
In Vitro Propagation of Manihot Esculenta Crantz ‘Pirun 2’
Supakit Vichayanan, Nattapol Phanmadee, Puwarit Khowean, and Noppamart Lokkamlue*Published Date : 2022-01-21
DOI : https://doi.org/10.12982/CMUJNS.2022.016
Journal Issues : Number 1, January-March 2022
Abstract In Thailand, cassava (Manihot esculenta Crantz ‘Pirun 2’) is an important economic crop. The tissue culture technique is used to produce true-to-type cassava plantlets on a large scale within a short period. Therefore, the objective of this study was to investigate the optimum culture medium for cassava axillary bud in vitro propagation. Stem nodes were treated with a solution of 20% (v/v) commercial bleach (25% w/v NaOCl) for 10 minutes. Then, the sterilized buds were cultured on three different types of culture media: Murashige and Skoog (MS), Driver and Kuniyaki Walnut (DKW), and MS supplemented with NAA and BA. Various parameters were evaluated for their effects on plantlet development (height of shoot and numbers of leaves, roots, and shoots after 1 month). The results showed that the mean (± standard deviation) numbers of leaves (6.67 ± 0.82) and roots (2.50 ± 0.55) were significantly higher when they were cultured on MS medium. In addition, the highest number of shoots (3.67±0.82) was obtained on MS medium supplemented with 0.05 mg/l NAA+1 mg/l BA. The cut shoots of plantlets cultured on MS medium without hormones developed into mature plantlets after 1 month. Thus, the MS medium was the optimum for mature cassava Pirun 2 culture. This protocol would be useful for plantlet induction on MS medium supplemented with NAA and BA hormones to produce more shoots and for transfer to MS medium without rooting hormones. Moreover, in vitro derived plantlets were successfully acclimatized on a peat moss which gave the high percentage of transplanting survival (100%).
Keywords: Axillary bud, Cassava, Stem node, Surface sterilization, Tissue culture
Funding: The authors are grateful for the SCiUS funding provided by Ministry of Higher Education, Science, Research and Innovation, Thailand.
Citation: Vichayanan,S., Phanmadee, N., Khowean, P., and Lokkamlue, N. 2021. In vitro propagation of Manihot esculenta Crantz ‘Pirun 2’. CMU J. Nat. Sci. 21(1): e2022016.
INTRODUCTION
Cassava (M. esculenta Crantz) production in Thailand has been ongoing for more than 40 years (Malik et al., 2020). The ‘Pirun 2’ variety of cassava (Huay Bong 60 × Hanatee) was developed through cooperation between the Department of Agriculture, Mahidol University’s Institute of Molecular Biosciences, and the National Science and Technology Development Agency. Pirun 2 is both an edible and industrial variety that has a moderate hydrocyanic acid content and can be used to produce tapioca flour with a starch content of 24.7% (Malik et al., 2020). This variety has the characteristics of an upright stem, light green shoot tip, red petiole, dark brown peel, and low-fiber flesh. In general, seed propagation results in uncontrolled genetic variation in heterozygous cassava, with a slow rate of multiplication propagation. Consequently, it is propagated traditionally using propagation of cassava cuttings aged 8–12 months; however, this is labor-intensive and time-consuming for large-scale production and is a slow process compared to establishing grain crops (Santana et al., 2009). Furthermore, the stem cuttings are beset by diseases such as leaf mosaic disease that contributes to the spread of diseases in the field (Ntawuruhunga and Legg, 2007), often resulting in the transmission of systemic infections from one generation to another and leading to poor yields in successive seasons (Roca and Mroginski, 1991). Thus, the tissue culture technique provides a method to mass-produce true-to-type and disease-free cassava plants within a short period and requires less space than that required for conventional methods (Aladele and Kuta, 2008). The reported cassava Pirun 2 in vitro culture protocol is seldom published in Thailand. With a few studies reporting on the Rayong 5, Rayong 7 and Rayong 72 varieties (Ubonprasit et al., 2015). In addition, the plant genetic variety may vary in different plant tissue culture media. Thus, the aim of this study was the establishment of a simple protocol for axillary bud in vitro culture and the investigation of the optimum culture medium for cassava Pirun 2 propagation.
