
Effect of Nocturnal and Diurnal Moderate-intensity Swimming Exercise on Increasing Irisin Level of Female Mice (Mus musculus)
Muhamad Fauzi Antoni, Purwo Sri Rejeki*, Sulistiawati, Adi Pranoto, Kristanti Wanito Wigati, Gadis Meinar Sari, Ronny Lesmana, and Yoshio YamaokaPublished Date : 2022-03-31
DOI : https://doi.org/10.12982/CMUJNS.2022.033
Journal Issues : Number 2, April-June 2022
Abstract This study aims to compare moderate-intensity swimming exercise in the morning and at night towards the increment of irisin levels in female mice (Mus musculus). This research is a real experiment with the randomized control group post-test-only design. A total of 24 female mice (Mus musculus), aged 3 months old, and weighing 20-40 grams (Lee’s Index Value > 0.3) were randomized into three groups, namely G1 (n = 8, control without intervention), G2 (n = 8, moderate-intensity swimming exercise in the morning), and G3 (n = 8, moderate-intensity swimming exercise at night). Swimming exercises were carried out 3x/w for 4 weeks with an intensity of 6% of body weight and the duration was 70% of maximum swimming time. Irisin levels were measured using the ELISA method. The data analysis techniques used were one-way ANOVA test and Tukey's HSD post-hoc test. The results that were obtained from this experimental study were mean levels of irisin in G1 (1.86 ± 0.06 ng/mL), G2 (2.66 ± 0.12 ng/mL), G3 (3.43 ± 0.35 ng/mL), and (P ≤ 0.001). The results of Tukey's HSD post-hoc test showed that there was a significant difference in
the mean post-training irisin levels between G2 and G1 (P ≤ 0.05), G3 and G1 (P ≤ 0.001), and G3 and G2 (P ≤ 0.05). Based on the results of this study, it was concluded that moderate-intensity swimming exercises in the morning and at night were able to increase irisin levels. However, moderate-intensity swimming exercise at night showed a higher effectiveness in increasing irisin levels of female mice (Mus musculus).
Keywords: Obesity, Irisin levels, Morning exercise, Night exercise
Citation: Antoni, M.F., Rejeki, P.S., Sulistiawati, Pranoto, A., Wigati, K.W., Sari, G.M., Lesmana, R., and Yamaoka, Y. 2022. Effect of nocturnal and diurnal moderate-intensity swimming exercise on increasing irisin level of female mice (Mus musculus). CMU J. Nat. Sci. 21(2): e2022033.
INTRODUCTION
Exercise is not only an active and preferred way to improve health but also an effective way to reduce the harm of metabolic diseases (Smith and Adams, 2011; Badawy et al., 2020), such as obesity and type 2 diabetes mellitus (T2DM) (Sigal et al., 2006; Fatouros, 2018). In addition, exercise also has a positive impact on body health because it promotes muscle contractions. Muscle contraction activates molecular pathways and myokine pathways that do not only act on muscles via autocrine or paracrine but also mediate interactions between muscles and other organs through endocrine mechanisms (Pedersen and Febbraio, 2008). One of the myokines that play a role in preventing metabolic syndrome is irisin. Irisin is one of the myokine types that is secreted by the proteolytic cleavage of membrane protein fibronectin type III domain-containing protein 5 (FNDC5) and it is regulated by peroxisome proliferation-activated receptor coactivator-1α (PGC1α) (Boström et al., 2012). Irisin acts on white fat cells to stimulate protein-1 uncoupling (UCP-1) and in the browning process of fat tissue (Fatouros, 2018). Irisin is released into the bloodstream to elevate energy expenditure, therefore irisin might be used to treat obesity problems and maintain glucose homeostasis (Boström et al., 2012).
Exercise is one of the safe non-pharmacological approaches to increase irisin levels, which is a mediator of increasing energy metabolism (Fatouros, 2018). Irisin is involved in white adipose tissue browning and energy metabolism which plays an important role in increasing insulin sensitivity (Yang et al., 2016). Lower levels of irisin indicate a low metabolic rate, resulting in an increase in energy stores in the body and an increased risk of obesity. Obesity and low metabolic rate conditions in individuals with low levels of physical activity (sedentary lifestyle) will have a negative impact on health (Yang et al., 2016).
Common exercise barriers include lack of time, long work hours, and demanding study during the day that causes the right time to do exercise to be at night to avoid a sedentary lifestyle. However, according to Algul et al. (2017), the exact time to exercise for humans according to lifestyle (circadian rhythm) for health is still unknown, nor the time of exercise to burn body fat. Likewise, the exact time to exercise in mice is also not known (Mendoza et al., 2021). Nocturnal mice, unlike humans, sleep during the day and behave at night (Cajochen et al., 2003; Perreau-Lenz et al., 2004; Garidou-Boof et al., 2005). The biological and psychological circadian rhythm times have a major influence on physical performance, which is most often observed in the early night by considering individual chronotypes and using timed training could be an effective method of improving physical performance at a given point in time, which will promote subcutaneous browning of white adipocytes (Teo et al., 2011; Norheim et al., 2014). In addition to the time factor, exercise intensity can also affect changes in irisin levels (Winn et al., 2017). Moderate-intensity exercise is relatively easy to do because it does not require great effort compared to high-intensity exercise (Omar et al., 2021).
