
Chromosome Y Analysis of Long-Term Frozen Extract from Sperm-Negative Stains on Lower Undergarments in Sexual Abuse Cases
Manoch Chockjamsai, Supakit Khacha-ananda, Sukon Prasitwattanaseree, Pattarach Rattaracheewakul, and Karnda Mekjaidee*Published Date : 2022-01-21
DOI : https://doi.org/10.12982/CMUJNS.2022.019
Journal Issues : Number 1, January-March 2022
Abstract Y-short tandem repeat (Y-STR) typing is very beneficial for identifying offenders in cases of sexual assault. Even though vaginal swabs from female victims are routinely taken for DNA analysis, penile penetration does not occur in every case. Therefore, in such cases the victim’s lower undergarment is also obtained, along with vaginal and/or anal swabs. In accordance with each country’s statutory guidelines, the positive legal prescription for sexual offences is rather extensive. So, cases that have occurred prior to DNA analysis being applied routinely may now be typed for Y-STR, even after the specimens have been frozen for so long. The aim of this study was to investigate the quality and quantity of Y-STRs that we derived from fluids extracted from sperm-negative specimens. Twenty-eight frozen stain-extracted-fluids from lower undergarments samples were available. Their Y-STR profiles were amplified, and typed, using commercial Y-STR kits. The results demonstrated that 35.7% of stain-extracted-fluids on lower undergarments that had been stored in -20°C for 16-53 months showed complete typing of Y-STRs, with an average DNA peak height of each sample occurring within a range of 90.60 - 2,905.92 RFUs. Nor was there a significant difference in the number of loci and average DNA peak height found among the samples kept frozen from < 36 months, as compared to those of > 36 months. The results of this study support our assertion that even extremely long-term preservation of extracted fluids at -20 °C can maintain high-quality and quantity Y-STR segments.
Keywords: DNA peak height, Frozen extract, Sexual assault, Y-STR, Y-STR loci
Funding: This work was supported by the Faculty of Medicine Research Fund, Chiang Mai University, Chiang Mai, Thailand (Research ID: 5823 / Study code: FOR-2561-05823).
Citation: Chockjamsai, M., Khacha-ananda, S., Prasitwattanaseree, S., Rattaracheewakul, P., and Mekjaidee, K. 2022. Chromosome Y analysis of long-term frozen extract from sperm-negative stains on lower undergarments in sexual abuse cases. CMU J. Nat. Sci. 21(1): e2022019.
INTRODUCTION
DNA typing for individual identification is based on the amplification of the unique region of DNA strands known as short-tandem repeats (STRs). These regions can be found on both autosomal and sex chromosomes. Y-STRs, which are found in the DNA of male sex chromosomes, have been widely used as a crucial step in forensic investigations since 1990 (Roewer and Epplen, 1992). It is a male-specific marker that is most beneficial in the DNA identification of males when confronted with a mixed sample including predominantly female DNA (Shewale et al., 2003; Roewer, 2009; Kayser, 2017), such as can be found in vaginal swabs from rape cases. Y-STRs are useful in many investigations of other alleged crimes as well, not just in sexual assault cases. They can be used in the identification of missing persons, and in the discrimination of paternal kinship. (Ambers et al., 2018; Kumar et al., 2018; Roewer, 2019).
Sexual assault investigations often find Y-STRs upon analyzing a child’s lower undergarments and genital-region swabs since they are present not only in spermatozoa, but in immature germ cells, leukocytes, and epithelial cells (Fedder, 1996). Therefore, Y-STRs are of great value in proving whether male sexual contact has happened. Another key factor is that there are many cases involving the sexual abuse of children in which the offenders do not achieve penile penetration. Yet with an effective method for collecting Y-STRs from these other kinds of cells, it does not matter whether penile penetration has occurred, or whether ejaculate is present, or whether the specimen contains spermatazoa (Delfin et al, 2005; Sween et al., 2005; McDonald et al., 2015; Owers et al., 2018; Tozzo et al., 2018).
