
A Review on Potential of Plant Growth Promoting Microorganisms in Silviculture of Neolamarckia cadamba to Industrial Timber Production Areas (ITPAs) in Malaysia
Alan Chua Yee Quan, Peter Morin Nissom and Tan Lee Tung*Published Date : 2022-03-31
DOI : https://doi.org/10.12982/CMUJNS.2022.027
Journal Issues : Number 2, April-June 2022
Abstract Sarawak had around 62% forest area coverage in 2018, contributed 40% of forest area in Malaysia. Two million hectares of licensed forest area was designated as Industrial Timber Production Areas (ITPAs) to sustain industrial timber production. Neolamarckia cadamba was one of the tropical indigenous timber species selected for the development of ITPAs. The selection is based on its local environment adaptation, pest and disease resistance, fast growing properties, soil maintenance contributions, its wood quality suitable for plywood industry. Tropical soils are infamous for poor plant available nutrients profile, contributed by high precipitation rate in tropical climate and acidic soil orders. Plant growth promoting microorganisms in wild environment would be a feasible solution for N. cadamba planting activities. Such studies on N. cadamba were limited whilst drafting this review paper. Nonetheless, potential strains, i.e Nitrogen fixing microorganisms, phosphate solubilising microorganisms and potassium solubilising microorganisms, were discussed. They could potentially provide three major plant available nutrients. For industrial production, their processing with solid, liquid or solid-liquid integrated carrier materials would provide long shelf-life, better survivability in the field and easy handling in different application strategies.
Keywords: Biofertilisers, Neolamarckia cadamba, plant growth promoting microorganisms, Malaysia, tropical soils
Funding: The authors are grateful for the research funding provided by the National Higher Education Science Research and Innovation Policy Council.
Citation: Chua, A.Y.Q., Nissom, P.M., and Tan, L.T. 2022. A Review on Potential of Plant Growth Promoting Microorganisms in Silviculture of Neolamarckia cadamba to Industrial Timber Production Areas (ITPAs) in Malaysia. CMU J. Nat. Sci. 21(2): e2022027.
INTRODUCTION
In Malaysia, the total forest area coverage was 68.1% in 1990 but declined to 58.5% in 2018. As such, about three-fifth or 19.3 million hectares of land was still under forest coverage in Malaysia (D’enghien, 2016; The World Bank, 2019). Sarawak, the largest state in Malaysia, plays an important role in maintaining the forest coverage. With a landmass of 12.4 million hectares, Sarawak had about 62% of the land covered by forest in 2018, contributed 7.72 million hectares or 40% of forest area in Malaysia. 70,863 hectares of these forest areas were industrial timber production areas (ITPAs), created by replanting Neolamarckia cadamba on 2,368,858 hectares of grossed license area with a rotation period of 13 years (Krisnawati et al., 2011; Sarawak Forest Department, 2021). Neolamarckia cadamba, tropical indigenous species, has advantages in local environment adaptation, pests and disease resistance (Vincent, 2002). For industrial aspects, it is an important lightweight hardwood source for Sarawak plywood industry with a production amount of 2.74 million m3 annually (Lissem, 2013).
Tropical soils are well known for their properties of high porosity, acidic and poor plant available nutrients (Igwe, 2011). Nevertheless, N. cadamba is widely found along the riverbank of secondary forests in Southeast Asia, including Sarawak. It prefers well-aerated and fertile soil conditions to support optimal growth (Krisnawati et al., 2011). To resolve the poor available nutrient properties of tropical soils, plant growth promoting microorganisms, associated to host rhizospheres, can acquire plant available nutrients from atmospheric nitrogen and mineral deposits to sustain growth of host plants (Vessey, 2003). These microorganisms are potential strains to mimic the wild environment in ITPAs. Hence, this review included (1) Introduction of Neolamarckia cadamba (2) tropical soil properties (3) interactions of plant growth promoting microorganisms and host plants, focusing on nitrogen fixing microorganisms, phosphate solubilising microorganisms, potassium solubilising microorganisms (4) Application of plant growth promoting microorganisms as biofertilisers, including their incorporation with carrier materials and reduced rate chemical fertilisers (5) concluding statement for the implications of plant growth promoting microorganisms to silviculture of N. cadamba in Malaysia.
INTRODUCTION TO NEOLAMARCKIA CADAMBA
Neolamarckia cadamba has a former botanical name called Anthocephalus cadamba Miq. It belongs to family Rubiaceae. In Malaysia, its common local names are Kelampayan, Laran and Selimpoh. This tropical and subtropical species is native to South Asia and Southeast Asia. It is known to have fast growth characteristic, preference in alluvial regions and resistance to serious pests and diseases. Therefore, N. cadamba is suitable for both reforestation and industrial plantations (Krisnawati et al., 2011). It is one of the selected indigenous species for Sarawak ITPAs.
Neolamarckia cadamba is a tropical pioneer species found in the deep and moist alluvial regions. They are also found growing along the riverbanks in the secondary forest, permanently and periodically flooded areas. It would not be unusual to find N. cadamba mother trees in the primary forests too. Although N. cadamba was reported growing on various types of soils, well-accelerated fertile soils would be preferred over the leached and poorly aerated soils (Krisnawati et al., 2011). The growth of N. cadamba is not only influenced by soil conditions but also the climate and landscape of its local environment. It is a light demanding species and sensitive to frost. The temperature in the natural habitat of N. cadamba ranges from 3°C to 42°C. Since it prefers moist soil environments, the annual rainfall should be in the range of 1500-5000 mm. However, some N. cadamba were reported to grow in regions with only 200 mm annual rainfall regions such as the central parts of South Sulawesi. N. cadamba usually grows at an altitude range of 300-800 m above the sea level (Krisnawati et al., 2011; Orwa et al., 2009). However, there are some exceptions that the trees were found to grow at 1,000 m above sea level in highlands of the equator region (Krisnawati et al., 2011). In South Asia region, N. cadamba is found in deep and moist deciduous forests of Maharashtra, India where can support the living requirements of N. cadamba (Maharashtra State Forest Department, [date unknown]). Sarawak would fulfil the living requirements of N. cadamba with its local tropical climate and landscapes, except for the tropical soils that are generally poor in plant nutrients.
