
Antibacterial, Sperm Immobilization and Spermicidal Activities of Bacteriocins Produced by Vaginal Lactobacilli
Siriwoot Sookkhee*, Sarawuth Manonuek, Chatchawann Apichartpiyakul, and Choompone SakonwasunPublished Date : 2022-01-21
DOI : https://doi.org/10.12982/CMUJNS.2022.015
Journal Issues : Number 1, January-March 2022
Abstract Lactobacillus bacteriocin is an interesting compound playing a dual spermicidal/bacteriocidal role so that this compound can be developed as an agent that can be able to replace the unsafety chemical, nonoxynol-9. The present study aimed to investigate the antibacterial, sperm immobilization, and spermicidal activities of bacteriocins that were produced from vaginal lactobacilli. Nine of 932 cell-free supernatants exhibited potent antibacterial activities against the standard bacterial strains and the pathogenic Niesseria gonorrhoeae. Ion exchange-purified bacteriocins B84/7 F2 demonstrated the lowest minimum inhibitory concentration and the highest bacteriocin activity against the tested pathogens. The isolate B84/7 was then identified as Lactobacillus crispatus. The rapid killing activity was exerted within 15 minutes after being exposed to the tested bacteria, and also significantly inhibited the exponential growth of the tested pathogen. Among 48 semen of volunteers, the median time of sperm immobilization of ion-exchange purified B84/7 F2 bacteriocin (18.70 mg/mL) was 30.42 seconds. Within 60 seconds, spermicidal activities of the partial fractionated and ion-exchange purified B84/7 F2 at the undiluted concentration were 83.67 and 80.75%, respectively. Its activity will significantly decline at pH > 8.0 or after being treated with alkaline and proteolytic enzymes. Two peptide bands of this bacteriocin were 7.08, and 8.81 kDa after being detected on a tricine SDS-PAGE gel. In-depth characteristics of this bacteriocin should be further investigated regarding the mode of action and purification. These findings can be applied to develop as the protecting agent of sexually transmitted infections especially gonorrhea, and unwanted pregnancy.
Keywords: Antibacterial activity, Bacteriocins, Neisseria gonorrhoeae, Sperm immobilization, Spermicidal activity, Vaginal lactobacilli
Citation: Sookkhee, S., Manonuek, S., Apichartpiyakul, C., and Sakonwasun, C. 2022. Antibacterial, sperm immobilization and spermicidal activities of bacteriocins produced by vaginal lactobacilli. CMU J. Nat. Sci. 21(1): e2022015.
INTRODUCTION
Nowadays, Sexually Transmitted Infections (STI) are a major worldwide reproductive health concern. Gonorrhea is one of the most common STI caused by Neisseria gonorrhoeae, an obligate human causative agent. If undiagnosed, and untreated, gonococcal infections can lead to severe disseminated symptoms, pelvic inflammatory disease, ectopic pregnancy, and sterility. Therefore, the reduction of gonococcal infections along with antibiotic use, and resistance should be in major focus to prevent and treat gonorrhea. One of the effective ways is by using an alternative treatment such as healthy vaginal flora and their bacteriocins. Lactobacilli plays an important role by reducing the risk of gonorrhea and protecting women from colonization and infection by incoming pathogens (Antonio et al., 1999; Martin et al., 1999) Epidemiological evidence suggested that women with high numbers of vaginal lactobacilli have reduced susceptibility to gonorrhea and chlamydial infections following the exposure (Wiesenfeld et al., 2003).
Contraceptives, especially condoms are important tools that have been used for preventing unwanted pregnancy and STI (Starke et al., 1994). Microbicidal contraceptives offer a suitable alternative to condoms as the most viable, women-controlled method for dual protection. Nonoxynol-9 (N-9) and benzalkonium chlorides have been used for these purposes (Méndez et al., 1986). Several studies have indicated that N-9 and other nonionic detergents are potent in vitro inhibitors of vaginal lactobacilli (Klebanoff, 1992; McGroarty et al., 1992). However, N-9 not only failed to offer any protection against STI and HIV but also increased the incidence of susceptibility, and enhanced the risk of STI (Stephenson, 2000; Fichorova et al., 2001; Inácio  et al., 2011). It is important to discover new compounds that have effective antimicrobial and spermicidal activities, and are safe for vaginal epithelial cells. Antimicrobial peptides (AMPs) may offer as an alternative or enhancement to the existing chemical options. To date, several antimicrobial peptides with spermicidal activity have been documented including LL-37 or cathelicidin from neutrophils of Homo sapiens (Srakaew et al., 2014), maximin-1 and maximin-3 from the skin of Chinese red belly toad (Bombina maxima) (Lai et al., 2002), magainin-2 from the skin of African clawed frog (Xenopus laevis) (Chen et al., 1988), dermaseptin S1 and dermaseptin S4 from the skin of Sauvage’s leaf frog (Phyllomedusa sauvagii) (Zairi et al., 2005), Sarcotoxin Pd from rove beetles (Paederus dermatitis) (Zare-Zardini et al., 2016), subtilosin A from Bacillus subtilis (Sutyak et al., 2008), Pediocin CP2 from Pediococcus acidilactici (Kumar, 2012), fermenticin HV6b from Lactobacillus fermentum (Kaur, 2013), nisin A from Lactococcus lactis (Gupta, 2009), and lacticin 3147 from Lactococcus lactis DPC3147 (Silkin et al., 2008). Bacteriocins are bactericidal proteinaceous molecules that could be defined into four distinct classes elucidated by their probable structures; Class I lantibiotics, the membrane-active peptide (Lactococcus lactis (Aranha et al., 2004; Chatterjee et al., 2005) has been reported. McGroarty isolated antibacterial proteins from L. acidophilus and L. casei obtained from urethral specimens that were active against Escherichia coli. (McGroarty et al., 1988). The growth of N. gonorrhoeae was inhibited by a heat-resistant peptide which was extracted from a vaginal isolate, L. salivarius (Ocaña et al., 1999). It seems to be that bacteriocins produced by vaginal lactobacilli are generally safe for such environment and have antibacterial activity against a number of vaginal pathogens. Besides their antimicrobial activity, their spermicidal function was also fascinating. However, the reports related to the antibacterial and spermicidal activity of bacteriocin produced from vaginal lactobacilli as the bactericidal agent to the urogenital pathogen, have been limited. Therefore, the present study aimed to discover the new bacteriocins produced from vaginal lactobacilli, and to provide evidence showing that bacteriocins possess anti-Neisseria gonorrhoeae, sperm immobilization, and spermicidal activities. Lactobacillus dual functions, revealed from this study, may be applied to the further development of this bacteriocin as a potential anti-gonorrheal and contraceptive agent.