Based on the description of the background described above, the purpose of this study was to compare moderate-intensity swimming exercises which were carried out in the morning and at night towards the increment of irisin levels in female mice (Mus musculus).
MATERIALS AND METHODS
Experimental Design
This research is a real experiment with the randomized control group post-test-only design. A total of 24 female mice (Mus musculus), aged 3 months old, and weighing 20-40 grams (Lee’s Index Value > 0.3) were randomized into three groups, namely G1 (n = 8, control without intervention), G2 (n = 8, moderate-intensity swimming exercise in the morning), and G3 (n = 8, moderate-intensity swimming exercise in the night). The research was conducted at the Virology Laboratory of Veterinary Medicine Faculty within 1.5 months (28 February 2021 – 5 April 2021). The mice were placed at room temperature of 26 ± 2°C with 50-60% humidity and the lighting was regulated by a light-dark cycle with 12-hour light and 12-hour dark cycles (08:00–20:00). The cages for the mice were 30 x 45 x 20 cm, made of plastic covered with wire gauze, and equipped with food containers and drinking bottles. Each cage contained 1 group (3–4 mice). Food and drink were given at 07.00 AM with a dose of 20 grams/mice/day. This research has followed animal welfare principles published by the European Convention for the Protection of Vertebrate Animals. All research procedures have been approved by the Health Research Ethics Commission, Faculty of Medicine, Universitas Airlangga, Surabaya with registration number: 49/EC/KEPK/FKUA/2021.
Protocol of Exercise
An acclimatization process was done before the swimming exercise intervention for 7 days. During the acclimatization process, the mice were swimming without using weights. Swimming exercises were carried out 3x/w for 4 weeks with an intensity of 6% of body weight and the duration was 70% of maximum swimming time. Morning swimming exercise was held at 08.00–09.00 AM, while night swimming exercise was held at 20.00–21.00 PM (Kwak et al., 2020; Pranoto et al., 2020). When the mice were given intervention of moderate-intensity swimming exercise in the morning and at night, the water temperature was maintained
at 30 ± 2°C (Wierzba et al., 2006).
Data collection
Body weight measurements were carried out pre-training and post-training for 4 weeks using a digital harnic HL-3650 heles scale (0-5kg scale). Measurements of Lee’s index pre-training and post-training for 4 weeks were carried out using the cube root formula of body weight in grams divided by naso-anal length in mm and multiplied by 10,000. Blood sampling was carried out 24 hours after the last exercise and was taken from the left ventricle of mice as much as 1-2 mL. Then, the blood was centrifuged for 10 minutes at 3,000 rpm to separate the serum and then it was stored at –80°C for analysis of irisin levels in the next day. Irisin levels were measured using BT-Lab Enzyme-Linked Immunosorbent Assay kit BT-E1479Mo (Biossay Technology Laboratory, Inc., Shanghai, China P.R.) with standard curve range: 0.05 – 30 ng/mL and sensitivity level: 0.024 ng/mL.
Statistical Analysis
Statistical analysis was conducted using SPSS software version 16 (SPSS Inc., Chicago, IL, USA). The normality test used was the Shapiro-Wilk test, while the homogeneity test used was the Levene test. The difference test used was One-Way ANOVA, followed by Tukey's Honestly Significant Difference (HSD) post-hoc test and linear correlation with Pearson product-moment model. All data are displayed with Mean ± Standard Error of the Mean (SEM). All statistical analyzes used a significance level (P ≤ 0.05).
RESULTS
Study Library Selection
Research data including body weight data (pre-training, post-training, and delta), Lee’s obesity index (pre-training, post-training, and delta) and irisin level data (post-training) are presented in Figures 1–3. Based on Figure 1, the result of one-way ANOVA test indicates that there is no significant difference in the mean weight of the pre-training mice in each group (P ≥ 0.05), while the post-training and Δ (post–pre) show significant difference in the mean weight of mice (P ≤ 0.001). The results of the Tukey's HSD post-hoc test shows that there is a significant difference in the mean body weight of post-training mice between G2 and G1 (P ≤ 0.05), G3 and G1 (P ≤ 0.001), and G3 and G2 (P ≤ 0.05). Likewise, Δ (post–pre) shows a significant difference in the mean weight of mice between G2 and G1 (P ≤ 0.05) and G3 and G1 (P ≤ 0.05), while G2 and G3 do not show a significant difference (P ≥ 0.05).