Neolamarckia cadamba is a large tree with a broad umbrella-shaped crown and straight cylindrical stem (Figure 1). The branches are arranged in layers. It can grow to a height of 45 m with a trunk diameter of 100-160 cm. The bark appearance can differentiate the age of trees. Young trees have grey, smooth and very light bark while old trees have rough and longitudinal fissured bark (Krisnawati et al., 2011). The heartwood is white in colour with a yellow tinge darkening to creamy yellow on the exposure that cannot be clearly differentiated from sapwood. The wood is fine to medium in texture and low lustre (Figure 2). It has a density range of 290-560 kg/m3 at 15% moisture content (Krisnawati et al., 2011). The wood is moderately strong in strength and can be treated with preservatives easily. The treated wood is durable but its durability does not cause an issue in wood processing operations (Bijalwan et al., 2014). Neolamarckia cadamba is a lightweight hardwood could be an important timber supply in the plywood industry. Sarawak is the largest plywood producer in Malaysia with a production amount of 2.74 million m3 (Lissem, 2013).

Figure 1. The flowering Neolamarckia cadamba (Bijalwan et al., 2014).

Figure 2. The wood texture of Neolamarckia cadamba (Krisnawati et al., 2011).
Besides its lightweight hardwood timber, the other parts of N. cadamba could also be useful products. Its dried bark can be used to relieve fever, or to be consumed as a tonic (Orwa et al., 2009). The green, glossy leaves have the dimension sizes of 15-50 cm long by 8-25 cm wide (Krisnawati et al., 2011). Fresh leaves can be treated as fodder to feed cattle. Shedded leaves and non-leaf litters are good soil improver. The decomposition process improves soil physical and chemical properties of the canopy (Orwa et al., 2009). Neolamarckia cadamba has many fleshy fruitlets with four hollow or solid structures on each fruitlet upper parts. The fruitlets are small in size and the packed by fleshy capsules to form a fleshy yellow-orange infructescence. Each fruitlet may contain approximately 8000 seeds which are trigonal or irregular in shapes without wings attached (Krisnawati et al., 2011). The ripened fruitlets and inflorescences are edible (Orwa et al., 2009).
TROPICAL SOIL PROPERTIES
Soils are unconsolidated mineral material on the earth surface and a growing medium for various plants. Soils consist of mineral matters, organic matters, water, gases and organisms (Igwe, 2011). Soils are formed by the weathering process of rocks or materials deposited from water bodies and wind blows. The soil formation process is slow that forming a few centimetres of soil could cost thousands of years. The soil formation process is controlled by climate, organisms, parent material, topography and time. Hence, soil formation rates in different locations are variable due to these factors (Loganathan, 1987).
The tropical soils are mainly containing coarse particles because the fine particles like silt and clay would be removed by heavy rainfall and excessive leaching. Therefore, the soil textures are mostly sandy loam to sandy clay. Those soil textures are relatively low in silt and clay contents (Igwe, 2011). Silt and clay are responsible for the storage of plant nutrients because the plant nutrients in silt and clay are more resistant to leaching process by soil water infiltration. Meanwhile, sand is mainly responsible for soil water regulation and soil aeration. It has little effect on the retention of plant nutrients (Sheard, 1991). Hence, tropical soils do not have a large capacity to store plant nutrients due to lower silt and clay contents. When the organic matters are degraded by tropical climate, the tropical soils cannot retain the plant nutrients well and the rainwater would wash the plant nutrients away from soil particles easily (Loganathan, 1987). Hence, the sandy soil texture that is low in silt and clay is one of the main causes of low plant nutrients in the tropical soils.
Besides that, there are three acidic soil orders identified from tropical soils including Alfisols, Oxisols and Ultisols (Igwe, 2011). The pH of those three soil orders ranges from 4.0 to 5.5 (Schulte and Ruhiyat, 1998). In addition, the high precipitation rate in tropical climate leaches the basic ions into water bodies and causes soil acidification. Due to acidic condition, plant macronutrients including nitrogen, phosphorus, potassium, calcium and magnesium are less available in tropical soils (Truog, 1946). Therefore, low soil pH is another root cause of poor nutrients in the tropical soils.
Even though the tropical soils are low on plant nutrients, they can still support forestry and agriculture developments. The plant growth promoting microorganisms in tropical rhizospheres may have played a key role. Dipterocarpaceae native to Southeast Asia are known to tolerate acidic tropical soils with low nutrients due to the plant growth promoting microorganisms that help to provide available nutrients for plant growth (Fujii, 2014). Besides that, a study in Malaysia showed that plant growth promoting microorganism inoculated to rice seedlings would render the seedlings to gain significant height and biomass increments without any chemical fertiliser supplement (Tan et al., 2014).