It is true that complete DNA profiles of STRs are more effective than partial profiles in offender identification, since insufficient numbers of STRs can lead to false positive identification (Martin et al., 2001); yet the practical limitations of forensic practice make the retrieval of specimens with incomplete Y-STRs unavoidable. It has been proved that the quality of DNA in seminal stains is sufficient for inclusion in use as forensic evidence even years after the incident has occurred (Nakanishi et al., 2014; Hady et al., 2021). However, not all evidentiary undergarments or swabs are well-enough preserved before reaching the laboratory; and some are even delayed before being submitted. Moreover, some specimens are inadequately harvested, and even others are contaminated with polymerase chain reaction (PCR) inhibitor (Putkonen et al., 1996). Some specimens for analysis are not fresh, but rather are presented as extracted fluids which have been stored in a frozen state. This usually occurs when more strategic and effective technology emerges years after the incident being investigated has occurred, or when new corroborating evidence is sought, or when a DNA data bank has a substantial number of samples for comparison (Clark, 2018; Strokes, 2019).
Previous researchers have demonstrated that environments possessing optimal levels of humidity and temperature, as needed for biological preservation, also accelerate the growth of microorganisms in biological evidence which can result in DNA degradation. In the case of specimens used for DNA analysis, it has been suggested that storage at 4° C, or −20°C, will decrease the growth of microorganisms and minimize DNA degradation (Caputo et al., 2011). To the knowledge of the authors, a properly defined period of freezing of bodily fluids extracted from stains for Y-STR typing has never been reported.
The aim of this study is to investigate the quality and quantity of Y-STRs in bodily fluids extracted from stains that have been frozen and stored long-term at -20°C.
MATERIAL AND METHODS
Samples
The samples in this study were composed of fluid extracted from stains on the lower undergarments worn by victims of sexual assault. All the samples were frozen within the 5 years previous to the study’s inception. The only specimens included for analysis were those that had detectable Y-STRs, yet under microscopic examination yielded negative results for sperm. The DNA analysis of the specimens was done under the ethical auspices of the Research Ethics Committee of the Faculty of Medicine, Chiang Mai University (Research ID: 5823 / Study code: FOR-2561-05823).
DNA extraction
After removing the extracted frozen fluid from the -20°C conditions under which it had been stored, it was left to defrost at room temperature. Next, it was centrifuged in order to collect cell pellets. Then the DNA was extracted using a Qiagen® QIAamp DNA Investigator kit (Qiagen, Germany) in keeping with the manufacturer’s instructions. The cell pellets were incubated at 56°C for 60 minutes, with buffer ATL, proteinase K, and dithiothreitol (DTT). After centrifugation, the supernatant was mixed with buffer ALT, and absolute ethanol, and transferred in the QIAamp MinElute column. Then the column was centrifuged, and buffer AW1 was added into the column. Next, absolute ethanol was put into the column to dry the membrane. Finally, the DNA was eluted by adding sterile distilled water and subjecting the mixture to further centrifugation. The DNA template was kept at -20°C throughout the experiments.
DNA amplification and typing
Then, DNA amplification was accomplished by mixing template DNA with the master mix, which consists of deoxynucleotide (dNTP) solution mix, MgCl2, bovine serum albumin, primer mix, and multi Taq2 DNA polymerase. Typing of Y-STRs was performed using the commercial Investigator Argus Y- 12 QS (Qiagen, Germany). The DNA templates for Y-STRs were amplified 28 cycles using a specific primer and an automated thermal cycler (Applied Biosystems, CA, USA) as specified by the manufacturer’s instructions. The amplified product was typed with an ABI Prism 3130 Genetic Analyzer (Applied Biosystems, CA, USA). The Y-STR profile was analyzed using the Gene-Mapper™ ID Software (v3.2) program.
In order to ascertain the quantity of the DNA template, the average peak height was theoretically defined for all Y-STR loci. It was calculated by dividing the sum of the heights of the observed peaks in both homozygous and heterozygous Y-STRs, by the number of observed peaks (Debernardi et al., 2011). The peak heights in the electropherogram are directly proportional to the amount of DNA template in the sample (Timken et al., 2014).
Statistical analysis
The data was summarized using descriptive statistics. The Man-Whitney U test was used to compare the number of loci and the average DNA peak height between the 2 groups with different freezing periods (less than or equal to 36 months vs. more than 36 months). All statistical analysis was performed using SPSS 18. A significance level of 0.05 was prescribed for use in this study.
