
Comparison of Phytochemicals in Sesame and Perilla (Seeds and Oils) Grown at Mae Hong Son Thailand
Arunee Apichartsrangkoon*, Autchara Tiampakdee, Warangkana Tameeya, Danchai Kreungngern, Siriwan Srisajjalertwaja, and Wissanee SupraditarepornPublished Date : 2022-03-31
DOI : https://doi.org/10.12982/CMUJNS.2022.029
Journal Issues : Number 2, April-June 2022
Abstract Sesame and perilla seeds were analyzed for fat, protein, moisture and ash. Their mineral contents, lignan, γ-tocopherol and fatty acid profiles were also determined. The results showed that sesame seeds had higher protein, lipid and mineral contents but lower fiber contents than perilla seeds, accounting for 17.38-20.76%, 42.70-46.18%, 3.07-6.47%, 18.00-21.88%, respectively. Major minerals in the seed were 0.26-0.95% calcium and 0.11-0.23% magnesium. Perilla oil showed lower pH than sesame oil giving it higher acid value. Most lignans were more concentrated in sesame than perilla. Sesamin was the predominant lignin, followed by sesamolin. The sesamin and sesamolin contents in sesame were ranged for 148.30-183.68 mg/100g and 33.96-43.54 mg/100g, respectively. Although black sesame oil had low content of sesamin and sesamolin, it performed high physiological functions due to its high contents of γ-tocopherol and unsaturated fatty acid. Gamma tocopherol of the oils was ranged from 35.81 to 51.64 mg/100g, and perilla oil showed the highest γ-tocopherol content. Fatty acid profiles revealed that the major fatty acids in sesame oils were 44.43-47.21% linoleic followed by 36.40-38.39% oleic, 7.07-9.64% palmitic and 5.09-7.72% stearic acids, giving them high polyunsaturated fatty acids (PUFA), followed by monounsaturated fatty acids (MUFA) and saturated fatty acids (SFA). White sesame oil had higher linoleic acid but lower oleic acid than other sesame oils. Perilla oil contained high amounts of 55.27% α-linolenic followed by 19.75% linoleic, 12.70% oleic and 7.38% plamitic acids, giving it high PUFA, but low SFA and MUFA.
Keywords: Chemical composition, Minerals, Fatty acid profiles, Lignans, Gamma-tocopherol
Citation: Apichartsrangkoon, A., Tiampakdee, A., Tameeya, W., Kreungngern, D., Srisajjalertwaja, S., and Supraditareporn, W. 2022. Comparison of Phytochemicals in Sesame and Perilla (Seeds and Oils) Grown at Mae Hong Son Thailand. CMU J. Nat. Sci. 21(2): e2022029.
INTRODUCTION
Mae Hong Son province is a major source of sesame (Sesamum indicum L.) and perilla (Perilla frutescens L.) seeds and their products in the northern Thailand. As a hilly region, Mae Hong Son is most suitable for growing these plants. During the rainy season water can easily flow through the plants, which can diminish some plant diseases. Moreover, most sesame and perilla grown in this area do not need treatment for pest control, enabling them to be grown naturally with little attention. In general, sesame seed is rich in lipid, protein, fiber and minerals, its protein has a good amino acid profile with nutritional value comparably to soy protein (López et al., 2003). Linoleic acid, one of the predominant fatty acid in sesame lipid, can lower triglyceride and cholesterol contents and improve cardiovascular health (Clarke, 2000). Oleic acid, another major fatty acids, is beneficial in lowering blood sugar, and protecting against heart disease (Junpeng et al., 2019). The main minerals of sesame are calcium and magnesium assisting in teeth and bone development. Apart from that, magnesium is essential for enzyme activity, and regulates the acid-alkaline balance in the body (Khan et al., 2011).
The most abundant phytochemicals in sesame are oil-soluble lignans in the form of aglycon, such as sesamin, sesamolin, sesamol, sesaminol and sesamolinol, etc (Dar and Arumugam, 2013). Other major lignan glycosides such as sesaminol triglucoside, sesaminol diglucoside and sesaminol monoglucoside are discharged along with the oil free meal (Othman et al., 2015). Dar and Arumugam (2013) stated that sesaminol glucosides could be hydrolyzed by intestinal β-glucosidase to sesaminol which could provide strong antioxidant activity. Generally, lignans have several physiological functions, such as antihypertensive and hypocholesterolemic activities, γ-tocopherol bioavailability and protecting liver against damage caused by oxidation (Wu, 2007; Dar and Arumugam, 2013; Imran et al., 2020). Sesamin, the major lignan is relatively heat-stable phytoestrogen (Ji et al., 2019). This lignan can inhibit the proliferation of several types of tumour cells and exert antitumour effects (Meng et al., 2021); as well as improve intestinal health by promoting the proliferation and adhesion of intestinal probiotics leading to modulating gut microbiota (Wang et al., 2021).
Gamma tocopherol, another important bioactive compound is abundant in both sesame and perilla. Although it has little vitamin E activity, this tocol can protect polyunsaturated fatty acids (PUFA) from lipid peroxidation in food matrices and in the human body. As a result, it is capable to inhibit the formation of some secondary products of lipid oxidation in food (Delgado et al., 2020). Regarding health benefits, γ-tocopherol has an antiproliferative effects on human cancer cells (Yokota et al., 2007) and anti-inflammatory activity (Jiang et al., 2001). Apart from its health-promoting effects, sesame oil shows remarkable stability and high resistant to rancidity due to the presence of numerous antioxidants such as sesamin, sesamolin, sesamol, tocopherol and polyphenolic compounds (Wu, 2007).
Perilla seed has 35-45% lipid comprising several unsaturated fatty acids such as α-linolenic (ω-3 fatty acid), linoleic (ω-6 fatty acid) and oleic (ω-9 fatty acid), accounting for 60, 13 and 16%, respectively (Lee et al., 2020). In vivo ω-3 fatty acids are transformed to docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which could assist in preventing abnormal clotting, reducing inflammation and relaxing blood vessels (Asif, 2012). Other health benefits of α-linolenic acid include prevention of cardiovascular and cerebrovascular diseases, lowering cholesterol, inhibiting the occurrence and metastasis of cancer, as well as providing anti-aging and anti-inflammatory effects (Albert, 2005). Apart from that perilla oil is known to have anti-bacterial, antiallergic properties and immunomodulatory action (Žekonis et al., 2008 and Sirilun et al., 2016) due to its abundance in γ-tocopherols, polyphenols, unsaturated fatty acids fiber and phytosterols. Total tocopherols of perilla are in the range of 40-78 mg/100 g oil, and the most abundant is γ-tocopherol, followed by α-tocopherol and δ-tocopherol, respectively (Park et al., 2018). Perilla seeds also contain polyphenols or flavones such as rosemarinic acid, luteolin, chrysoeriol, quercetin and catcehin (Meng et al. 2009). These antioxidants along with ω-3 fatty acid contribute to allergy, cancer and cardiovascular prevention (Gediminas et al. 2008).
This study was designed to determine the content of phytochemicals with potentially health benefits, such as lignans, γ-tocopherol, fatty acids and minerals in these plants. Their chemical composition and physical characteristic were also conducted.
MATERIAL AND METHODS
Seeds and oil preparation
Sesame (white, brown and black) and perilla seeds were procured from several villages of Mae Hong Son province, Thailand. The seeds were cleaned and approximately 3,000 g seeds were properly dried for around 30 min or until their moisture decreased to 6-7% with hot-air rotary drier. For health concerns, cold-pressed process was applied to liberate the oil. The dried seeds were squashed through a small screw press (Friend Energy Ltd. Part, Chiang Mai, Thailand) at an extraction rate of 5 kg/h. The temperature of the screw during operation was adjusted to less than 70°C and the process produced about 30-35 g of oil per 100 g of seed.