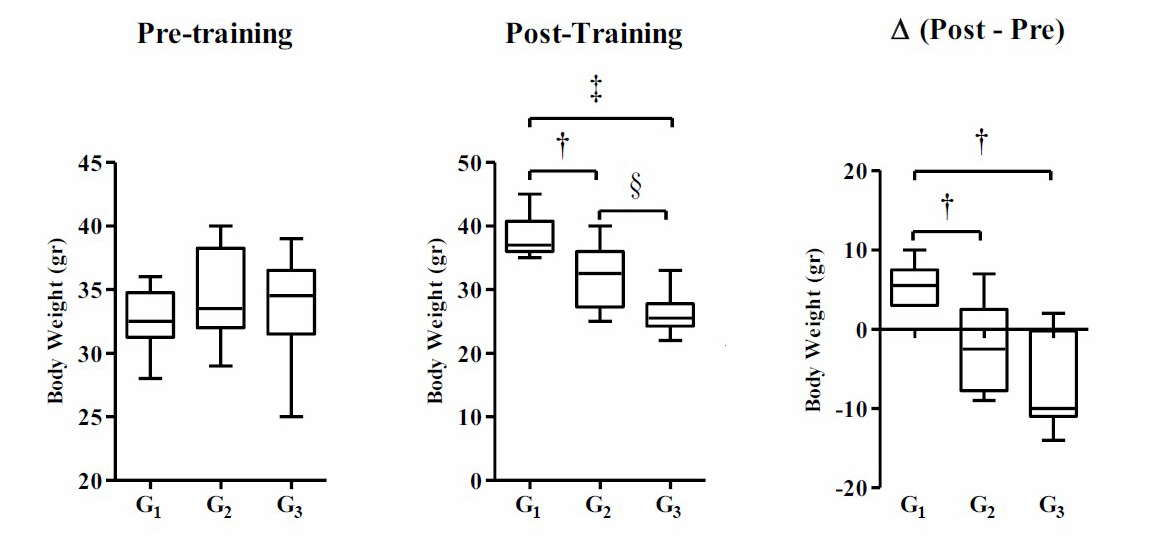
Figure 1. Body weight are modulated among group post-training. Body weight data were presented as average of Mean with Standard Error of the Mean (SEM). Significant was considered with P ≤ 0.05.
Note: G1 (control without intervention), G2 (moderate-intensity swimming exercise in the morning), and G3 (moderate-intensity swimming exercise in the night). (†) significant vs G1 (P ≤ 0.05), (‡) significant vs G1 (P ≤ 0.001), and (§) significant vs G2 (P ≤ 0.05).
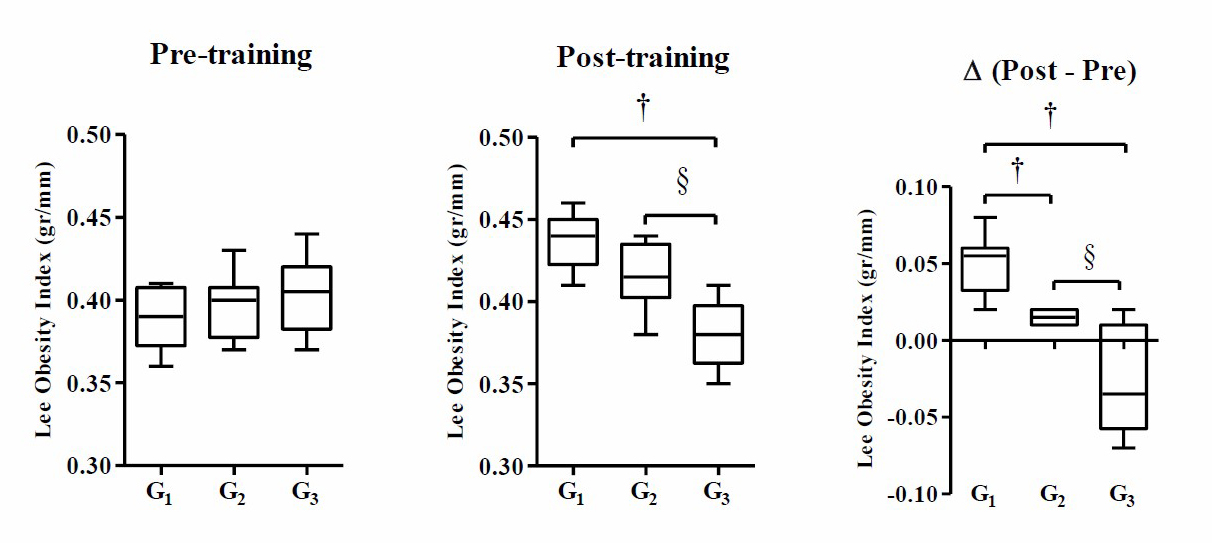
Figure 2. Moderate-intensity swimming exercise in the evening significantly reduced Lee's obesity index of post-training, and delta mice. Data were presented as average of Mean with Standard Error of the Mean (SEM). Significant was considered with P ≤ 0.05.