RESULTS
There were 28 samples that met the criteria for the 5-year timeframe. The period of freezing for all samples were between 16-53 months, with the median at 44 months, and the mode at 45 months. Complete Y-STR profiles possessing all 11 loci were found in 10 samples (35.7%). Frozen period of this group ranged from 23 to 53 months. For the samples with partial profiles, in which less than 11 loci were detected, 13 (72.2%) contained more than 5 loci. The frequency of samples by number of loci is shown in Figure 1.
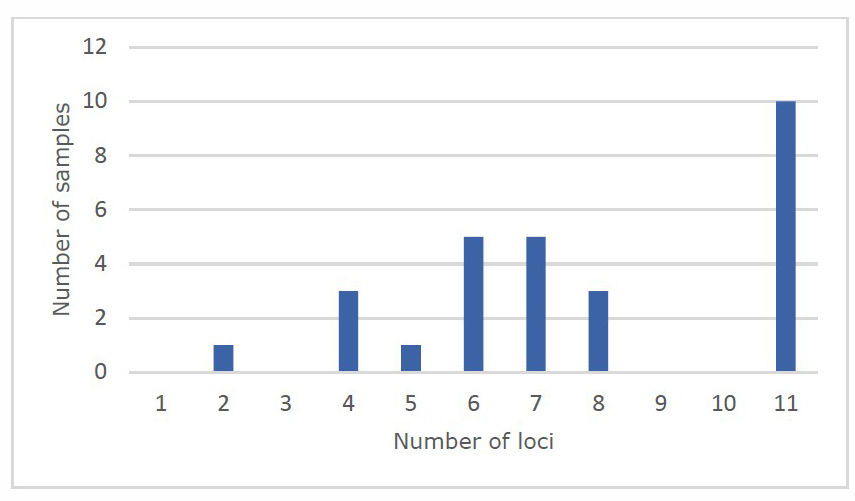
Figure 1. The number of samples possessed complete (11 loci) and partial STR profiles (1-10 loci).
The average peak height of Y-STR occurrence was between 90.60 to 2,905.92 RFUs. The SD, median, and mean were found at 671.50, 150.03, and 431.09 RFUs, respectively. Scatter plots of the average peak height of Y-STR in different freezing periods appear in Figure 2.
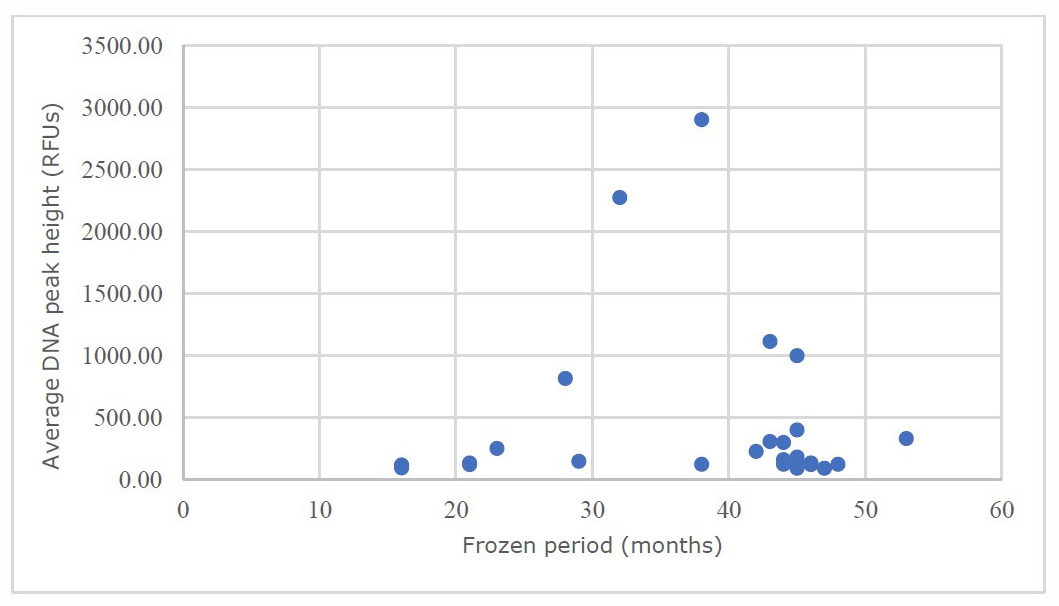
Figure 2. Average peak height of Y-STR vs. different freezing periods.
