
Incidence of Death from Stroke and Brains of Stroke Victim Cadavers in Northern Thailand
Pornnarez Thaweekhotr, Chanida Thongsopha, Surut Tanprawate, Paiwan Sudwan, Kanokkan Bumroongkit, Kewalee Seeharach, Chatchadaporn Jaiyen, and Ranida Quiggins*Published Date : 2022-03-31
DOI : https://doi.org/10.12982/CMUJNS.2022.028
Journal Issues : Number 2, April-June 2022
Abstract Stroke is a leading cause of death in Thailand. However, there are varying numbers of regional stroke patients. This study aims to report the ranking of stroke as the cause of death in Northern Thai cadavers and to investigate the brains of stroke victim cadavers. A retrograde study was used to obtain data about cadavers and gross examinations were performed on the brains of the stroke victim cadavers housed in the Department of Anatomy, Faculty of Medicine, Chiang Mai University in the years 2019-2020. The results showed that death from stroke among the cadavers in the Northern Thailand increased during 2016-2018. Stroke was ranked as the fourth and fifth leading cause of death. The stroke victims were primarily aged over 60 years. The incidence of stroke in males was significantly higher than that of females. Approximately 92.7% of total stroke brains were the intracerebral hemorrhagic type stroke. About 41.7% of the intracerebral hemorrhagic brains had occurred with intraventricular hemorrhage and 16.7% of those occurred with subarachnoid hemorrhage. The hemorrhagic sizes varied from 5.66 to 260.43 ml. The territories of the cortical branches of the middle cerebral artery were the most common hemorrhages, making up 22.9% of total vascular territories. In conclusion, stroke was the fourth and fifth leading cause of death in the self-donated population in the Northern Thailand. Intracerebral hemorrhagic stroke was the main cause the death. Additionally,
the vascular territories of the stroke might be related to the likelihood of death.
Keywords: Stroke, Cerebrovascular disease, Cadavers, Intracerebral hemorrhage, Northern Thailand
Funding: This study was funded by the Faculty of Medicine Research Fund, grant no. 046/2563, Chiang Mai University, Chiang Mai, Thailand.
Citation: Thaweekhotr, P., Thongsopha, C., Tanprawate, S., Sudwan, P., Bumroongkit, K., Seeharach, K., Jaiyen, C., and Quiggins, R. 2022. Incidence of death from stroke and brains of stroke victim cadavers in Northern Thailand. CMU J. Nat. Sci. 21(2): e2022028.
INTRODUCTION
Stroke is a non-communicable disease. It is defined as “a rapid development of local, or at times, global disturbances of cerebral function lasting 24 hours, or leading to death with no apparent cause other than that of vascular origin” (Hatano, 1976). The causes of death from cerebrovascular disease (CVD) are coded as I60-I69 which is based on the International Classification of Diseases, 10th version system, (ICD-10) (Thrift et al., 2017). Stroke is considered to be the second leading cause of death around the world including South East Asia (WHO, 2001, 2008, 2017, 2018, Naghavi et al., 2015). In Thailand, stroke is the leading cause of death and the second cause of death in Thai males (Pongvarin, 2007). According to a report obtained from the World Health Organization, during 2000-2010, stroke is the third leading cause of death in Thailand after lower respiratory infection and ischemic heart disease (WHO, 2014). During 2015-2016, stroke became the second leading cause of death of Thai people after ischemic heart disease (WHO, 2018). The ratio of male to female was 1.2: 1. The trend for men's deaths will likely continue to increase. People over 70 years of age was the highest in deaths for both sexes. The number of Thai people who died from hemorrhagic stroke was higher than from ischemic stroke (WHO, 2016, 2018). Hemorrhagic stroke results from a weakness of the vessel wall and abnormal high blood pressure that consequently causes the blood vessels to rupture (Pansky and Allen, 1980). Whenever blood rushes into the brain parenchyma it is called an intracerebral hemorrhage. When it rushes into the cerebrospinal fluid in the subarachnoid space it is a subarachnoid hemorrhage (Aguilar and Freeman, 2010). The cerebrum is supplied by three cerebral arteries: the anterior, middle, and posterior cerebral arteries. The cortical branches of the cerebral arteries supply the main part of the cerebral lobes while their perforating branches supply the deep structures of the cerebral hemispheres (Chanda et al., 2017). In 2008, Smithuis described the hemorrhagic lesion as the vascular territories of the cortical branch or the perforating branches of the cerebral artery (Smithuis, 2008). The highest prevalence of stroke patients in Thailand was found in Bangkok, followed by the Central, Southern, Northern, and Northeastern regions, respectively (Hanchaiphiboolkul et al., 2011; Kongbunkiat et al., 2015). The stroke patients were more likely to be male than female, and were mostly over 60 years of age (Areechokchai et al., 2017). Even though hemorrhagic stroke was found to have occurred less than ischemic strokes, it had caused more deaths than ischemic stroke (Guzik and Bushsnell, 2017). We would like to know whether the incidence of death from stroke of the Northern Thai cadavers is the same as the national figures. This research aims to determine the incidences of death caused by stroke using cadavers housed in the Department of Anatomy, Faculty of Medicine, Chiang Mai University and to analyze the brains of stroke victims by investigating the type of stroke and the vascular territories of the hemorrhagic brains affected by stroke.
MATERIAL AND METHODS
A retrospective study of the cadaveric data
We retrospectively studied the cadaveric data from the central cadaveric data obtained from the Department of Anatomy, Faculty of Medicine, Chiang Mai University, Thailand. The Department of Anatomy receives cadavers who have made self-donations and who have lived in Chiang Mai and other nearby provinces in Northern Thailand which are located less than 200 kilometers from the department. All cadavers were delivered to the department approximately 24-48 hours after death. The age, sex, and cause of death of cadavers received during 2016-2018 were analyzed. All cadavers were confirmed dead, and the cause of death was indicated by physicians in the hospitals. Ranking of the causes of death of cadavers included cerebrovascular disease (CVD). Trends of stroke victims during this period were then investigated.