Chemical compositions of the seeds
Analysis of the seeds for moisture, fat, protein, crude fiber, and ash was followed standard analytical methods (AOAC 2000). Moisture was determined by drying 2 g seeds in an oven at 105°C. Protein was determined by combustion method (Leco, model EP 528, MI, USA) with a nitrogen conversion factor of 6.25. Lipid was analyzed using Soxhlet apparatus (Soxtec Avanti, model 2055, Tecator, Sweden) and n-hexane as an extracted solvent (Kanu, 2011). Crude fiber was assessed using a fiber digester (Fibertec 8,000, Scanco analytical instruments, Fl, USA). Ash was determined by incinerating the seeds at 550°C.
Minerals of the seeds
Determination of mineral in the seeds was followed Nookabkaew et al. (2006) and Krajčová et al. (2009). A weight of 1 g ground sample was added to 10 ml mixture of nitric : percloric acid at a 6:1 ratio. The mixture was subsequently digested at 180°C for 2 h, then diluted to 50 ml and filtered through filter paper (Whatman No. 42). The filtrate was used to analyze for Ca, and Mg by flame atomic absorption spectrophotometer (AAS, Analytik Jena, model 700PC, Jena, Germany). The AAS was equipped with a hollow cathode lamp as a light source and used air/acetylene as a nebulizing gas. Absorbances were then taken at λmax 427.7 nm for Ca, 285.2 nm for Mg and 372 nm for Fe. Each mineral was quantified from the standard calibration curves.
Lignans of the oils
Determination of lignin content in the oils was followed Rankadilok et al. (2010) and Kosińsk et al. (2011). Briefly, 3.5-4.5 g of oils was extracted with 5 ml 80% methanol for 30 min, then centrifuged at 2,000 × g, for 5 min. The supernatant was adjusted with 80% methanol, and filtered through 0.45 µm nylon membrane for high-performance liquid chromatographic (HPLC) analysis. The analysis for sesamin and sesamolin were performed by an external standard method using a HPLC system (Shimadzu Automatic sampler, SIL-10Axl, MD, USA) equipped with a thermostatically controlled column, a quaternary pump (LC-10ADvp), and an UV-visible spectrophotometric detector (SPD-M10Avp). A reversed-phase column, Hypersil 150 × 4 mm, 5 µm (Thermo Electron Co., Southern-on-Sea, UK), was used for the separation. The mobile phase was a mixture of deionized water : methanol (20:80 v/v) with an isocratic flow rate of 0.8 ml/min. The injection volume was 10 µl and detected at λmax 290 nm. The retention times for sesamin and sesamolin were 5.80 and 7.20 min, respectively. Peak areas were used to quantify the amounts based on the peak areas of each standard lignan. A typical chromatogram of lignans in white sesame oil is shown in Figure 1.
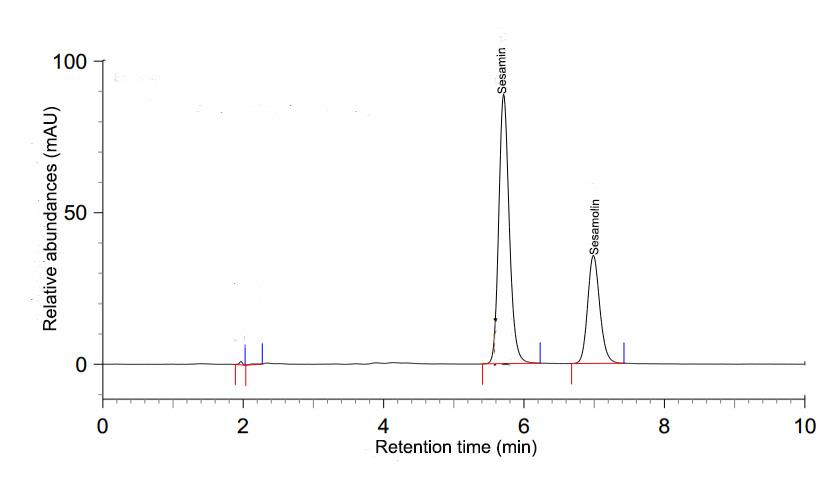
Figure 1. Chromatogram of lignans in white sesame oil.
Gamma-tocopherol of the oils
Determination of γ-tocopherol was followed Rankadilok et al. (2010) and Pestana-Bauer et al. (2012). Purposely, 100 mg of the oil sample were dissolved in 5 ml ethanol and filtered through a 0.45 µm nylon membrane for HPLC analysis. HPLC analysis of γ-tocopherol was performed by external standard method using a HPLC system (Shimadzu Automatic sampler, SIL-10Axl, MD, USA) with a thermostatically controlled column, a quaternary pump (LC-10ADvp), and a fluorescence detector (RF-10Axl). The system was equipped with Pinnacle®II PAH (150 × 3.0 mm, 4 µm) column (Restek®, USA). The mobile phase consisted of acetonitrile : methanol : isopropanol as 50:40:10 v/v (solvent A) and acetonitrile : methanol : isopropanol as 30:65:5 v/v (solvent B) with a gradient elution: 0-15 min, 100%A and 16-20 min, 100%B. Flow rate of the mobile phase was 1 ml/min at 25°C with an injection volume of 10 µl. The fluorescence detector was used with an emission wavelength of 330 nm and an excitation wavelength at 290 nm. The retention time of γ-tocopherol was 52.20 min. Peak areas were used to quantify the amount based on the peak areas of standard γ-tocopherol. A typical chromatogram of γ-tocopherol in black sesame oil is shown in Figure 2.
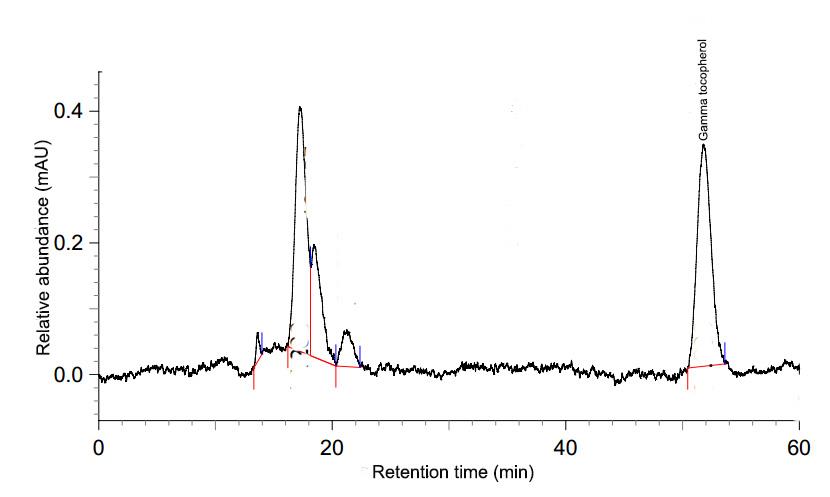
Figure 2. Figure 2 Chromatogram of γ-tocopherol in black sesame oil
Fatty acids of the oils
Determination of fatty acids was followed AOAC (2003). For esterification, 0.5 g oil was refluxed with 0.5 mol/l NaOH in 5 ml methanol for 5 min then 5 ml boron trifluoride-methanol was added and refluxed for a further 5 min. The solution was then cooled and added 10 ml NaCl plus 5 ml hexane. The top clear solution was filtered through 0.45 µm nylon membrane and the fatty acid methyl esters (FAMEs) in the filtrate were used for gas chromatographic (GC) analysis. The FAMEs were separated by GC (Brucker 436-GC, Munich, Germany) and determined by flame ionization detector. The GC was equipped with a biscyanopropyl-polysilozane column, 0.25 mm internal diameter, 100 m length and 0.20 µm film thickness (Restek®-2560, USA). Column injector and detector temperatures were 225 and 250°C, respectively, and nitrogen was used as a carrier gas. Contents of fatty acids were calculated based on the peak area relative to standards. Typical chromatograms of fatty acid profiles for black sesame and perilla oils are shown in Figures. 3 and 4, respectively.