MATERIAL AND METHODS
Vaginal lactobacillus isolates
Vaginal lactobacilli were collected from 100 specimens of healthy patients who came for routine checks at the Gynecologic OPD Clinic, Maharaj Nakorn Chiang Mai Hospital, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand between 2008-2015. Then diagnosed by Amsel criteria under the light microscope for confirmation as a healthy vaginal fluid at the Diagnostic Laboratory Unit of the hospital. Each collected fluid was freshly recovered on De Mann Rogosa Sharpe agar (MRS; Difco™, Becton Dickinson, Sparks, MD.), bacteria selected and kept at -70°C freezer at Department of Microbiology, Faculty of Medicine, Chiang Mai University until use.
The population size of lactobacilli was calculated from the formula, n = (Z2*pq)/d2. Our previous study exhibited that 33.2% of the tested lactobacilli demonstrated as the antimicrobial isolates. So,
p = 0.332
q = 1 – p = 0.668
Z constant value at α = 0.05 is 1.96
and d = 0.1 *p = 0.0332.
The final calculation showed the population size of tested lactobacilli was 773 isolates.
Preparations of lactobacillus
In the present study, these isolates were grown on MRS agar, and then incubated at 37°C, 5% CO2 for 48 hrs before being investigated in the present study. MRS broth was the culture broth for producing bacteriocin. After 36 hours of each culture, the cell-free supernatant was separately harvested by using a cross-flow hollow fiber unit (QuixStandTM benchtop system, GE Healthcare Life Science, Uppsala, Sweden) according to the laboratory scale-ultrafiltration. A commercial hollow-fiber cartridge membrane with a molecular weight cut-off of 5,000 Da (Amersham Biosciences AB, Uppsala, Sweden) was performed. The membrane effective surface area is 650 cm2. Tris-HCl buffer solution at 0.5 M, pH 6.8 was used for determining whether the membrane was clogged or fouled. The permeate flux with the flow rate at 0.64 L/min was constantly maintained.
Preparation of nisin
Commercial nisin (N5764; Sigma-Aldrich, St.Louis, MO) in a form of 2.5% (w/w) nisin lyophilized powder from Lactococcus lactis was utilized in the present study. The nisin was prepared according to the previous study (Abts et al., 2011). Briefly, 130 mg of powder (corresponding to 3.25 mg nisin) was dissolved in 10 mL, 50 mM lactic acid pH 3, then filtered through a 0.45 μm membrane filter, and eluted through a HiTrap SP HP cation exchange column (GE Healthcare Life Science) at a flow rate of 2 mL/min. The column was subsequently washed with 50 mM lactic acid pH 3 until a stable baseline was reached to remove nonspecifically bound material. Peptides were eluted by increasing the NaCl concentration stepwise with a flow rate of 1 mL/min (with 200, 400, 600, 800, and 1,000 mM of NaCl). To remove NaCl, protein in elution fractions was precipitated with 20% (v/v) trichloroacetic acid (TCA) overnight at 4°C. Precipitated protein was washed two times with ice-cold acetone to remove residual TCA. Finally, the protein pellet was suspended in 50 mM lactic acid pH 3. Nisin concentrations were determined by Qubit® 2.0 fluorometer and Qubit™ protein assay kit (Molecular Probe™, Eugene, OR) according to the protocol of the manufacturer. Four hundred µg/mL of nisin solution was used as a positive control for the antibacterial and sperm immobilization studies.
Selection of bacteriocin-producing lactobacilli
Four reference indicators including Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, and Neisseria gonorrhoeae ATCC 49226 were performed as the tested strains to determine the antimicrobial activity by using agar-cup diffusion (Gordon et al., 1980). For the preparation of these indicators, they were separately grown in Tryptic Soy Broth (TSB; Difco™) at 37°C for 24 hours, then equilibrated turbidity of the cell suspension to be equal to McFarland No. 0.5. Except for N. gonorrhoeae, all indicators were tested on Mueller Hinton Agar (MHA; Difco™) for 24 hours. N. gonorrhoeae was grown on Chocolate agar at 37°C, 5% CO2 for 48 hours before adjusting turbidity to equal as McFarland No. 0.5. Each tested strain was separately swabbed on the tested plate. Two hundred μl cell-free supernatant of each lactobacillus isolate at neutralized pH condition were filled into a cup that was placed on the agar plates. The plates were then incubated at 37°C. The experiments were done triplicately. The diameter of the inhibition zone was measured. The tested lactobacillus supernatant gave a wider inhibitory diameter than a positive control, nisin. It was suggested to possess the antibacterial activity for performing further study. Antibacterial lactobacillus isolates were then identified by using their biochemical characteristics, and their proteomic profiles according to the API-50 CHL test kit (bioMérieuxTM, Marcy l'Etoile, France), and VITEK MS (bioMérieuxTM), respectively.
Partial purification of potent bacteriocins
Bacteriocins of these potent supernatants were partially purified. According to ultrafiltration, the cell-free supernatant of each selected lactobacillus isolate was fractionated by using vivaflow-50™ ultrafiltration at the cut-off 5, 10, and 30 kDa (Sartorius Stedem Lab, Stonehouse, UK). Four fractions in a range of 3-5 (F1), 5-10 (F2), 10-30 (F3), and > 30 (F4) kDa were harvested and then concentrated. Each bacteriocin was separately purified by anion exchange chromatography using Fast Protein Liquid Chromatography (FPLC; AKTA® Explorer, GE Healthcare Life Science). HitrapTM Capto S, a strong sulfoethyl cation exchanger (Amersham Biosciences AB) was performed as a stationary phase. Bacteriocin fractions after elution with 20 mM Tris-HCl pH 8.0 were separately collected and desalted. Their concentrations were determined by Qubit fluorometric assay.
