
Determination of Proximate Composition, Phytochemical Contents, Amino Acid Profile, and Functional Characteristics of Landolphia togolana Root Bark as a Potential Functional Food
Deborah O. Opaleke*, Lilian I. Salami, Ekemini E. Uko-Aviomoh, Oluwatosin A. Ijabadeniyi, and Abimbola K. ArisePublished Date : 2022-01-21
DOI : https://doi.org/10.12982/CMUJNS.2022.020
Journal Issues : Number 1, January-March 2022
Abstract Overtime, consumers have become more concerned with eating a nutritious diet in order to improve their well-being. In this regard, plant bioactive chemicals with functional activity might play a crucial role in preventing acute and chronic health diseases in addition to their usual nutritional functions. As a result, researchers, health experts, and regulatory agencies are seeking known and yet to be identified plants for potential use as functional foods. Therefore, this study evaluated the nutritional composition of Landolphia togolana. L. togolona root procured from a commercial farm was extracted using methanolic extraction and subjected to laboratory analyses to check for its proximate composition, phytochemical screening, mineral composition, amino acid profiling, and functional characteristics. Results showed that L. togolona is a good source of carbohydrate (40.03). The highest and lowest minerals were iron (36.3 mg/100g) and calcium (0.86 mg/100g) respectively, and the higher contents of thiamine (39.28 µg/100g) and α-tocopherol (52.98 µg/100g). Secondary plant metabolites found were flavonoids (392.49 mg QE/g), steroids (126.00 g/100 mL), triterpenes (95.68 g/mL), alkaloids (0.41 g/100mL), saponins (0.821 g/100 mL), tannins (148.8 mgTA/g), and glycosides (0.04 g/100mL). Amino acid profiling revealed significant levels of essential amino acids such as glutamine (7.8 mg/100g), histidine (3.90 mg/100g), and proline (3.60 mg/100g). Further results showed a high swelling index (SI = 2.21) and water absorption capacity (WAC = 3.10). Analysis of color indicated values for L* a* b*. L. togolana is a rich source of phytochemicals, vitamins, and amino acids and could be well integrated in local diets.
Keywords: Functional food, Landolphia togolana, Phytochemical, Proximate, Thickeners
Citation: Opaleke, D.O., Salami, L.I., Uko-Aviomoh, E.E., Ijabadeniyi, O.A., and Arise, A.K. 2022. Determination of proximate composition, phytochemical contents, amino acid profile, and functional characteristics of Landolphia togolana root bark as a potential functional food. CMU J. Nat. Sci. 21(1): e2022020.
INTRODUCTION
Plants high in phytonutrients and bioactive chemicals have been used in many civilizations, particularly in developing countries where herbal medicine is the primary source of healthcare for 80 percent of the population due to its low cost and widespread availability (Ekor, 2014; Ahmed et al., 2021). Plant seeds, leaves, and other plant parts are employed in local diets and in medication used for treating a variety of illnesses, thus making them highly valued as a panacea to treat several illnesses such as hemorrhage, diabetes, and cardiovascular disease (Sofowora et al., 2013; Shaito et al., 2020). Plant-based bioactive plants could serve as natural remedies to the management of some diseases such as hyperuricemia and hyperlipidemia (Gong et al., 2020; Jiang et al., 2020). Similarly, Mohamad et al. (2020) asserted that bioactive plants are still in extensive use in traditional applications, particularly in childbirth. Furthermore, phytochemical investigations of medicinal plants have shown that extracts and fractions exhibit significant pharmacological activity against malaria parasites, leishmaniasis, and trypanosomiasis (Imieje et al., 2017, Ogbeide et al., 2018). There are a good number of medicinal plants used as antifertility agents in different tribes and regions of the world (Daniyal and Akram, 2015), and particularly, the abortifacient, antipyretic, and antiasthmatic activities of extracts of Piper nigrum have been studied (Mishra and Singh, 2009).
Recent reports have emphasized that plant-based phytochemicals have positive effects on numerous postpartum effects of nursing mothers when supplied and incorporated into their diets. These plants are often used as soup thickeners and as concoctions to improve postpartum health. Amornlerdpison et al. (2020) observed that banana inflorescence is consumed as a traditional Thai cuisine for milk lactation in maternal breastfeeding. The researchers affirmed that banana inflorescence is a beneficial health food supplement for general consumers and could be an alternative drink for postpartum mothers who breastfeed their infants. Besides lactation in postpartum mothers, bioactive plants such as aegle marmelos contain diverse molecules such as essential oils, coumarins, furoquinoline, alkaloids, triterpenoids, tannins, and sterols (Jain et al. 2011). Similarly, cinnamomum zeylanicum is used in uterine hemorrhage, stomachache, as an antiseptic and an astringent. Some of these properties of the plant have been validated scientifically, though some are particular to a specific place. Another prominent functional food is Landolphia togolana, known for its nutritional and antioxidant potential (Kossivi et al., 2015). However, little or no scientific information exists on the nutritional composition of this plant.
L. togolana is a climber that belongs to the family of Apocynaceae, commonly called vine rubber. Landolphia has about 60 species that are found throughout Africa, and it is known locally in Nigeria by several names, including Ibo – Eso/utu, Yoruba–Mba/Alakitipa, and Hausa–Ciwa. It is commonly found in the rainforest region of Nigeria and other parts of the African countries of Tanzania and Madagascar. Medicinally, the leaves are used as purgatives and to cure malaria, ulcers, and the root, when soaked in local gin for a week, is used to treat gonorrhea, and a decoction is applied to relieve sprains (Owoyele et al., 2001). L. togolana root powder is usually used in soup preparation as a thickener by women in western Nigeria to cure and prevent diseases associated with the postpartum period, such as dizziness, hormonal disorders, weakness of internal organs, blood loss, relieving pain and stress undergone during child birth, and maintaining the wellness of postpartum mothers.