INTERACTIONS OF PLANT GROWTH PROMOTING MICROORGANISMS AND HOST PLANTS
The plant growth promoting microorganisms refer to a wide variety of soil microorganisms which are found in rhizosphere or associated to host plants and able to promote their growth. The host plants release root exudates to attract plant growth promoting microorganisms by changing the physical and chemical compositions of the soil in the rhizosphere including soil pH, soil moisture content and partial pressure of oxygen (Vessey, 2003). In addition, they also provide carbon sources, amino acids, organic acids and other small molecules in the forms of exudates to increase the microbial population around the root surfaces and in the rhizosphere (Glick, 2012). At the meantime, the plant growth promoting microorganisms promote the growth of host plants in by increasing the uptake of plant nutrients (Abhilash et al., 2016).
There are five mechanisms used by plant growth promoting microorganisms to enhance the nutrient uptake of host plants. These mechanisms are the biological nitrogen fixation, acquisition of the unavailable nutrients in the rhizosphere, increase of the root surface area, aiding effect on other beneficial symbioses of the host, and lastly a combination of these mechanism (Vessey, 2003). The nitrogen fixing microorganisms, phosphate solubilising microorganisms and potassium solubilising microorganisms were discussed in this review paper. They covered the biological nitrogen fixation, fixed phosphate and potassium solubilisation.
Nitrogen fixing microorganisms
Nitrogen (N) is a key plant nutrient that promoting plant growth because it is an essential raw material for many important organic compounds. Plants need nitrogen to synthesise amino acids and form proteins, nucleic acids, alkaloids, chlorophyll, purine bases and enzymes (El-Ramady, 2014). The nitrogen element is abundant in the earth’s atmosphere as the form of nitrogen gas. However, nitrogen gas is not the available nitrogen that plants can uptake. Plants can only uptake nitrogen in the forms of ammonium ion (NH4+) and nitrate ion (NO3-). The tropical soils are deficient in available nitrogen as high rainfall would wash off available nitrogen from the soil. There are two pathways to fix atmospheric nitrogen to available nitrogen. The first pathway is non-biological fixation that fixes atmospheric nitrogen to nitrate ion via thunder lightning. Non-biological fixation only fixes a small amount of atmospheric nitrogen. The second pathway is the biological fixation which contributes 60% of total nitrogen fixation (Buerdass and Hurst, 2002). The biological fixation activities are carried out by symbiotic and free living nitrogen fixing microorganisms (Orr et al., 2011). The nitrogen fixing microorganisms fix atmospheric nitrogen to form ammonia via nitrogenase catalysis.
Nitrogenase would utilise a metal-based catalyst and high input of adenosine triphosphate (ATP) to convert atmospheric nitrogen to ammonia molecules. There are three types of nitrogenases which are encoded by a set of specific genes and combined with different metals at their active sites. The molybdenum dependent (Mo-dependent) enzyme is the most abundant and widely reported nitrogenase group. It has a metallo-cofactor called FeMo-cofactor in its active site (Santos et al., 2012; Seefeldt et al., 2009). The cofactor contains molybdenum, iron, sulphur, (R)-homocitrate and an unknown light atom, X (Seefeldt et al., 2009). The nitrogen fixing reaction catalysed by Mo-dependent nitrogenase is shown in the equation below (Seefeldt et al., 2009):
N2 + 8 H+ + 16 MgATP + 8 e- → 2 NH3 + H2 + 16 MgADP + 16 Pi
The Mo-dependent nitrogenase consisted of two metalloprotein components which as known as iron (Fe) protein and molybdenum-iron (MoFe) protein. The nitrogen fixation process involves a series of reactions between the two metalloprotein components, electrons, MgATP and protons (Seefeldt et al., 2009). The Fe-protein is an exclusive electron donor with high reducing ability whereas the MoFe-protein receives the energy of electrons and convert atmospheric nitrogen molecule into two ammonia molecules. The nitrogen fixing microorganisms utilise organic molecules from the soil environment to support the high ATP input in the biological fixation process (Rashid et al., 2016).
The ammonia molecules produced from biological nitrogen fixation are assimilated in the nitrogen fixing microorganisms by the enzyme glutamine synthase (GS) (Figure 3). However, the excess concentration of ammonia can inhibit the expression of nif gene and thus cause negative impacts on the synthesis and activity of nitrogenase (Colnaghi et al., 1997). Therefore, the excess ammonia molecules are excreted from nitrogen fixing microorganisms to overcome the inhibitory effect on nitrogenase activity (Colnaghi et al., 1997; Hartono et al., 2016). The first ammonia excretion experiment was carried out on mutated Klebsiella pneumoniae. The mutant strains were grown in the nitrogen free medium and found excreting excess amount of ammonia into the medium (Colnaghi et al., 1997). On the other hand, a study of nitrogen fixing Lysobacter sp. strain reported that the ammonia excretion was caused by the low carbon source in the environment and the bacteria cell lost its capability to retain ammonia (Iwata et al., 2010). The excreted ammonia molecules are either direct available for plant absorption or converted to nitrate as another source of available nitrogen by nitrifying bacteria via nitrification (Hartono et al., 2016).