Determination of minimal inhibitory concentration
Each cell-free supernatant, fractionated protein, and purified bacteriocin showed the inhibitory effect against N. gonorrhoeae ATCC 49226. The potent fractionated proteins and partially purified bacteriocins were determined by comparing their MIC toward the tested N. gonorrhoeae. The bacteriocin unit was determined by using two-fold serial dilution against N. gonorrhoeae at OD600. One bacteriocin arbitrary unit was defined as the lowest concentration of bacteriocins which inhibited 90% growth of the N. gonorrhoeae ATCC 49226 after comparing it with the untreated control.
Determination of the rapid growth inhibition and killing times
Rapid growth inhibition of the selected bacteriocin against N. gonorrhoeae was determined. Fresh culture of N. gonorrhoeae was adjusted to be equal to McFarland standard No. 0.5, and separately mixed with bacteriocins in a ratio of 1:1 at various times before being plated on Chocolate agar, and incubated at 37°C under CO2 condition, then collected every three hours. The numbers of residual cells were counted. Killing time was also determined. Fresh culture of N. gonorrhoeae was adjusted to be equal to McFarland standard No. 0.5, and mixed with the bacteriocins, collected every 3 hours before being plated on chocolate agar, and incubated at 37°C under CO2 condition. Bacteriocins were omitted from the negative control that volume was replaced with sterile MRS broth. Experiments were carried out in duplicate.
Volunteer recruitment and ethical approval
Volunteers must be healthy men and 18 to 40 years old at the time of enrollment. They must be non-smokers or have no alcoholic dependence. Volunteers who have suffered from one of the systemic diseases, sexually transmitted diseases, dermatitis or skin lesion, fever in the three months before preceding sample collection, any clinical manifestation of the lower genital tract infection, abnormalities of the reproductive organs, infertility problem, vasectomy, azoospermia, chronic prostatitis, urethritis, and erectile dysfunction were excluded. All research methodologies regarding volunteers of the present study were approved by the Research Ethic Committee of the Faculty of Medicine, Chiang Mai University (Human ethic issue No. CMU032-53). Informed consent was obtained from all volunteers.
For the population size calculation for volunteers recruited in the present study, we referred to the study of Lopez-Teijon (2008) that reported the 42.2% as normozoospermic semen and 90.2% volume qualified semen in 1239 volunteers. In the formula below,
N = [(Zα+Zβ)2*(2PQ)]/D2
where, α = 0.05, Zα=1.64; β = 0.1, Zβ = 1.28
P = (P1+P2)/2 = (0.422+0.902)/2 = 0.662
Q = (1-P) = 1-0.662 = 0.338
D = P2-P1 = 0.902-0.422 = 0.480
Therefore, the population size which possessed the normozoospermic semen was more than 16.56 or 17 cases.
Semen collection and evaluation
Volunteers were instructed to abstain from ejaculation for 48 to 72 hours before the semen collection. Semen was obtained by their voluntary masturbation in a close and private room, then collected into a sterile wide-mouth polypropylene container with a screw-top cap, and kept in the incubator adjusted to body temperature. After liquefaction within 15-25 minutes, the volume of semen was measured. Semen qualification was evaluated according to the World Health Organization guidelines (World Health Organization, 2010). INCYTO disposable hemocytometer for semen analysis. (C-Chip™ DHC-S022) was performed for assessment of sperm count and motility (Halvaei et al., 2011). Inclusion criteria were progressive motility of more than 95% of spermatozoa. Each sample was assayed three times for the spermicidal effect. At least 200 spermatozoa, normal sperm morphology was reported as a percentage value.
Sperm immobilization
Each cell-free supernatant was also screened for the inhibitory effect against sperm motility within 4 minutes under high magnification fields. Ten μl of a tested supernatant was mixed with 20 μl of whole semen in a disposable hemocytometer for semen analysis. Sperm immobilization time or the exposure time which mostly inhibited the sperm motility was assessed. No inhibitory activity was interpreted if the sperm motility was still detected at the four minutes of exposure time. Negative and positive controls were MRS broth and nisin, respectively. The potent tested supernatants that exhibited the shortest sperm immobilization time were selected for further study. Experiments were performed using semen samples from three donors. Three individual experiments were tested. The fractionated proteins, and purified bacteriocins of the selected supernatants were conducted to prove the inhibitory effect. The highest dilution of bacteriocin that demonstrated 50 and 100% of sperm immobilization within 60 sec was taken as the minimum effective concentration at 50 and 100%, EC50 and EC100, respectively.
Spermicidal activity
A two-step eosin-nigrosin assay (World Health Organization, 2010) was performed. The selected bacteriocin and semen were mixed in a ratio of 1:1 and placed for 60 seconds. Then, the mixture and eosin 0.67% (w/v) were mixed in a ratio of 1:2 and placed for 30 seconds. A volume of nigrosin 10% (w/v) in 0.9% NaCl was added in a ratio of 1: 1 and placed for 30 seconds. Sperm smears were done on the slide and were observed at high-magnification fields (400x). The percentage of viable sperm was determined. The spermicidal activity was then determined as the percentage of dead sperm. The potent bacteriocins which exhibited the faster sperm immobilization time and higher spermicidal activity than the activity of 320 µg/mL nisin solution were called the spermicidal bacteriocins.
Characterization of potent bacteriocins
For pH sensitivity, each aliquot of bacteriocin was resuspended in the buffer solution ranging from pH 1-14 and incubated for two hours at 37°C. For heat sensitivity, bacteriocin was separately heated at 60°C, 80°C, 100°C for 30 min, and 121°C for 15 minutes. The residual antibacterial and spermicidal activities were determined and compared with that of the untreated controls. For proteolytic enzyme sensitivity, bacteriocins were separately treated with the final concentration of 1 mg/mL of protease, trypsin, and pepsin. Following incubation at 37°C for two hours, enzyme activity was terminated by adding the excess fetal bovine serum and remaining activities were determined.
One-dimentional polyacrylamide electrophoresis
The selected antibacterial and spermicidal bacteriocins were analyzed on glycine or tricine sodium dodecylsulfate-polyacrylamide gel (Mini-Protein™ IV, BIO-RAD laboratory, Hercule, CA) with multicolor low range protein ladder (Spectra™, Fermentus, Vilnius, Lithuania). They were visualized by PlusOne Silver Staining Kit (PlusOne™, Amersham Biosciences AB). The molecular weights of protein bands were calculated.
Statistical analysis
Data were expressed as means ± SE, and median value. Repeated measures were employed for statistical analysis of the data. Differences were considered statistically significant at a value of P = 0.05.