Nwokonkwo (2014) and Matemu et al. (2017) studied the nutritional and phytochemical contents of some plant-based medicinal plants and found out that Landolphia contained some proximate and phytochemical components which could be a potent tool for solving several health-related problems. In a more recent study, Odoh and Agbachi (2020) evaluated the quantitative phytochemical analysis of Landolphia owariensis. Their results indicated the presence of carbohydrates, alkaloids, glycosides, saponins, flavonoids, resins, proteins, steroids and terpenoids. Despite the wide applications of using L. togolana, and previous reports on L. owariensis; thus, in this study, L. togolana root was extracted to determine its proximate composition, phytochemical screening, amino acid profiling, and proximate parameters determination in order to validate its ethnomedicinal claims as a functional food.
MATERIAL AND METHODS
Materials
The plant was collected from Adelabu Salami’s farm in Owo (7°11'46.32" N 5°35'12.52" E) Owo Local Government Area of Ondo State, Nigeria. It was identified at the Department of Plant Biology and Biotechnology, Faculty of Life Science, University of Benin, Benin City, Nigeria.
Sample preparation
The roots of the plant were properly washed under running tap water and the root bark removed and air dried for 72 hours. The bark was then oven dried for 2 hours at 50 °C and pulverized with a Hammer mill (Retsch GmbH 5657, Germany). The pulverized plant sample was sieved with a 60mesh sieved. The sample was kept in an airtight container until ready for use.
Proximate analysis
The quantitative parameters were carried out using the Association of Analytical Chemists (AOAC) standard procedures.
Moisture content
The powdered sample (2 g) was weighed into a clean, dry crucible of known weight. The crucible with its content was oven dried at 105°C until a constant weight was reached. The moisture content was estimated by average percentage weight loss as shown in equation 1 as
![]()
Total ash
Twelve crucibles were washed thoroughly, dried in a hot oven at 100°C. While still warm, they were marked numbers 1 to 12 and subsequently cooled in desiccator and weighed. 2 g of the powdered sample was weighed into each crucible. The crucibles with its content were ashed in the furnace at 600°C for 6 hours. After which the furnace was switched off and the temperature allowed to drop. Thereafter the crucibles were removed, cooled in a desiccator and reweighed. The percentage ash is calculated using equation 2 as.
![]()
Where W is the weight of the crucible and ash; Z is the weight of empty crucible; and N is the weight of sample.
Crude fibre
The dried plant material was ground to uniform fineness and was passed through a stainless-steel mesh sieve (No. 20). 3 g of the sample was defatted with petroleum ether in a Soxhlet apparatus. The defatted sample was digested with 1.25% H2SO4 and 1.25% NaOH solutions. After digestion, the sample was dried in an oven at 130°C for 2 hours and then ignited at 600°C for 30 minutes. The loss in sample weight on ignition and the weight of the ground sample before defatting were utilized to determine the % crude fibre content from equation 3.
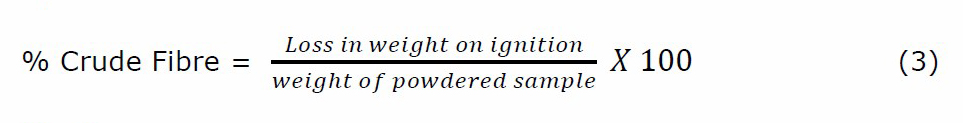
Lipid
The sample was extracted with petroleum ether (100 mL) under reflux for 3 hours by heating on an electric hot plate at 50ºC. After exhaustive extraction, petroleum ether was evaporated to dryness, and the lipid was recovered by cooling the flask in a desiccator, and its value was calculated by reweighing the flask and its content. The percentage of lipid content in the plant sample was calculated using equation 4
![]()
Protein
The nitrogen value which is the precursor for protein of a substance was determined by Micro-Kjeldahl method
Carbohydrate
Carbohydrate was determined by difference method as stated in the procedures of Odoh and Agbachi (2020). The sum total of the moisture, fat, protein, and ash content of each part of the samples were subtracted from 100 as follows: Carbohydrate content = 100 – [protein (%) + fat (%) + moisture (%) + ash (%)].
Phytochemical screening
100 g of the powdered sample was macerated with hot water and filtered with Whatman No.1 filter paper. The filtrate was used to test for different plant secondary metabolites such as alkaloids, flavonoids, saponins, tannins, triterpenes, steroids using standard procedures stated in AOAC (2005) and Odebiyi and Sofowora (1978).
Extraction of plant material
3 kg of the powdered plant sample was extracted with 7.5 L of 95% methanol by maceration at room temperature of 25°C for 72 hours. The extract was filtered and the filtrate concentrated in vacuo with a rotary evaporator (RE 300, Bibby Scientific, UK) at 40°C, and the crude extract yield was noted.
Quantitative phytochemicals determination
Flavonoids
The flavonoid content of the L. togolana root bark sample was determined according to the method described by Ebrahimzadeh et al. (2008). In this method, 0.5 mL of the extract solution (1 mg/mL) was mixed with 1.5 mL of methanol, followed by 0.1 mL of 10% aluminum chloride, 0.1 mL of 1M potassium acetate and 2.8 mL of distilled water. The mixture was incubated at room temperature for 30 minutes. The absorbance was measured with a spectrophotometer at 415 nm. A triplicate determination was carried out. The standardized curve was prepared using Quercetin as a standard agent. The result was expressed as milligram Quercetin per gram of extract (mg QE/g extract).
Alkaloids
This was done according to the method of Harborne (1973). Exactly 200 mL of 10% acetic acid in ethanol was added to each 2.5 g of the powder sample in a 250 mL beaker and allowed to stand for 4 hours. The extract was concentrated in a water bath to one-quarter of the original volume, and concentrated ammonium hydroxide was added dropwise until precipitate formation was complete. After 3 hours of mixture sedimentation, the supernatant was discarded and the precipitates were washed with 20 mL of 0.1 M ammonium hydroxide and then filtered. After drying the residue in an oven, the percentage yield of alkaloid was calculated using equation 5.