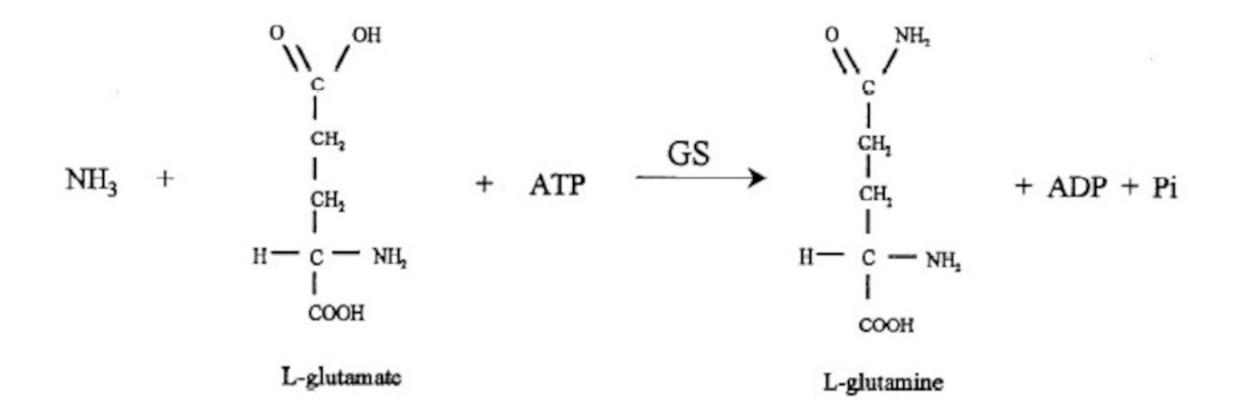
Figure 3. The reaction of ammonia molecules with L-glutamate (Colnaghi et al., 1997).
The studies of nitrogen fixing microorganisms on timber species are relatively scarce. Only some legume and actinorhizal species were reported to contain effective nitrogen fixing microorganisms. Acacia mangium, Acacia auriculiformis and their hybrid (Legume plants) were inoculated with rhizobium strains in a greenhouse experiment. The two rhizobium strains, Aust13c and AH12c could significantly increase shoot biomass of A. mangium and Acacia hybrid by 79% and 78% respectively (Le Roux et al., 2009). Casuarina spp., the actinorhizal species, develop an endosymbiosis relationship with nitrogen fixing soil actinomycetes Frankia. In root nodules, Frankia provides fixed nitrogen to host plant in exchange for carbon source. Casuarina equisetifolia, found on Africa salt-affected soil, fixed an average of 15 kg N/ha/year. In temperate regions, its nitrogen fixation activity was on par to legume species at 300 kg N/ha/year (Santi et al., 2013). A pot trial study showed Casuarina cunninghamiana increased nitrogen fixation activity from 125.48 g N/plant in monoculture to 152.69 g N/plant in mixed planting with Eucalyptus globulus. The increment indicated competition of soil nitrogen between actinorhizal species and non-nitrogen fixing plants (Forrester et al, 2006).
Phosphate solubilising microorganisms
Phosphorus (P) is another important plant nutrient besides nitrogen. It plays a central role in plant energy transfer and protein metabolism. It is also the component of RNA and DNA (El-Ramady, 2014). Although phosphorus is abundant in soil, most of it is in unavailable forms and could not be absorbed by the plant. In fact, only 0.1% of total phosphorus may exist as available forms (Sharma et al., 2013). The available forms are soluble orthophosphate ions such as dihydrogen phosphate ion (H2PO4-), hydrogen phosphate ion (HPO42-) and phosphate ion (PO43-). The most direct way to replenish soil available phosphorus is applying fertiliser. However, major available phosphorus released from the fertiliser could be promptly immobilised by highly reactive Al3+ and Fe3+ in the acidic soil, or Ca2+ in calcareous or normal soils (Yadav and Verma, 2012). Some microorganisms such as phosphate solubilising fungi and bacteria can extract phosphorus from this immobilised phosphorus pool in the soil. In the soil, there are organic and inorganic phosphorus sources which are equally important for plant growth. The phosphate solubilising microorganisms release mineral dissolving compounds to solubilise inorganic phosphorus sources. As for organic phosphorus sources, they could be released by extracellular enzyme secretion and phosphorus substrates degradation (Sharma et al., 2013).
There are two groups of inorganic phosphorus source in the soils. They are calcium phosphate from calcium dominant soils and iron and aluminium phosphates from iron and aluminium dominant soils (Yadav and Verma, 2012). These phosphorus sources are insoluble and unavailable for plant root absorption. Calcium phosphate is derived a mineral called apatite which is commonly found in calcareous and neutral soils. The phosphate solubilising microorganisms secrete different types organic acids to lower the pH of rhizosphere, and the solubility of calcium phosphate is thus enhanced via proton substitution or release of calcium ions. In addition, phosphate solubilising microorganisms could also solubilise calcium phosphate by releasing carboxylic anions to chelate the calcium ions due to its high affinity to calcium. Hence, the solubilisation of calcium phosphate could be accomplished by the combined effect of lowering of rhizosphere pH and carboxylic acid synthesis (Sharma et al., 2013; Yadav and Verma, 2012). Iron and aluminium phosphates are another inorganic phosphorus sources. The iron phosphate is originated from strengite whereas aluminium phosphate is derived from variscite. Those two minerals are abundant in the acidic soils (Yadav and Verma, 2012). The phosphate solubilising microorganisms release carboxylic acids to solubilise the iron and aluminium phosphates. The carboxylic acids can dissolve the mineral phosphate via anion exchange of phosphate ions by acid anions or chelation of both iron and aluminium ions (Sharma et al., 2013; Yadav and Verma, 2012).
The solubilisation or mineralisation of organic phosphorus process plays an important role in recycling soil organic phosphorus. The organic phosphorus sources may contribute 4-90% of total soil phosphorus content depending on the soil types. Four types of enzymes are known to catalyse this process: phosphatases, phytase, phosphonatases and C–P lyases (Sharma et al., 2013). The phosphatases can be classified into acid and alkaline phosphatases and are known to be released from the cell exterior (Khan et al., 2014). Acid phosphatases are commonly found in the acidic soils, while alkaline phosphatases are predominant in neutral and alkaline soils (Sharma et al., 2013). These two phosphatases could release phosphorus from organic phosphorus compounds such as inositol hexaphosphate (Khan et al., 2014). Phytase is another widely studied enzyme in the mineralisation of phytate, the major component of organic phosphorus in soil (Khan et al., 2014). Lastly, phosphonatases and C-P lysases are another two enzymes known to cleave the carbon and phosphorus bonding in organophosphonates, thus releasing the phosphorus content (Khan et al., 2014).