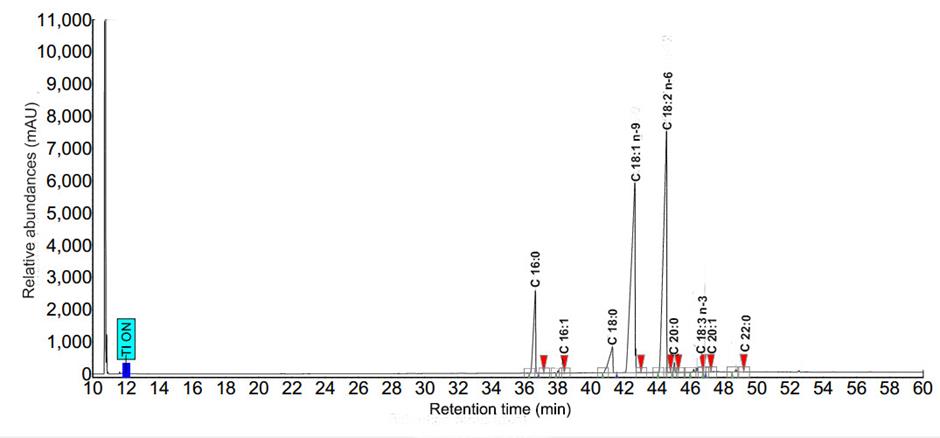
Figure 3. Chromatograms of fatty acid profile for black sesame oil.
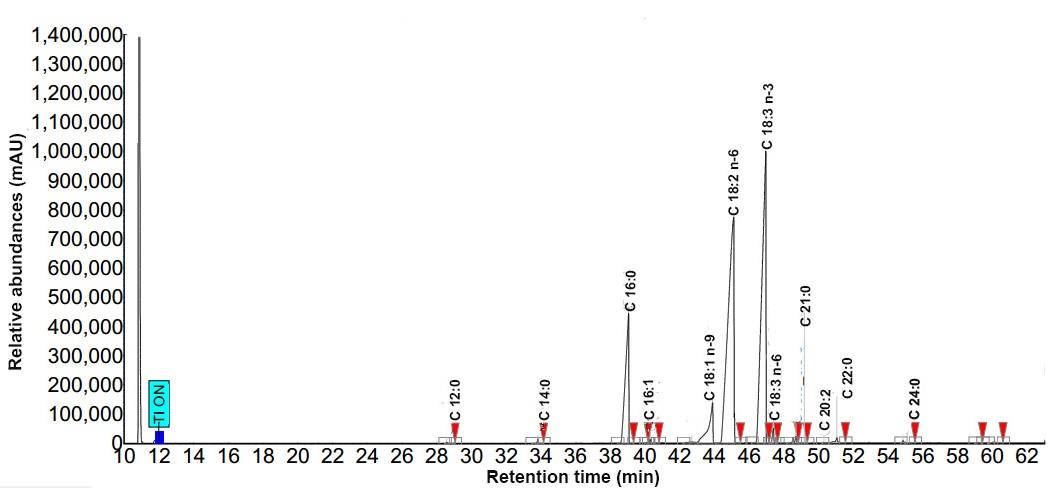
Figure 4 Chromatograms of fatty acid profile for perilla oil
Statistical analysis
All variables were determined for one-way analysis of variance (ANOVA) using SPSS 17 software (SPSS Inc., IL, USA). Duncan’s multiple range test (DMRT) was applied to compare the differences among means (P ≤0.05).
RESULTS
Chemical composition of sesame and perilla seeds
Table 1 illustrated that black, brown and white sesame seeds contained similar protein contents (~ 20%), while perilla seed was significantly less (P ≤0.05). White and brown sesame yielded significantly higher (P≤0.05) lipid contents than other seeds. Perilla seeds had significantly less (P ≤0.05) lipid than those sesame. Moreover, white sesame seed contained significantly less (P ≤0.05) fiber, while perilla had significantly more (P ≤0.05) fiber than the other seeds. In addition, Perilla seed contained significantly more (P ≤0.05) moisture and carbohydrate than the other sesame seeds, whereas white and brown sesame seeds had the least moisture and carbohydrate.
Table 1. Chemical and mineral compositions of sesame and perilla seeds.
|
Chemical compositions (%) |
Types of sesame |
Perilla |
||
|
White |
Brown |
Black |
|
|
|
Protein |
20.76 ± 0.81a |
19.29 ± 1.07ab |
19.72 ± 0.56a |
17.38 ± 1.12b |
|
Lipid |
45.63 ± 0.43a |
46.18 ± 0.51a |
44.01 ± 0.66b |
42.70 ± 0.39c |
|
Fiber |
18.00 ± 0.72c |
19.06 ± 0.53bc |
19.39 ± 0.19b |
21.88 ± 0.30a |
|
Moisture |
6.21 ± 0.10c |
6.55 ± 0.17b |
6.32 ± 0.13bc |
7.57 ± 0.19a |
|
Carbohydrate* (excluded fiber) |
4.29 ± 0.17b |
3.20 ± 0.14c |
4.09 ± 0.12b |
7.40 ± 0.20a |
|
Ash |
5.11 ± 0.14c |
5.72 ± 0.22b |
6.47 ± 0.21a |
3.07 ± 0.30d |
|
Calcium |
0.77 ± 0.11ab |
0.61 ± 0.16b |
0.95 ± 0.13a |
0.26 ± 0.09c |
|
Magnesium |
0.21 ± 0.02a |
0.19 ± 0.02a |
0.23 ± 0.02a |
0.11 ± 0.01b |
|
Iron |
0.01 ± 0.00 |
0.01 ± 0.00 |
0.02 ± 0.00 |
- |
Note: Variables with the same superscript letters in the same row are not significantly different (P >0.05). The experiments were performed for triplication (n=6). *Calculated by difference
Black sesame seed showed the highest ash/mineral contents including calcium, magnesium and iron, while perilla seed had the lowest ash contents (Table 1). Overall, sesame seeds contained much more minerals than perilla seeds, the predominant mineral was calcium followed by magnesium and iron. It was worth noting that calcium in black sesame was the significantly highest (P ≤0.05) content.
Physical characteristics of sesame and perilla oils
Table 2 showed that perilla oil had the highest water activity, while white sesame oil had the lowest water activity. Black sesame oil showed the highest viscosity followed by white and brown sesame oils, perilla had the lowest viscosity. For color, white sesame and perilla oils displayed the highest L* parameter. White and black sesame oils exhibited significantly greater (P ≤0.05) a* parameter than the other oils, perilla oil had the lowest a* parameter. On the other hand, perilla oil showed the highest b* parameter followed by brown, black and white sesame oils. Overall, the whiteness indices demonstrated that perilla oil was significantly darker (P ≤0.05) than the sesame oils. Among three types of sesame oils, white and black sesame oil showed lighter than brown sesame oil.