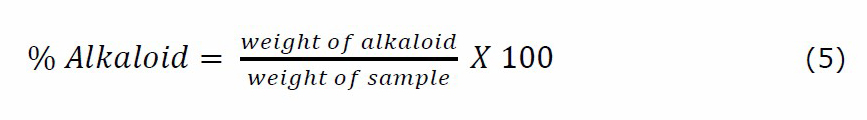
Tannins
The tannin content of the powdered sample was determined by a modified method of Peri and Pompei (Roghini and Vijayalakshmi, 2014). From a 1 mg/mL solution of the extract, 1mL was transferred into a test tube and 1 mL of distilled water was added. This was followed by the addition of 0.5 mL of Folin’s phenol reagent (1:2) and 5 mL of 35% sodium carbonate. The mixture was allowed to stand at room temperature for 5 min. The absorbance of the resulting solution was measured at 640 nm. Gallic acid (0.1-1 mg/mL) was used to generate a calibration curve from which the tannin content of the extract was determined. The total tannin content was expressed in mg/g of extract.
Saponins
The saponin content was determined by the method of Obadoni and Ochuko (2002). 20 g of the powdered sample was transferred into a conical flask to which 100 mL of 20% ethanol was added. The sample was heated over a hot water bath for 4 hours with continuous stirring at about 55°C. The mixture was filtered and the residue re-extracted with another 200 mL of 20% ethanol. The combined extracts are reduced to 40 mL over a hot water bath (90°C). The concentrate is then transferred into a 250 mL separating funnel and extracted with 20 mL x 4 of diethyl ether. The aqueous layer was recovered while the diethyl ether layer was discarded. To the aqueous layer, 60 mL of n-butanol was added, and the combined n-butanol extracts were washed twice with 10 mL of 5% sodium chloride. The remaining solution was then evaporated over hot water and finally dried in an oven to a constant weight. Values are expressed as mg/g of extract.
Terpenoids content
The terpenoid content of the plant was determined using the method of Indumathi et al. (2014). 100 mg was soaked in 9 mL of ethanol for 24 hours. The extract was filtered and the filtrate was extracted with 10 mL of petroleum ether using a separating funnel. The ether extract was separated in pre-weighed glass vials and allowed to dry completely. The percentage content of the terpenoids was calculated from the equation 6
![]()
Where PS and DE are the is the powdered dried samples respectively.
Amino acid profile
The amino acid profile of the sample was determined according to the method described by Vázquez-Ortiz et al. (1995). 3 mg of L. togolona was hydrolysed with 6 M HCl at 150°C for 6 hours. After hydrolysis, the acid was removed under vacuum with a rotary evaporator (RE500 Yamato Scientific America Inc.). The sample was resuspended in 2 mL of sodium citrate buffer, pH 2.2. Sample derivatization was carried out by adding o-phthalaldehyde (OPA) 7.5 mM to the sample in citrate buffer (OPA reagent contains β-mercaptoethanol and Brij 35). The amino acid constituents of the sample were evaluated by HPLC using external and internal standards. The amino acid reference standard consisting of fifteen amino acids (0.05 μmoles mL-1 each amino acid) was utilised to determine the retention times for each amino acid. An internal standard, α- aminobutyric (0.05μmoles mL-1), was added to the amino acid reference standard and the plant sample to normalize and quantify the amino acid content. A gradient mobile phase of sodium acetate 0.1 M pH 7.2 and methanol (9:1) was used to elute the sample for amino acid separation through C18 column reversed-phase octadecyl dimethylsilane particles (100 x 4.6 mm x 1/4" Microsorb 100-3C18). Fluorescence detection was used at an excitation-emission wavelength of 360 and 455 nm, respectively. Star Chromatography work station (Varian version 5.51) software was used to achieve amino acid peak integration.
Functional properties of the root powder of L. togolana
Water and oil absorption capacity
Water and oil absorption capacity was determined using Suraiya et al. (2016) method. 1 g of the sample was weighed into a dry centrifuge tubed in triplicate, and then distilled water/oil was added to the sample powder to make up to 10 mL. It was vortexed for 30 seconds and then centrifuged at 4000 rpm for 40 min using a centrifuge (5810 R, 15-amp version, eppendorf AG, Barkhausenweg 1, 22339 Hamburg, Germany). The supernatant was discarded while the tube with its content was reweighed. The gain in mass is the water/oil absorption capacity of the sample. It was done in triplicates. The water and oil absorption capacity (%) was calculated using equation 7.
![]()
Where W1 is the weight of residue in a tube after centrifuge; W2 is the weight of empty tube before centrifuge Ws is the weight of sample used.
Bulk density
This was determined by the method described by Al Shammary et al. (2018). 3 g of the sample was placed in a 10 mL graduated cylinder. The cylinder was tapped several times with rubber tubing. The volume of the starch powder after tapping was recorded, and bulk density (g/mL), calculated as the weight of the powdered sample (g) per unit volume (mL).
Statistical analysis
The statistical analysis was carried out using Microsoft Excel software (Version 2015). Experimental observations were carried out in triplicate, while the results were expressed as mean ± standard error of the mean (SEM).