Note: G1 (control without intervention), G2 (moderate-intensity swimming exercise in the morning), and G3 (moderate-intensity swimming exercise in the night). (†) significant vs G1 (P ≤ 0.05), and (§) significant vs G2 (P ≤ 0.05).
Based on Figure 2, the result of the one-way ANOVA test indicates that there is no significant difference in the mean Lee’s obesity index of pre-training mice in each group (P ≥ 0.05), while post-training and Δ (post–pre) mice show significant differences in Lee’s obesity index (P ≤ 0.001). The results of Tukey's HSD post-hoc test show that there is a significant difference in the Lee’s obesity index of post-training mice between G3 and G1 (P ≤ 0.001) and G3 and G2 (P ≤ 0.001), while G2 and G1 do not show a significant difference (P ≥ 0.05). The results of Δ analysis (post–pre) show that there is a significant difference in the mean Lee’s obesity index between G2 and G1 (P ≤ 0.05), G3 and G1 (P ≤ 0.001), G3 and G2 (P ≤ 0.001).
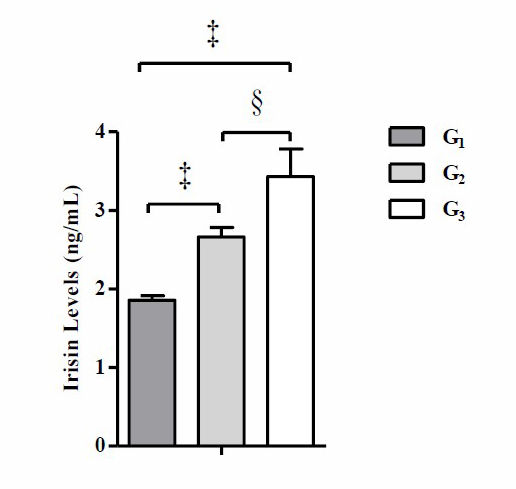
Figure 3. Moderate-intensity swimming exercise in the evening significantly increased post-training irisin levels compared to control group. Data were presented as average of Mean with Standard Error of the Mean (SEM). Significant was considered with P ≤ 0.05.
Note: G1 (control without intervention), G2 (moderate-intensity swimming exercise in the morning), and G3 (moderate-intensity swimming exercise in the night). (‡) significant vs G1 (P ≤ 0.001), and (§) significant vs G2 (P ≤ 0.05).
Based on Figure 3, the result of the one-way ANOVA test shows that there is a significant difference in the mean post-training irisin levels (P ≤ 0.001). The results of the Tukey's HSD post-hoc test show that there is a significant difference in the mean post-training irisin levels between G2 and G1 (P ≤ 0.05), G3 and G1 (P ≤ 0.001), and G3 and G2 (P ≤ 0.05). The results of the correlation analysis of post-training irisin levels with body weight, Δ body weight, Lee’s obesity index, and Δ Lee’s obesity index of post-training mice are presented in Figure 4.
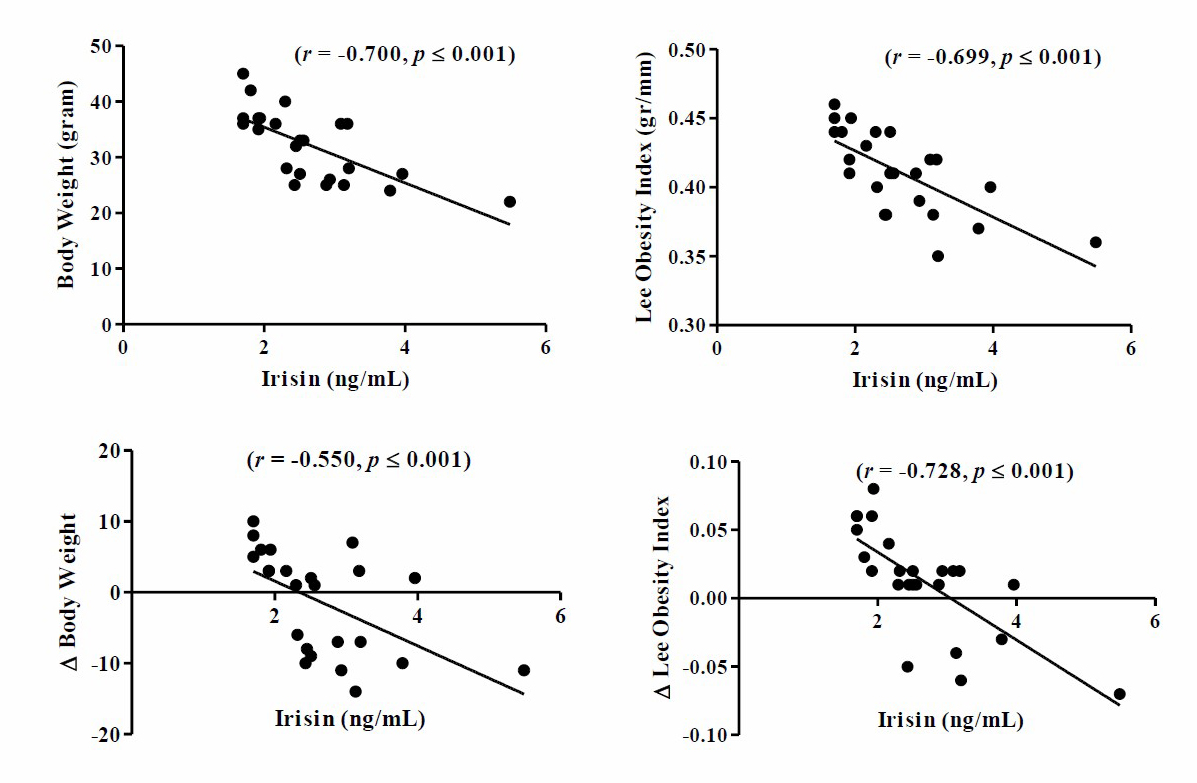
Figure 4. The negative correlation between irisin levels with body weight and Lee’s obesity index of post-training mice. The significant linear correlation between parameters is visualized in the plot model (P ≤ 0.001).