Table 2. Physical characteristics of sesame and perilla oils.
|
Characteristics of |
Types of sesame |
Perilla |
||
|
White |
Brown |
Black |
||
|
aw |
0.46 ± 0.03b |
0.50 ± 0.03ab |
0.49 ± 0.02ab |
0.52 ± 0.02a |
|
Viscosity (cP) |
5.54 ± 0.24ab |
5.18 ± 0.17b |
5.84 ± 0.32a |
4.49 ± 0.26c |
|
Color L* parameters |
91.27 ± 1.53a |
82.96 ± 1.96b |
85.78 ± 1.48b |
89.20 ± 2.07ab |
|
a* parameters |
6.02 ± 0.29ab |
5.57 ± 0.18b |
6.11 ± 0.26a |
3.32 ± 0.22c |
|
b* parameters |
47.06 ± 1.64c |
53.25 ± 2.49b |
49.13 ± 2.93bc |
75.02 ± 3.11a |
|
Whiteness indices |
52.14 ± 2.63a |
43.82 ± 2.15b |
48.00 ± 1.71a |
24.18 ± 1.90c |
|
pH |
6.46 ± 0.13a |
6.71 ± 0.21a |
6.11 ± 0.17b |
5.5 ± 0.09c |
|
Acid values (mg KOH/g) |
2.20 ± 0.21ab |
1.32 ± 0.14c |
2.05 ± 0.08b |
2.4 ± 0.15a |
|
Peroxide values (mmol/kg) |
3.07 ± 0.15c |
4.15 ± 0.22a |
4.54 ± 0.19a |
3.62 ± 0.27b |
Note: Variables with the same superscript letters in the same row are not significantly different (P >0.05). The experiments were performed for triplication (n=9)
Regarding chemical characteristics, perilla oil had significantly lower pH (P ≤0.05) than sesame oil. In addition, brown sesame oil showed significantly lower acid value
(P ≤0.05) than the other oils, and perilla oil showed the highest acid value. Peroxide values illustrated that brown and black sesame oil had significantly higher peroxide values than the other two oils (Table 2).
Lignans and tocopherol in sesame and perilla oils
Table 3 shows the lignans and γ-tocopherol present in the cold-pressed oils. Among two types of lignans, sesamin was predominant. White and brown sesame oil had significantly higher contents (P ≤0.05) of sesamin, sesamolin and total lignans than the black sesame oils. On the other hand, perilla oil had the least concentration of lignans.
Table 3. Lignans and γ-tocopherol of sesame and perilla oils.
|
Active components (mg/100 g) |
Types of sesame |
Perilla |
|
||||
|
White |
Brown |
Black |
|
|
|||
|
Sesamin |
183.68 ± 13.75a |
175.07 ± 10.18a |
148.30 ± 11.39b |
13.61 ± 3.35c |
|||
|
Sesamolin |
43.54 ± 4.37a |
37.63 ± 5.92ab |
33.96 ± 4.11b |
- |
|||
|
Total lignans |
227.22 |
212.70 |
182.26 |
13.61 |
|||
|
γ-tocopherol |
35.81 ± 2.37b |
38.93 ± 3.79b |
40.66 ± 5.45b |
51.64 ± 3.60a |
|||
Note: Variables with the same superscript letters in the same row are not significantly different (P >0.05). The experiments were performed for triplication (n=6)
Apart from lignans, γ-tocopherol is also an essential phytochemical found in sesame and perilla oil. Three types of sesame oil in Table 3 showed non-significant difference (P ≤0.05) with γ-tocopherol contents. However, significantly higher (P ≤0.05) concentrations of γ-tocopherol were found in perilla than in sesame oils.
Fatty acid profiles of sesame and perilla oils
Table 4 shows that black sesame and perilla oil contained more varieties of fatty acids than brown and write sesame oils. Among 21 fatty acids quantified in the oils, two SFA palmitic and stearic acids were significantly more (P ≤0.05) abundant in sesame oil than in perilla oil. The major USFA in these oils were oleic acid (MUFA) and linoleic (PUFA) which were also significantly greater (P ≤0.05) in sesame than in perilla oil; accounting for ~37.66% and ~45.62% in sesame oil as well as ~12.70% and 19.75% in perilla oil. On the other hand, α-linolenic acid (PUFA) was the highest (55.27%) content in perilla oil, whereas sesame oil contained less than 1%. These fatty acids determined the concentrations of SFA, MUFA and PUFA in sesame and perilla oils.
Table 4. Fatty acid profiles of cold pressed sesame and perilla oils.
|
Fatty acids (%) |
Types of sesame oil |
Perilla oil |
|||
|
|
White |
Brown |
Black |
|
|
|
C 12:0 |
- |
- |
0.02 ± 0.00 |
0.04 ± 0.00 |
|
|
C 14:0 |
- |
- |
0.13 ± 0.01 |
0.12 ± 0.01 |
|
|
C 16:0 |
9.64 ± 0.62a |
7.07 ± 0.32b |
7.73 ± 0.45b |
7.38 ± 0.25b |
|
|
C 16:1 |
0.15 ± 0.00b |
0.11 ± 0.00c |
0.12 ± 0.01c |
0.22 ± 0.04a |
|
|
C 17:0 |
- |
- |
0.04 ± 0.00 |
0.05 ± 0.00 |
|
|
C 17:1 |
- |
- |
0.26 ± 0.03 |
- |
|
|
C 18:0 |
7.72 ± 0.52a |
7.04 ± 0.63a |
5.09 ± 0.47b |
3.09 ± 0.40c |
|
|
C 18:1 n-9 |
36.41 ± 0.75b |
38.39 ± 0.94a |
38.17 ± 1.12a |
12.70 ± 0.54c |
|
|
C 18:2 n-6 |
47.21 ± 0.41a |
45.22 ± 0.84b |
44.43 ± 0.62b |
19.75 ± 0.50c |
|
|
C 20:0 |
- |
- |
0.20 ± 0.01 |
- |
|
|
C 18:3 n-6 |
0.57 ± 0.04b |
0.82 ± 0.07a |
0.48 ± 0.06b |
0.24 ± 0.01c |
|
|
C 18:3 n-3 |
0.99 ± 0.11b |
0.73 ± 0.18b |
0.44 ± 0.09c |
55.27 ± 2.10a |
|
|
C 20:1 |
0.28 ± 0.02a |
0.14 ± 0.03b |
0.08 ± 0.01c |
0.19 ± 0.02b |
|
|
C 20:2 |
0.07 ± 0.01 |
0.06 ± 0.00 |
0.08 ± 0.00 |
0.06 ± 0.01 |
|
|
C 21:0 |
0.05 ± 0.00 |
0.05 ± 0.00 |
0.09 ± 0.01 |
0.05 ± 0.00 |
|
|
C 22:0 |
0.29 ± 0.02a |
0.26 ± 0.02a |
0.21 ± 0.01b |
0.19 ± 0.02b |
|
|
C 20:3 |
- |
- |
0.10 ± 0.01 |
0.04 ± 0.00 |
|
|
C 20:4 |
- |
- |
0.06 ± 0.00 |
- |
|
|
C 24:0 |
0.09 ± 0.01c |
0.12 ± 0.01b |
0.09 ± 0.02bc |
0.25 ± 0.02a |
|
|
C 20:5 |
0.05 ± 0.00 |
- |
0.03 ± 0.00 |
- |
|
|
C 24:1 |
0.48 ± 0.03 |
- |
0.13 ± 0.02 |
0.36 ± 0.03 |
|
|
SFA |
17.79 |
14.54 |
15.61 |
11.39 |
|
|
MUFA |
37.32 |
38.64 |
38.77 |
13.48 |
|
|
PUFA |
44.89 |
46.82 |
45.62 |
75.35 |
|
Note: Variables with the same superscript letters in the same row are not significantly different (P >0.05). The experiments were performed for duplication (n=4)
Footnote: C 12:0 = lauric acid, C 14:0 = myristic acid, C 16:0 = palmitic acid, C 16:1= palmitoleic acid, C 17:0 = margaric acid, C 17:1 = heptadecenoic acid, C 18:0 = stearic acid, C 18:1 n-9 = oleic acid, C 18:2 n-6 = linoleic acid, C 18:3 n-3 = α-linolenic acid, C 18:3 n-6 = γ-linolenic acid, C 20:0 = arachidic acid, C 20:1 = gadoleic acid, C 20:2 = eicosadienoic acid, C 20:3 = eicosatrienoic acid, C 20:4 = arachidonic acid, C 20:5 = timnodonic acid, C 21:0 = heneicosanoic acid, C 22:0 = behenic acid, C 24:0 = lignoceric acid, C 24:1 = nervonic acid
Accordingly, sesame oil had higher content of SFA and MUFA than perilla oil, accounting for ~16%, ~38.24% and 11%, 13%, respectively. PUFA more concentrated in perilla oil (75.35%) than in sesame oil (~45.78%). White sesame oil had significantly less oleic than other sesame oils, it had though a higher linoleic content. Overall, white sesame oil contained higher SFA, but lower MUFA and PUFA than the other sesame oils. Perilla oil had lower SFA and MUFA than sesame oil, however, it had a higher PUFA content.