RESULTS
Proximate and mineral compositions of L. togolana
Table 1 shows the proximate composition of the dried pulverized root powdered of methanol extract. The result shows that carbohydrates (40.03 ± 0.25) and fibre (33.67%) form a major component of L. togolana root because of their high content (Table 1). Ash, protein, fat, and moisture content were 11.05%, 8.00%, 4.52%, 2.73% respectively (Table 1)
Table 1. Proximate composition of L. togolana root bark.
|
Proximate Composition |
Amount (%) |
|
Moisture Content |
2.73 ± 0.01 |
|
Total Ash |
11.05 ± 0.02 |
|
Crude Fibre |
33.67 ± 0.04 |
|
Lipid |
4.52 ± 0.01 |
|
Carbohydrate |
40.03 ± 0.25 |
|
Protein |
8.00 ± 0.01 |
Note: Values are expressed as Mean ± SEM, n = 3.
The mineral composition of the root of L. togolana is presented in Table 2. Iron (Fe) had the highest (36.3 mg/100g) value among the minerals, followed by sodium (19.24 mg/kg). Fluorine had the lowest mineral content (0.11%). Other observed minerals were sulphur (0.52%), nitrogen (0.98%), calcium (0.86%).
Table 2. Mineral content of L. togolana root extract.
|
Element |
Amount |
Element |
Amount |
|
Sulphur |
0.52 % |
Copper |
1.70 mg/kg |
|
Nitrogen |
0.98 % |
Iron |
36.30 mg/kg |
|
Calcium |
0.86 % |
Fluorine |
0.11 % |
|
Magnesium |
0.79 % |
Zinc |
13.00 mg/kg |
|
Potassium |
3.94 % |
Manganese |
13.30 mg/kg |
|
Sodium |
19.24 mg/kg |
Aluminium |
2.40 mg/kg |
Vitamins and phytochemical compositions of L. togolana
Vitamin E (α-tocopherol) was estimated to have the highest concentration of (52.98 µg/100g) in the sample, followed by thiamine (39.28 µg/100g). Niacin had the lowest (0.02 µg/100g) concentration (Table 3). The γ-carotenoid concentration (16.097 µg/100g) was higher compared to α and β carotenoid. Niacin and riboflavin, a major component of vitamin B contents were significantly much lower (less than 0.10 µg/100g). It was, however, observed that axerophtol was not present in L. togolana (Table 3).
Table 3. Estimation of the vitamin contents of Landolphia togolana root extract.
|
Vitamins |
Properties |
Amount (µg/100g) |
|
A |
α-carotenoid |
0.36 |
|
β-carotenoid |
0.87 |
|
|
γ-carotenoid |
16.10 |
|
|
Axerophtol |
ABS |
|
|
B |
Niacin |
0.02 |
|
Pyrindoxine |
20.86 |
|
|
Thiamine |
39.28 |
|
|
Folic Acid |
15.90 |
|
|
Cyanocobalamin |
10.33 |
|
|
Riboflavin |
0.06 |
|
|
E |
α-tocopherol |
52.98 |
Note: A ‒ vitamin A; B ‒ vitamin B; E ‒ vitamin E; ABS - Absent
Phytochemical analysis showed higher total flavonoids (392.49 ± 0.67 mg/g) followed by tannins (148.80 ± 0.59 mg/g) while saponin, alkaloids, and glycosides showed low concentrations. Phlobatannin and fixed oil were obviously absent in the phytochemical analysis.
Table 4. Determination of qualitative and quantitative phytochemical analysis of methanol extract of L. togolana root
|
Phytochemicals |
Quantitative concentration |
|
Saponins |
0.82 ± 0.03 g/100 ml |
|
Steroid |
126.00 ± 0.39 g/100 ml |
|
Phlobatannin |
ABS |
|
Tannins |
148.80 ± 0.59 mg TA/g extract |
|
Flavonoids |
392.49 ± 0.67 mg QE/g extract |
|
Coumarins |
35.14 ± 0.17 mg GA/g extract |
|
Terpenoids |
59.36 ± 0.19 g/100 ml |
|
Glycosides |
0.03 ± 0.01 g/100 ml |
|
Triterpene |
95.68 ± 0.84 g/100 ml |
|
Fixed oil |
ABS |
|
Alkaloids |
0.41 ± 0.02 g/100 ml |
Note: Values are expressed as Mean ± SEM, n =3, ABS = Absent
Amino acid profile
The result of the amino acid profile showed glutamate to be the highest (7.8 mg/100g) concentration, followed by histidine (3.90 mg/100g) (Figure 1). Indicative properties of the amino acids show they are significantly different from each other, except for leucine (LEU) and phenylalanine (PHE).
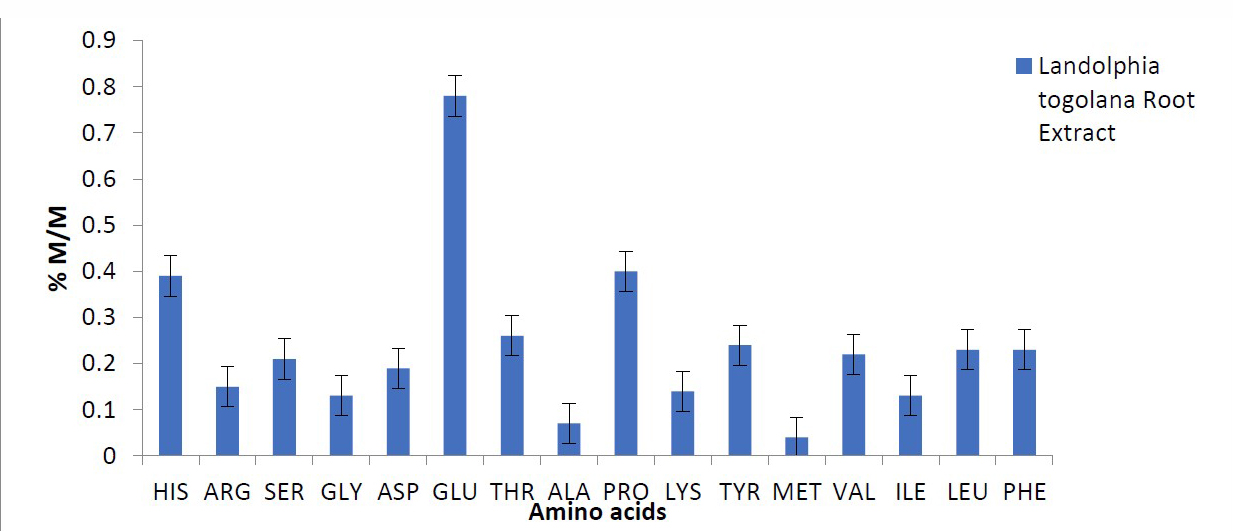
Figure 1. Amino acid profiling.