MATERIAL AND METHODS
Plant material
Stem cuttings of cassava (M. esculenta Crantz ‘Pirun 2’) were collected from Chaiyo Bio Power Company Limited, Chaiyo district, Ang thong province, Thailand.
Stem cultivation
The Pirun 2 stems aged 8 months were processed into cuttings 25 cm in length and then submerged in 0.2 g/l thiamethoxam (C8H10ClN5O3S) solution to prevent insect pests.
Surface sterilization of cassava explant
The stem nodes derived from the Pirun 2 plants aged 3 months were collected and cut into pieces having one axillary bud/explant. They were washed under running tap water for 1 min to remove dust particles. Then surface sterilization was accomplished by soaking in a solution of 20% (v/v) commercial bleach (25% w/v NaOCl) for 10 min with continuous agitation, followed by rinsing three times with sterile distilled water to remove all traces of NaOCl. Then, damaged tissue was excised from each explant to obtain an axillary bud 1 cm in length. Petri dishes with 30 ml of culture media containing nine explants and three replicates were used. The cultures were incubated at 25 ± 2°C under a light intensity of 1,500–2,300 lux using a 16 h photoperiod.
Transplanting into shoot induction medium
Under aseptic conditions, sterilized axillary buds were transferred into shoot induction media. Three treatments were used to evaluate the germination and development of cassava shoots (MS, DKW and MS + 0.05 mg/l NAA + concentrations of BA, as follow: 0.5 mg/l, 1 mg/l and 1.5 mg/l). Each treatment consisted of six replicates (glass bottles) and each replicate contained one axillary bud. After 1 month, the morphological parameters were recorded: numbers of shoots, leaves, roots and shoot height (cm). Then, the multiple shoot tips were cut from cassava cultivated in MS+0.05 mg/l NAA+1 mg/l BA medium with three replicates (glass bottles), where each replicate contained one axillary bud. The tips were subcultured on MS medium without hormones. Then, all cultures were incubated at 25 ± 2°C under a light intensity of 1,500–2,300 lux using a 16 h photoperiod.
Acclimatization
The six-month-old ten in vitro cassava plantlets were washed to remove medium residues and then transferred to peat moss for the acclimatization process. The pots were covered with transparent polyethylene bags and incubated in a greenhouse for 3 days. After 1 week of acclimatization, the polyethylene bags were removed and the rates of survival (%) were recorded.
Statistical analysis
All data were analyzed using one-way ANOVA and significant differences among treatment means were determined using Duncan's multiple range test at P <0.05, using the statistical software package SPSS version 14.0. Values were presented as mean ± standard deviation.
RESULTS
Shoot development of cassava Pirun 2
The sterilized Pirun 2 axillary buds on the MS and DKW media were able to grow into plantlets after 1 month (Figures 1A and B). There were significant differences between treatments for the numbers of leaves, shoots and roots (Table 1); however, there were no significant differences for shoot height. The MS medium produced the maximum numbers of leaves (6.67 ± 0.82) and roots (2.50 ± 0.55). Furthermore, the results showed that the addition of 0.05 mg/l NAA and 1 mg/l BA in MS media was necessary to achieve high numbers of Pirun 2 shoots (Figure 1C). There were significant differences among treatments as shown in Table 1 with the highest number of shoots being 3.67 ± 0.82. However, the addition of 0.05 mg/l NAA + 0.5 mg/l BA or 1.5 mg/l BA in MS media represented the lower rates of proliferation at the same time. Furthermore, the shoots were unable to grow into plantlets due to the lack of root development. Instead, two types of calli (friable or compact), approximately 1.56 ± 0.29 cm in diameter, were formed at the base of these shoots (Figure 1C).