DISCUSSION
Protein, lipid and fiber of sesame and perilla seeds
Most sesame proteins are storage proteins comprising 67.3% globulins, 8.6% soluble albumins, 1.4% prolamines, and 6.9% glutelin (Gómez-Arellanoa, et al., 2017). In this study, sesame had higher protein than perilla, and other sesame seeds had similar contents, such as sesame from Congo-Brazzaville 19-26% (Nzikou et al., 2009), from Cameroon ~23% (Tenyang et al., 2017) and from Morocco 22% protein (Gharby et al., 2017). In addition, Morris et al., (2021) obtained 13.92-21.76 % protein from Chinese and Thai sesame seeds, which were relatively equivalent to this observation. On the other hand, Rizki et al. (2014) found higher 26-28% protein in Moroccan sesame, which could be due to applying different extraction method (Hao et al., 2021). Tenyang et al. (2017) stated that white and brown sesames had identical protein contents, which was compatible with this study. However, variations in chemical composition of sesame and perilla depend on variety, origin, color and size of the seeds (Lee et al., 2018).
Color or cultivars could be influent on the lipid contents, Tenyang et al. (2017) found 53 and 49.6% lipid in white and brown sesame seeds, respectively. Similarly, 52.17 and 42.48% lipid in light and dark sesame seeds, respectively were obtained by Morris et al., (2021). According to this finding, white sesame seed was likely to contribute more lipid than black seed. Nevertheless, variation of lipid in the oil seeds is associated with the differences in variety of plant cultivar, cultivation climate, ripening stage, harvesting time and the extraction method used (Achouri et al., 2012). In addition, sesame like olive lipid is relatively resistant to oxidation as compared to other plant lipids (Gharby, et al., 2017). Though perilla seeds had less lipid than those sesame, this was in accordance with Sargi et al. (2013) who found 40 and 42.3% lipid in white and brown perilla seeds, respectively. Fibers are also incorporated in several physiological functions. The concentration of 4-19% sesame fiber from several countries was reported by Nzikou et al. (2009) and Tenyang et al. (2017). Hence the fiber contents in both sesame and perilla is considered high. Generally, carbohydrate including fiber of perilla seed has around 23% (Dhyani et al., 2019) which is compatible with this investigation.
Ash and mineral contents of sesame and perilla seeds
Ash in different sesame seeds is ranged from 4.6 to 5.6% (Nzikou et al., 2009) also 4.54-4.71% (Alyemeni et al., 2011) and 4.5% (Gharby et al., 2017) which are slightly lower than this finding. Kanu (2011) stated that minerals in sesame vary with cultivation area and seed color or cultivar. Black sesame seed had the highest ash/mineral contents which was complied with the results of Tenyang et al. (2017) who found 6.03% in brown and 4.62% in white seeds. On the other hand, Alyemeni et al. (2011) obtained similar amount of ash (~4.6%) in black and white Saudi sesame seeds. Minerals such as calcium and magnesium tended to be more concentrate in black rather than white sesame, Tenyang et al. (2017) also found 0.98, 2% calcium and 0.44, 0.47% magnesium in white and brown Cameroon sesame, respectively. Alyemeni et al. (2011) obtained 1.16-1.45% calcium, 0.18-0.52% magnesium, 0.01% iron in several color sesames which were much higher than this finding, probably due to the variation of genotypes and cultivation areas (Morris, 2021). Perilla yielded the least minerals (3%), similarly, Dhyani et al. (2019) also found 3.33% ash and 0.25% calcium and 0.26% magnesium in Indian perilla seed,
Physical and chemical characteristics of sesame and perilla oils
Variation of aw and viscosity might be due to some extent of the variation of moisture in the seed, perilla oil had the highest moisture content, reflected in low viscosity. Regarding the color of the oils, white and black sesame oils yielded the highest whiteness indices (lighter color) due to their high L* and low b* parameters. On the other hand, perilla oil exhibited the lowest whiteness index (darkest color) with high b* and L* parameters, presumably caused by Maillard browning during oil extraction.
Hydrolytic rancidity and breakdown of triacylglycerol by lipase activity increase the levels of free fatty acids, acid value, and peroxide value in edible oil during oil extraction or storage (Lee et al., 2020). In general, Peroxide value is a measurement of hydroperoxide occurring at the initial state of lipid oxidation, as a result of free radicals interacting with unsaturated fatty acids (Cao et al, 2017). These hydroperoxide might be further broken down into non-volatile secondary products (Wąsowicz et al., 2004). In addition, an existence of some volatile aldehydes such as hexanal, 2-heptenal and little nonanal 2-decenal is also associated with linoleic acid oxidation (Kiralan et al., 2016), which could be a good index of lipid rancidity. The acid value is frequently used to determine the initial state of lipid oxidation. According to Codex Alimentarius, FAO/WHO (2009), the non-refined oil quality remains acceptable until the acid and peroxide values reaches 4 mg/KOH g and 15 meq/kg oil, respectively. Both values in this investigation were well complied with the acceptable margin, indicating good resistance to peroxidation. Sirilun et al. (2016) applied moist heat under pressure to extract perilla oil and found higher acid and peroxide values for 3.52 mg/KOH g and 6.56 mmol/kg, respectively than the control. Ji et al. (2019) roasted sesame seeds prior to oil extraction and noticed that roasting temperature could rise the acid values but reduce the peroxide values. Rising of acid values could be caused by the appearance of some acids such as phytic, oxalic, and phenolic acids, whereas, reducing of peroxide values could be due to decomposition of peroxide when the temperature reached a certain level (Ji et al., 2019).
Lignans in sesame and perilla oils
Fat soluble aglycon-lignans such as sesamin, sesamolin and sesamol are abundant in sesame. Sesamin is relatively stable on heating, while sesamolin could be reduced by heat and some converted to sesamol (Ji et al., 2019). Rangkadilok et al. (2010) reported that the contents of 167 mg/100g sesamin was greater than 58 mg/100g sesamolin in Thai sesame oils, relatively similar to this finding. On the other hand, Morris et al. (2021) found much higher 898 mg/100g sesamin and 279 mg/100g sesamolin in Thai sesame oils. The variation of lignan concentrations might be due to environmental stresses, genetics, cultivars, as well as agronomic and extraction conditions (Kim et al., 2014). Other researchers obtained sesamin and sesamolin in the range of 8–258 mg/100g and 28–252 mg/100g (Ajit et al., 2019), 500-600 mg/100g and 190-240 mg/100g (Imran et al., 2020), 550-898 mg/100g and 104–630 mg/100g (Morris et al., 2021) as well as 491 mg/100 g and 300 mg/100 g (Wu, 2007), respectively.
This study, white cultivar contained more lignans than brown and black cultivars. Kim et al. (2014) found similar results with 520 and 198 mg/100 g of sesamin in white and black Korean sesame oils, as well as 401 mg/100g and 172 mg/100g sesamin were found in light and dark sesame seeds, respectively (Morris et al., 2021). Rangkadilok et al. (2010) also obtained similar results of white and black Thai sesames from several provinces. Moreover, Rangkadilok et al. (2010) emphasized that genetic, environmental, soil conditions, water, nutritional, climate, extraction method and detection techniques might cause some variation of sesamin and sesamolin in the oil. According to this accomplishment, it could be presumable that white sesame is likely to gain more benefits to health than black in the respect of lignan. Although black sesame exhibits low content of sesamin and sesamolin, it has high antioxidative and antiproliferative activities (Kancharla & Arumugam, 2020) probably due to its high contents of γ-tocopherol, phenolic components and flavonoids along with the unsaturated fatty acid.