Stroke mortem brain examination
A total of 13 stroke cadaveric brains during 2019-2020 were used in this study. After the brains were extracted from the skulls, they were fixed in a fixative solution containing 8% paraformaldehyde and 20% glycerin. The brains were fixed for one week, or until they were firm. All brains were sliced in a horizontal plane having a 10 mm thickness by a ham slicer. All brain sections in each brain were photographed. The brains of stroke cadavers were investigated for type of stroke to determine whether it was a hemorrhagic or ischemic stroke. The cerebral vascular territories of the hemorrhagic lesion were also evaluated as being either the cortical branch or perforating branches of the cerebral artery. The lesion volumes were calculated by using an image J program. The total hemorrhagic volume was collected from all hemorrhagic sections.
Statistical analysis
The incidence of the death caused by CVD during three years including 2016-2018, was reported as a percentage of stroke death in the sampled population, and was used to establish a ranking of death caused by CVD. The incidence of the specific vascular territory of the hemorrhagic area was also reported as a percentage of all territories. The Chi-squared test was used to compare between two groups. The Wilcoxon signed ranks test was used to compare the hemorrhagic volume between that the left and right cerebral hemispheres. The values of P < 0.05 was considered statistically significant.
Ethics
This study was approved by the Ethical Review Committee for Research in Human Subjects, Faculty of Medicine, Chiang Mai University (CMU) (No. Exemption-6589/2562).
RESULTS
Causes of death in the cadavers housed in the Department of Anatomy, Faculty of Medicine, Chiang Mai University.
The number of cadavers in 2016, 2017, and 2018 were 486, 421, and 527, respectively. Causes of death in cadavers housed in the Department of Anatomy of each year during 2016-2018 were ranked as shown in Figure 1. Neoplasm was the most common cause of death in these cadavers. The deaths from CVD ranked fifth after neoplasm, respiratory disease, sepsis, and heart disease in 2016; were ranked fourth in 2017, then returned to a fifth ranking in 2018. The trend of percentage of stroke cadavers during 2016-2018 was slightly increased as shown in the Figure 2.
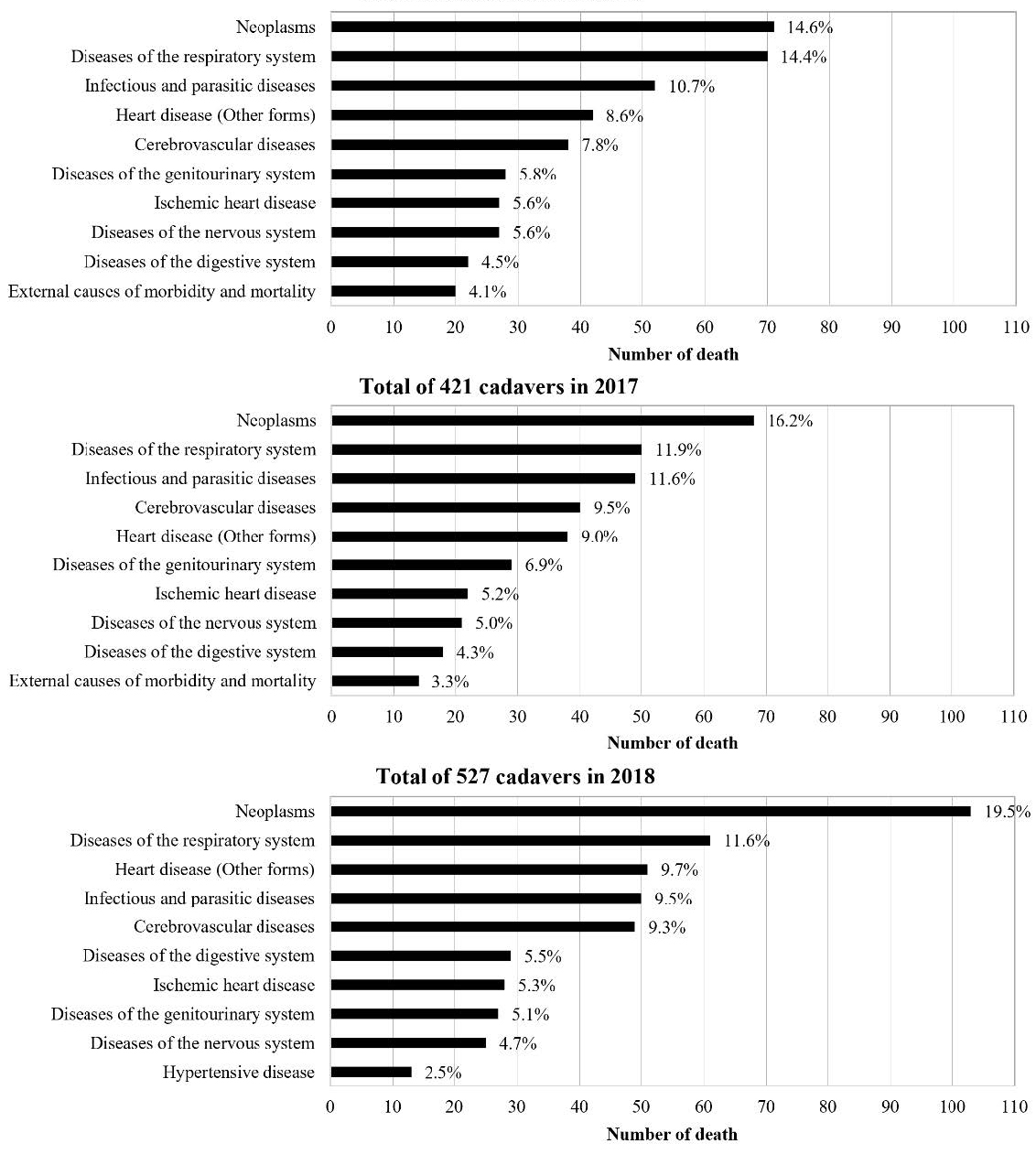
Figure 1. The top ten causes of death classified by ICD-10 in cadavers housed in the Department of Anatomy, Faculty of Medicine, Chiang Mai University in 2016-2018.
