
The Effect of Pangasius Djambal Gelatin Treatment on the Lymphocyte Cells after Tooth Extraction
Muhammad Chair Effendi*, Fredy Mardiyantoro, Novi Khila Firani, Ahmad Taufiq, and Ibnu Putra NugrahaPublished Date : 2022-03-31
DOI : https://doi.org/10.12982/CMUJNS.2022.022
Journal Issues : Number 2, April-June 2022
Abstract Inflammation is a phase of the wound healing process that occurs after tooth extraction which is influenced by the immune system. Lymphocytes are one type of cell in the immune system that have an important role in the inflammation process. The purpose of this study was to investigate the effect of Pangasius djambal gelatin treatment on the enhancing lymphocyte cell count after tooth extraction in Rattus norvegicus. In this study, the in vivo true experimental laboratories with a post-test group design was maintained. In this study, we performed 3 control groups without treatment by the Pangasius djambal gelatin and 3 treatment groups with 100 mg/mL Pangasius djambal gelatin on the post-extraction wound of the mandibular left lateral incisor of Rattus norvegicus. Each group consists of 5 rats. The lymphocyte cells count in the tooth socket tissue was evaluated on days 3, 5, and 7. The control groups' lymphocyte cells count decreased significantly from 32 to 17.2 from day 3 to day 7. Interestingly, the lymphocyte cells of treatment groups increased from 92.2 to 114.6 from day 3 to day 7. Furthermore, a very strong positive correlation between the observation time and lymphocyte cell count was observed in this work (r = 0.838; very strong). Therefore, the Pangasius djambal gelatin opens new potency to increase the lymphocyte cells count after tooth extraction.
Keywords: Pangasius djambal fish, Lymphocytes, Tooth extraction, Intraperitoneal anesthesia
Citation: Effendi, M.C., Mardiyantoro, F., Firani, N.K., Taufiq, A., and Nugraha, I.P. 2022. The effect of pangasius djambal gelatin treatment on the lymphocyte cells after tooth extraction. CMU J. Nat. Sci. 21(2): e2022022.
INTRODUCTION
In general, tooth extraction is one of the most common procedures performed by dentists to solve infection problems. After tooth extraction, the wound healing process occurs in several phases such as hemostasis, inflammation, proliferation, and maturatin/remodeling (Schultz et al., 2011; Khoswanto, 2020). Inflammation is an essential response for the maintenance of normal tissue homeostasis provided by the immune systems during tissue injury and infection (Ahmed, 2011). Besides neutrophils, monocytes, eosinophils, basophils, and platelets, lymphocytes also play an essential role in the inflammation process (Oki et al., 2018). Experimentally, lymphocytes consist of many cells in human blood with spherical shape and a diameter range of 7 to 8 μm. Furthermore, lymphocytes contain approximately 35% of leukocytes in normal blood with a spherical nucleus that almost fills the entire cell (Prinyakupt and Pluempitiwiriyawej, 2015).
Lymphocytes in the form of gelatin could be produced from several types of plants and animals that are often used as traditional medicines. In the pharmaceutical industry, gelatin has been used for the manufacture of hard and soft capsule shells (Gullapalli, 2010). Theoretically, gelatin is an insoluble protein produced by hydrolysis collagen. Collagen is a basic structure of animal bodies such as skin, tendons, bones, and connective tissues (Hanani, 2016). During the last years, commercial gelatin has been generally obtained from mammals. One of the animals that become an alterative source to produce gelatin on a large scale is fish because it contains high collagen content (Alfaro et al., 2014).