Tocopherol of sesame and perilla oils
Tocopherol is another bioactive compound found in both sesame and perilla oils. Tocopherol homologues of sesame comprise 2.2% α-tocopherol, 7.3% δ-tocopherol, and 90.5% γ-tocopherol with small amount of tocotrienols (Gharby et al., 2017), thus γ-tocopherol is the major component in sesame. Generally, total tocopherols in sesame oil range from 40.36 to 46.10 mg/100g (Durazzo et al., 2021; Gharby et al., 2017) and 17.25-23.95 mg/100g (Morris et al., 2021). Kanu (2011) found white sesame seed yielded greater γ-tocopherol than black sesame seed, while Morris et al. (2021) obtained equivalent content of ~20 mg/100g γ-tocopherol in light and dark seed, agreed with this finding. Rangkadilok et al. (2010) noted that Thai sesame oils exhibited wide-ranging of 30.4-64.7 mg/100 g γ-tocopherol, irrespectively their colors. However, variation of γ-tocopherol in sesame seeds and oils is possibly due to differences in genetic and environmental situations such as soil, irrigation, and phenology influences as well as extraction method used (Williamson et al., 2008). Ji, et al. (2019) observed that roasting process could decrease tocopherol due to oxidation reactions and cell damage on roasting, whereas Vujasinovic et al. (2012) found an increase in tocopherol of roasted sesame seed because of better releasing from the broken cell membranes. Perilla oil yielded the highest γ-tocopherol content which was coincident with the results of Yang et al. (2018) and Zhao et al. (2012) who detected 45.4 and 40.9 mg/100 g of γ-tocopherol in perilla oils, respectively.
Fatty acid profiles of sesame and perilla oils
The most abundant fatty acid in sesame oil was linoleic acid followed by oleic, plamitic and stearic acids, indicating high contents of PUFA followed by MUFA and SFA. In general, linoleic and oleic acids are the primary fatty acids in sesame accounting for 80% of the total fatty acids (Mondal et al., 2010). Similar to this study, Nzikou et al. (2009) found 46.34% linoleic and 38.81% oleic acids in Congo-Brazzaville sesame oil, while Junpeng et al. (2019) found 40.82% linoleic and 34.43% oleic acids in Chinese sesame oil. In addition, Kiralan and Ramadan (2016) obtained 45.99% linoleic, 38.12% Oleic, 9.79% palmitic, 4.86% stearic acids in Turkish sesame oil, whereas Morris et al. (2021) detected 44.97% linoleic and 37.24% oleic acids in Thai sesame oil, agreed well with this study. Equal quantity of 42% linoleic and oleic were also found by Gharby et al. (2017). In contrary, sesame oil with less linoleic (0.3-45%) but high oleic (40-50%) was reported by Imran et al. (2020). Asghar and Majeed (2013) stated that oil content variability might be caused by variety differences, planting season, maturing stage, harvesting date and extraction method, which would affect the fatty acid concentrations. Imran et al. (2020) pointed out that PUFAs such as linoleic and α-linolenic acids could increase high-density lipoprotein (HDL) cholesterol, while decreasing low-density lipoprotein (LDL) cholesterol.
It is noteworthy that white sesame oil had more linoleic but less oleic than black sesame oil, while Tenyang et al. (2017) found less linoleic and oleic acid in white than brown sesame oils for 41.18 and 47.21% linoleic acid and 43.14 and 46.04% oleic acid, respectively. Morris et al. (2021) also found less linoleic acid in light (46.14 %) than dark (48.46%) sesame oil, but more oleic acid for 36.68 % and 34.78 %, respectively. However, it was clarified that white sesame oil yields less MUFA and PUFA than black sesame oils. Furthermore, white sesame oil in this study was inclined to be more SFA and less USFA, owing to the existence of high palmitic and stearic acids contents, while similar content of SFA in light and dark sesame oils was found by Morris et al., (2021). Additionally, Ji et al (2019) observed that fatty acid contents of sesame were relatively stable upon roasting the seeds.
Perilla oil yielded larger quantities of ~55% ω-3 fatty acid (α-linolenic) than sesame oil (< 1%), while lesser amount of other fatty acids being observed. This made it be more abundant in PUFA than SFA and MUFA. Major fatty acids of perilla oil found by different researchers are as follows: 60% α-linolenic, ~13% linoleic, and ~16% oleic in Korean perilla oil (Lee et al, 2021), 61.7% α-linolenic and 14% linoleic acids in Korean perilla oil (Jung et al., 2012), 60.4% α-linolenic and 16% linoleic acids in Thai perilla oils (Sirilun et al., 2016), 59.8% α-linilenic and 9.7% linoleic acids in Chinese perilla oils (Zhao, 2012). The highest fatty acid profile was found by Torri et al., (2019) for 60.13% α-linolenic, 18.35% linoleic and 12.63% oleic in Japanese perilla oil. However, variation of the fatty acid contents might be due to soil and growth conditions, climate and latitude of planting (Ding et al., 2012). With its high PUFA content, perilla oil tends to be engaged in oxidative development. To subside this phenomenon, some researchers recommended for either blending with other oxidatively stable oil (Torri et al., 2019) or pretreatment the seed prior to the extraction process (Lee et al., 2010). In overall, sesame oil showed higher concentration of SFA and MUFA than perilla oil, while perilla oil displayed the highest PUFA content.
CONCLUSION
Sesame seeds tended to have higher protein, lipid and mineral (Ca and Mg) contents but lower fiber than perilla seeds. Black sesame seeds contained more ash/minerals than other sesame seeds, while white and brown sesame seeds had more lipid contents than black seeds. Sesamin was the major lignan in sesame, followed by sesamolin. White sesame showed larger lignans than black sesame. Perilla oil yielded higher γ-tocopherol content than sesame. Black sesame oil displayed higher content of γ-tocopherol than white sesame. The major fatty acids in sesame oils were linoleic acid, followed by oleic, palmitic and stearic acids, giving them high PUFA contents followed by MUFA and SFA. White sesame oil had less PUFA and MUFA than other sesame oil. Perilla oil contained high amounts of α-linolenic acid giving it high PUFA, but low SFA and MUFA. It seemed that perilla had a superior nutrient value, in the respect of its lipid profile. However, its high PUFA content and the presence of lipase activity could make it more susceptible to oxidatively rancid development than other oil seeds. Therefore, this should be taken in account along with the storage stability. To minimize this degradation, some researchers recommended for either applying pretreatment processes or blending with other oxidatively stable oil.
In comparison with high temperature or solvent extraction oils, there was a limitation of cold-pressed oil on lowering the product quantity. The process could liberate only a certain levels of the oil leading to reducing some oil soluble phytochemicals, thus different concentrations of bioactive components were obtained from different extraction techniques. Despite the impact of this limitation enabled inevitably supplying high cost products, this circumstance was not applied to the health conscious consumers.
ACKNOWLEDGEMENTS
This research were supported by Office of Research Administration, Chiang Mai University and Agricultural Research Development Agency, Bangkok, Thailand for whose efforts were greatly appreciated.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Achouri, A., Nail, V., and Boye, J.I. 2012. Sesame protein isolate: fractionation, secondary structure and functional properties. Food Research International. 46: 360–369.
Ajit, G., Uma, D., Manonmani, S., Vinothkumar, B., Rajesh, S. 2019. Diversity analysis of sesame lignans in 40 sesame collections in Tamil Nadu. International Journal of Current Microbiology and Applied Sciences. 8: 2329–2336.Albert, C.M., Oh, K., Whang, W., Manson, J.E., Chae, C.U., and Stampfer, M.J. 2005. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation. 112: 3232–3238.