A limited number of samples did not adequately fit the criteria for statistical analysis and led us to divide the freezing period data into 2 groups. Group 1, which had a freezing period < 36 months, included 9 samples. Group 2, whose freezing period was > 36 months, had more than enough available data, with 19 samples.
Of the samples that had complete Y-STR profiles, there were 3 out of 9 samples (33.3%) in group 1, and 7 out of 19 samples (36.8%) in group 2. Among the samples with partial Y-STR profiles that contained more than 5 loci, there were 4 out of 6 samples (66.7%) in group 1, and 9 out of 12 samples (75.0%) in group 2.
Descriptive statistics for the number of Y-STR loci and the average DNA peak height are in Table 1. Statistical analysis shows no significant difference in the number of loci and average peak height of Y-STR between the samples kept in -20°C for < 36 months, and those kept for > 36 months (P =0.612 and 0.476, respectively).
Table 1. Descriptive statistics of number of loci and average peak height of Y-STR.
|
|
Number of STR loci |
|
Average peak height (RFUs) |
||
|
< 36 m |
> 36 m |
|
< 36 m |
> 36 m |
|
|
Min. |
2.00 |
4.00 |
|
94.67 |
90.60 |
|
Max. |
11.00 |
11.00 |
|
2,275.86 |
2,905.92 |
|
Mean |
7.33 |
8.00 |
|
451.70 |
421.33 |
|
SD |
3.24 |
2.58 |
|
721.24 |
666.97 |
|
Median |
7.00 |
7.00 |
|
132.75 |
159.15 |
|
IQR |
6.00 |
5.00 |
|
419.69 |
207.86 |
Note: RFUs= relative fluorescence units; min = minimum; max = maximum; SD = standard deviation, IQR = interquartile range
DISCUSSION
This study showed that a high yield of Y-STRs could be retrieved from the frozen extracted fluid present in stains on lower undergarments used as supportive forensic evidence of sexual contact, even though the specimen had been stored in -20 °C for long and has been subjected to the aforementioned DNA extraction protocols.
While there exist some studies that have reported substantial reduction of DNA in specimens stored in -20°C (Lee et al., 2010; Romanazzi et al., 2015), the result of this study demonstrates a high yield of Y-STR fractions can be extracted to support evidence of DNA transfer in male sexual-contact cases, in spite of the stain-extracts being stored at -20°C for more than 36 months. We found that the average peak height of RFU levels from Y-STR does not differ between samples stored at - 20°C for < 36 months, and for samples stored at > 36 months. The conclusions of the 2021 study by Hady et al. support this current study. Hady et al. found no significant reduction of DNA concentration in seminal stains on the cotton fabrics which were stored at -20°C. (Hady et al., 2021).
Moreover, it was found in this study that almost 37% of the samples presented a complete profile, even though they were stored in a frozen condition for more than 36 months. Furthermore, 75% of the samples with partial profiles contained Y-STRs at more than 5 loci. These findings reveal a significant opportunity in forensics since re-examining cases that occurred before the era of routine DNA investigation can now potentially provide fresh evidence of illicit male sexual contact.
In 2005, Delfin et al., reported the analysis of vaginal and anal swabs from sexually abused children within 72 hours after the incidents had occurred. They found that only partial profiles were demonstrated in those specimens (Delfin et al., 2005). Even though the samples of this study were kept for years, it was found that 35.7% of Y-STR analysis on the stains from lower undergarments provided complete profiles, and 72.2% of those with partial profiles provided more than 5 loci. This revealed the advantage to gain more evidence of male DNA on victim’s lower undergarment over vaginal and anal swabs. This is not surprising since even though many of the offenders did not achieve penile penetration of their victim’s vagina or anus, penile skin cells and/or epithelial cells can be deposited on lower undergarments in a way that endures over time. This encourages the supposition that lower undergarments worn by victims should be collected, especially in child sexual abuse cases, and Y-STR can be an effective and useful forensic tool.