RESULTS
Antibacterial activities of lactobacillus bacteriocins
Among 932 isolates of vaginal lactobacilli, nine isolates provided cell-free supernatants that possessed the strongest antibacterial activity against four reference and three clinical tested bacteria as shown in Table 1. According to identification results of API-50 CHL and VITEK MS, they were Lactobacillus plantarum 36/14 and D4, L. jensennii B16 and F36, L. crispatus B84/7, L. acidophilus A68, L. paracasei B89/4, and L. rhamnosus 18/6 and B69. Because of their promising antibacterial activity, it may produce antimicrobial peptides or proteins, bacteriocins, to inhibit the tested bacteria. These bacteriocins may probably be applied to prevent the gonococcal infection. The result of the antibacterial activities against N. gonorrhoeae ATCC 49226 revealed that the activity of some fractionated proteins and some purified bacteriocins remained (Table 2, Figure 1). They were the fraction B16 F3, B16 F4, B84/7 F1, B84/7 F2, B84/7 F3, B69F2, and B69 F4. Four ion-exchange purified bacteriocins, B16 F3, B84/7 F1, B84/7 F2 and B84/7 F3, exerted their lowest MIC to the tested pathogens, 1.95, 0.98, 0.49 and 0.49 µg/mL, respectively (Table 3). The strongest bacteriocin activity of B84/7 F2 was found. Total protein concentrations and bacteriocin units at each purification for the B84/7 F2 were shown in Table 4.
Volunteer recruitment and serum evaluation
Fifty healthy volunteers were enrolled. No volunteer was excluded from the recruitment. Their qualified semen were investigated for sperm immobilization and spermicidal activity. Demographic data and some semen quality parameters were shown in Figure 2.
Table 1. Antibacterial activity of the selected supernatant of vaginal lactobacilli against 7 tested strains. All data exhibit the average diameter ± standard deviation of a clear zone in millimeter unit (n = 3).
|
Tested strains |
B. subtilis ATCC 6633 |
S.aureus ATCC 25923 |
E. coli ATCC 25922 |
N. gonorrhoeae ATCC 49226 |
N. gonorrhoeae CM03 |
N. gonorrhoeae CM04 |
N. gonorrhoeae CM05 |
|||
|
36/14 |
23.67 ±0.58 |
23.67 ±0.29 |
20.33 ±0.76 |
20.50 ±0.87 |
21.00 ±1.00 |
20.33 ±0.58 |
20.17 ±0.29 |
|||
|
D4 |
24.17 ±0.29 |
23.83 ±0.29 |
20.17 ±0.76 |
20.17 ±0.76 |
20.67 ±0.58 |
21.33 ±0.58 |
20.83 ±0.76 |
|||
|
B16 |
24.17 ±0.76 |
24.17 ±0.76 |
19.83 ±1.26 |
20.33 ±0.58 |
21.50 ±0.50 |
21.67 ±0.58 |
22.00 ±1.00 |
|||
|
F36 |
23.33 ±0.58 |
25.33 ±0.76 |
20.67 ±0.58 |
20.83 ±0.29 |
21.33 ±1.15 |
21.50 ±0.50 |
21.50 ±0.50 |
|||
|
B84/7 |
25.17 ±0.29 |
24.83 ±0.29 |
21.67 ±0.58 |
21.50 ±0.50 |
21.50 ±0.50 |
22.17 ±0.29 |
22.33 ±0.58 |
|||
|
A68 |
21.67 ±0.58 |
23.50 ±0.87 |
19.33 ±0.58 |
20.00 ±1.00 |
19.67 ±0.58 |
20.83 ±0.76 |
18.67 ±1.15 |
|||
|
B89/4 |
21.33 ±0.58 |
23.33 ±0.58 |
18.33 ±0.58 |
19.33 ±1.15 |
19.67 ±0.58 |
20.67 ±0.58 |
19.50 ±0.87 |
|||
|
18/6 |
22.67 ±0.58 |
22.17 ±0.29 |
19.67 ±0.58 |
19.17 ±0.76 |
19.33 ±0.58 |
20.50 ±0.50 |
19.67 ±0.58 |
|||
|
B69 |
22.17 ±0.29 |
22.33 ±0.58 |
18.33 ±0.58 |
18.83 ±0.29 |
18.33 ±0.58 |
20.67 ±0.58 |
19.83 ±0.76 |
|||
|
Nisin* |
22.33 ±0.58 |
23.67 ±1.15 |
20.67 ±0.58 |
19.17 ±0.29 |
17.67 ±0.58 |
17.67 ±0.58 |
18.17 ±0.29 |
|||
Note: *positive control
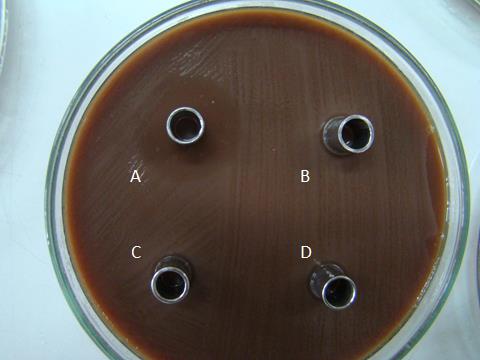
Figure 1. Inhibition zones of some cell-free supernatants (A, B84/7, the selected isolates; B, B85/7; C B86/7, D, B87/7) against N. gonorrhoeae ATCC 49226.
Table 2. Antimicrobial activities of each fractionated protein (x/) and its purified bacteriocin (/x) of the selected vaginal lactobacilli against N. gonorrhoeae ATCC 49226
|
Lactobacilli |
Diameter of clear zone (mm) of |
|||
|
F1 |
F2 |
F3 |
F4 |
|
|
L. plantarum 36/14 |
-/- |
-/- |
++/- |
++/+ |
|
L. plantarum D4 |
+/- |
-/- |
++/- |
++/+ |
|
L. jensennii B16 |
++/+ |
++/- |
++/++ |
++/++ |
|
L. jensennii F36 |
-/- |
-/- |
-/- |
++/- |
|
L. crispatus B84/7 |
++/++ |
++/++ |
++/++ |
++/+ |
|
L. acidophilus A68 |
++/+ |
-/- |
-/- |
+/- |
|
L. paracasei B89/4 |
++/- |
++/- |
-/- |
+/- |
|
L. rhamnosus 18/6 |
-/- |
-/- |
+/- |
++/- |
|
L. rhamnosus B69 |
++/- |
++/++ |
+/- |
++/++ |
Note: -, lesser activity; +, equal activity; ++, more activity than positive control. Nisin solution was performed as the positive control. Its diameter of clear zone, 18.0, 17.0 mm, respectively.