Note: Significant with P ≤ 0.001 by Pearson’s product-moment correlation test.
Based on research results it was found that there was a significant negative relationship between post-training irisin levels with body weight and Lee’s obesity index of post-training mice. The results of the parametric Pearson product-moment linear correlation analysis showed that post-training irisin levels were negatively correlated with body weight (r = –0.605, P ≤ 0.001), Δ body weight (r = –0.550, P ≤ 0.001), Lee’s obesity index (r = –0.665, P ≤ 0.001), and Δ Lee’s obesity index (r = –0.728, P ≤ 0.001).
DISCUSSION
The results of the analysis showed that there was a difference in the mean body weight of mice between pre-training and post-training (4 weeks) ones and they had a tendency to lose weight in G2 and G3, while in G1, the mean weight increased (Figure 1). Weight losses in G2 and G3 were probably due to metabolic factors and the effects of exercise (Kurdanti et al., 2015). Exercise has a positive effect in reducing body fat stores (Giolo De Carvalho and Sparks, 2019). During exercise, there is a reduction and a decrease in food intake as an energy source, which causes the increment in the utilization of stored energy sources in the body, especially body fat stores. Another factor that determines weight losses in G2 and G3 is the increased use of muscle glycogen and fat as an energy source during exercise (Ivy, 2004). At G2 and G3, the body increased the usage of fat as an energy source if glycogen stores in the muscles were depleted (Cochran, 2010). During exercise, triacylglycerol which is the main energy reserve in adipose tissue will be hydrolyzed into free fatty acids (FAs), then released into the circulation which is used as a fuel source for muscle contraction and other tissues (Giolo De Carvalho and Sparks, 2019; Mika et al., 2019). Thus, regular exercise causes a reduction in adipose tissue mass and increases metabolism (Mika et al., 2019).
The analysis results showed that there was a difference between the group of moderate-intensity swimming in the morning (G2) and the control group (G1) and the group of moderate-intensity swimming at night (G3) and the control group (G1) (Figure 3). These results are in line with the research conducted by Lu et al. (2016) which concluded that swimming exercise significantly increased irisin levels compared to the control group. Likewise, the research conducted by Kang et al. (2019) showed that swimming exercise significantly increased post-training irisin levels compared to the control group. The elevation of irisin levels in the group of moderate-intensity swimming in the morning is possibly due to exercise factor.
Exercise leads to increased activation of peroxisome proliferation-activated receptor coactivator-1α (PGC-1α) (Boström et al., 2012) to stimulate expression of fibronectin type III domain-containing protein 5 (FNDC-5) (Fatouros, 2018) and proteolytic membrane protein FNDC-5 cleavage occurs in skeletal muscle, thereby causing the release of irisin into the blood circulation (Moreno-Navarrete et al., 2013). Irisin released into the bloodstream might also be caused by an increase in energy requirements for muscle contraction during exercise, therefore stored energy in muscles decreases, which has an impact on increasing the release of irisin in bloodstream to maintain energy balance during exercise. The release of irisin in bloodstream will stimulate the browning process in white adipose tissue by inducing the expression of UCP-1 through signaling p38 mitogen-activated protein kinase (p38-MAPK) and extracellular-signal regulated kinase (ERK) (Perakakis et al., 2017; Fatouros, 2018). Irisin also increases lipolysis through the cylic adenosine monophosphate (cAMP) and protein kinase A (PKA) pathways, which leads to a decrease in fat accumulation (Perakakis et al., 2017).
