenetics in transformation of inflammation into colorectal cancer. World Journal of Gastroenterology. 25: 2863-2877.
Yao, C.H., Tsai, H.M., Chen, Y.S., and Liu, B.S. 2005. Fabrication and evaluation of a new composite composed of tricalcium phosphate, gelatin, and Chinese medicine as a bone substitute. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 75: 277-288.
Yin, H., Udomsom, S., Kantawong, F. 2020. Fabrication of Blended Gelatin–Polyvinyl Alcohol–Chitosan Scaffold for Wound Regeneration. Chiang Mai University Journal of Natural Sciences. 19: 930-952.
Yoo, S.H., Kim, J.G., Kim, B.S., Lee, J., Pi, S.H., Lim, H.D., Shin, H.I., Cho, E.S., and You, H.K. 2016. BST2 mediates osteoblast differentiation via the BMP2 signaling pathway in human alveolar-derived bone marrow stromal cells. PLoS One. 11: e0158481.
Zhang, N., Ying, M.D., Wu, Y.P., Zhou, Z.H., Ye, Z.M., Li, H., and Lin, D.S. 2014. Hyperoside, a flavonoid compound, inhibits proliferation and stimulates osteogenic differentiation of human osteosarcoma cells. PLoS One. 9: e98973.
Zhang, W., Liang, X., Gong, Y., Xiao, C., Guo, B., Yang, T. 2019. The signal transducer and activator of transcription 5B (STAT5B) gene promotes proliferation and drug resistance of human mantle cell lymphoma cells by activating the Akt signaling pathway. Medical Science Monitor. 25: 2599-2608.
Zhao, B., Xiong, Y., Zhang, Y., Jia, L., Zhang, W., and Xu, X. 2020. Rutin promotes osteogenic differentiation of periodontal ligament stem cells through the GPR30-mediated PI3K/AKT/mTOR signaling pathway. Experimental Biology and Medicine. 245: 552-561.
Zhu, L., Han, M.B., Gao, Y., Wang, H., Dai, L., Wen, Y., and Na, L.X. 2015. Curcumin triggers apoptosis via upregulation of Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes. Molecular Medicine Reports. 12: 1151-1156.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Fahsai Kantawong1, *, Suraiya Sadeeyamoo1, Peeraya Wongsit1, Montree Tungjai2, Phenphichar Wanachantararak3, Suruk Udomsom4, Jianghua Yang1, 5, and Ataya Sathirachinda6
1 Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
2 Department of Radiologie Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
3 The Dental Research Center, Faculty of Dentistry, Chiang Mai University, Chiang Mai 50200, Thailand
4 Biomedical Engineering Institute, Chiang Mai University, Chiang Mai 50200, Thailand
5 Orofacial Reconstruction and Regeneration Laboratory, the Affiliated Stomatology Hospital of Southwest Medical University, Luzhou 646000, China
6 Chemical and Systems Biology, School of Medicine Stanford University, Stanford, California 94305, United States
Corresponding author: Fahsai Kantawong, E-mail: fahsai.k@cmu.ac.th
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: July 19, 2021;
Revised: March 5, 2022;
Accepted: March 8, 2022;
Published online: March 15, 2022