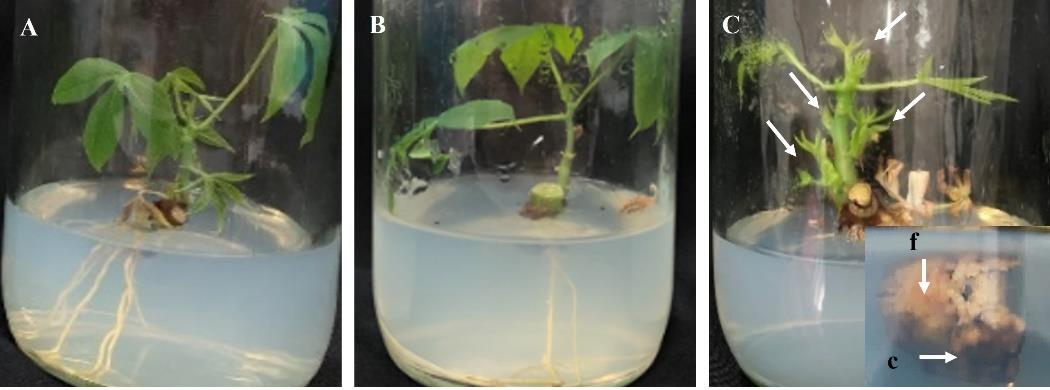
Figure 1. Development of Pirun 2 axillary bud at 1 month: (A) MS medium; (B) DKW medium; (C) MS+0.05 mg/l NAA+1 mg/l BA medium, showing multiple shoots (arrows) with two types of calli (f = friable callus and c = compact callus).
Table 1. Effects of culture media on numbers of leaves, shoots, and roots, and shoot height of cassava Pirun 2.
|
Treatment |
Morphological parameter |
|
|||
|
No. of leaves |
No. of shoots |
No. of roots |
Shoot height (cm) |
||
|
DKW |
5.33 ± 0.82b |
1.00 ± 0.00d |
1.83 ± 0.75b |
2.08 ± 0.67a |
|
|
MS |
6.67 ± 0.82a |
1.00 ± 0.00d |
2.50 ± 0.55a |
2.33 ± 0.26a |
|
|
MS+0.05 mg/l NAA+0.5 mg/l BA |
3.50 ± 1.26c |
2.33 ± 0.52b |
0.00 ± 0.00c |
2.08 ± 0.38a |
|
|
MS+0.05 mg/l NAA+1.0 mg/l BA |
5.17 ± 0.98b |
3.67 ± 0.82a |
0.00 ± 0.00c |
2.67 ± 0.68a |
|
|
MS+0.05 mg/l NAA+1.5 mg/l BA |
1.50 ± 0.50d |
1.67 ± 0.52c |
0.00 ± 0.00c |
2.17 ± 0.26a |
|
Note: Mean ± standard deviation values with different lowercase superscripts within a row are significantly different at P <0.05.
Development of the cut shoot and root
Shoot tips cut from shoots cultivated in MS+0.05 mg/l NAA+1 mg/l BA medium were then able to be developed into plantlets with roots on MS medium after 1 month (Figure 2A). These plantlets had a normal root system consisting of tap roots and lateral roots (Figures 2B and C, respectively). The root development of the cut shoots could produce more lateral roots after 2–6 months (Figures 2C and D).
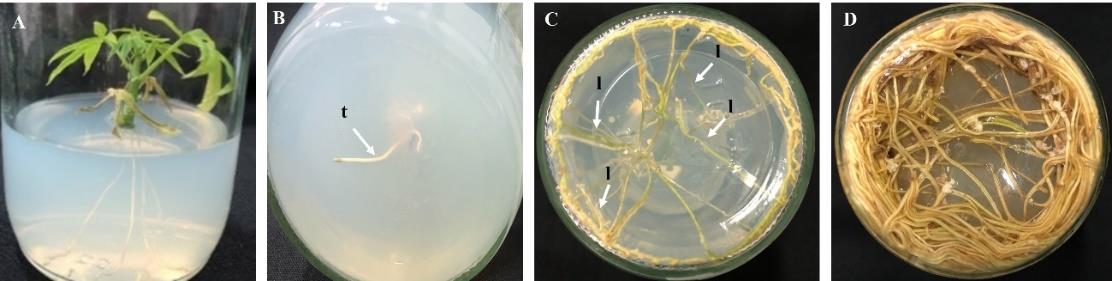
Figure 2. Cut shoot with root development on cassava Pirun 2 plantlet: (A) plantlet aged 1 month; (B) root germination after 1 month (t = tap root); (C) root development after 2 months (l = lateral root); (D) root development after 6 months.