The released phosphorus from both inorganic and organic sources would be available for plant absorption. A few studies showed effective phosphate solubilising microorganisms isolated from rhizosphere of woody plants and those isolates were proven to significantly promote growth of timber species. dos Santos et al. (2015) isolated 35 phosphate solubilising bacterial isolates and 10 phosphate solubilising fungal isolates from rhizosphere of Calophyllum brasiliense, a notable reforestation species in Brazil. Bacterial isolates could mobilise higher concentration phosphate from FePO4 at the range of 2.01-3.35 mg P/mL, while 1.18- 3.78 mg P/mL was reported from fungal isolates. Nevertheless, all isolates were found to be positive on indoleacetic acid (IAA) synthesis, which indicated extensive root growth for better nutrient uptake. Another study in Brazil reported phosphate solubilising rhizobia strain BHCB-PL19 from Platymenia reticulata nodules incorporated with Gigaspora margarita and Glomus etunicatum, mycorrhizal fungal strains, effectively increased height and diameter of Eucalyptus camaldulensis after 8 months of transplanting to experimental sites (Scotti et al., 2007). In Taiwan, mixed inoculation of phosphate solubilising bacteria and Glomus spp. from Taiwan tropical soil enhanced the growth of Acacia confuse and Acacia mangium by 14-63% and 7-88% respectively (Young, 1990).
Potassium solubilising microorganisms
Potassium (K) is usually considered as the third important plant nutrient that affects plant growth. It helps plants to maintain osmotic pressure and ionic concentration. It is also a cofactor or activator of many carbohydrate enzymes and protein metabolism (El-Ramady, 2014). There are four different forms of potassium in the soil: mineral potassium, non-exchangeable potassium, exchangeable potassium and soil solution potassium. Nonetheless, only 1-2% are available for plants in the forms of exchangeable potassium and soil solution potassium in the soils while the remaining 98% are unavailable forms such as mineral potassium and non-exchangeable potassium (Zarjani et al., 2013). Application of a low amount of chemical-based potassium fertiliser may slightly convert mineral potassium to available potassium but this method is considered not efficient (Meena et al., 2015). On the other hand, potassium solubilising microorganisms can dissolve mineral potassium in the soils and release the soluble potassium into the soil solution.
Potassium solubilising microorganisms utilise organic acid production and acidic exo-polysaccharide secretion mechanisms to solubilise mineral potassium. Feldspar and mica are the major mineral potassium sources with high potassium content and abundant in most soils. They are in insoluble crystalline forms thus unavailable for plant root absorption. The weathering process of mineral potassium is time consuming and not able to immediately support the plant growth (Meena et al., 2016). In the widely reported organic acid production mechanism by potassium solubilising microorganisms studies, the organic acids would be produced from organic matter degradation and potassium solubilising microorganisms (Shanware et al., 2014). These organic acids acidify the microbial cells, rhizosphere and surroundings of mineral potassium. When the rhizosphere pH is lowered, the potassium solubilising microorganisms can dissolve mineral potassium by chelating the aluminium and silicon cations bound to potassium in the mineral potassium and thus the fixed potassium is solubilised into soil solution potassium (Rashid et al., 2016). The secretion of acidic exo-polysaccharide is the second mechanism to solubilise the mineral potassium. The exo-polysaccharides absorbs the organic acids in the soil and bound on the surface of mineral potassium. Hence, the exo-polysaccharides can absorb the silicon dioxide molecules in the mineral potassium and release the soluble potassium into soil solution (Jaiswal et al., 2016).
The soluble potassium released from both mechanisms is directly available for plant root absorption. Rather than agricultural crops, little studies of potassium solubilising microorganisms was conducted on timber species. A distribution studies of potassium solubilising bacteria was carried out in Myanmar forest and plantation rhizosphere soils. Dong et al. (2019) reported that potassium solubilising bacteria were more abundant in forest soils. The key difference was interference of human agricultural activity. Intact forest soils could reuse potassium from environmental materials in a long-term cycle, whereas such cycle was broken in plantation soils. Richness of plant species and plant functional diversity have a positive impact on the overall metabolism activity and metabolism diversity of the bacterial community. In the study, research team successfully isolated 14 bacterial strains from both soil samples with potassium solubilisation activities of 78.43-109.03 mg K/L aqueous medium (Dong et al., 2019). On the other hand, Sun et al. (2020) isolated three potassium solubilising bacterial strains from Mikania micrantha, a tropical plant known as bitter vine, rhizospheric soil, named GZ18, HZ18 and SZ5. After 30-day inoculation, total biomass, nitrogen, phosphorus and potassium of inoculated Mikania micrantha seedlings were significantly higher than control group (Sun et al., 2020).