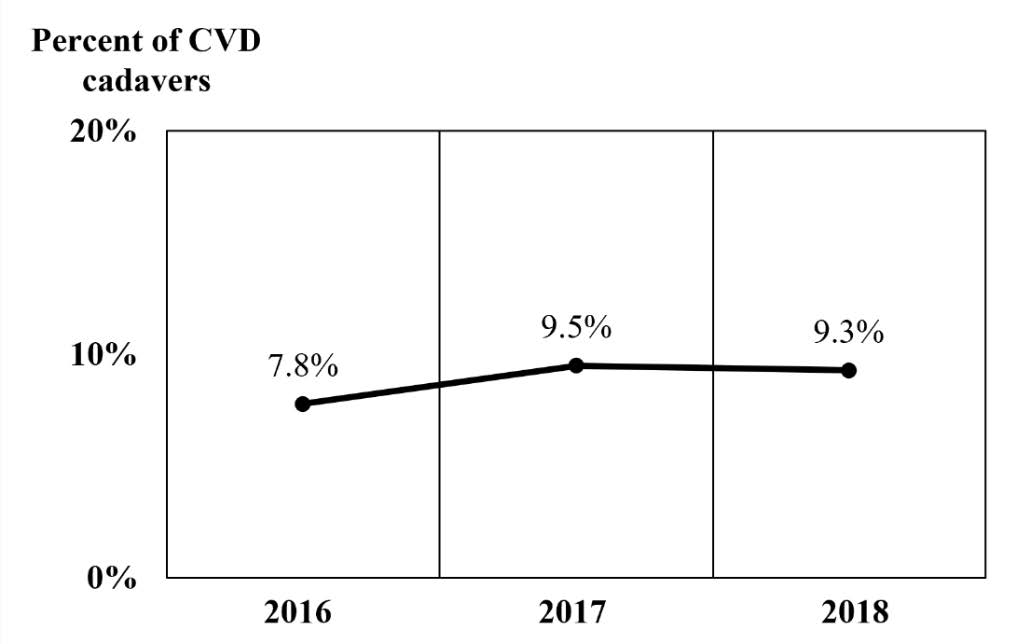
Figure 2. The trend of CVD cadavers housed in the Department of Anatomy, Faculty of Medicine, Chiang Mai University in 2016-2018.
The data of the CVD cadavers over three years were retrogradely analyzed in terms of gender and age as shown in Figure 3. The youngest CVD cadavers was 22 while the oldest one was 94 years old. The main population of stroke cadavers were in the 60-69 age group. Interestingly, the number of the male cadavers in the 60-69 and 70-79 age groups, were significantly higher than that of the females (P < 0.05).
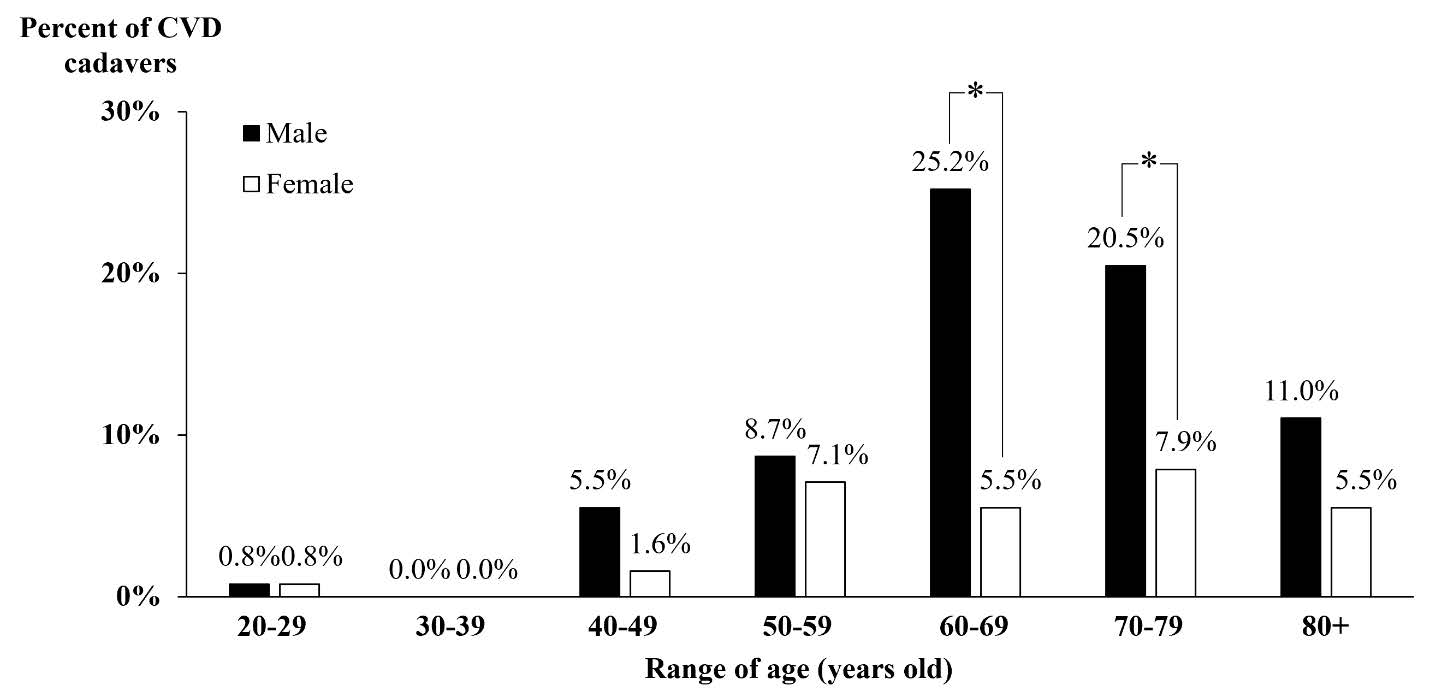
Figure 3. The population of the CVD cadavers in the Northern Thailand, classified by age and gender in 2016 - 2018. * P < 0.05, the male versus the female.