Functional properties of L. togolana
The mean score of the functional properties of two concentrations of Landolphia togolana root extract (20% and 80%) is presented in Table 5. The bulk density and oil absorption capacity both decreased from 0.73 to 0.67 g/ml and 1.73 to 1.70g/g, respectively. Conversely, the mean score for water absorption capacity, pH, swelling index, and solubility of Landolphia togolana powdered root increased from 3.04 to 3.10 g of water/g flour, 6.72 to 6.84, 1.53 to 2.21 g/ml and 29.18 to 29.30 respectively, with higher concentration of L. togolana powdered root (20% to 80%).
Table 5. Mean score of the functional properties of different concentrates of Landolphia togolana root extract.
|
Samples |
BD |
WAC |
OAC |
pH |
SI |
Solubility |
|
20 |
0.73 ± 0.00 |
3.04 ± 0.10 |
1.73 ± 0.03 |
6.72 ± 0.01 |
1.53 ± 0.01 |
29.18 ± 0.01 |
|
80 |
0.67 ± 0.00 |
3.10 ± 0.03 |
1.70 ± 0.03 |
6.84 ± 0.01 |
2.21 ± 0.01 |
29.30 ± 0.01 |
Note: BD ‒ Bulk density; WAC ‒ Water absorption capacity; OAC ‒ Oil absorption capacity; pH ‒ Potential of hydrogen; SI ‒ Swelling index.
Color determination
The result for the color determination of L. togolana is presented in Table 6. The color showed that the average values for L, a and b were 48.66, 6.60 and 9.57 respectively.
Table 6. Color determination.
|
Color Indices |
20% |
80% |
|
L* |
48.52 ± 0.00 |
48.80 ± 0.00 |
|
a* |
6.46 ± 0.00 |
6.74 ± 0.02 |
|
b* |
9.42 ± 0.10 |
9.72 ± 0.06 |
DISCUSSION
The presence of fibre in any given food helps to regulate bowel movements, which is important for digestive health. In addition, findings from this study also indicated that the presence of carbohydrates in L. togolana makes it a source of energy. The fibre content of L. togolana obtained in this study was higher than that of L. Owariensis seed in the report of Evelyn et al. (2019). Likewise, Odoh and Agbachi (2020) reported a higher difference in the fibre content of L. owariensis. Carbohydrate, protein, and ash contents of L. owariensis seed reported by Odoh and Agbachi (2020) were higher compared to values obtained in this study.
Iron is an essential trace metal and plays numerous biochemical roles in the body. This may be the reason for its wide consumption in the study area among the post-partum mothers, because their main aim of consumption was to replenish the lost blood during delivery. Iron is required for hemoglobin formation and its deficiency causes anemia, weakness, poor resistance to infection and in women, may cause infertility (Nikzad et al, 2018). 36.3 mg/100 g, which was above the recommended daily allowance (RDA) of 8 mg/day for adult males and up to 28 mg/day for pregnant women (Akesson et al., 1998) and also the FAO/WHO (1988) recommended 20-24 mg of iron per day. The macronutrients varied considerably in the plant sample with several biological functions. They are required in large amounts to provide the energy needed to maintain body functions and carry out the activities of daily life during the post-partum period. The presence of magnesium in the sample was 0.79 g/100g and it is involved in the formation and function of bones and muscles. It also prevents high blood pressure and heart diseases (Jahnen and Ketteler, 2012).
Vitamins are essential components of food which are shown to have anti-infective properties, promote wound healing, and may boost the immune system (Wright, 2002). Vitamin E is a well-known antioxidant that helps reduce pain and blood loss during the post-partum period and protects the cell membrane from oxidative damage and maintains cellular functioning. This vitamin also protects the food from oxidative damage during storage and processing (Guyton and Hall, 2006). Findings from this study show that Landolphia togolana is a rich source of vitamin A compared to standard vitamin A (Axerophtol) with a ratio of 1:4. Vitamin A is an essential nutrient needed in small amounts by humans for the normal functioning of the visual system, growth and maintenance of epithelial cellular integrity, immune function, and reproduction (FAO, 2004). Tardy et al. (2019) and Adeyemi et al. (2018) reported that some vitamins contribute to the prevention and maintenance of well-being of humans and other diet-related diseases. In the current study, the determination of a broad spectrum of water-soluble vitamins (B-Complex) revealed that thiamine (vitamin B1), which functions as the coenzyme thiamine pyrophosphate (TPP) in the metabolism of carbohydrates and branched-chain amino acids, is present. Deficiency of thiamine can cause beriberi (Attaluri et al., 2018).
Phytochemical qualitative and quantitative investigation of L. togolana root demonstrates the presence of nine secondary metabolites (saponins, phenols, steroids, cumarins, terpenoids, glycosides, and triterpenes) as presented in Table 4. However, phlobatannin and fixed oil were not observed in the methanol extract of L. togolana root. Flavonoids act as a preventative measure by balancing inflammation and lowering the risk of certain diseases. These phytochemicals are known to exhibit medicinal and physiological activities, confirming their usefulness in traditional soup thickeners for treating, preventing, and managing some diseases associated with well-being during the post-partum period. This further affirmed the reasons for using L. togolana as a soup thickener during the post-partum period among some natives. The presence of saponins in the methanol extract of L. togolana root supports its use as an additive agent, anti-inflammatory and anti-tumor activities. Steroids are a group of hormones derived from cholesterol that act as chemical messengers in the body. They regulate a wide range of physiological activities, development, and the function of the reproductive system. The results obtained for steroids in this study are higher than the daily recommended allowance of 40-60 mg and 100 mg (Desai et al., 2013). More so, the presence of terpenoids and steroids gives an indication that this L. togolana could be active against bacteria such as Staphylococcus aureus (Uddin et al., 2012; Ahmad et al., 2018). Notably, plants containing these two compounds have the potential as soup thickeners for people that suffer from low steroid hormones.