Alyemeni, M.N., Basahy A.Y., and Sher, H. 2011. Physico-chemical analysis and mineral composition of some sesame seeds (Sesamum indicum L.) grown in the Gizan area of Saudi Arabia. Journal of Medicinal Plants Research. 5: 270–274.
AOAC. 2000. Official methods of the association of official analytical chemists. Association of Official Analytical Chemists, Washington D.C.
AOAC. 2003. Official methods of the association of official analytical chemists. Association of Official Analytical Chemists, Washington D.C.
Asghar, A. and Majeed, M.N. 2013. Chemical characterization and fatty acid profile of different sesame varieties in Pakistan. American Journal of Scientific and Industrial Research. 4: 540–545.
Asif, M. 2012. Nutritional importance of monounsaturated and polyunsaturated fatty acids of perilla oil. International Journal of Phytopharmacy. 2: 154–161.
Cao, G., Ruan, D., Chen, Z., Hong, Y., and Cai, Z. 2017. Recent developments and applications of mass spectrometry for the quality and safety assessment of cooking oil. TRAC Trends in Analytical Chemistry. 96: 201–211.
Clarke, S.D. 2000. Polyunsaturated fatty acid regulation of gene transcription: a mechanism to improve energy balance and insulin resistance. British Journal of Nutrition. 83: S59–S66.
Codex alimentarius, FAO/WHO. 2009. CL 2009/6-FO, Alinorm 09/32/17: Report of the 21st session of the codex alimentarius committee on fats and oils. Kota Kinabalu, Malaysia.
Dar, A.A. and Arumugam, N. 2013. Lignans of sesame: Purification methods, biological activities and biosynthesis, A review. Bioorganic Chemistry. 50:1–10.
Delgado, A.E., Al-Hamimi, S., Ramadan, M.F., Wit, M.D., Durazzo, A., Nyam, K.L., and Issaoui, M. 2020. Contribution of Tocols to Food Sensorial Properties, Stability, and Overall Quality. Journal of Food Quality. 2020:1–8.
Dhyani, A., Chopra, R., and Garg, M. 2019. A review on nutritional value, functional properties and pharmacological application of perilla (Perilla Frutescens L.). Biomedical & Pharmacology Journal. 12: 649–660.
Ding, Y., Neo, M.C., Hu, Y., Shi, L., Chao M.A., and Liu, Y.J. 2012. Characterization of fatty acid composition from five perilla seed oils in China and its relationship to annual growth temperature. Journal of Medicinal Plants Research. 6: 1645–1651.
Durazzo, A., Nazhand, A., Lucarini,M., Delgado, A.M., De Wit, M., Nyam, K.L., Santini, A., and Ramadan, M.S. 2021. Occurrence of tocols in foods: An updated shot of current databases. Journal of Food Quality. 2021: 1−7.
Gediminas, Z., Jonas, Z., Renata, S., Genovaitė, S. and Egidijus, K. 2008. Effect of Perilla frutescens aqueous extract on free radical production by human neutrophil leukocytes. Medicina (Kaunas). 44: 699–705.
Gharby, M.S., Harhar, H., Bouzouba, Z., Asdadi, A., Yadini, A.E. and Charrouf, Z. 2017. Chemical characterization and oxidative stability of seeds and oil of sesame grown in Morocco. Journal of the Saudi Society of Agricultural Sciences. 16: 105–111.
Gómez-Arellanoa, A., Jiménez-Islasa, H., Castrejón-Gonzáleza, E.O., Medina-Torresb, L., Dendoovenc, L., and Escamilla-Silvaa, E.M. 2017. Rheological behaviour of sesame (Sesamum indicum L.) protein dispersions. Food and Bioproducts Processing. 106: 201–208.
Hao, L., Lv, C., Cui, X., Yi, F. and Su, C. 2021. Chang Su Study on biological activity of perilla seed oil extracted by supercritical carbon dioxide. LWT - Food Science and Technology. 146: 1–7.
Imran, M., Khan, M.K., Ali, M., Nadeem, M., Mushtaq, Z., Ahmad, M.H., Arshad, M.S., Ahmad, N. and Rahim, M. A. 2020. Chapter 10 - Cold pressed sesame (Sesamum indicum) oil, Editor(s): M.F., Ramadan, Cold Pressed Oils, Academic Press, pp 105-111.
Ji, J., Liu, Y., Shi, L., Wang, N. and Wang, X. 2019. Effect of roasting treatment on the chemical composition of sesame oil. LWT-Food Science and Technology. 101: 191–200.
Jiang, Q., Christen, S., Shigenaga, M. K., and Ames, B. N. 2001. γ-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. American Journal of Clinical Nutrition. 74: 714–722.
Jung, D.M., Yoon, S.H. and Jung, M.Y. 2012. Chemical properties and oxidative stability of perilla oils obtained from roasted perilla seeds as affected by extraction methods. Journal of Food Science. 77: C 1249–1255.
Junpeng, Y., Qi, Z., Xin, L., Xinsheng, W., Bing, L. and Wenxue, Z. 2019. Steam explosion technology based for oil extraction from sesame (Sesamum indicum L.) seed. Journal of the Saudi Society of Agricultural. 18: 1–6.
Kancharla, P.K. and Arumugam, N. 2020. Variation of Oil, Sesamin, and Sesamolin Content in the Germplasm of the Ancient Oilseed Crop Sesamum indicum L. Journal of the American Oil Chemists’ Society. 97: 475–483.
Kanu, P.J. 2011. Biochemical analysis of black and white sesame seeds from china. American Journal of Biochemistry and Molecular Biology. 1: 145–157.
Khan, k.y., Khan M.A., Niamat, R., Munir, M., Fazal, H. Mazari, P., Seema, N., Bashir, T., Kanwal, A., and Ahmed, S.N. 2011. Element content analysis of plants of genus Ficus using atomic absorption spectrometer. African Journal of Pharmacy and Pharmacology. 5: 317–321.
Kim, J.H., Seo, W.D., Lee, S.K., Lee, Y.B., Park, C.H., Ryu, H.W., and Lee, J.H. 2014. Comparative assessment of compositional components, antioxidant effects, and lignan extractions from Korean white and black sesame (Sesamum indicum L.) seeds for different crop years. Functional Foods 7: 495–505.
Kiralan, M. and Ramadan, M.F. 2016. Volatile oxidation compounds and stability of safflower, sesame and canola cold-pressed oils as affected by thermal and microwave treatments. Journal of Oleo Science. 65: 825−833.
Kosińsk, A., Urbalewicz, A. Penkacik, K., Karamac, M., and Amarowicz, R. 2011. SE-HPLC-DAD analysis of flaxseed lignan macromolecule and its hydrolysates. Polish Journal of Food and Nutrition Sciences. 61: 263–271.
Krajčová, A., Schulzová, V., Hajšlová, J., and Bjelková, M. 2009. Lignans in Flaxseed. Czech Journal of Food Science. 27: 252–255.
Lee, S.W., Jeung, M.K., Park, M.H., Lee, S.Y., and Lee, J.H. 2010. Effects of roasting conditions of sesame seeds on the oxidative stability of pressed oil during thermal oxidation. Food Chemistry. 118: 681–685.
Lee, K.Y., Rahman, M.S., Kim, A.N., Kim, H.J., Son, Y., Gu, S., Lee, M.H., Kim, J.I., Ha, T.J., Kwak, D., et al. 2020. Effect of freeze-thaw pretreatment on yield and quality of perilla seed oil. LWT - Food Science and Technology. 122: 1–7.
Lee, J.H., Kim, M.J. and Jung, M.Y. 2018. Seed oil (sesame seed, perilla seed). In K.Y. Park, D.Y. Kwon, K.W. Lee, and S. Park (Eds.), Korean functional foods: composition, processing and health benefits (chapter 12). Taylor & Francis Group LLC.