One type of fishes that can be used to produce gelatin is Pangasius djambal because it contains 68.6% protein (Andriani et al., 2019). The fish has a silver and white elongated body shape with a bluish back. It has no scales; its head is relatively small, with its mouth located at the end of the head, which is slightly down, and its body length can reach 120 cm (Kordik, 2005). Therefore, based on the above explanation, it is essential to investigate the potential of the Pangasius djambal gelatin treatment to increase the lymphocyte count in post-tooth-extraction wounds. (Wolfensohn and Lloyd, 2013) In this study, researchers used an easily available source of gelatin, so the advantage was that the production of gelatin for wound healing can be developed easily by utilizing sources that are easily obtained and processed. The weakness of this research and which can be developed is the sample preparation technique. In this study, researchers used immunohistochemistry (IHS) which uses sections of whole tissue. In future studies, immunocytochemistry (IHC) can be used which can provide more accurate/specific results by using cells that have been isolated from tissue or grown in culture and lymphocyte cells count will be more clearly visible.
MATERIAL AND METHODS
In this study, an in-vivo true experimental laboratory study was employed with a post-test group design. We performed 3 control groups (K1, K2, K3), which were not treated by Pangasius djambal gelatin, and 3 treatment groups (P1, P2, P3) which were treated by the fish gelatin with a concentration of 100 mg/mL. The experimental animals used were male wistar strain Rattus norvegicus (Physiology Laboratory, Faculty of Medicine, University of Brawijaya, Indonesia). Wistar strain Rattus norvegicus were chosen as the population since they were relatively docile experimental animals, easy to maintain, their metabolic function is similar to humans, and can be obtained in large quantities. The grouping of the research sample was as follows: the control group (K1) and the treatment group (P1) were observed on day 3; the control group (K2) and the treatment group (P2) were observed on day 5; the control group (K3) and the treatment group (P3) were observed on day 7. The observations were carried out on the increase in the lymphocyte count in the wound healing process after the extraction of the mandibular left lateral incisor of Rattus norvegicus.
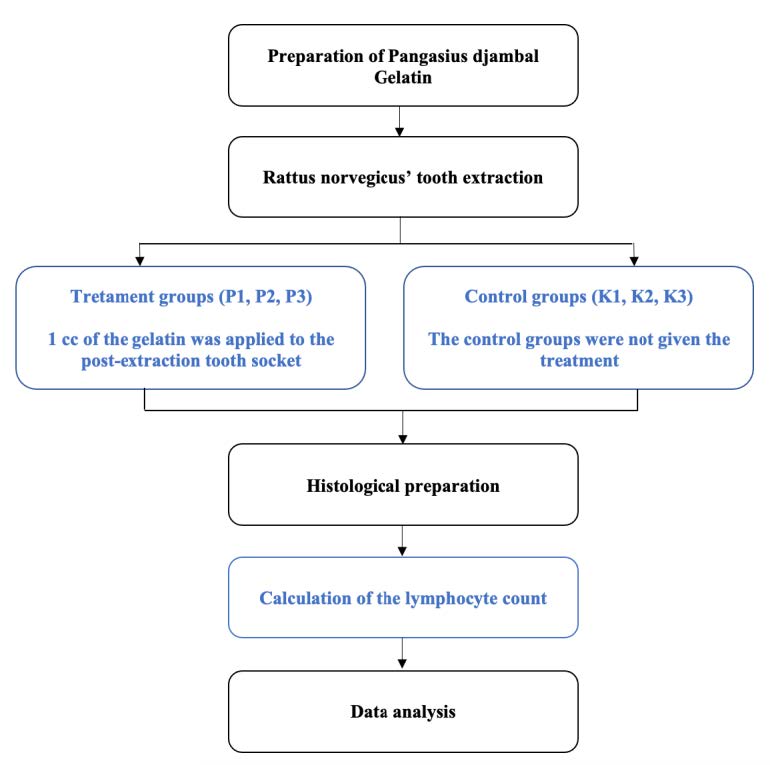
Figure 1. Research workflow.