Acclimatization of cassava plantlets
The in vitro cassava Pirun 2 plantlets aged 6 months produced more leaves and lateral roots (Figure 3A). The result of the cassava plantlets showed production of normal leaves and stems as shown in Figure 3B. Cassava plantlets that were transferred to the greenhouse after acclimatization had a 100% survival rate. We found that some leaves were wilted but survived; however, the surviving shoots of cassava could develop to produce new leaves (arrows) (Figure 3C). The cassava plantlets produced did not show abnormalities which might indicate the occurrence of variation or mutation during the micropropagation process, and all plants appeared as true-to-type and reached about 16 cm in length after 1 month (Figure 3D).

Figure 3. Acclimatized cassava Pirun 2 plantlet transplanting on peat moss: (A) in vitro cassava plantlet aged 6 months; (B) dissected in vitro cassava plantlet; (C) cassava plantlet after 1 week produced new leaves (arrows); (D) cassava plantlet after 1 month.
DISCUSSION
The MS medium produced the maximum numbers of leaves (6.67 ± 0.82) and roots (2.50 ± 0.55) for the cassava Pirun 2 plantlets. This result suggested that the plantlets with numerous well-developed leaves were more efficient photosynthetically and therefore could adapt quickly to the natural environment compared to those with smaller and fewer leaves (Ogero et al., 2012). In addition, roots have an essential role and function in plant life and development, supplying water and nutrients to the plant from the environment (Schiefelbein et al., 1997). Regeneration of roots from the axillary bud explants occurred easily without any rooting hormone in the medium. This was consistent with Yona et al. (2010) who reported that cassava explants developed roots naturally roots without the addition of auxins.
The highest number of shoots (3.67 ± 0.82) was obtained from MS supplemented with 0.05 mg/l NAA and 1 mg/l BA. This was consistent with Alla et al. (2013) who observed that cassava shoot induction for an American cultivar in MS medium supplemented with 0.05 mg/l NAA and 1 mg/l BA, produced the greatest number of shoots (5.67). On the other hand, the best cytokinin for shoot induction of cassava mosaic disease-resistant cultivars was BAP on its own at 10 μM, producing the highest number of shoots/explant (3.60 ± 0.03), although the regenerated multiple shoots were stunted (Sessou et al., 2020). Combining BAP and NAA produced the best shoot elongation for some cassava varieties (Shiji et al., 2014). Moreover, the use of these growth regulators during growth initiated from the meristem culture of different cassava varieties was also recommended by Razdan (2005). However, we found that the callus formation occurred at the base of the cassava Pirun 2 shoot due to the ratio of auxin-to-cytokinin in the cassava cells. Furthermore, the presence of cytokinins affects the callogenesis process by reducing cell wall lignification and assists callus initiation from the cut surface of the explant (Kumlay and Ercisli, 2015). The types of callus produced were consistent with a study using Nigerian cassava culture where both compact and friable calli produced somatic embryos due to their varieties (Nkaa et al., 2015). We found a more compact callus than friable callus (data not shown) due to the NAA hormone with BAP addition in the medium. In contrast, callus inoculated on the medium enriched with 2 ppm 2,4-D + 5 ppm BAP showed a highly friable structure in kaffir lime (Tunjung et al., 2021). Therefore, the callus with the 2,4-D hormone (auxin group) had a more friable texture than the NAA hormone (Harahap et al., 2019).