APPLICATIONS OF PLANT GROWTH PROMOTING MICROORGANISMS AS BIOFERTILISERS
At production aspect, formation of biofertilisers or microbial innoculants from combinations of plant growth promoting microorganisms with suitable carrier materials is a common practice (Pindi and Satyanarayana, 2012). Suitable carrier materials should meet a few criteria: (1) non-toxic to biofertilisers and plants, (2) capable of retaining moisture, (3) easy to process uniform materials, (4) suitable for bulk sterilisations, (5) low cost and readily available materials, (6) adhesive to plant seeds, and (7) maintain viability of biofertilisers in the formulations (Senoo, 2006). There are four basic dispersal formulations available in the market including powder, granules, slurry and liquid. The powder formulation is suitable for spore-producing microorganisms which tolerate to heat and desiccation. The formulation is formed by ground peat and soil carriers with microorganisms at size range of 0.075 to 0.25 mm for better adhesion to the plant seeds (Sahu and Brahmaprakash, 2016; Zayed, 2016). Another solid formulation is granule which can be made of peat, prill, small marble, calcite or silica grains. The microorganisms can be impregnated into the granular materials and get protected from soil stress conditions like low pH, desiccation, low temperature and waterlogging. The granule formulation can be applied to the furrow directly and thus facilitate the lateral root interactions (Sahu and Brahmaprakash, 2016; Zayed, 2016). The slurry form is derived from powder formulation by suspending the powder in liquid like water. Then, the slurry formulation can be directly applied to the plant seeds or furrow (Zayed, 2016). The last formulation is liquid formulation which are formed by dissolving microbial consortia in the water, mineral or organic oils. Liquid formulations can be added with some additives to improve the viabilities of microbial cells (Sahu and Brahmaprakash, 2016; Zayed, 2016). Common additives are growth suppressants, contaminants suppressant (i.e sodium azide, sodium benzoate, butanol, acetone, fungicides and insecticides etc.) and cell protectants (i.e polyvinylpyrrolidone, polyethylene glycol, gum arabic, sodium alginate and glycerol etc.) (Pindi and Satyanarayana, 2012; Santhosh, 2015). In addition, the microorganisms are not clumping in the liquid medium after prolonged storage and can be directly used for resuspension (Pindi and Satyanarayana, 2012). In general, solid carrier based and liquid formulations can keep biofertilisers up to six months and two years respectively (Brar et al., 2012).
Furthermore, application of biofertilisers can be incorporated with lower proportions of chemical fertilisers for enhancing the plant nutrients uptake, improving soil properties and preventing the plant available nutrients from leaching process (Agamy et al., 2012). This integrated application has been conducted on woody plants, agricultural crops and ornamental flowers with 50%-75% reduction in chemical fertiliser usage. Those treated plant samples gained similar growth rate to plant samples treated with full strength chemical fertilisers (Kumar et al., 2009; Agamy et al., 2012; Farahat et al., 2014).
CONCLUSION
As the nutrient-deficit tropical soils could not be altered by any sorts of modern technologies, the assistance of plant growth promoting microorganisms in the wild environments of tropical climates would be a key solution. Nitrogen fixing microorganisms, phosphate solubilising microorganisms and potassium solubilising microorganisms act as carriers of available nitrogen, available phosphorus and available potassium to support optimal growth of N. cadamba in ITPAs as in wild environments. In order to prolong the shelf-life and sustain the environmental challenges, plant growth promoting microorganisms can be incorporated with carrier materials in every application strategy, either plant seeds or furrows. Solid-based, liquid-based and solid-liquid integration are able to provide shelf life from six months up to two years.
The research of plant growth promoting microorganisms on tropical timber species, particularly N. cadamba, is relatively scarce at the writing moment. Long-term research commitment and high risk on the return of investment could be the primary challenges to bring motivations for funding bodies and young scientists. Nevertheless, researchers could be more diligent to engage plant growth promoting microorganisms research on tropical timber species to produce promising results for commercial farmers, thus pushing plant growth promoting microorganisms into industrial production based on the growing demand. In a nutshell, plant growth promoting microorganisms would be hoped to aid large-scale planting of N. cadamba.
ACKNOWLEDGEMENTS
Authors would like to express great appreciation to Sarawak Timber Association for the Research and Development Funding (Adjudication No. C01K966016-Q040), Sarawak Forestry Corporation (SFC) (Adjudication No. G01L003313-Q040) for research collaboration and many other organizations involved in forest plantation and timber industry.
CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
REFERENCES
Abhilash, P.C., Dubey, R.K., Tripathi, V., Gupta, V.K., and Singh, H.B., 2016. Plant growth-promoting microorganisms for environmental sustainability. Trends in Biotechnology. 34: 847‒850.
Agamy, R.A., Mohamed, G.F., and Rady, M.M., 2012. Influence of the application of fertilizer type on growth, yield, anatomical structure and some chemical components of wheat (Triticum aestivum L.) grown in newly reclaimed soil. Australian Journal of Basic and Applied Sciences. 6: 561‒570.
Bijalwan, A., Dobriyal, M.J.R., and Bhartiya, J.K., 2014. A potential fast growing tree for agroforestry and carbon sequestration in India: Anthocephalus cadamba (Roxb.) Miq. American Journal of Agriculture and Forestry. 2: 296‒301.
Brar, S.K. et al., 2012. Shelf-life of Biofertilizers: An Accord between Formulations and Genetics. Journal of Biofertilizers & Biopesticides 3 (5): 1‒3.
Buerdass, D. and Hurst, J., 2002. Rhizobium, root nodules & nitrogen fixation. Microbiology Society. Available at [access date: 04.06.2021]: http://labs.bio.unc.edu/Vision/pmabs/rhizobium.activity2.pdf.
Colnaghi, R., Green, A., He, L., Rudnick, P., Kennedy, C., 1997. Strategies for increased ammonium production in free-living or plant associated nitrogen fixing bacteria. Plant and Soil. 194: 145‒154.
D’enghien, P.B., 2016. Malaysia is green and growing. The Star. 22 January. Available at [access date: 19.09.2017]: http://www.thestar.com.my/news/nation/2016/01/22/
malaysia-is-green-and-growing-new-data-from-the-un-fao-disproves-ngos-accusations-that-oil-palm-caus/
Dong, X. et al. 2019. Differences in distribution of potassium-solubilizing bacteria in forest and plantation soils in Myanmar. International Journal of Environmental Research and Public Health. 16: 700.
dos Santos, L.C.R. et al., 2015. Isolation and selection of P-solubilizing and IAA synthesizing microorganisms from the rhizosphere of Guanandi (Calophyllum brasiliensis). African Journal of Agricultural Research. 10: 4455‒4460.