Research samples
The randomization technique for grouping research samples used a completely randomized design. The number of samples is calculated using the Resource Equation Approach formula, which is a maximum of n = 20/k + 1 (Arifin & Zainuddin, 2017) with a group of 6. Based on the calculation, a maximum sample of each group is 5 samples for each group. There were 6 research groups with 5 Rattus norvegicus for each group that would be decapitulated and their jaw samples were taken at different times. Therefore the total samples were 30 Rattus norvegicus. The inclusion criteria for the Rattus norvegicus were: male (to avoid the dominant hormonal effect on female Rattus norvegicus); 200-300 g of weight; 2-3 months old; healthy characterized by active movements, clear eyes, and thick, shiny white lashes; 7-day adaptation. Furthermore, the exclusion criteria were: having an infection during the study; experiencing a drastic weight loss when compared from the beginning of the study to the time of the decapitation (
Preparation of Pangasius djambal Gelatin
The Pangasius djambal's skin was taken and separated from the meat and the fat attached to the skin, and it then stored at -20°C. The fish skin that had been stored was thawed at room temperature and then cut into about 1 cm3 in size. Next, it was rinsed with lemon water to remove any material other than the fish skin. Next, 100 g of Pangasius djambal skin was rinsed and immersed in a citric acid solution for 12 h to liquefy the collagen fibers into fibers/fibrils so that they could be easily extracted. After that, it was neutralized by washing it several times until the washing water is at a neutral pH (6-7). The skin was then extracted using a shaker water bath with distilled water at 60°C for 6 h. The gelatin solution was separated from the remaining skin using a filter cloth. The gelatin solution was cooled at room temperature until the gelatin gel was formed.
Rattus norvegicus’ tooth extraction
Tooth extraction was done to all groups. Before the Rattus norvegicus’ mandibular left lateral incisor was extracted, the tissue disinfection was given in the abdominal area using 70% alcohol to perform intraperitoneal anesthesia. Then, it was given an anesthetic injection using ketamine 1000 mg/10 mL, so the Rattus norvegicus did not feel pain and easy to handle. The anesthetic was injected intra-peritoneally into the abdomen, and it was aspirated. If the needle had been placed in the correct place, then it was injected. The anesthesia worked approximately 3 min after the injection and the effects would be gone after an hour or so. Separation of the roots of the Rattus norvegicus’ teeth from the gingiva used a modified lecron, and removal of the teeth from the gingiva used a modified needle holder. Tooth extraction was carried out in the same direction as the tooth socket and carefully with the same force to prevent tooth fractures (Widyastomo et al., 2013). The Rattus norvegicus was given Novalgin 500 mg/mL once at a dose of 0.3 mL intra-peritoneally after the tooth extraction to avoid complications in the form of pain.
Pangasius djambal gelatin
Before giving the Pangasius djambal gelatin, the bleeding was stopped by using some gauze after the extraction to get space to apply the gelatin. Using a pipette, 1 cc of the gelatin was applied to the post-extraction tooth socket using a syringe which was inserted into the bottom of the socket to prevent air bubbles trapping in the tooth socket once after the extraction and after homeostasis occurs. Meanwhile, the control groups were not given the treatment. After that, each control and treatment group was decaputated and their jaw samples were taken.
Post extraction care
After the tooth extraction, the Rattus norvegicus were fed by diluting the food using a nasogastric tube to go straight to the stomach without passing through the mouth to prevent healing problems in the tooth socket. Feeding was carried out 2 times a day, every morning and evening, so the food intake for the Rattus norvegicus was fulfilled. For the fluid intake, the Rattus norvegicus was still given enough water. Sampling was carried out on days 3, 5, and 7 and the increase in the lymphocyte count in the tissue in the tooth socket was observed. Prior to decapitated and removal of the mandible, anesthesia was performed using ether inhalation with a lethal dose of 0.9 mL. Confirmation of the death of the Rattus norvegicus had to be done by looking at the aspiration; if the aspiration was not visible, then the mandibular decaputated could be done using scalpel no.11 in the socket where the tooth was extracted. The mandible was then put into a tube containing 10% formalin for tissue fixation and labeled. The Rattus norvegicus are then buried in the ground with the help of experts from the laboratory.