The cut shoots derived from MS+0.05 mg/l NAA+1 mg/l BA medium were able to be developed into plantlets with roots on MS medium. This result revealed that cells containing the auxin endogenous hormone that induced root primordia and cell enlargement. Wounding is usually required to achieve rooting perhaps because the wounding-related compounds play a main role in the dedifferentiation phase (de Klerk et al., 1999). The cassava plantlets produced normal roots because cassava is a root crop and does not necessarily require any growth hormones (Zimmerman et al., 2007; Yona et al., 2010). In addition, roots are important role to absorb water and nutrients. Therefore, in vitro root development usually enhances transplanting success because functioning roots relate to a favorable plant water balance (Díaz-Pérez et al., 1995). This result suggested that normal roots would promote cassava Pirun 2 growth and subsequently use axillary buds for in vitro multiplication. Then, in vitro cassava plantlets were utilized for commercial scale propagation by acclimatization in peat moss for 1 week.
CONCLUSION
All sterilized axillary buds successfully developed into shoots. The optimum MS medium produced the maximum numbers of leaves (6.67 ± 0.82) and roots (2.50 ± 0.55) on cassava Pirun 2 plantlets after 1 month. MS supplemented with 0.05 mg/l NAA and 1 mg/l BA produced more shoots (3.67 ± 0.82) than the MS medium (1.00 ± 0.00) after 1 month. This protocol would be useful for plantlet propagation on MS medium supplemented with NAA and BA hormones to produce more shoots and for transfer to MS medium without requiring any rooting induction medium. This approach could reduce the cost of cassava plantlet production. Moreover, in vitro cassava Pirun 2 plantlets could develop in peat moss after acclimatization and showed true-to-type plants.
ACKNOWLEDGEMENTS
This project was supported by Science Classroom in University Affiliated School (SCiUS) with its funding provided by the Ministry of Higher Education, Science, Research and Innovation. The authors gratefully acknowledge the necessary facilities provided by the Department of Genetics, Kasetsart University.
AUTHOR CONTRIBUTIONS
Supakit Vichayanan, Nattapol Phanmadee and Puwarit Khowean assisted in performing the experiments and data statistical analysis. Noppamart Lokkamlue designed, conducted all of the experiments, and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Alla, N.A.A., Ragab, M.E., El-Miniawy, S.El-D.M., and Taha, H.S. 2013. In vitro studies on cassava plant micropropagation of cassava (Manihot esculenta Crantz). Journal of Applied Sciences Research. 9: 811–820.
Aladele, S.E. and Kuta, D.D. 2008. Environmental and genotypic effects on the growth rate of in vitro cassava plantlet (Manihot esculenta Crantz). African Journal of Biotechnology. 7: 381–385.
Díaz-Pérez, J.C., Shackel, K.A., and Sutter, E.G. 1995. Effects of in vitro-formed roots and acclimatization on water status and gas exchange of tissue cultured apple shoots. Journal of the American Society for Horticultural Science. 120: 435–440.
de Klerk, G.-J., van der Krieken, W., and de Jong, J.C. 1999. Review the formation of adventitious roots: new concepts, new possibilities. In Vitro Cellular & Developmental Biology - Plant. 35: 189–199.
Harahap, F., Diningrat, D.S., Poerwanto, R., Nasution, N.E.A., and Hasibuan, R.F.M. 2019. In vitro callus induction of sipahutar pineapple (Ananas comosus L.) from North Sumatra Indonesia. Pakistan Journal of Biological Sciences. 22: 518–526.
Kumlay, A.M., and Ercisli, S. 2015. Callus induction, shoot proliferation and root regeneration of potato (Solanum tuberosum L.) stem node and leaf explants under long-day conditions. Biotechnology and Biotechnological Equipment. 29: 1075–1084.
Malik, A.I., Kongsil, P., Nguyễn, V.A., Ou, W., Sholihin, W., Srean, P., Sheela, M.N., López-Lavalle, L.A.B., Utsumi, Y., Lu, C., et al. 2020. Cassava breeding and agronomy in Asia: 50 years of history and future directions. Breeding Science. 70: 145–166.