DNA on clothes has been proved to be useful in identification of the contact individuals, especially of the offenders, in sexual assault cases. (Kumar et al., 2018; Ruan et al., 2018). However, the type of fabric also plays an important role in reducing DNA quantity in stains on the clothes (Brayley-Morris et al., 2015). Sethi et al., in 2013, reported that touch DNA on cotton, polyester, and polyester-blend fabrics was quantitatively insufficient (Sethi et al., 2013). This could be an explanation for the low Y-STR peak heights, and for the fewer numbers of loci found in the extracts from some lower undergarments in this study.
Although the number of samples available in our study were somewhat limited because of the time-period, our results are good enough to instill confidence in using
Y-STR analysis as a valid tool for determining male sexual contact from frozen bodily fluid extracts that have been kept in storage for many years. We suggest further analysis of the quality and quantity of Y-STRs in other types of forensic investigation (for example, on samples from blood-soaked weapons used during bodily assaults on female victims) will expand the usefulness of Y-STRs in forensic practice.
CONCLUSION
In summary, this study was undertaken in order to investigate the yield of Y-STR fractions in the fluid extracted from the stains on lower undergarments submitted for seminal investigation and stored in -20°C for 16 to 53 months. The results demonstrate substantial Y-STR fractions, both in terms of the locus number and the DNA peak height, which are enough to be counted as evidence of male sexual contact in most of the specimens, even though they were stored for more than 36 months.
ACKNOWLEDGEMENTS
The authors would like to thank the staffs of the Forensic genetic unit of the Department of Forensic Medicine, from the Faculty of Medicine at Chiang Mai University, for their assistance during this project. We are grateful to the department of Forensic Medicine and the Faculty of Medicine for providing the instruments and the location for our experiments.
AUTHOR CONTRIBUTIONS
Manoch Chockjamsai designed and wrote the manuscript. Supakit Khacha-ananda contributed to data collection, statistical analysis and the writing of the manuscript. Sukon Prasitwattanaseree performed statistical analysis and contributed to the writing of the manuscript. Pattarach Rattaracheewakul conducted the experiments. Karnda Mekjaidee contributed to statistical analysis, the designing, and the writing of the manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
Ambers, A., Votrubova, J., Vanek, D., Sajantila, A., and Budowle, B. 2018. Improved Y-STR typing for disaster victim identification, missing persons investigations, and historical human skeletal remains. Internal Journal of Legal Medicine. 132:1545-1553.
Brayley-Morris, A., Sorrell, A., Revoir, A.P., Meakin, G.E., and Court, D.S. 2015. Persistence of DNA from laundered semen stains: Implications for child sex trafficking cases. Forensic Sciences International: Genetics supplement series. 19: 165-171.
Caputo, M., Bosio, L.A., and Corach, D. 2011. Long-term room temperature preservation of corpse soft tissue: an approach for tissue sample storage. Investigative Genetics. 2: 17.
Clark, M., Gill, J., Sasinouski, K., and McGuire, A. 2019. Cold Case Homicides: DNA Testing of Retained Autopsy Sexual Assault Smears. Journal of Forensic Sciences. 64: 1100-1104.
Debernardi, A., Suzanne, E., Formant, A., Pène, L., Dufour, A.B., and Lobry, J.R. 2011. One year variability of peak heights, heterozygous balance and inter-locus balance for the DNA positive control of AmpFSTR© Identifiler© STR kit. Forensic Science International: Genetics. 5: 43-49.
Delfin, F.C., Madrid, B.J., Tan, M.P., Maria, and De Ungria, M.C. 2005. Y-STR analysis for detection and objective confirmation of child sexual abuse. Internal Journal of Legal Medicine. 119:158-163.
Fedder, J. 1996. Nonsperm cells in human semen: with special reference to seminal leukocytes and their possible influence on fertility. Archives of Andrology. 36: 41-65.
Hady, R.H.A., Thabet, H.Z., Ebrahem, N.E, and Yassa, H.A. 2021. Thermal effects on DNA degradation in blood and seminal stains: forensic view. Academic Forensic Pathology. March 19.
Kayser, M. 2017. Forensic use of Y-chromosome DNA: a general overview. Human genetics. 136: 621–635.
Kumar N., Maitray A., Gupta R., Sharma D, and SK S. 2018. Importance of Y- STR profiling in sexual assault cases with mixed DNA profile. International Journal of Molecular Biology: Open Access. 3: 42-45.