The results showed that there was a significant difference in irisin levels between the moderate-intensity swimming exercise group in the morning (G2) and the moderate-intensity swimming exercise group at night (G3). These results are in line with the results of a research conducted by Algul et al. (2017) which concluded that night-time exercise increased irisin levels higher than morning exercise. The different results may be due to the factor of training time. Doing exercise in the morning might increase cortisol hormone, thereby suppressing the melatonin hormone receptors and having an impact on decreasing the production of melatonin hormone. The reduction production of melatonin hormone might disrupt body's metabolic processes and cause the reduction of irisin production. Doing exercise at night could reduce the production of hormone cortisol, thereby increasing the production of melatonin hormone to increase the release of irisin in bloodstream. Irisin has a circadian rhythm with the lowest level at 6:00 AM and highest level at 9:00 PM (Anastasilakis et al., 2014). However, exercise also has a significant impact on irisin release. Exercise induces irisin release through peroxisome proliferator-activated receptor-γ (PPAR-γ) and PGC-1α (Norheim et al., 2014). PPAR-γ and PGC-1α are multispecific transcriptional coactivators which are capable in regulating several genes in response to nutritional and physiological signals in tissues. PPAR-γ and PGC-1α are expressed in skeletal muscle, brown adipose tissue, liver and heart (Moreno-Navarrete et al., 2013; Xu, 2013; Norheim et al., 2014; Gizaw et al., 2017).
Interval training increases the activation of PGC-1α, especially in heart and skeletal muscles, and increases various metabolic parameters, such as insulin sensitivity and signaling, and promotes AMPK activation as well as phosphorylation of PGC1α and FNDC5 production followed by FNDC5 cleavage to produce irisin which will be released into the blood circulation (Moreno-Navarrete et al., 2013; Xu, 2013; Norheim et al., 2014). Previous studies reported that the release of irisin in bloodstream significantly increased energy consumption and oxidative metabolism (Swick et al., 2013; Vaughan et al., 2015), which caused reduction in body fat accumulation (Perakakis et al., 2017). Thus, exercise can be used as a potential therapeutic target in the future to prevent the increasing prevalence of obesity (Anastasilakis et al., 2014).
The results of our study reported that the average irisin level in our study was lower than the average irisin level in previous studies (Mazur-Bialy et al., 2017; Zhang et al., 2017). This difference in irisin levels is probably due to the difference in the linear range and sensitivity of the ELISA Kit used. In the research of Zhang et al. (2017) Irisin levels were measured using the ELISA Kit (#EK-067-16, Phoenix Pharmaceuticals, Inc.) with a linear range: 6.8 – 96.1 ng/mL and sensitivity: 6.8 ng/mL, while in our study irisin levels were measured using ELISA. Kit BT-E1479Mo (Biossay Technology Laboratory, Inc., Shanghai, China PR) with linear range: 0.05 – 30 ng/mL and sensitivity: 0.024 ng/mL. In addition, the irisin concentration in the study of Mazur-Bialy et al. (2017) examined on blood plasma, and in the study of Zhang et al. (2017) irisin levels was examined in bone serum, while in our study irisin levels were examined in blood serum. Therefore, irisin levels in our study could not be compared with irisin levels in other studies, eg Zhang et al. (2017) and Mazur-Bialy et al. (2017) because there are differences in the examination sample. The results of this study can be compared if the linear range and sensitivity of the ELISA kit used are the same and the samples used for examination of irisin levels are the same.
Limitations to this current study include 1) small sample size, 2) high drop-out rate, 3) one parameter measured. Firstly, in this study, we only use a small sample size with a total sample of 24 female mice (Mus musculus). Therefore, the future study should include more female mice (Mus musculus) samples. Secondly, the high dropout rate is due to the high mortality rate of the sample during the intervention. Thirdly, we only use one parameter to measure irisin levels. Meanwhile, it required another parameter measurement, such as peroxisome proliferation-activated receptor γ coactivator-1α (PGC-1α), fibronectin type III domain-containing protein 5 (FNDC-5), markers of browning such as uncoupling protein 1 (UCP1), PR domain containing 16 (PRDM16), and other cytokines that might be related to circulating irisin levels such as interleukin-6 (IL-6).
CONCLUSION
Based on the results of this study, it was concluded that moderate-intensity swimming exercises in the morning and at night which were carried out 3x/w for 4 weeks increased irisin levels. However, moderate-intensity swimming exercise at night was more effective in increasing irisin levels of female mice (Mus musculus) compared to moderate-intensity swimming exercise in the morning.
ACKNOWLEDGEMENTS
This study was supported by the internal research grant of the Faculty of Medicine (Penelitian Unggulan Fakultas), Universitas Airlangga, Surabaya, Indonesia, under Grant Number: 219/UN/3.1.1/PT/2021. We also thank to Virology Laboratory, Faculty of Veterinary Medicine, Universitas Airlangga to help in conducting this research.