Lee, K.Y., Rahman, M.S., Kim, A.N., Jeong, E.J., Kim, B.G., Lee, M.H., Kim, H.J., and Choi, S.G. 2021. Effect of superheated steam treatment on yield, physicochemical properties and volatile profiles of perilla seed oil. LWT - Food Science and Technology. 135: 1–8.
López, G., Flores, I., Gálvez, A., Quirasco, M., and Farrés, A. 2003. Development of a liquid nutritional supplement using a Sesamum indicum L. protein isolate. LWT-Food Science and. Technology. 36: 67–74.
Meng, L., Lozano, Y. F., Gaydou, E. M., and Li, B. 2009. Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules. 14: 133–140.
Meng, Z., Liu, H., Zhang, J., Zheng, Z., Wang, Z., Zhang, L., Jia, Z. and Sui, Y. 2021. Sesamin promotes apoptosis and pyroptosis via autophagy to enhance antitumour effects on murine T-cell lymphoma. Journal of Pharmacological Sciences. 147: 260–270.
Mondal, N., Bhat, K.V. and Srivastava, P.S. 2010. Variation in fatty acid composition in Indian germplasm of sesame. Journal of the American Oil Chemists' Society. 87: 1263–1269.
Morris, J.B., Wang, M.L. and Tonnis, B.D. 2021. Variability for oil, protein, lignan, tocopherol, and fatty acid concentrations in eight sesame (Sesamum indicum L.) genotypes. Industrial Crops & Products. 164: 1–12.
Nookabkaew, S., Rangkadilok, N., and Satayavivad, J. 2006. Determination of trace elements in herbal tea products and their infusions consumed in Thailand. Journal of Agricultural and Food Chemistry. 54: 6939–6944.
Nzikou, J.M., Matos, L., Bouanga-Kalou, G., Ndangui, C.B., Pambou-Tobi, N.P.G., Kimbonguila, A., Silou, T., Linder, M., and Desobry, S. 2009. Chemical composition on the seeds and oil of sesame (Sesamum indicum L.) grown in Congo-Brazzaville. Advance Journal of Food Science and Technology. 1: 6–11.
Othman, S.B., Katsuno, N., Kanamaru, Y., and Yabe, T. 2015. Water-soluble extracts from defatted sesame seed flour show antioxidant activity in vitro. Food Chemistry. 175: 306–314.
Park, K.Y., Kwon, D.Y., Lee, K.W., and Park, S. 2018. Korean functional foods: Composition, processing and health benefits in Functional foods and nutraceuticals series. London, England: Taylor & Francis Group, CRC Press
Pestana-Bauer, V., Zambiazi, R., Mendoca, C., Beneito-Cambra, M., and Ramis-Ramos, G. 2012. Gamma-oryzanol and tocopherol contents in residues of rice bran oil refining. Food Chemistry. 134: 1479–1483.
Rangkadilok, N., Pholphana, N., Mahidol, C., Wongyai, W., Saengsooksree, K., Nookabkaew, S., and Satayavivad, J. 2010. Variation of sesamin, sesamolin and tocopherols in sesame (Sesamum indicum L.) seeds and oil products in Thailand. Food Chemistry. 122: 724–730.
Rizki, H., Kzaiber, F., Elharfi, M., Nablousi, A., Hanine, H. 2014. Chemical composition and morphological markers of 35 cultivars of sesame (Sesamum indicum L.) from different areas in Morocco. International Journal of Science and Research. 3: 2306–2311.
Sargi S.C., Silva, B.C., Santos, H.M.C., Montanher, P.F., Boeing, J.S., Santos Júnior, O.O., Souza, N.E., and Visentainer, J.V. 2013. Antioxidant capacity and chemical composition in seeds rich in omega-3: chia, flax, and perilla. Ciência e Tecnologia de Alimentos. 33: 541–548.
Sirilun, S., Sivamaruthi, B.S., Pengkumsri, N., Saelee, M., Chaiyasut,K., Tuntisuwanno, N., Suttajit, M., Peerajan, S., and Chaiyasut, C. 2016. Impact of different pre-treatment strategies on the quality of fatty acid composition, tocols content and metabolic syndrome related activities of Perilla frutescens seed oil. Journal of Applied Pharmaceutical Science. 6: 1–8.
Tenyang, N., Ponka, R., Tiencheu, B., Djikeng, F.T., Azmeera, T., Karuna, M.S.L., Prasad, R.B.N., and Womeni, H.M. 2017. Effects of boiling and roasting on proximate composition, lipid oxidation, fatty acid profile and mineral content of two sesame varieties commercialized and consumed in far-north region of Cameroon. Food Chemistry. 221: 1308–1316.
Torria, L., Bondiolib, P., Folegattib, L., Rovellinib, P., Piochia, M., and Morinia, G. 2019. Development of Perilla seed oil and extra virgin olive oil blends for nutritional, oxidative stability and consumer acceptance improvements. Food Chemistry. 286:584–591.
Vujasinovic, V., Djilas, S., Dimic, E., Basic, Z., and Radocaj, O. 2012. The effect of roasting on the chemical composition and oxidative stability of pumpkin oil. European Journal of Lipid Science and Technology. 114: 568–574.
Wang, M., Liu, P., Kong, L., Xu, N., and Lei, H. 2021. Promotive effects of sesamin on proliferation and adhesion of intestinal probiotics and its mechanism of action. Food and Chemical Toxicology. 149: 1–7.
Wąsowicz, E., Gramza, A., Hęś, M., Jeleń, H.H., Korczak, J., Małecka, M., Mildner-Szkudlarz, S., Rudzińska, M., Samotyja, U., and Zawirska-Wojtasiak, R. 2004. Oxidation of lipids in food. Polish Journal of Food Nutrition Science. 13: 87–100.
Williamson, K.S., Morris, J.B., Pye, Q.N., Kamat, C.D., and Hensley, K. 2008. A survey of sesamin and composition of tocopherol variability from seeds of eleven diverse sesame (Sesamum indicum L.) genotypes using HPLC-PAD-ECD. Phytochemical Analysis. 19: 311–322.
Wu, W.H. 2007. The contents of lignans in commercial sesame oils of Taiwan and their changes during heating. Food Chemistry. 104: 341–344.
Yang, R., Zhang, L., Li, P., Yu, L., Mao, J., Wang, X., and Zhang, Q. 2018. A review of chemical composition and nutritional properties of minor vegetable oils in China. Trends in Food Science & Technology. 74: 26–32.
Yokota, T., Matsuzak, Y., Koyama, M., Hitomi, T., Kawanaka, M., Enoki Kochini, M., and Okuyama, Y. 2007. Sesamin, a lignan of sesame, down regulates cyclin DL protein expression in human tumor cells. Cancer Science. 98: 1447–1453.
Žekonis, G., Zekonis, J., Sadzeviciene, R., Simoniene, G., and Kevelaitis, E. 2008. Effect of Perilla frutescens aqueous extract on free radical production by human neutrophil leukocytes. Medicina Kaunas. 44: 699–705.
Zhao, T.T., Hong, S.I., Lee, J., Lee, J.S., and Kim, I.H. 2012. Impact of roasting on the chemical composition and oxidative stability of perilla oil. Journal of Food Science. 77: C1273–1278.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Arunee Apichartsrangkoon1, *, Autchara Tiampakdee1, Warangkana Tameeya1, Danchai Kreungngern2, Siriwan Srisajjalertwaja3, and Wissanee Supraditareporn4
1 Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand
2 Faculty of Science and Technology, Kamphaeng Phet Rajabhat University, Kamphaeng Phet 62000, Thailand
3 Faculty of Science and, Chiang Mai Rajabhat University, Chiang Mai 50300, Thailand
4 Faculty of Agricultural technology, Chiang Mai Rajabhat University, Chiang Mai 50300, Thailand
Corresponding author: Arunee Apichartsrangkoon, E-mail: arunee.a@cmu.ac.th
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: October 16, 2021;
Revised: January 29, 2022;
Accepted: February 2, 2022;
Published online: February 15, 2022