El-Ramady, H.R., Alshaal, T.A., Amer, M., Domokos-Szablocsy, É., Elhawat, N., Prokisch, J., Fári, M., 2014. Soil quality and plant nutrition. Sustainable agriculture reviews. 14: 354‒447.
Farahat, M.M., Fatma El-Quesni, E.M., El-Khateeb, M.A., El-Leithy, A.S., and Hashish, K.I., 2014. Impact of combined chemical and biofertilizers on vegetative growth and chemical composition of Paulownia kawakamii seedlings. Middle East Journal of Agriculture Research. 3: 852‒858.
Forrester, DI. et al., 2006. Mixed-species plantations of Eucalyptus with nitrogen-fixing trees: A review. Forest Ecology and Management. 233: 211‒230.
Fujii, K., 2014. Soil acidification and adaptations of plants and microorganisms in Bornean tropical forests. Ecological Research. 29: 371‒381.
Glick, BR., 2012. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica. 2012: 1‒15.
Hartono, H., Nurfitriani, Asnawati, F., Citra, H., Handayani, N.I., Junda, M., Ali, A., Hala, Y., and Jumadi, O., 2016. Ability of ammonium excretion, indol acetic acid production, and phosphate solubilization of nitrogen-fixing bacteria isolated from crop rhizosphere and their effect on plant growth. ARPN Journal of Engineering and Applied Sciences. 11: 11735‒11741.
Igwe, C.A., 2011. Tropical soils, physical properties. Encyclopedia of Agrophysics. 1: 934‒937.
Iwata, K., Azlan, A., Yamakawa, H., and Omori, T., 2010. Ammonia accumulation in culture broth by the novel nitrogen-fixing bacterium, Lysobacter sp. E4. Journal of Bioscience and Bioengineering 110: 415‒418.
Jaiswal, D.K., Verma, J.P., Prakash, S., Meena, V.S., and Meena, R.S., 2016. Potassium as an important plant nutrient in sustainable agriculture: a state of the art. Potassium Solubilizing Microorganisms for Sustainable Agriculture. 1: 21‒29.
Khan, M.S., Zaidi, A., and Ahmad, E., 2014. Mechanism of phosphate solubilization and physiological functions of phosphate-solubilizing microorganisms. Phosphate Solubilizing Microorganisms: Principles and Application of Microphos Technology. 1: 31‒62.
Krisnawati, H., Kallio, M., and Kanninen, M. 2011. Anthocephalus cadamba Miq.: Ecology, silviculture and productivity. Center for International Forestry Research. Available at [access date: 20.01.2015]: http://www.cifor.org/publications/pdf_files/Books/BKrisnawati1105.pdf
Kumar, D., Singh, B.P., and Singh, V.N., 2009. Effect of integrated nutrient management on growth, flowering behaviour and yield of African marigold (Tagetes erecta L.) cv. African Giant Double Orange. Journal of Horticulture Sciences. 4: 134‒137.
Le Roux, C. et al., 2009. Bradyrhizobianodulating the Acacia mangium X A. auriculiformis interspecific hybrid are specific and differ from those associated with both parental species. Applied and Environmental Microbiology. 75: 7752‒7759.
Lissem, N.A., 2013. Status of timber industry in sarawak. in: international conference of timber. 7‒8 June 2013. Kuching, Sarawak, Malaysia.
Loganathan, P., 1987. Composition of soil. Food and Agriculture Organization of the United Nations. Available at [access date: 18.04.2017]: http://www.fao.org/docrep/field/003/ac172e/AC172E03.htm#ch3
Loganathan, P., 1987. Soil formation, soil profile and soil classification. Food and Agriculture Organization of the United Nations. Available at [access date: 18.04.2017]: http://www.fao.org/docrep/field/003/ac172e/AC172E07.htm.
Maharashtra State Forest Department, [date unknown]. Neolamarckia cadamba (Roxb.) Bosser (Kadamb). Available at [access date: 14.12.2017]: http://www.mahaforest.
nic.in/fckimagefile/kadamb.pdf
Meena, V.S., Bahadur, I., Maurya, B.R., Kumar, A., Meena, R.K., Meena, S.K., Verma, J.P., 2016. Potassium-solubilizing microorganism in evergreen agriculture: An overview. Potassium Solubilizing Microorganisms for Sustainable Agriculture 1: 1‒20.
Meena, V.S., Maurya, B.R., Verma, J.P., Aeron, A., Kumar, A., Kim, K., and Bajpai, V.K., 2015. Potassium solubilizing rhizobacteria (KSR): Isolation, identification, and K-release dynamics from waste mica. Ecological Engineering 81: 340‒347.
Orr, C.H., James, A., Leifert, C., Cooper, J.M., and Cummings, S.P., 2011. Diversity and activity of free-living nitrogen-fixing bacteria and total bacteria in organic and conventionally managed soils. Applied and Environmental Microbiology. 77: 911‒919.
Orwa, C., Mutua, A., Kindt, R., Jamnadass, R., and Simons, A., 2009. Anthocephalus cadamba, World Agroforestry Centre. Available at [access date: 15.01.2016]: http://www.worldagroforestry.org/treedb/AFTPDFS/Anthocephalus_cadamba.PDF
Pindi, P.K. and Satyanarayana, S.D.V., 2012. Liquid microbial consortium- A potential tool for sustainable soil health. Journal of Biofertilizers and Biopesticides. 3: 1‒9.