Lee, S.B., Crouse, C.A., and Kline, M.C. 2010. Optimizing storage and handling of DNA extracts. Forensic Science Review. 22: 131-44.
Martin, P.D., Schmitter, H., and Schneider, P.M. 2001. A brief history of the formation of DNA databases in forensic science within Europe. Forensic Science International. 119: 225-31.
McDonald, A., Jones, E., Lewis, J., and O'Rourke, P. 2015. Y-STR analysis of digital and/or penile penetration cases with no detected spermatozoa. Forensic Science International: Genetics. 15: 84-89.
Nakanishi, H., Hara, M., Takahashi, S., Takada, A., and Saito, K. 2014. Evaluation of forensic examination of extremely aged seminal stains. Legal Medicine (Tokyo, Japan). 16: 303-307.
Owers, R., McDonald, A., Montgomerie, H., and Morse, C. 2018. A casework study comparing success rates and expectations of detecting male DNA using two different Y-STR multiplexes on vaginal swabs in sexual assault investigations where no semen has been detected. Forensic Science International: Genetics. 37: 1-5.
Putkonen, M.T., Palo, J.U., Cano, J.M., Hedman, M., and Sajantila, A. 2010. Factors affecting the STR amplification success in poorly preserved bone samples. Investigative Genetics. 1: 9.
Roewer, L., and Epplen, J. T. 1992. Rapid and sensitive typing of forensic stains by PCR amplification of polymorphic simple repeat sequences in case work. Forensic Science International. 53: 163– 171.
Roewer, L.Y. 2009. Chromosome STR typing in crime casework. Forensic Science, Medicine, and Pathology. 5: 77–84.
Roewer, L. 2019. Y-chromosome short tandem repeats in forensics—sexing, profiling, and matching male DNA. Wiley Interdisciplinary Review. 1: e1336.
Romanazzi, V., Traversi, D., Lorenzi, E., and Gilli, G. 2015. Effects of freezing storage on the DNA extraction and microbial evaluation from anaerobic digested sludges. BMC Res Notes. 8: 420.
Ruan, T., Barash, M., Gunn, P., and Bruce, D. 2018. Investigation of DNA transfer onto clothing during regular daily activities. International Journal of Legal Medicine. 132: 1035–1042.
Sethi, V., Panacek, E.A., Green, W.M., NG, J., and Kanthaswamy, S. 2013. Yield of male touch DNA from fabrics in an assault model. Journal of Forensic Research. T1: 001.
Shewale, J.G., Sikka, S.C., Schneida, E., and Sinha, S.K. 2003. DNA profiling of azoospermic semen samples from vasectomized males by using Y-PLEX 6 amplification kit. Journal of Forensic Sciences. 48: 127-129.
Stokes, J. 2019. Technical note: Next generation identification - A powerful tool in cold case investigations. Forensic Science International. 299: 74-79.
Sween, K.R., Quarino. L.A., and Kishbaugh, J.M. 2015. Detection of male DNA in the vaginal cavity after digital penetration using Y-chromosome short tandem repeats. Journal of Forensic Nursing. 11: 33-40.
Timken, M.D., Klein, S.B., and Buoncristiani, M.R. Stochastic sampling effects in STR typing: Implications for analysis and interpretation. Forensic Science International: Genetics. 11: 195-204.
Tozzo, P., Ponzano, E., Spigarolo, G., Nespeca, P., and Caenazzo L. 2018. Collecting sexual assault history and forensic evidence from adult women in the emergency department: a retrospective study. BMC Health Services Research. 18: 383.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Manoch Chockjamsai1, Supakit Khacha-ananda1, Sukon Prasitwattanaseree2, Pattarach Rattaracheewakul3, and Karnda Mekjaidee1,*
1 Department of Forensic Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, 50200, Thailand.
2 Department of Statistics, Faculty of Science, Chiang Mai University, Chiang Mai, 50200, Thailand.
3 Phrae hospital, Phrae, 50400, Thailand.
Corresponding author: Karnda Mekjaidee, E-mail: Karnda.me@gmail.com
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: September 29 2021;
Revised: November 19, 2021;
Accepted: November 23, 2021