The result showed that the plant root contains essential amino acids that are of biological importance in a relatively small amount. Glutamate helps with metabolism and brain function. (Akram et al., 2011). Amino acids play a key regulatory role in numerous metabolic processes. They act as potent hormone secretors, stimulating the growth hormone. However, some amino acids have specific functions, with glutamate playing a key role in the biosynthesis and degradation of amino acids (Walker and van der Donk, 2019). In the present study, it was observed that L. togolona contained essential amino acids in relatively large amounts.
Low bulk density would be an advantage in the formulation of complementary foods (Akapata and Akubor, 1999), while the higher bulk density of Landolphia togolana (20%) suggests its suitability to be used as a thickener in soup preparation since it helps to reduce paste thickness. (Chandra et al., 2015). The result on bulk density is similar to that reported by Eltayeb (2011). The water absorption capacity in this study agreed with the findings of Ashraf et al. (2012). Low water absorption observed in the sample obtained from L. togolana could be due to the high proportion of hydrophilic groups and polar amino acids on the surface of the protein molecules (Ashraf et al., 2012) and the increase may be due to the water binding properties of the sample. Butt and Batool (2010) reported that the increase in the water absorption capacity from the sample with 20% of L. togolana powdered root to the one with 80% was due to the increase in the amylose leaching and solubility, and loss of starch crystalline structure. There is also the likelihood that the sample with an 80% concentration of L. togolana powdered root has more hydrophilic constituents such as polysaccharides. As such, samples with high water absorption capacity may prove useful in products where good viscosity is required, such as soups. Solubility is directly proportional to the water absorption capacity. Solubility of Landolphia togolana extract is consistent with the findings of Butt and Batool (2010). The observed difference in the solubility of samples may be due to the degree of interaction with water. The low oil absorption capacity seen in the sample could be due to the presence of a hydrophilic group from L. togolana incorporated into the sample. Protein concentration and their conformational properties in foods also influence fat absorption (Ashraf et al., 2012).
Swelling capacity and solubility represent the extent of interaction between starch chains within the amorphous and crystalline domains of the starch granule (Ratnayake et al., 2002). In this study, it was estimated that the sample had a high amount of carbohydrates, 40.03%, which could be the source of starch responsible for the swelling index. Furthermore, it is influenced by amylose and amylopectin characteristics (Chan et al., 2009). The low swelling capacity of the sample obtained from L. togolana root suggests the presence of stronger bonding forces within the interiors of starch granules and more amylose lipid complex. This could be responsible for the low swelling capacity of the sample. Consequently, the solubility of the sample was 29.24%, which is relatively high. The solubility of the sample in this study may also be due to the presence of a high amount of protein in the powder that could form inclusion complexes with amylose. The potential of hydrogen (pH) of the sample is very important because it affects most of the functional properties of food (Eleazu and Ironua, 2013). The mean score of pH is consistent with the findings of Okonkwo et al. (2014) who reported a pH of 6.60 ± 0.06 at a significant value of 5% for L. owariensis. The pH revealed very weak acidity, which correlates well with the 6.78% very low acid value of the samples. This shows that L. togolona is a non-toxic substance and hence safe for human consumption.
Higher b* value has been reported to be an indication of presence of higher ash content (Kaur and Singh, 2007) which correlate with our study of ash contents was 11.05%.
CONCLUSION
The present study has provided some biochemical information on nutritional composition and functional properties of Landolphia togolana root specie. Findings revealed that the root of L. togolana could serve as a cheap supplement for vitamins B1, B2 and E, iron, carbohydrate and fiber for vulnerable population if properly utilized. Also, L. togolana is a rich source of phytochemicals, vitamins, and amino acids and could be well integrated in local diets.
ACKNOWLEDGEMENTS
The authors wish to thank Betty Ajibade, Ibilola ltiolu, and Opeyemi Alabi for their contributions during the laboratory work. This research was funded by the TETFUND Scholarship grant through the University of Ilorin, Nigeria and the National research foundation of South Africa through the research program of Professor Ijabadeniyi Oluwatosin of the Department of Food Technology, Durban University of Technology, Durban, South Africa.
REFERENCES
Adeyemi, D., Temitope, K., Johnson, O., and Ayoola, G. 2018. HPLC quantification of ascorbic acid, thiamine and nicotinamide in some local edible fruits. West African Journal of Pharmacy. 29: 140–146.
Ahmad, M., Butt, M.A., Zhang, G., Sultana, S., Tariq, A., and Zafar, M. 2018. Bergenia ciliata: A comprehensive review of its traditional uses, phytochemistry, pharmacology and safety. Biomedicine & Pharmacotherapy. 97: 708–721.
Ahmed, S.R.., Rabbee, M.F., Roy, A., Chowdhury, R., Banik, A., Kubra, K., Hassan-Chowdhury, M. M., and Baek, K. -H. 2021. Therapeutic promises of medicinal plants in Bangladesh and their bioactive compounds against ulcers and inflammatory diseases. Plants, 10: 1-30.
Akapata, M.I., and Akubor, P.I. 1999. Chemical composition and selected functional properties of sweet orange (Citrus sinensis) seed flour. Plant Food and Human Nutrition, 54: 353–362.