Histological Preparation
Tissue fixation was done by immersing the tissue in 10% formalin for 18-24 h, then the tissue was washed with aquadest for 15 min and the tissue embedding was done by putting the tissue in the liquid, namely acetone for 1 h × 4, Xylol for ½ h × 4 , liquid paraffin for 1 h × 3 and implantation of skin tissue on paraffin blocks. Tissue slicing was done by means of an embedded block and the tissue was placed on an ice block for about 15 min, then the block was attached to a rotary microtome disc (MSLK221, China). After that, it incised the ulcer tissue vertically with a size of 4 microns. The tissue incision, which was formed a ribbon, was taken using a small brush and placed in a water bath with a temperature of 36°C. The incision was taken using an object-glass and left for 24 h. Staining the object-glass by placing the object-glass in Xylol for 15 min was repeated for 3 times, and in 96% alcohol for 15 min was also repeated for 3 times, then washed with running water for 15 min. After that, the object-glass was put in Haematoxylin stain for 15 min and washed with running water for 15 min. The object-glass was put on Lithium carbonate for 20 seconds and washed with running water for 15 min. The object-glass was put on the Eosin stain for 15 min, then put in 96% alcohol for 15 min and Xylol for 15 min, each repeated 3 times. Finally, the preparations were closed using an entellant deck glass.
Calculation of the lymphocyte count
Samples with replication of 2 pieces of wound tissue were placed in the object-glass. The tissue specimens were viewed using a digital microscope (Olympus, Japan) with 400× magnification to ascertain the location of the specimens to be counted, then a photo of the specimens was made. The sample on the preparation was divided into 5 visual fields of the same size. Each piece of tissue was calculated the lymphocyte count systematically starting from the lower-left corner, then shifted to the right and pulled upwards and so on so that all visual fields were read. The mean of lymphocytes of the five tissue pieces was calculated.
Data analysis
The ANOVA test was used to determine differences in the lymphocytes count in all treatment groups. Pearson’s test was employed to determine the correlation between the observation time and the lymphocyte count in the treatment groups.
Ethics
The present research was declared to have passed the ethics feasibility by the Ethics Commission of the Faculty of Medicine, Universitas Brawijaya, Indonesia, with Ethical Certificate No.339A/EC/KEPK-FKG/12/2017.
RESULTS
The lymphocyte count of 5 specimens fo the control group (K1) without the Pangasius djambal gelatin treatment has a mean lymphocyte count of 34 on day 3. The treatment group (P1) treated by the Pangasius djambal gelatin has a mean lymphocyte count of 92.2 on day 3 (Figure 2). In the observation of 5 specimens on day 5, the control group (K2) without the Pangasius djambal gelatin treatment has a mean lymphocyte count of 19.2. The treatment group (P2) given the gelatin has a mean lymphocyte count of 106.6 (Figure 3). Furthermore, the observation on day 7 for 5 specimens, the control group (K3) without Pangasius djambal gelatin treatment, has a mean lymphocyte count of 17.2. The treatment group (P3) given Pangasius djambal gelatin treatment has a mean lymphocyte count of 114.6 (Figure 4). The lymphocyte count of the control groups (K) decreased, while the lymphocyte count of the treatment groups (P) increased.