Brains of the stroke cadavers
A total of thirteen brains of stroke cadavers had been collected during 2019-2020. They were ten males and three females and were 38-79 years old (average 60.2 years). About 92% of the total brains (12 of 13) were found to be cerebral hemorrhage and the other 8% (1 of 13) was found to be a cerebral ischemia. All twelve cerebral hemorrhage brains were intracerebral hemorrhage. About 42% of the intracerebral hemorrhage (5 of 12; S620801, S620802, S621101, S630701, and S631101) had occurred with intraventricular hemorrhage (Figure 4-B), 17 % of the intracerebral hemorrhage (2 of 12; S621101 and S630302) had occurred with subarachnoid hemorrhage (Figure 4-A) and 8% (1 of 12; S630801) had occurred with subarachnoid and intraventricular hemorrhage. The intracerebral hemorrhagic lesions were obviously noticeable, and we were able to detect the outlines of lesions.

Figure 4. The horizontal sections of the intracerebral hemorrhagic brains. (A) The intracerebral hemorrhage occurred with a subarachnoid hemorrhage (arrows). (B) The intracerebral hemorrhage occurred with an intraventricular hemorrhage (arrow heads). The scale bar in B is 5 cm. and applies to panel A; Lt. and Rt. in A represent the left and the right hemispheres and apply to panel B.
The intracerebral hemorrhagic lesions are seen as the dark areas containing blood or hematoma in the parenchymal brain tissue. Fifty percent of all hemorrhagic brains (6 of 12) were found to be hemorrhagic lesions occurring in both hemispheres, and 33.3% (4 of 12) were found to have occurred in the right hemisphere and 16.7 % (2 of 12) in the left (Figure 5). The hemorrhagic lesions in the right cerebral hemisphere were found to be 1.25 times than that in the left. The hemorrhagic lesions were also found in the left cerebellar, the pons, and the midbrain.
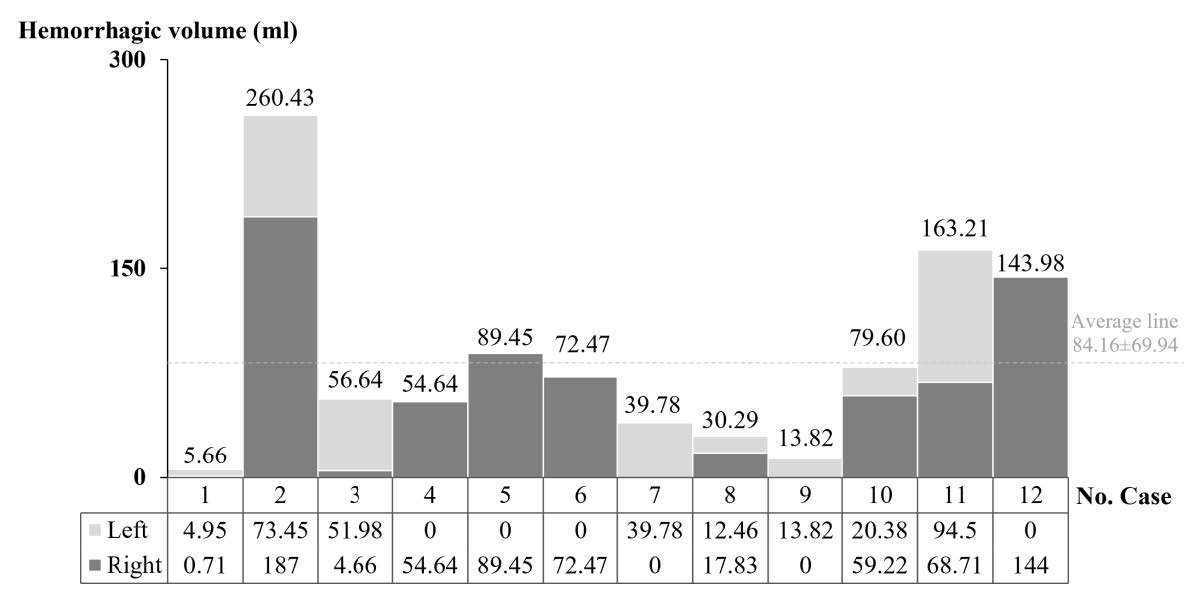
Figure 5. The hemorrhagic volume in all twelve brains. The hemorrhagic lesions were occurred in both hemisphere (No. 1; S620801, 2; S620802, 3; S620901, 8; S630303, 10; S630701 and 11; S630801), in the left (No. 7; S630302 and 9; S63002) and the right hemisphere (No. 4; S621901, 5; S621101, 6; S630301 and 12; S631107).
The total hemorrhagic volume in each brain varied from 5.66 to 260.43 ml, with the average being 84.16 ml as shown in Figure 5. After comparing the average hemorrhagic volume between each hemisphere, the volume in the right hemisphere (69.9 ± 56.3 ml) was found to be not significantly larger than that in the left (38.9 ± 30.2 ml) as shown in Figure 6. These various size hemorrhagic lesions were found in several structures including the cerebral lobes, basal ganglia, internal capsule, thalamus, cerebellum, and brain stem. The effected structures were vascular territories of the cortical and the perforating branches of cerebral arteries, and other vessels.