Akesson A., Bjellerup P., Berglund, M., Bremme, K., and Vahter, M. 1998. Serum transferring receptor: A specific marker of iron deficiency in pregnancy. American Journal of Clinical Nutrition, 68: 1241–1246.
Akram, M., Asif, H.M, Uzair, M., Akhtar, N., Madni, A., Shah, S.M.A., Hasan, Z., and Ullah, A. 2011. Amino acids: A review. Journal of Medicinal Plants Research. 5: 3997–4000.
Al-Shammary, A.A.G., Kouzani, A.Z., Kaynak, A., Khoo, S.Y., Norton, M., and Gates, W. 2018. Soil bulk density estimation methods: A review. Pedosphere, 28: 581–596.
Amornlerdpison, D., Choommongkol, V., Narkprasom, K., and Yimyam, S. 2021. Bioactive compounds and antioxidant properties of banana inflorescence in a beverage for maternal breastfeeding. Applied Science, 11: 1-8.
AOAC. 2005. Official methods of the association of analytical chemists. Association of Official Analytical Chemists, Washington D.C.
Ashraf, S., Saeed, S.M.G., Sayeed, S., and Ali. R. 2012. Impact of microwave treatment on the functionality of cereal and legumes. International Journal of Agriculture and Biology, 14: 356–370.
Attaluri, P., Castillo, A., Edriss, H., and Nugent, K. 2018. Thiamine deficiency: An important consideration in critically ill patients. American Journal of Medical Science, 356: 382‒390.
Butt, M.S., and Batool, R. 2010. Nutritional and functional properties of some promising legumes proteins isolates. Pakistan Journal of Nutrition. 9: 373–379.
Chan, H. T., Bhat, R., and Karim, A. A. 2009. Physicochemical and functional properties of ozone-oxidized starch. Journal of Agriculture and Food Chemistry. 57: 5965–5970.
Chandra, S., Singh, S., and Kumari, D. 2015. Evaluation of functional properties of composite flours and sensorial attributes of composite flour biscuits. Journal of Food Science and Technology. 52: 3681–3688.
Daniyal, M., and Akram, M. 2015. Antifertility activity of medicinal plants. Journal of the Chinese Medical Association, 78: 382-388.
Desai, T., Sudhalkar, A., Vyas, U., and Khamar, B. 2013. Methanol poisoning predictors of visual outcomes. Jama Ophthalmology. 131: 358‒364.
Ebrahimzadeh, M.A, Pourmorad F., and Bekhradnia A.R. 2018. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. African Journal of Biotechnology. 7: 3188–3189.
Ekor, M. 2014. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in Pharmacology, 4: 1-10.
Eleazu C.O., and Ironua C. 2013. Physicochemical composition and antioxidant properties of a sweet potato variety (Ipomoea batatas L.) commercially sold in South Eastern Nigeria. African Journal of Biotechnology. 12: 720–727.
Eltayeb, A.R.S.M., Ali, A.O., Abou-Arab, A.A., and Abu-Salem, F.M. 2011. Chemical composition and functional properties of flour and protein isolate extracted from Bambara groundnut (Vigna subterranean). African Journal of Food Science. 5: 82–90.
Evelyn, A.K, Yeboah, M.A, Frimpong, B.M., and Adu, A.G. 2019. Quality control standardization of leaves and root of Landolphia owariensis (Apocynaceae). The Journal of Phytopharmacology. 8: 185-191.
FAO. 2004. Vitamin and mineral requirement in human nutrition. Report of a joint FAO/WHO expert consultation. Bangkok, Thailand,
FAO/WHO. 1988. Requirements of vitamin A, iron, folate and vitamin B12. Report of a joint FAO/WHO expert consultation. Rome, Italy.
Gong, X., Li, X., Xia, Y., Xu, J., Li, Q., Zhang, C., and Li, M. 2020. Effects of phytochemicals from plant-based functional foods on hyperlipidemia and their underpinning mechanisms. Trends in Food Science & Technology, 103: 304-320.
Guyton, A., and Hall, J. 2006. Textbook of medical physiology, Philadelphia (PA): Saunders.
Imieje, V., Zaki, A.A., Fasinu, P.S., Ali, Z., Khan, I.A., Tekwani, B., Khan, S.I., Nosa, E.O. and Falodun, A., 2017. Antiprotozoal and cytotoxicity studies of fractions and compounds from Enantia chlorantha. Tropical Journal of Natural Product Research, 1: 89.
Indumathi, C., Durgadevi, G., Nithyavani, S., and Gayathri, P.K. 2014. Estimation of terpenoid content and its antimicrobial property in Enicostemma litorrale. International Journal of ChemTech Research, 6: 4264-4267.
Jahnen-Dechent, W., and Ketteler, M. 2012. Magnesium basics. Clinical Kidney Journal, 5(Suppl 1): 3–14.
Jain, N., Sharma, V., and Ramawat, K.G. 2011. Therapeutic potentials of medicinal plants traditionally used during postpartum period and their molecular targets. Journal of Ecobiotechnology. Available Online: http://journal-ecobiotechnology.com/
Jiang, L.-L., Gong, X., Ji, M.-Y., Wang, C.-C., Wang, J.-H., and Li, M.-H. 2020. Bioactive compounds from plant-based functional foods: A promising choice for the prevention and management of hyperuricemia. Foods, 9: 1-24.
Kaur, M., and Singh, N. 2007. Characterization of protein isolates from different Indian chickpea (Cicer arietinum L.) cultivars. Food Chemistry. 102: 366–374.
Kossivi, D., Amegnona, A., and Messanvi, G. 2015. Antioxidant and toxicological studies of ethanolic root extract of Byrsocarpus coccineus. Journal of Medicinal Plants Research, 9: 940–949.