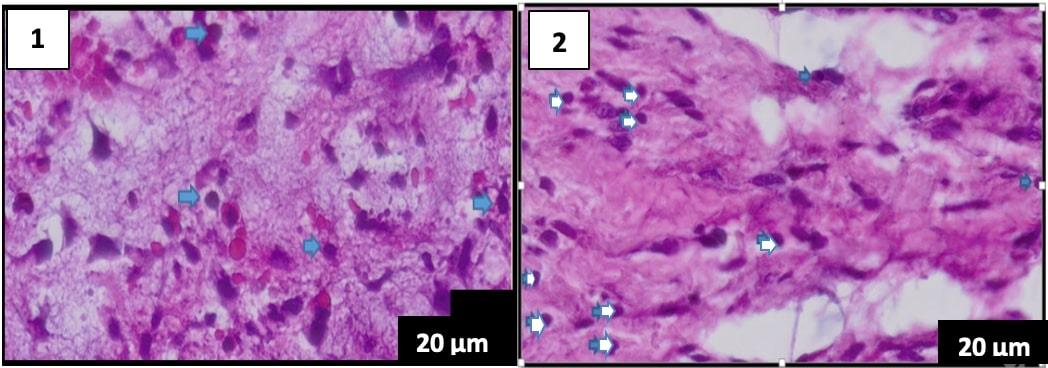
Figure 2. Comparison of histological features on day 3 of the hematoxylin-eosin staining, the lymphocyte count is blue. (A) group K1 (B) group P1 (400× magnification), scale bar 20 µm.
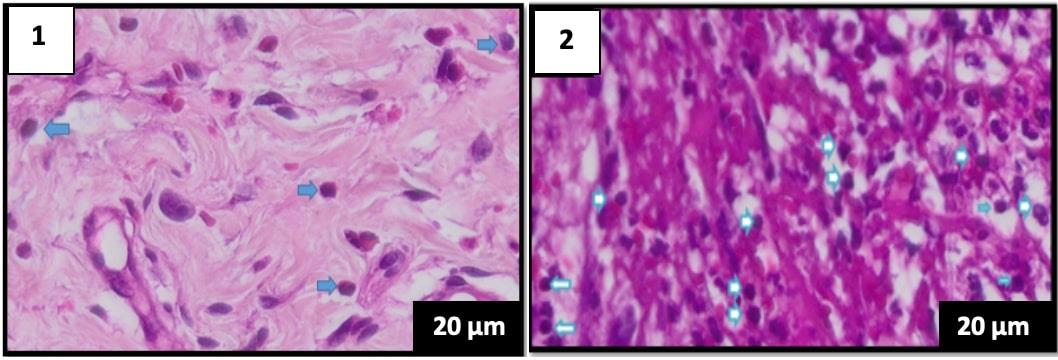
Figure 3. Comparison of histological features on day 5 of the hematoxylin-eosin staining, the lymphocyte count is blue. (A) group K1 (B) group P1 (400× magnification), scale bar 20 µm.
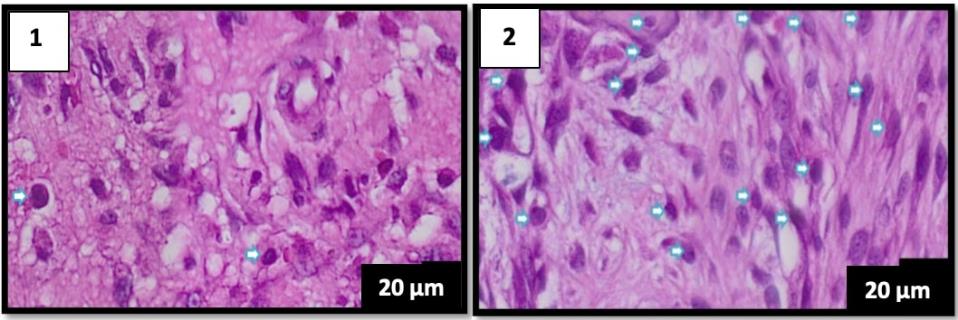
Figure 4. Comparison of histological features on day 7 of hematoxylin-eosin staining, the lymphocyte count is blue. (A) group K1 (B) group P1 (400× magnification), scale bar 20 µm.
The results of the ANOVA test (Figure 5) showed no significant difference between the control groups K1-K2-K3, and K2-K3 (P > 0.05). Between the treatment groups P1-P2-P3 as well as P2-P3 there were also no significant differences (P > 0.05). However, all control groups had very significant differences in lymphocyte counts with all treatment groups (P < 0.01). Furthermore, as shown in Table 1, the Pearson's correlation test displays a robust correlation but insignificant relationship between the observation time and the increase in the lymphocyte count after treatment by the Pangasius djambal gelatin (r = 0.838; P = 0.077).