AUTHOR CONTRIBUTIONS
Conceiving and designing the experiments: MFA PSR. Performing the experiments: MFA PSR S. Analyzing the data: AP RL. Contributing reagents/materials/analysis tools: YY KWW GMS. Writing the paper: MFA PSR S AP KWW GMS YY RL.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
REFERENCES
Algul, S., Ozdenk, C., and Ozcelik, O. 2017. Variations in leptin, nesfatin-1 and irisin levels induced by aerobic exercise in young trained and untrained male subjects. Biology of sport, 34: 339–344.
Anastasilakis, A.D., Polyzos, S.A., Saridakis, Z.G., Kynigopoulos, G., Skouvaklidou, E.C., Molyvas, D., Vasiloglou, M.F., Apostolou, A., Karagiozoglou-Lampoudi, T., Siopi, A. et al. 2014. Circulating irisin in healthy, young individuals: day-night rhythm, effects of food intake and exercise, and associations with gender, physical activity, diet, and body composition. The Journal of clinical endocrinology and metabolism, 99: 3247–3255.
Badawy, E., El-laithy, N.A., Morsy, S.M. et al. 2020. Role of swimming on muscle PGC-1α, FNDC5 mRNA, and assessment of serum omentin, adropin, and irisin in high carbohydrate high fat (HCHF) diet induced obesity in rats. Egyptian Journal of Medical Human Genetics, 21: 37.
Boström, P., Wu, J., Jedrychowski, M.P., Korde, A., Ye, L., Lo, J.C., Rasbach, K.A., Boström, E.A., Choi, J.H., Long, J.Z., et al. 2012. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature, 481: 463–468.
Cajochen, C., Kräuchi, K., and Wirz-Justice, A. 2003. Role of melatonin in the regulation of human circadian rhythms and sleep. Journal of neuroendocrinology, 15: 432–437.
Cochran, A.J., Little, J.P., Tarnopolsky, M.A., and Gibala, M.J. 2010. Carbohydrate feeding during recovery alters the skeletal muscle metabolic response to repeated sessions of high-intensity interval exercise in humans. Journal of applied physiology (Bethesda, Md.: 1985), 108: 628–636.
Fatouros I.G. 2018. Is irisin the new player in exercise-induced adaptations or not? A 2017 update. Clinical chemistry and laboratory medicine, 56: 525–548.
Garidou-Boof, M.L., Sicard, B., Bothorel, B., Pitrosky, B., Ribelayga, C., Simonneaux, V., Pévet, P., and Vivien-Roels, B. 2005. Environmental control and adrenergic regulation of pineal activity in the diurnal tropical rodent, Arvicanthis ansorgei. Journal of pineal research, 38: 189–197.
Giolo De Carvalho, F., and Sparks, L.M. 2019. Targeting white adipose tissue with exercise or bariatric surgery as therapeutic strategies in obesity. Biology, 8: 16.
Gizaw, M., Anandakumar, P., and Debela, T. 2017. A review on the role of irisin in insulin resistance and type 2 diabetes mellitus. Journal of pharmacopuncture, 20: 235–242.
Ivy J.L. 2004. Regulation of muscle glycogen repletion, muscle protein synthesis and repair following exercise. Journal of sports science & medicine, 3: 131–138.
Kang, Y.S., Kim, J.C., Kim, J.S., and Kim, S.H. 2019. Effects of swimming exercise on serum irisin and bone FNDC5 in rat models of high-fat diet-induced osteoporosis. Journal of sports science & medicine, 18: 596–603.
Kurdanti, W., Suryani, I., Syamsiatun, N.H., Siwi, L.P., Adityanti, M.M., Mustikaningsih, D., Sholihah, K.I. 2015. Factors that influence the incidence of obesity in adolescents. Indonesian Journal of Clinical Nutrition, 11: 179-190.
Kwak, J.J., Yook, J.S., and Ha, M.S. 2020. Potential biomarkers of peripheral and central fatigue in high-intensity trained athletes at high-temperature: a pilot study with momordica charantia (Bitter Melon). Journal of immunology research, 2020: 4768390.
Lu, Y., Li, H., Shen, S.W., Shen, Z.H., Xu, M., Yang, C.J., Li, F., Feng, Y.B., Yun, J. T., Wang, L. et al. 2016. Swimming exercise increases serum irisin level and reduces body fat mass in high-fat-diet fed Wistar rats. Lipids in health and disease, 15: 93.
Mazur-Bialy, A.I., Bilski, J., Wojcik, D., Brzozowski, B., Surmiak, M., Hubalewska-Mazgaj, M., Chmura, A., Magierowski, M., Magierowska, K., et al. 2017. Beneficial effect of voluntary exercise on experimental colitis in mice fed a high-fat diet: the role of irisin, adiponectin and proinflammatory biomarkers. Nutrients, 9: 410.
Mendoza, J. 2021. Nighttime light hurts mammalian physiology: what diurnal rodent models are telling us. Clocks & sleep, 3: 236–250.