Matemu, A., Adeyemi, D., Nyoni, H., Mdee, L., Tshabalala, P., Mamba, B., and Msagati, T. 2017. Fatty acid composition of dried fruits of Sclerocarya birrea, Diospyros blancoi and Landolphia kirkii. International Journal of Environmental Research and Public Health, 14: 1-9.
Mishra, R.K., and Singh, S.K., 2009. Antispermatogenic and antifertility effects of fruits of Piper nigrum L. in mice. Indian Journal of Experimental Biology, 47:706-714.
Mohamad, N.E., Romli, M.F., Alitheen, N.B., Abu, N., Yeap, S.K., Lim, K.L., et al. 2020. Apoptosis and metastasis inhibitory potential of pineapple vinegar against mouse mammary gland cells in vitro and in vivo. Nutrition and Metabolism, 1649.
Nikzad, Z., Iravani, M., Abedi, P., Shahbazian, N., and Saki, A. 2018. The relationship between iron deficiency anemia and sexual function and satisfaction among reproductive-aged Iranian women. PLOS ONE. 13:
Nwokonkwo, D.C. 2013. Phytochemical screening, antimicrobial properties and proximate analysis of Landolphia owariensis P. Beauv seeds. International Journal of Chemistry; 6: 48-54.
Obadoni, B.O. and Ochuko, P.O. 2002. Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in Edo and Delta states of Nigeria. Global Journal of Pure Applied Sciences, 8: 203-208.
Odebiyi, O.O., and Sofowora, E.A. 1978. Phytochemical screening of Nigerian medicinal plants II. Lloydia, 41: 234–246.
Odoh, U.E., and Agbachi, B.C. 2020. Phytochemical, proximate and nutritive composition analyses of the seed of Landolphia owariensis P. Beauv. (Apocynaceae). World Journal of Innovative Research (WJIR). 8: 104–109.
Ogbeide, O.K., Dickson, V.O., Jebba, R.D., Owhiroro, D.A., Olaoluwa, M.O., Imieje, V.O., Erharuyi, O., Owolabi, B.J., Fasinu, P., and Falodun, A. 2018. Antiplasmodial and acute toxicity studies of fractions and Cassane-Type diterpenoids from the stem bark of Caesalpinia pulcherrima (L.). Tropical Journal of Natural Product Research, 2: 179-184.
Okonkwo, T.J.N., Obonga, W.O., and Okonkwo, C.J.O. 2014. Fatty acid profile and physicochemical properties of Landolphia owariensis P. Beauv stringy seed oil. Tropical Journal of Pharmaceutical Research. 13(8): 1347–1352.
Owoyele, B.V., Olaleye, S.B., Oke, J.M., and Elegbe, R.A. 2001. Anti-inflammatory and analgesic activities of leaf extract of Landolphia owariensis. African Journal of Biomedical Research, 4: 131–133.
Ratnayake, W. S., Hoover, R., and Tom, W. 2002. Pea starch: Composition, structure, and properties. Starch/Starke. 54: 217–234.
Roghini, R., and Vijayalakshmi, K. 2018. Phytochemical screening, quantitative analysis of flavonoids and minerals in ethanolic extract of Citrus paradise. International Journal Pharmaceutical Sciences & Research, 9: 4859-4864.
Shaito, A., Thuan, D.T.B., Phu, H.T., Nguyen, T.H.D., Hasan, H., Halabi, S., …and Pintus, G. 2020. Herbal medicine for cardiovascular diseases: Ffficacy, mechanisms, and safety. Frontiers in Pharmacology, 11: 1-32.
Sofowora, A., Ogunbodede, E., and Onayade, A. 2013. The role and place of medicinal plants in the strategies for disease prevention. African Journal of Traditional, Complementary and Alternative Medicines, 10: 210-229.
Suraiya, J., Qazi, I.M., and Ahmed, I. 2016. Comparative studies on flour proximate compositions and functional properties of selected Pakistani rice varieties. Proceedings of Pakistan Academy of Sciences: B. Life and Environmental Sciences. 53: 47–56.
Tardy, A.L., Pouteau, E., Marquez, D., Yilmaz, C., and Scholey, A. 2020. Vitamins and minerals for energy, fatigue and cognition: A narrative review of the biochemical and clinical evidence. Nutrients. 12(1): 1–35.
Uddin, G., Rauf, A., Arfan, M., Ali, M., Qaisar, M., Saadiq, M., and Atif, M. 2012. Preliminary phytochemical screening and antioxidant activity of Bergenia caliata. Middle-East Journal of Scientific Research. 11: 1140–1142.
Vázquez-Ortiz, F.A., Caire, G., Higuera-Ciapara, I. and Hernández, G., 1995. High-performance liquid chromatographic determination of free amino acids in shrimp. Journal of Liquid Chromatography & Related Technologies, 18: 2059-2068.
Walker, M. C., and van der Donk, W. A. 2015. The many roles of glutamate in metabolism. Journal of Industrial Microbiology & Biotechnology. 43: 419–430.
Wright, K. 2002. Healing foods. In Wu G. 2009. Amino acids: metabolism, functions, and nutrition. Amino Acids (1-17). Scotland: Geddes and Grosset.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Deborah O. Opaleke1,2,3,*, Lilian I. Salami, Ekemini E. Uko-Aviomoh2, Oluwatosin A. Ijabadeniyi3, and Abimbola K. Arise1
1 Department of Home Economics and Food Science, Faculty of Agriculture, University of Ilorin, Kwara State, Nigeria.
2 Department of Vocational and Technical Education, Faculty of Education, University of Benin, Benin City, Nigeria.
3 Department of Food Technology, Durban University of Technology, Steve Biko Campus, Durban, South Africa.
Corresponding author: Deborah O. Opaleke, E-mail: oluyemisi@unilorin.edu.ng
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: August 6 2021;
Revised: October 22, 2021;
Accepted: December 15, 2021