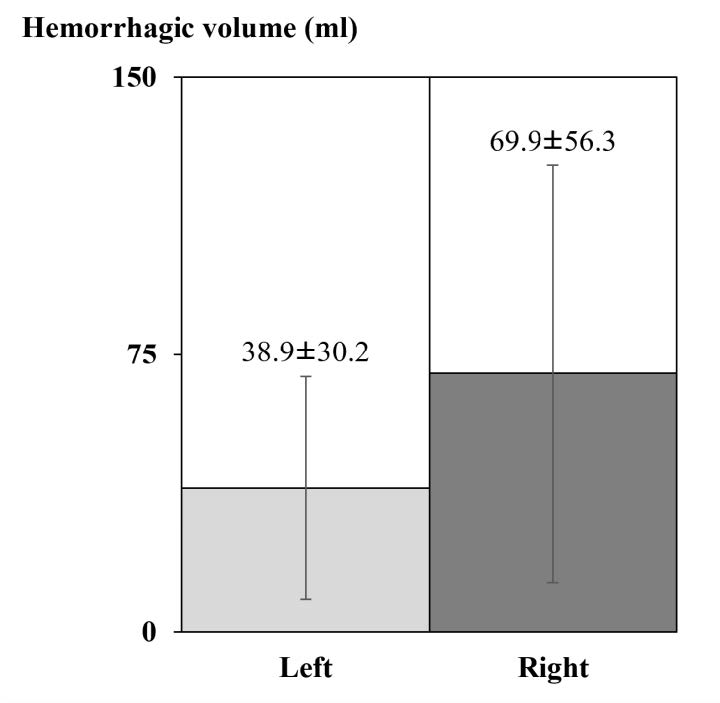
Figure 6. Comparing the hemorrhagic volume occurred in the left (n= 8) and the right (n=10) hemispheres.
Figure 7-A demonstrates the territories of cortical branches of the anterior (ACA), middle (MCA), and posterior (PCA) cerebral arteries which are in the medial part of the frontal lobe (yellow), the lateral part of cerebral hemisphere (red), and occipital pole (blue), respectively. The territories of the lateral lenticulostriate arteries (lLSA), a deep perforating branch of the MCA, are the lateral part of the lenticular nucleus, most of the caudate nucleus, and the anterior internal capsule (purple). The territory of the anterior choroidal artery (AChA) is the posterior limbs of the internal capsule (pink). The thalamus (blue) is supplied by the perforating branches of PCA. Figure 7-B shows a large hemorrhagic lesion in the lateral part of the right cerebral hemisphere which is in the territory of cortical branches of the right MCA. The hematoma in the medial part of left frontal lobe (Figure 7-C) and in the left cerebellum (Figure 6-D) are in the territories of the cortical branches of ACA and of the posterior inferior cerebellar artery (PICA), respectively.
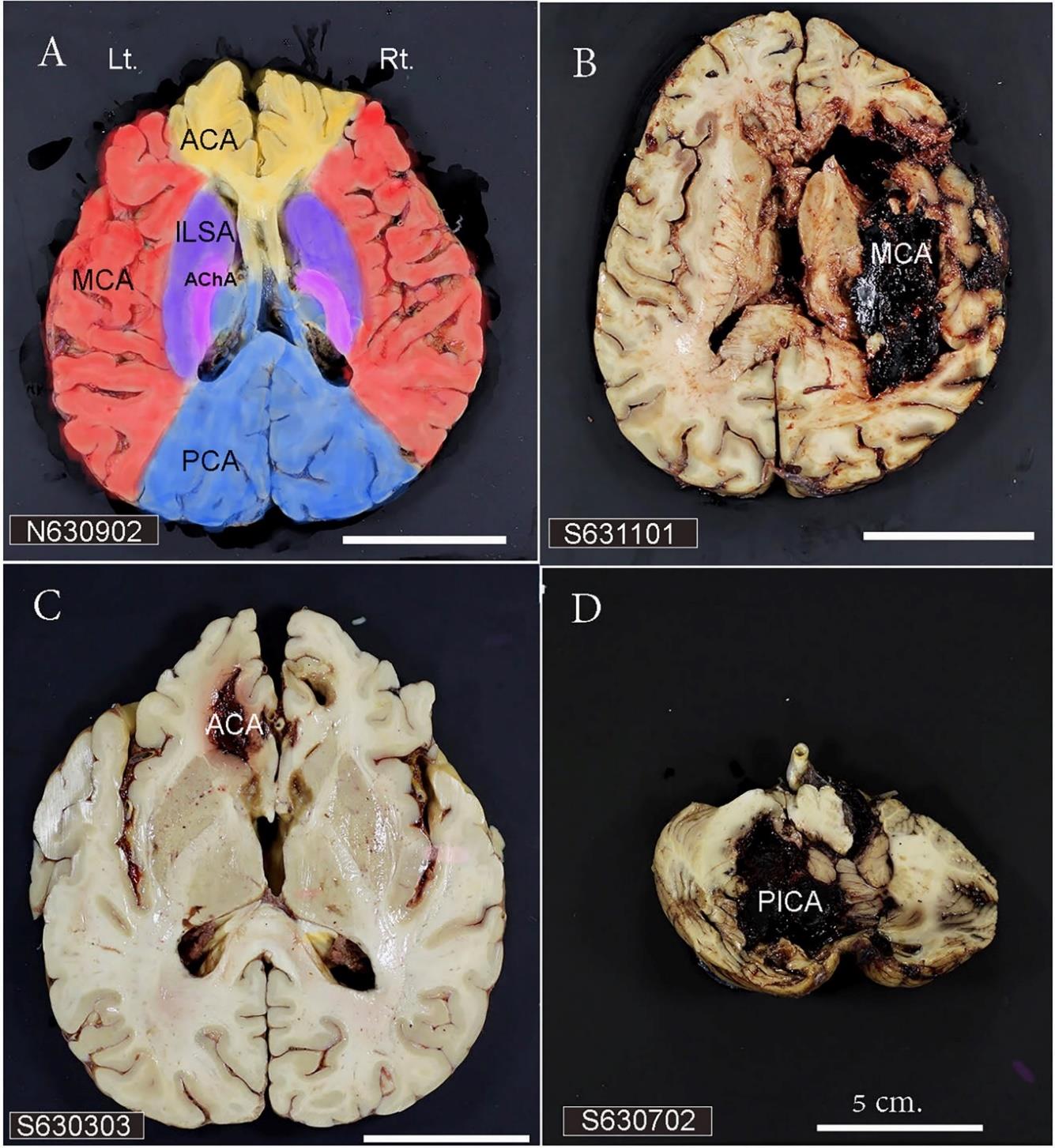
Figure 7. A) The vascular territories of the cortical and perforating branches of the cerebral arteries. B) The intracerebral hemorrhagic brain with the lesion supplied by the cortical branches of MCA. C) The hemorrhagic brain with the lesion supplied by the cortical branches of ACA. D) The hemorrhagic cerebellum with the lesion supplied by the PICA. The scale bar in D is 5 cm. and applies to all panels. Lt. and Rt. represent the left and the right hemispheres and apply to all panels. ACA= anterior cerebral artery; AChA= anterior choroidal artery; lLSA = lateral lenticulostriate artery; MCA = middle cerebral artery; PCA = posterior cerebral artery; PICA = posterior inferior cerebellar artery.