Mika, A., Macaluso, F., Barone, R., Di Felice, V., and Sledzinski, T. 2019. Effect of exercise on fatty acid metabolism and adipokine secretion in adipose tissue. Frontiers in physiology, 10: 26.
Moreno-Navarrete, J.M., Ortega, F., Serrano, M., Guerra, E., Pardo, G., Tinahones, F., Ricart, W., and Fernández-Real, J.M. 2013. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. The Journal of clinical endocrinology and metabolism, 98: E769–E778.
Norheim, F., Langleite, T.M., Hjorth, M., Holen, T., Kielland, A., Stadheim, H.K., Gulseth, H.L., Birkeland, K.I., Jensen, J., and Drevon, C.A. 2014. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. The FEBS journal, 281: 739–749.
Omar, J.S., Jaradat, N., Qadoumi, M., and Qadoumi, A.N. 2021. Regular swimming exercise improves metabolic syndrome risk factors: a quasi-experimental study. BMC sports science, medicine & rehabilitation, 13: 22.
Pedersen, B.K. and Febbraio, M.A. 2008. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiological reviews, 88: 1379–1406.
Perakakis, N., Triantafyllou, G.A., Fernández-Real, J.M., Huh, J.Y., Park, K.H., Seufert, J., and Mantzoros, C.S. 2017. Physiology and role of irisin in glucose homeostasis. Nature reviews. Endocrinology, 13: 324–337.
Perreau-Lenz, S., Kalsbeek, A., Pévet, P., and Buijs, R.M. 2004. Glutamatergic clock output stimulates melatonin synthesis at night. The European journal of neuroscience, 19: 318–324.
Pranoto, A., Wahyudi, E., Prasetya, R.E., Fauziyah, S., Kinanti, R.G., Sugiharto, S., and Rejeki, P.S. 2020. High intensity exercise increases brain derived neurotrophic factor expression and number of hippocampal neurons in rats. Comparative Exercise Physiology, 16: 325-332.
Sigal, R.J., Kenny, G.P., Wasserman, D.H., Castaneda-Sceppa, C., and White, R.D. 2006. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes care, 29: 1433–1438.
Smith, B.W. and Adams, L.A. 2011. Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nature reviews. Endocrinology, 7: 456–465.
Swick, A.G., Orena, S., and O'Connor, A. 2013. Irisin levels correlate with energy expenditure in a subgroup of humans with energy expenditure greater than predicted by fat free mass. Metabolism: clinical and experimental, 62: 1070–1073.
Teo, W., Newton, M.J., and McGuigan, M.R. 2011. Circadian rhythms in exercise performance: implications for hormonal and muscular adaptation. Journal of sports science & medicine, 10: 600–606.
Vaughan, R.A., Gannon, N.P., Mermier, C.M., and Conn, C.A. 2015. Irisin, a unique non-inflammatory myokine in stimulating skeletal muscle metabolism. Journal of physiology and biochemistry, 71: 679–689.
Wierzba, T.H., Olek, R.A., Fedeli, D., and Falcioni, G. 2006. Lymphocyte DNA damage in rats challenged with a single bout of strenuous exercise. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society, 57: 115–131.
Winn, N.C., Grunewald, Z.I., Liu, Y., Heden, T.D., Nyhoff, L.M., and Kanaley, J.A. 2017. Plasma Irisin Modestly Increases during Moderate and High-Intensity Afternoon Exercise in Obese Females. PloS one, 12: e0170690.
Xu B. 2013. BDNF (I)rising from exercise. Cell metabolism, 18: 612–614.
Yang, X.Q., Yuan, H., Li, J., Fan, J.J., Jia, S.H., Kou, X.J., and Chen, N. 2016. Swimming intervention mitigates HFD-induced obesity of rats through PGC-1α-irisin pathway. European review for medical and pharmacological sciences, 20: 2123–2130.
Zhang, J., Valverde, P., Zhu, X., Murray, D., Wu, Y., Yu, L., Jiang, H., Dard, M.M., Huang, J., Xu, Z. et al. 2017. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone research, 5: 16056.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Muhamad Fauzi Antoni1, Purwo Sri Rejeki1, 2, *, Sulistiawati3, Adi Pranoto4, Kristanti Wanito Wigati2, Gadis Meinar Sari2, Ronny Lesmana5, and Yoshio Yamaoka6
1 Sport Health Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
2 Department of Medical Physiology and Biochemistry, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
3 Department of Public Health, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
4 Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
5 Department of Biomedical Science, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia
6 Department of Environmental and Preventive Medicine, Faculty of Medicine, Oita University, Yufu, Japan
Corresponding author: Purwo Sri Rejeki, E-mail: purwo-s-r@fk.unair.ac.id; purwo_faal@yahoo.com
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: September 21, 2021;
Revised: March 5, 2022;
Accepted: March 8, 2022;
Published online: March 10, 2022