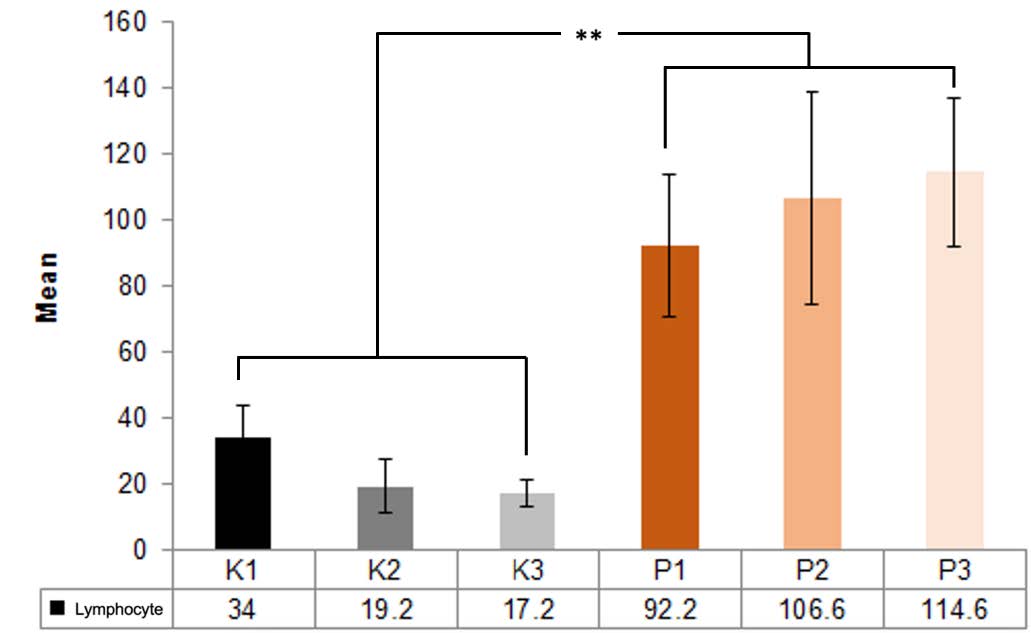
Figure 5. Mean lymphocyte count of the control (K) and treatment groups (P) on days 3, 4 and 5; **, P < 0.01; error bars = standard deviation.
Table 1. Correlation between the observation time and the lymphocyte count after tretament by the Pangasius djambal gelatin.
|
Variable correlation |
r |
P |
|
Observation time and lymphocyte count |
0.838 (very strong) |
Not significant |
DISCUSSION
The gelatin extracted from the Pangasius djambal has physicochemical properties with high amino acids close to the commercial gelatin. The Pangasius djambal can produce high-quality gelatin, which can be used to replace gelatin from mammals (Ratnasari et al., 2013). The role of lymphocytes in inflammation as a humoral and cellular response (Chaplin, 2010).
The study results reveal that the mean lymphocyte count in the control group (K1), which was not treated by the Pangasius djambal gelatin, observed on day 3 was 34. In the treatment group (P1) tretaed by the Pangasius djambal gelatin, observed on day 3, the mean lymphocyte count was 92.2 (Figure 2). The mean lymphocyte counts for the treatment groups (P2 and P3) treated by the Pangasius djambal gelatin on day 5 and day 7 were 106.6 and 114.6, respectively. For the control groups (K2 and K3) without the Pangasius djambal gelatin treatment, the mean of lymphocyte count on the observation day 5 and 7 decreased from 19.2 to 17.2 (Figures 3 and 4). The increasing lymphocyte count in the treatment groups was due to the post-extraction wounds were induced by giving the Pangasius djambal gelatin, which can stimulate an increase in the lymphocyte count. The amino acids contained in the Pangasius djambal affected wound healing. Arginine, a non-essential amino acid, plays an important role in the wound healing process and immune function (Chow and Barbul, 2014).