The vascular territories containing the hemorrhagic structures were analyzed and are shown in Figure 8. Among the territories of the cortical branches of cerebral arteries, those of the middle cerebral artery (MCA) had a higher incidence of intracerebral hemorrhage, being 22.9%. This was more than that of PCA and ACA, with those being 17.1% and 11.4%, respectively. All hemorrhagic areas found within the territory of cortical branch of MCA were in the lateral part of cerebral hemisphere. Most of the hemorrhagic areas found within those of PCA were mainly in the inferior medial part of temporal lobe (53.8%) and those of ACA were mainly in the genu of corpus callosum (70%).
The vascular territories of the perforating branch of the PCA, the lateral lenticulostriate artery (lLSA), and the medial lenticulostriate artery (mLSA) showed the same incidence of the intracerebral hemorrhage, that being 8.6%, as showed in Figure 8. The hemorrhagic structures found within the vascular territories of the perforating branch of PCA, lLSA, and mLSA were the thalamus, the lateral part of basal ganglia, and the anteromedial part of head of caudate nucleus, respectively (Figure 8).
The hemorrhage within the vascular territories of AChA, BA, and PICA were 5.7% of all hemorrhagic territories (Figure 8).
These hemorrhagic lesions were found in the posterior limb of internal capsule, hippocampus, amygdala, pons, and inferior occipital surface of cerebellum, respectively.
The hemorrhagic lesions within the territories of VA and SCA were found less than 5% of the total territories.
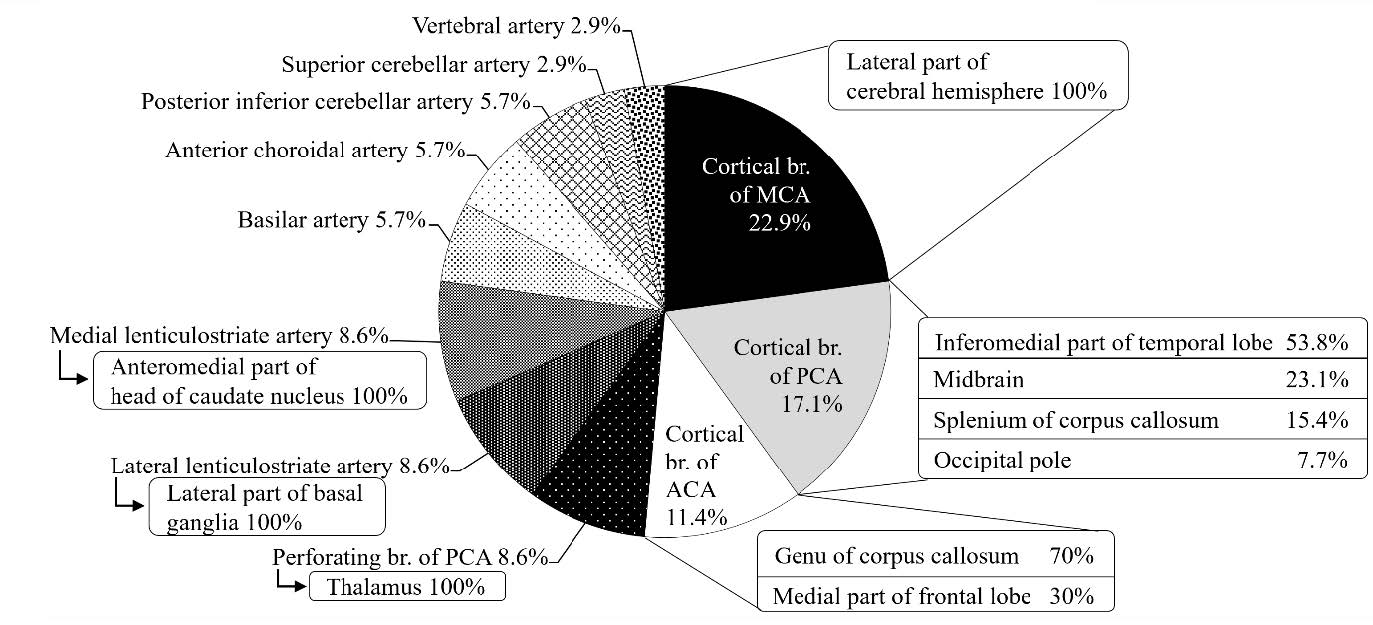
Figure 8. The percentage of vascular territories involving intracerebral hemorrhage. ACA = anterior cerebral artery; MCA = middle cerebral artery; PCA = posterior cerebral artery.