Rashid, M.I., Mujawar, L.H., Shahzad, T., Almeelbi, T., Ismail, I.M.I., and Oves, M., 2016. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiological Research. 183: 26‒41.
Sahu, P.K. and Brahmaprakash, G.P., 2016. Microbial inoculants in sustainable agricultural productivity. Springer, New Delhi.
Santhosh, G.P., 2015. Formulation and shelf life of liquid biofertilizer inoculants using cell protectants. Interntional Journal of Researches in Biosciences, Agriculture and Technology. 2: 243‒247.
Santi, C. et al., 2013. Biological nitrogen fixation in non-legume plants. Annals of Botany 111: 743‒767.
Santos, P.C.D., 2012. Distribution of nitrogen fixation and nitrogenase-like sequences amongst microbial genomes. BMC Genomics. 13: 1‒12.
Scotti, M.R. et al., 2007. Effect of plant species and mycorrhizal inoculation on soil phosphate-solubilizing microorganisms in semi-arid Brazil: Growth promotion effect of rhizospheric phosphate-solubilizing microorganisms on Eucalyptus camaldulensis. First International Meeting of Microbial Phosphate Solubilization 102: 167‒172.
Sarawak Forestry Department, 2021. Forest plantation development in Sarawak. Available at [access date: 03.06.2021]: https://forestry.sarawak.gov.my/page-0-362-1129-Forest-Plantation-Development-in-Sarawak.html
Sarawak Forestry Department, 2021. Progress of planting. Available at [access date: 03.06.2021]: https://forestry.sarawak.gov.my/page-0-246-1009-Progress-of-Planting.html
Sarawak Forestry Department, 2021. Types and categories of Sarawak’s forests. Available at [access date: 03.06.2021]: https://forestry.sarawak.gov.my/page-0-160-593-Types-and-Categories-of-Sarawak-s-Forests.html
Sattar, A. et al. 2019. Perspective of potassium solubilizing microbes in sustainable food production system: A review. Applied Soil Ecology. 133: 146‒159.
Schulte, A. and Ruhiyat, D., 1998. Soils of tropical forest ecosystems. Springer-Verlag Berlin Heidelberg, New York.
Seefeldt, L.C., Hoffman, B.M., Dean, D.R., 2009. Mechanism of Mo-dependent nitrogenase. Annual Review of Biochemistry 78: 701‒722.
Senoo, K., 2006. Carriers for Biofertilizers: Carrier materials. Biofertilizer Manual: 41‒44.
Shanware, A.S., Kalkar, S.A., and Trivedi, M.M., 2014. Potassium solubilisers: Occurance, mechanism and their role as competent biofertilizers. International Journal of Current Microbiology and Applied Sciences 3: 622‒629.
Sharma, S.B., Sayyed, R.Z., Trivedi, M.H. and Gobi, T.A., 2013. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus. 2: 1‒14.
Sheard, R.W., 1991. Understanding turf management. Available at [access date: 12.04.2017]: http://archive.lib.msu.edu/tic/stnew/article/1991sep4.pdf
Spaepen, S. and Vanderleyden, J., 2011. Auxin and plant-microbe interactions. Cold Spring Harbor Perspectives in Biology. 3: 1‒13.
Sun, F. et al. 2020. Isolation and identification of potassium-solubilizing bacteria from Mikania micrantha rhizospheric soil and their effect on M. micrantha plants. Global Ecology and Conservation. 23: 1‒9.
Tan, K.Z., Radziah, O., Halimi, M.S., Khairuddin, A.R., Habib, S.H., and Shamsuddin, Z.H., 2014. Isolation and characterization of rhizobia and plant growth-promoting rhizobacteria and their effects on growth of rice seedlings. American Journal of Agricultural and Biological Sciences. 9: 342‒360.
The World Bank, [date unknown]. Forest area (% of land area). Available at [access date: 03.06.2021]: https://data.worldbank.org/indicator/AG.LND.FRST.ZS
Truog, E., 1946. Soil reaction influence on availability of plant nutrients. Soil Science Society Proceedings. 305‒308.
Vessey, J.K., 2003. Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil. 255: 571‒586.
Vincent, A., 2002. Studies of native and exotic tree plantations in Sarawak. Master Thesis. Universiti Sarawak Malaysia, Sarawak. Malaysia.
Yadav, B.K. and Verma, A., 2012. Phosphate solubilization and mobilization in soil through microorganisms under arid ecosystems. The Functioning of Ecosystems. 1: 93‒108.
Young, C.C., 1990. Effects of phosphorus-solubilizing bacteria and vesicular-arbuscular mycorrhizal fungi on the growth of tree species in subtropical-tropical soils. Soil Science and Plant Nutrition. 36: 225‒231.
Zarjani, J.K., Aliasgharzad, N., Oustan, S., Emadi, M., Ahmadi, A., 2013. Isolation and characterization of potassium solubilizing bacteria in some Iranian soils. Archives of Agronomy and Soil Science. 59: 1713‒1723.
Zayed, M.S., 2016. Advances in formulation development technologies. Microbial Inoculants in Sustainable Agricultural Productivity. 1: 219‒237.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Alan Chua Yee Quan, Peter Morin Nissom and Tan Lee Tung*
Faculty of Engineering, Computing and Science, Swinburne University of Technology Sarawak Campus, Jalan Simpang Tiga, 93350 Kuching, Sarawak, Malaysia
Corresponding author: Tan Lee Tung, E-mail: ltdtan@swinburne.edu.my
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: August 12, 2021;
Revised: January 26, 2022;
Accepted: January 27, 2022;
Published online: February 2, 2022