Nkaa, F.A., Ene-Obong, E.E., Taylor, N.J., Fauquet, C.M., and Egesi, C.N. 2015. Evaluation of ten elite Nigerian cassava (Manihot esculenta Crantz) cultivars for somatic embryogenesis and regeneration potentials. Journal of Advances in Biology & Biotechnology. 2: 133–143.
Ntawuruhunga, P. and J. Legg. 2007. New spread of cassava brown streak virus disease and its implications for the movement of cassava germplasm in the East and Central African region. Crop Crisis Control C3P Project. Retrieved from https://www.crs.org/sites/default/files/tools-research/c3p-new-spread-of-cassava-brown-streak-virus-disease.pdf.
Ogero, K.O., Mburugu, G.N., Mwangi, M., Ombori, O., and Ngugi, M. 2012. In vitro micropropagation of cassava through low cost tissue culture. Asian Journal of Agricultural Sciences. 4: 205–209.
Razdon, M.K. 2005. Introduction to plant tissue culture (2nd ed.). Science publishers, USA.
Roca, W. and Mroginski, L.A. 1991. Tissue culture in agriculture: foundations and applications. International Center for Tropical Agriculture (CIAT), Cali, Colombia.
Santana, M.A., Romay, G., Matehus, J., Vicente-Villardón J.L., and Demey, J.R. 2009. A simple and low-cost strategy for micropropagation of cassava (Manihot esculenta Crantz). African Journal of Biotechnology. 8: 3789–3897.
Sessou, A.F., Kahia, J.W., Houngue, J.A., Ateka, E.M., Dadjo, C., and Ahanhanzo, C. 2020. In vitro propagation of three mosaic disease resistant cassava cultivars. BMC Biotechnology. 20: 1–13.
Schiefelbein, J.W., Masucci, J., and Wang, H. 1997. Building a root: the control of patterning and morphogenesis during root development. The Plant Cell. 9: 1089–1098.
Shiji, R., Geore, J., Sunitha, S., and Muthuraj, R. 2014. Micropropagation for rapid multiplication of planting material in cassava (Manihot esculenta Crantz). Journal of Root Crops. 40: 1–8.
Tunjung. W.A.S., Widyasari, A.F., Iskandar, A., Nurulita, A.J., Sasongko, A.B., Indrianto, A., Semiarti, E., and Maryani, E. 2021. Effect of 2,4-D and BAP on morphological characters and genetic stability of kaffir lime (Citrus hystrix DC.) callus cultures among generations. Chiang Mai University Journal of Natural Sciences. 20: e2021067.
Ubonprasit, B., Sanyuandeekul, S., Saisuwan, S., and Uncharisanggard, P. 2015. Effects of TDZ, BA and GA3 on tissue culture of cassava (Manihot esculenta Crantz) cv. Rayong 5, Rayong 72 and Rayong 7. 8: 7–16.
Yona, B., Kawuki, R., Otim, M., Masiga, C.W., and Mugoya, C. 2010. Cassava tissue culture techniques. Tissue culture, conservation biotechnology, virus indexing and seed systems for vegetative crops. In a training manual: ASARECA. Kampala, Uganda.
Zimmerman, T.W., Williams, K., Joseph, L., Wiltshire, J., and Kowalski, J.A. 2007. Rooting and acclimatization of cassava (Manihot esculenta) ex vitro. Acta Horticulturae. 738: 735–740.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Supakit Vichayanan1, Nattapol Phanmadee1, Puwarit Khowean1, and Noppamart Lokkamlue2,*
1 Kasetsart University Laboratory School Center for Educational Research and Development, Kasetsart University Kamphaeng Saen Campus, Nakhon Pathom 73140, Thailand
2 Department of Genetics, Faculty of Liberal Arts and Science, Kasetsart University Kamphaeng Saen Campus, Nakhon Pathom 73140, Thailand
Corresponding author: Noppamart Lokkamlue, E-mail: faasnmlo@ku.ac.th
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: August 1 2021;
Revised: November 2, 2021;
Accepted: November 9, 2021