DISCUSSION
The causes of death of cadavers housed in the Department of Anatomy, Faculty of Medicine, Chiang Mai University were determined by a retrograde study. The cadavers were collected from several provinces in Northern Thailand which could be considered as an appropriate sampling of the population of the entire Northern Region of Thailand. We found that stroke or cerebrovascular disease was the fourth and fifth leading causes of death in the Northern Thai cadavers. These results were different from WHO reporting stroke as the third leading cause of death in Thailand after lower respiratory infection and ischemic heart disease (WHO, 2014; 2016; 2018). In addition to those two leading causes, our results showed that neoplasm and infectious and parasitic diseases caused more deaths of cadavers in the Northern Thailand Region than cerebrovascular disease. A previous study also reported that the prevalence of stroke patients in the Northern Region was less than those other regions of Thailand (Pansky and Allen, 1980). It is likely that death by stroke in Northern Thailand does not equal that of the rest of Thailand in terms of its ranking as a leading cause of death in that region.
In this study, the stroke cadavers were found mainly in the ages over 60. The findings of this study were different from previous studies done on the global population which showed the highest numbers of stroke victims being over 70 years of age (WHO, 2018; Murray and Lopez, 1994). However, the number of male stroke cadavers were found to be higher than the female of all rankings of ages in both this study and previous studies. This study demonstrated that the younger age, 60-69 years, had a high tendency to have stoke. High blood pressure was known as the main risk factor of the intracerebral hemorrhage (Steinke et al., 1992). High blood pressure might be developed at a younger age in the Northern Thailand Region.
In this study, the death caused by hemorrhagic stroke was 92.3% percent. This data was consistent with previous studies which reported that hemorrhagic stroke caused more mortality than ischemic stroke (WHO, 2016; 2018). All the hemorrhagic stroke cadavers were intracerebral hemorrhage. This study found that the intracerebral hemorrhagic brains had intraventricular hemorrhaging, being 41.7%, and subarachnoid hemorrhaging, being 16.7%. The intraventricular and subarachnoid hemorrhages might have extended from the primary intracerebral hemorrhage. Our study showed that volume of intracerebral hemorrhage varied from 5.66-260.43 ml. The smallest hemorrhagic volume (in case no. 1; S620801) was composed of hemorrhages in the thalamus and at the head of the caudate nucleus. Intraventricular hemorrhaging was also found in this case. Our findings were consistent with a previous one which reported that the intrathalamic hemorrhage having a 3.3 cm diameter could cause intraventricular hemorrhaging and death (Steinke et al., 1992). Previous studies also reported that extended intraventricular and subarachnoid hemorrhages could lead to death or poor outcomes (Nieuwkam et al., 2000; Nogles and Galuska, 2021).
In this study, the territory of cortical branches of MCA was the most common area affected by intracerebral hemorrhage. The previous studies also reported that MCA stroke was found to be the most likely type leading to mortality compared to ACA and PCA strokes (Nogles and Galuska, 2021; Casano et al., 2021; Kuybu et al., 2021). The characteristics of stroked brains and which blood vessels were affected had been indicated by histopathologists (Simon and Allison, 2019). However, the characteristics of the main cerebral arteries leading to the hemorrhagic lesions led us to further investigate.
CONCLUSION
Stroke was in the fourth and fifth leading cause of death in the self-donated population in the Northern Thailand Region. It ranked lower than it does in the entire Thai national population statistics where it is ranked second. The cadavers who had died from stroke were primarily males with ages of 60-69 years. Intracerebral hemorrhage was the main cause stroke related death. The intracerebral hemorrhagic volume varied and was not different between that which took place in each hemisphere. Intracerebral hemorrhage with extended intraventricular hemorrhage causes more deaths than without any extended hemorrhage. The territory of the cortical branches of MCA or the lateral part of cerebral hemisphere was the region related to the likelihood of death.
ACKNOWLEDGEMENTS
The authors would like to thank the Faculty of Medicine, Chiang Mai University for grant support and the Neuromuscular Laboratory, Faculty of Medicine, Chiang Mai University for the cryostat sectioning.
REFERENCES
Aguilar, M.I. and Freeman, W.D. 2010. Spontaneous intracerebral hemorrhage. Seminars in Neurology. 30: 555-564.
Areechokchai, D., Vijitsoonthornkul, K., Pongpan, S., and Maeakhian, S. 2017. Population Attributable fraction of stroke risk factors in Thailand: Utilization of non-communicable disease surveillance systems. OSIR. 10: 1-6.
Casano, M.H.A., Tadi, P., and Ciofoaia, G.A. 2021. Anterior cerebral artery stroke. Treasure Island (FL): StatPearls Publishing; [updated 2021 July 21; accessed 2021 June 30]. https://www.ncbi.nlm.nih.gov/books/NBK537333/.
Chandra, A., Li, W., Stone, C., Geng, X., and Ding, Y. 2017. The cerebral circulation and cerebrovascular disease I: Anatomy. Brain Circulation. 3: 45-56.
Guzik, A. and Bushnell, C. 2017. Stroke epidemiology and risk factor management. Continuum (Minneap Minn). 23: 15-39.