The Anova test results show very significant differences in lymphocyte counts between all control groups that are not given the pangasius djambal fish gelatin and all treatment groups that are given the pangasius djambal fish gelatin. The Pearson's correlation results (Table 1) reveal a positive correlation coefficient in a very strong range between the observation time and the lymphocyte count but it is not significant.
The positive correlation means that the longer the observation time, the more increase in the lymphocyte count after the Rattus norvegicus’ tooth extraction. The result of this study is in line with the previous work presented that lymphocytes appear in significant numbers after day 5, and the peak appears on day 7 after the injury (Beauchamp et al., 2017). Lymphocytes play a fundamental role in the function of immune system. They influence responses of the immune system to foreign substances (Orakpoghenor et al., 2019). In the wound area, there are many antigens, and the body responds by producing lymphocytes to produce antibodies and create lymphokines to activate macrophages for wound healing (Guyton and Hall, 2014; Krzyszczyk et al., 2018). Furthermore, they critically involved in wound healing, from dampening inflammation to clearing debris and coordinating tissue repair (Kim and Nair, 2019). Therefore, as a final remark, it can be inferred that the Pangasius djambal gelatin produced in this work becomes a new potential candidate agent to repair the wound healing after toot extraction.
CONCLUSION
Based on the data analysis, it can be concluded that the lymphocyte cell count of the control groups without the Pangasius djambal gelatin treatment on the post tooth extraction decreased significantly from 34 to 17.2. Interestingly, the lymphocyte cell count of the groups treated by the Pangasius djambal gelatin in the post-tooth extraction increased from 92.2 to 114.6. Furthermore, the strong positive correlation was observed between the observation time and the lymphocyte cells count. Therefore, prepared gelatin obtained from Pangasius djambal in this work can be used as an alternative agent to solve wound healing problem after tooth extraction.
ACKNOWLEDGEMENTS
The authors would like to thank all the Biochemistry Laboratory, Parasitology Laboratory, Anatomical Pathology Laboratory of Faculty of Medicine, Faculty of Agricultural Technology, and Faculty of Dentistry Brawijaya University.
AUTHOR CONTRIBUTIONS
All authors conceived and designed the study. Muhammad Chair Effendi, Fredy Mardiyantoro, Novi Khila Firani, Ahmad Taufiq, and Ibnu Putra Nugraha performed material preparation, conducted the experiments, collected and analyzed the data. The final draft of the manuscript was written by Muhammad Chair Effendi and Ahmad Taufiq, and all authors commented on previous versions of the manuscript. All authors approved the final version of the manuscript and agree to held accountable for the content therein.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Ahmed, A.U. 2011. An overview of inflammation: mechanism and consequences. Frontiers in Biology. 6: 274-281
Alfaro, A.T., Balbinot, E., Weber, C.I., Tonial, I.B., Machado-Lunkes, A. 2014. Fish gelatin: characteristics, function properties, applications and future potentials. Food Engineering Reviews. 7: 33-44.
Andriani, Y., Zahidah, Dhahiyat, Y., Hamdani, H., Subhan, U. 2019. Growth of juvenile striped catfish (Pangasius hyphophtalamus) and water quality in aquaponics system. AJFAR 5: 1-7.
Arifin, W.N., Zahiruddin, W.M. 2017. Sample size calculation in animal studies using resource equation approach. Malaysian Journal of Medical Sciences. 24: 101-105.
Beauchamp, Evers, Mattox. 2017. Sabiston textbook of surgery. The biological basis of modern surgical practice. 20th ed. Elsevier, Philadelphia.
Chaplin, D.D. 2010. Overview of the immune response. Journal of Allergy and Clinical Immunology. 125: S3-23.