Determination of the rapid killing and time killing curve
In rapid kill study, 106 CFU/mL of the tested gonococci was killed in 15 minutes after bacteriocins were added at a concentration of 0.1 mg/mL of B84/7 F2 (Figure 2A). This bacteriocins that caused the growth inhibition of N. gonorrhoeae was significantly observed at 18 hours of incubation time (Figure 2B). It was suggested that this bacteriocin can kill the pathogen in a short time (15 minutes) of exposure and can also inhibit the growth of the pathogen within 18 hours.
Table 3. MIC of four selected bacteriocins demonstrated for lowest MIC to tested Neisseria, N. gonorrhoeae ATCC49226, and CM04.
|
Fractions |
Purified step* |
MIC (mg/mL) |
|
B16 F3 |
Supernatant |
7.81 |
|
|
Fractionation |
3.91 |
|
|
Ion exchange |
1.95 |
|
B84/7 F1 |
Supernatant |
7.81 |
|
|
Fractionation |
1.95 |
|
|
Ion exchange |
0.98 |
|
B84/7 F2 |
Supernatant |
7.81 |
|
|
Fractionation |
1.95 |
|
|
Ion exchange |
0.49 |
|
B84/7 F3 |
Supernatant |
7.81 |
|
|
Fractionation |
3.91 |
|
|
Ion exchange |
0.49 |
Note: The protein concentrations of each tested fraction were started at 1000 µg/mL before two-fold diluted. MIC is referred to as the minimal concentration which possesses the spermicidal activity of more than 50%.
Table 4. MIC, total protein, total activity, specific activity, purification folds, and % yields of each purification step of B84/7 F2.
|
Purification* |
Supernatant |
Fractionation |
Ion exchange |
|
Volume (mL) |
1,000 |
250 |
25 |
|
MIC (mg/mL) |
7.81 |
1.95 |
0.49 |
|
Total protein (mg) |
4,231.00 |
923.50 |
467.45 |
|
Bacteriocin unit (AU/mL) |
2,560 |
10,240 |
40,960 |
|
Total activity (AU) |
2,560,000 |
2,560,000 |
1,024,000 |
|
Specific activity |
605.06 |
2772.06 |
2190.61 |
|
Purification folds |
1 |
4.58 |
3.62 |
|
Yields (%) of total activity |
100 |
100 |
40 |
Note: *Fractionation, Cell-free supernatant were fractionated by membrane vivaflow. Ion exchange, purified bacteriocins were harvested from anion exchange chromatography. Specific activity is the ratio of total activity to that of total protein.
Sperm immobilization time
According to the inhibition of sperm motility, ninety-seven cell-free supernatants of a total of 932 lactobacillus isolates (10.41%) possessed the sperm immobilization activity within 4 minutes. Apart from 97 cell-free supernatants, four selected supernatants of L. plantarum 36/14, L. jensennii F36, L. rhamnosus 18/6, and L. rhamnosus B69 only demonstrated the antibacterial activity but the shorter sperm immobilization time was not detectable. However, only 12 cell-free supernatants (1.61% of total tested isolates) demonstrated faster sperm immobilization times than the sperm immobilization time (69.33 sec.) of nisin solution, the positive control. Seven of 12 isolates, L. plantarum A2, Lactobacillus paracasei A5, L. paracasei B280, L. acidophilus B34, L. johnsonii D68, L. crispatus F56, L. fermentum TD69 specifically resulted in only the sperm immobilization activity. Five of 12 cell-free supernatants which possessed both broad-spectrum antibacterial activity and sperm immobilization were selected, including L. plantarum D4, L. jensennii B16, L. crispatus B84/7, L. acidophilus A68, and L. paracasei B89/4. The sperm immobilization time of each fraction of these isolates was shown in Figure 3. After purification, the inhibitory activities of some bacteriocins of selected lactobacilli could not be observed at a faster time than nisin. The sperm immobilization time of seven potent bacteriocins namely, B84/7 F1, B89/4 F1, A68 F2, B84/7 F2, B16 F3, B84/7 F3, and B84/7 F4 were significantly detected and then selected to compare with the immobilization time of its cell-free supernatant as illustrated in Figure 4.
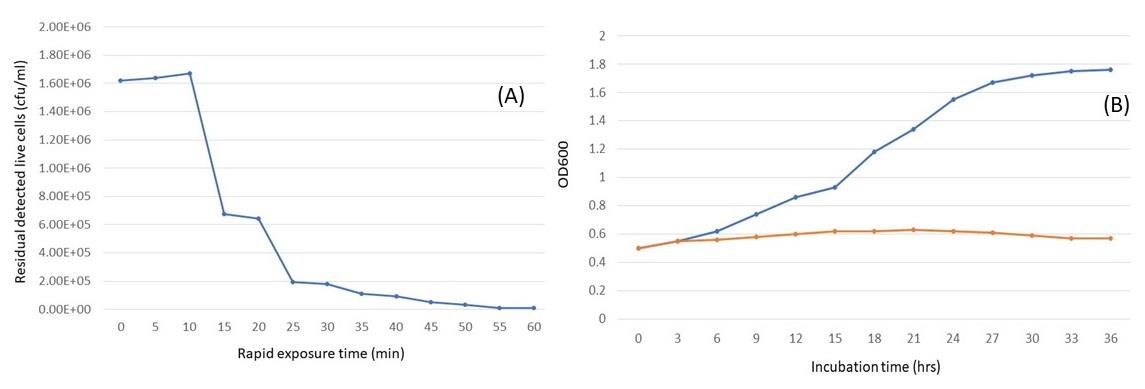
Figure 2. Rapid killing curve (a) and time killing curve (b) of the ion-exchange-purified B84/7 F2 bacteriocin against N. gonorrhoeae ATCC 49226 after comparison with the presence (orange) and absence (blue) of bacteriocin.