Hanchaiphiboolkul, S., Poungvarin, N., Nidhinandana, S., Suwanwela, N.C., Puthkhao, P., Towanabut, S., Tantirittisak, T., Suwantamee, J., and Samsen, M. 2011. Prevalence of stroke and stroke risk factors in Thailand: Thai Epidemiologic Stroke (TES) Study. Journal of the Medical Association of Thailand. 94: 427-436.
Hatano, S. 1976. Experience from a multicentre stroke register: A preliminary report. Bull World Health Organ. 54: 541-553.
Kongbunkiat, K., Kasemsap, N., Thepsuthammarat, K., Tiamkao, S., and Sawanyawisuth, K. 2015. National data on stroke outcomes in Thailand. Journal of Clinical Neuroscience. 22: 493-497.
Kuybu, O., Tadi, P., and Dossani, R.H. 2021. Posterior cerebral artery stroke. Treasure Island (FL): StatPearls Publishing; [updated 2021 August 11; accessed 2021 June 30]. https://www.ncbi.nlm.nih.gov/books/NBK532296/.
Murray, C.J.L., and Lopez, A.D. 1994. Global and regional cause-of-death patterns in 1990. Bull World Health Organ. 72: 447-480.
Naghavi, M., Wang, H., Lozano, R., Davis, A., Liang, X., Zhou, M., Vollset, S.E., Ozgoren, A.A., Abdalla, S., Abd-Allah, F., et al. 2015. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 385: 117-171.
Nieuwkamp, D.J., de Gans, K., Rinkel, G.J.E., and Algra, A. 2000. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. Journal of Neurology. 247: 117-121.
Nogles,T.E., and Galuska, M.A. 2021. Middle cerebral artery stroke. Treasure Island (FL): StatPearls Publishing; [updated 2021 August 13; accessed 2021 June 30]. https://www.ncbi.nlm.nih.gov/books/NBK556132/.
Pansky, B., and Allen, D.J. 1980. Review of Neuroscience. New York: Macmillan.
Pongvarin, N. 2007. Burden of Stroke in Thailand. International Journal of Stroke. 2: 127-128.
Simon, K., and Allinson, J. 2019. Deaths related to stroke and cerebrovascular disease. Diagnostic Histopathology. 25: 444-452.
Smithuis, R. 2008. Vascular territories. Leiderdorp (NL): Radiology department of the Alrijne Hospital; [accessed 2021 May 21]. https://radiologyassistant.nl/neuroradiology/brain-ischemia/vascular-territories.
Steinke, W.S., Sacco, R.L., Mohr, J.P., Foulkes, M.A., Tatemichi, T.K., Wolf, P.A., Price, T.R., and Hier, D.B. 1992. Thalamic stroke: presentation and prognosis of infarcts and hemorrhages. Archives of Neurology. 49: 703-710.
Thrift, A.G, Howard, G., Cadilhac, D.A., Howard, V.J., Rothwell, P.M, Thayabaranatha T, Feigin, V.L., Norrving, B., and Donnan, G.A. 2017. Global stroke statistics: An update of mortality data from contries using a broad code of "cerebrovascular diseases". International Journal of Stroke. 12: 796-801.
World Health Organization. 2001. The Global Burden of Disease: 2000 project: aims, methods and data sources [publication data]. Geneva (CH): WHO. [updated 2001; accessed 2021 November 10]. https://www.who.int/healthinfo/paper36.pdf.
World Health Organization. 2008. The Global Burden of Disease: 2004 update [publication data]. Geneva (CH): WHO. [updated 2008; accessed 2021 November 10]. https://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf.
World Health Organization. 2014. Global health estimates 2014 summary tables [dataset]. Geneva (CH): WHO; [updated 2014; accessed 2019 June 29]. https://www.who.int/healthinfo/global_burden_disease/GHE_Deaths_2000_country.xls.
World Health Organization. 2016 Global health estimates 2016 summary tables [dataset]. Geneva (CH): WHO; [updated 2016 December; accessed 2019 June 29]. https://www.who.int/healthinfo/global_burden_disease/GHE2015_Deaths-2005-country.xls.
World Health Organization. 2017 Global health estimates 2016 summary tables [dataset]. Geneva (CH): WHO; [updated 2017 March; accessed 2019 June 30]. https://www.who.int/healthinfo/global_burden_disease/GHE2015_Deaths-2010-country.xls.
World Health Organization. 2018. Global health estimates 2016 summary tables: deaths by cause, age, sex, by WHO region, 2000-2016 [dataset]. Geneva (CH): WHO; [updated 2018 April; accessed 2019 June 29]. https://www.who.int/healthinfo/global_burden_disease/GHE2016_Deaths_WHOReg_2000_2016.xls.
World Health Organization. 2018. Global health estimates 2016 summary tables [dataset]. Geneva (CH): WHO; [updated 2018 April; accessed 2021 November 11]. https://www.who.int/healthinfo/global_burden_disease/GHE2016_Deaths_2015-country.xls.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Pornnarez Thaweekhotr1, 2, 3, Chanida Thongsopha1, Surut Tanprawate4, Paiwan Sudwan1, Kanokkan Bumroongkit1, Kewalee Seeharach1, Chatchadaporn Jaiyen1 , and Ranida Quiggins1,*
1 Department of Anatomy, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand.
2 Graduate School, Chiang Mai University, Chiang Mai 50200, Thailand.
3 School of Integrative Medicine, Mae Fah Luang University, Chiang Rai 57100, Thailand.
4 Department of Medicine, Faculty of Medicine, Maharaj Nakorn Chiang Mai Hospital, Muang, Chiang Mai 50200, Thailand.
Corresponding author: Ranida Quiggins, E-mail: ranida.quiggins@cmu.ac.th
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: August 12, 2021;
Revised: January 26, 2022;
Accepted: January 27, 2022;
Published online: February 14, 2022