Chow, O., Barbul, A. 2014. Immunonutrition: role in wound healing and tissue regeneration. Advances Wound Care New Rochelle. 3: 46-53.
Gullapalli, R.P. 2010. Soft gelatin capsules (softgels). Journal of Pharmaceutical Sciences. 99: 4107-4148.
Guyton, A.C., Hall, J.E. 2014. A textbook of medical physiology. 12th ed. In M. Widjajakusumah, A. Tanzil, E. Ilyas (eds). Elsevier Singapore Pte Ltd, Singapore.
Hanani, Z.A.N. 2016. gelatin. p. 191-195. In B. Caballero, P.M. Finglas, F. Toldra (eds). Encyclopedia of Food and Health. American Press, Waltham.
Khoswanto, C. 2020. Hypoxia inducible factor 1α as key factor in wound healing post tooth extraction: an overview. Journal of International Dental and Medical Research. 13: 1191-1197.
Kim, S.Y. and Nair, M.G. 2019. Macrophages in wound healing: activation and plasticity. Immunology and Cell Biology. 97: 258-267.
Kordik, M.G.H. 2005. Patin fish cultivation, biology, hatchery and rearing. Yayasan Pustaka Nusantara, Yogyakarta.
Krzyszczyk, P., Schloss, R., Palmer, A., and Berthiaume, F. 2018. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Frontiers in Physiology. 9: 1-22.
Oki, A.S., Bimarahmanda, M.E., and Rahardjo, M.B. 2018. Increased number of fibroblasts and neovascularization after tooth extraction in wistar rats with moderate-intensity continuous exercise. Journal of International Dental and Medical Research. 11: 840-845.
Orakpoghenor, O., Avazi, D.O., Markus, T.P., and Olaolu, O.S. 2019. Lymphocytes: a brief review. Scientific Journal of Immunology & Immunotherapy. 3: 4-8.
Prinyakupt, J. and Pluempitiwiriyawej, C. 2015. segmentation of white blood cells and comparison of cell morphology by linear and naïve bayes classifiers. BioMedical Engineering OnLine. 14:1-19.
Ratnasari, I., Yuwono, S., Nusyam, H., and Widjanarko, S. 2013. Extraction and characterization of gelatin from different fresh water fishes as alternative sources of gelatin. International Food Research Journal. 20: 3085-3091.
Schultz, G.S., Chin, G.A., Moldawer, L., et al. 2011. Principles of wound healing. p. 23. In R. Fitridge, M. Thompson (eds). Mechanism of Vascular Disease: A reference book for vascular specialists. University of Adelaide Press, Adelaide.
Widyastomo, Wulan, K., and Sari, I. 2013. The effect of sweet star fruit juice (Averrhoa carambola linn.) on the increase in the number of fibroblasts in the socket of wistar strain rat after tooth extraction. Prodenta Journal of Dentistry. 1: 62-70.
Wolfensohn, S. and Lloyd, M. 2013. Handbook of laboratory animal management and welfare. 4th ed. Wiley-Blackwell, West Sussex.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Muhammad Chair Effendi1,*, Fredy Mardiyantoro2, Novi Khila Firani3, Ahmad Taufiq4, and Ibnu Putra Nugraha2
1 Department of Pediatric Dentistry, Faculty of Dentistry, Universitas Brawijaya, Malang 65145, Indonesia
2 Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Universitas Brawijaya, Malang 65145, Indonesia
3 Department of Oral Biology, Faculty of Dentistry, Universitas Brawijaya, Malang 65145, Indonesia
4 Department of Physics, Faculty of Mathematics and Natural Sciences, Universitas Negeri Malang 65145, Indonesia
Corresponding author: Muhammad Chair Effendi, E-mail: chair.fk@ub.ac.id
Total Article Views
Editor: Anak Iamaroon,
Chiang Mai University, Thailand
Article history:
Received: January 14, 2021;
Revised: August 19, 2021;
Accepted: December 27, 2021;
Published online: January 27, 2022