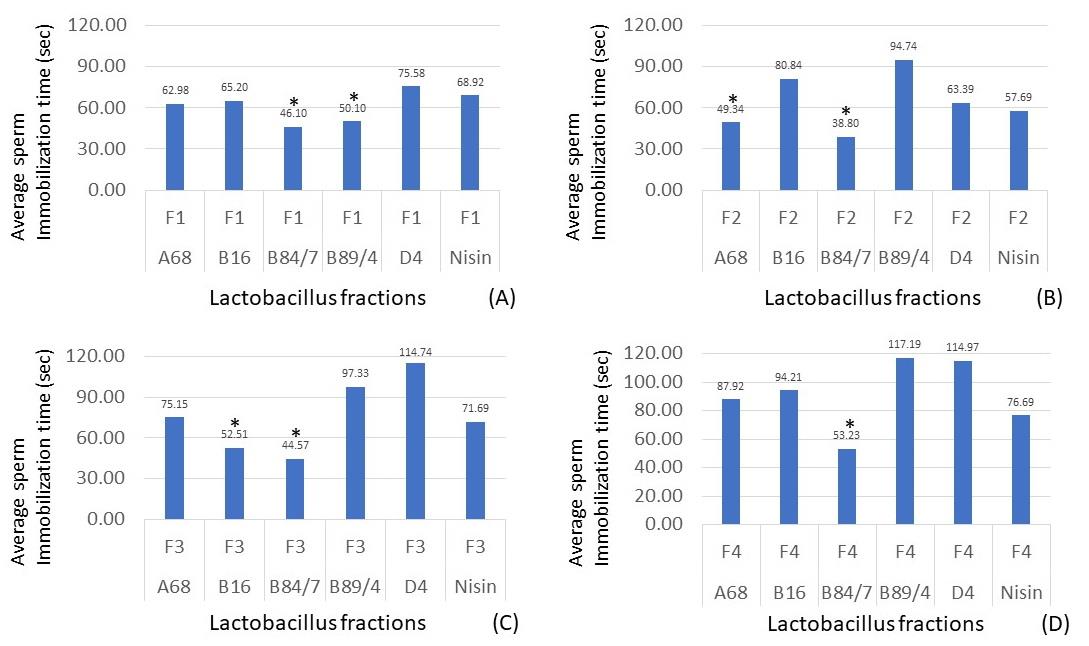
Figure 3. Sperm immobilization time of each protein fraction, F1 (A), F2 (B), F3 (C), and F4 (D), of selected antibacterial and spermicidal lactobacilli, compared with the nisin fraction. *, the sperm immobilization time of the selected bacteriocin was significantly shorter than the times of nisin, the positive control (P < 0.05). Data were from three individual trials.
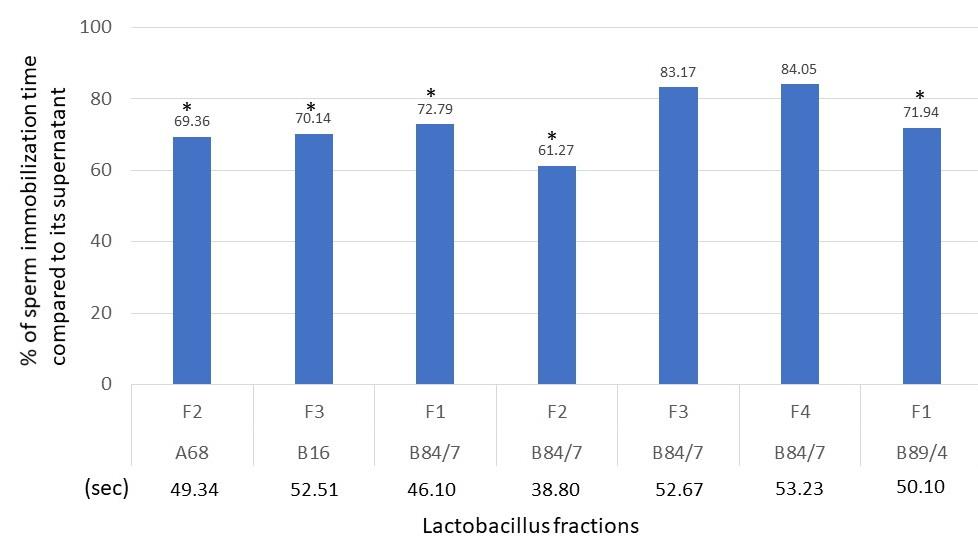
Figure 4. Percent of sperm immobilization time compared to its cell-free supernatant’s activity. Sperm immobilization times of cell-free supernatant A68, B16, B84/7, and B89/4 were 71.10, 74.90, 63.33, and 69.64 seconds, respectively.
Spermicidal Activity
Results shown in Figure 5 revealed that three tested bacteriocins, B84/7 F1, B84/7 F2, and B16 F3 exhibited a higher percentage of spermicidal activity than each nisin fraction. At the exposure time of 60 seconds, the percent spermicidal activity of these bacteriocins was demonstrated. They were selected as the spermicidal bacteriocins. It may be said that at EC100 of these interesting bacteriocins fraction possessed the strong spermicidal activity after exposure to 60 seconds. The spermicidal activity of these bacteriocins seem to be concentration-dependent after being determined at three concentrations including at undiluted, EC100, and EC50. Its spermicidal activity at various concentrations were shown in Figure 6. However, no morphological changes were found in the sperm head, mid-piece or tail when compared with untreated sperm. The eosin-nigrosin staining of each bacteriocin shows the morphology of sperm as shown in Figure 7. However, only B84/7 F2 possessed the spermicidal activity > 80% within 60 seconds of the exposure time to bacteriocin.
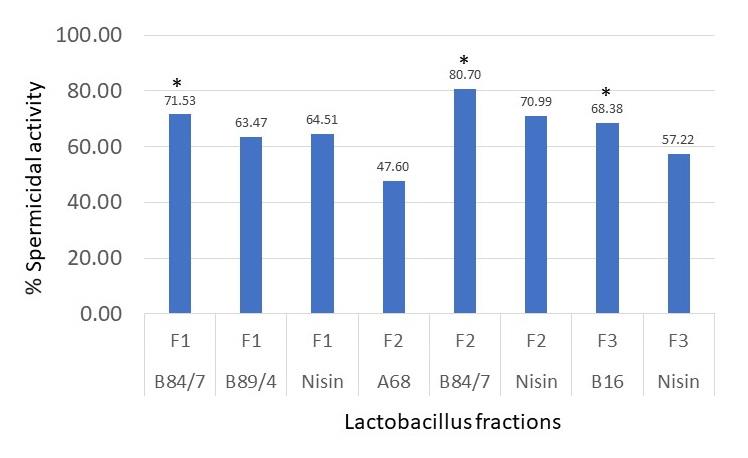
Figure 5. Percent of spermicidal activity at 60 seconds of exposure time to each selected protein fraction at EC100. *, the percentage of dead sperm affected from each selected fraction was significantly higher than the percentage of nisin, the positive control. Data were from 3 individual trials.
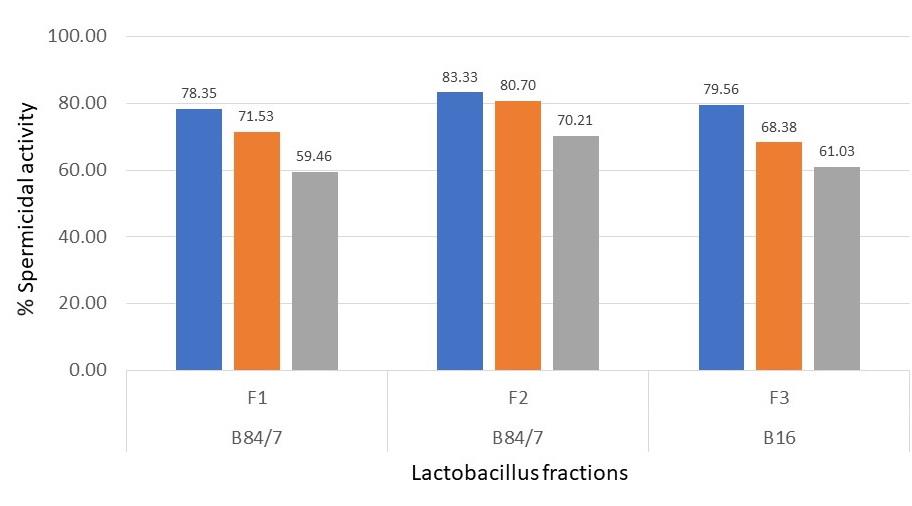
Figure 6. Percent of spermicidal activity at 60 seconds of exposure time to each selected protein fraction at the undiluted (blue bar), EC100 (orange bar), and EC50 (grey bar) concentrations. Data were from 3 individual trials.
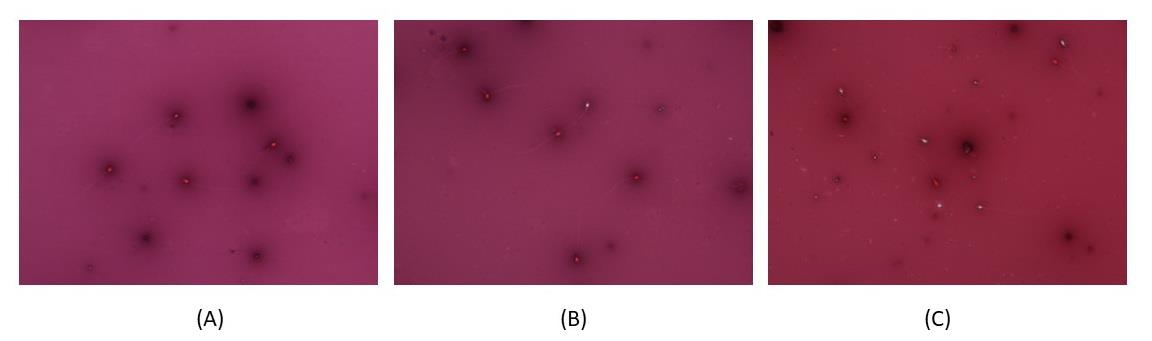
Figure 7. The spermicidal activity of bacteriocin fraction B84/7 F2 at doses of (A) undiluted, (B) EC100, and (C) EC50 after staining with nigrosin-eosin technique. Red cells, dead spermatozoa; White cells, viable spermatozoa. EC, minimal concentration of bacteriocin which affected sperm immobilization.
Among 48 voluntary semens, results demonstrated that the undiluted bacteriocin B84/7 F2 possessed the fastest sperm immobilization time with a median value at 30.42 seconds (average time ± SE.= 29.53 ± 0.70 seconds) as shown in Figure 8. The median time of other bacteriocins, B84/7F1, B16F3, and Nisin F2 were 45.53, 45.74, and 57.52 seconds. Their average time ± SE was 45.78 ± 1.16, 46.70 ± 1.23, and 56.96 ± 0.79 seconds.
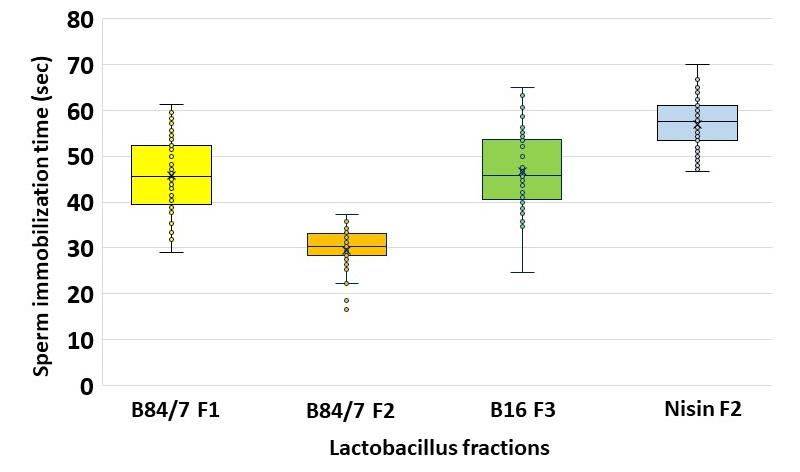
Figure 8. Sperm immobilization time of each selected crude protein against tested semen. The sperm immobilization time of these selected bacteriocins were significantly shorter than the times of nisin solution (P <0.05). (n=48)
One-Dimentional Polyacrylamide Electrophoresis
The protein bands on glycine or tricine gel were detected and calculated molecular weights as shown in Figure 9. Three selected bacteriocin fractions, B84/7 F1, B84/7 F2, B16 F3, and other fractions, B84/7 F3 were demonstrated. Two protein bands at 7.08 and 8.81 kDa were detected in B84/7 F2 bacteriocin after a run in tricine SDS-PAGE gel. This bacteriocin should be further analyzed and purified. Purification of this bacteriocin was also illustrated in Figure 10.
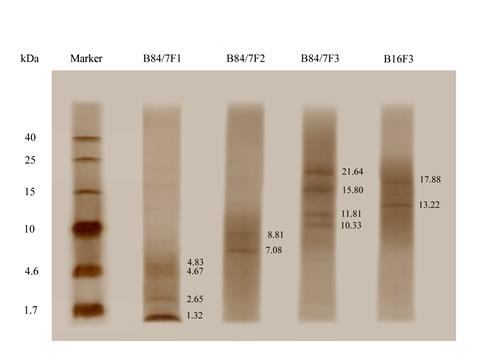
Figure 9. Protein bands of the fractionated proteins, including B84/7 F1, B84/7 F2, B84/7 F3, and B16 F3 bacteriocins detected on the tricine gel.
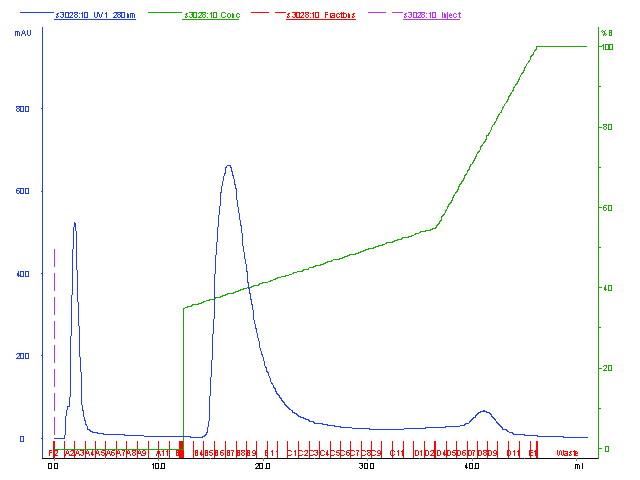
Figure 10. Partial purification of B84/7 F2 bacteriocin by anion exchange chromatography.
DISCUSSION
The present investigation highlights the isolation, selection, investigation of antibacterial, sperm immobilization and spermicidal agents produced from vaginal lactobacilli, and their characterization and partial purification. Because of the dual functions, antimicrobial and spermicidal activities were targeted for the protection of unwanted pregnancy and STI of Neisseria gonorrhoeae. Although a large number of these bacteriocins (or bacteriocin-like inhibitory substances) have been reported, a few groups of reports of extra-intestinal lactobacilli such as oral (Sookkhee et al., 2001) and vaginal lactobacilli (Aroutcheva et al., 2001; Kaewsrichan et al., 2006; Sabia et al., 2014; Maldonado-Barragán et al., 2016; Gaspar et al., 2018) have been studied in the broader spectrum against Gram-negative pathogens especially the inhibitory potential against N. gonorrhoeae. According to the antibacterial determinations, the screening of lactobacillus supernatants which possessed the promising antibacterial activity was performed in the present study by agar - well, and agar - cup diffusions for screening the standard strains and specific pathogens after comparing with the average inhibition zone of the nisin standard solution. Nisin has been well known that played a role as the bacteriocin and used as the positive control in the determination of bacteriocin. The supernatant of the nisin-producing strain must be determined for its activity by being tested with Lactobacillus sake ATCC 15521, the sensitive strain (Jozala, 2005). For the primary screening, S. aureus ATCC 25923, E. coli ATCC 25922, P. aeruginosa ATCC 27853 and N. gonorrhoeae ATCC 49226 were carried out as the standard indicators to determine the antibacterial activity. The interesting pathogen, N. gonorrhoeae was also selected as the tested indicator. Formerly, only in vitro inhibition of N. gonorrhoeae by L. acidophilus, the frequent strain of vaginal lactobacilli, has been reported (Zheng et al., 1994). However, this report also further comments that this inhibition was caused by the effects of its hydrogen peroxide and pH over than the effect of bacteriocins. Whereas the previous study reported that hydrogen peroxide-producing lactobacilli, such as L. crispatus and L. jensenii, could protect women against gonococci in both acidic and neutral conditions (St Amant et al., 2002). However, there were few studies of human spermicidal bacteriocins. We found that only five potent antibacterial bacteriocins which possessed the inhibitory effects against these standard indicators, and clinical strains of N. gonorrhoeae, also possessed sperm immobilization and spermicidal activity especially B84/7 F2 which was produced from L. crispatus B84/7. It was supported that L. crispatus and other species include L. rhamnosus, L. acidophilus, L. johnsonii play a role as the major flora and could protect the pathogenic bacteria by their bacteriocins. Their cell - free supernatants were selected as the antibacterial supernatant and further extracted the active protein for confirming the bacteriocin - producing isolates. These proteins still exhibited the antibacterial activity. In the present study, B84/7 F2 bacteriocin exhibited the lower MIC and faster killing time against N. gonorrhoeae than the previous study of other bacteriocins (Graver et al., 2011). This is the evidence on the vaginal lactobacilli which possessed the antibacterial activity, especially to N. gonorrhoeae. Moreover, the ability of lactobacilli to inhibit the vaginal epithelial adherence of N. gonorrhoeae was reported by Spurbeck et al. (Spurbeck et al., 2008).
According to the WHO guidelines on human semen, the recruitment of volunteers and the qualification of semen and sperm must be required. In the present study, forty-eight volunteers were recruited for semen donation. This group of volunteers were classified to be the normozoospermic volunteers. Three selected bacteriocins were also the potent sperm immobilization and spermicidal isolates in the group of volunteers. The fastest sperm immobilization could be detected nearly 30 seconds after being exposed to bacteriocin B84/7 F2. EC100 of this bacteriocin had a potent spermicidal effect with no changes in sperm morphology. It may be suggested that short-time exposure to certain concentrations of this bacteriocin was sufficient to induce a total loss of sperm motility. It was spermicidal bacteriocins that possessed significant activities to the tested bacteria and sperms. To compare the sperm immobilization time and spermicidal activity with the previously reported bacteriocins, the activity of B84/7 F2 was nearly close to lacticin 3147 which was approximately 30-35 seconds (Silkin et al., 2008). Bacteriocin B84/7 F2 exerted the complete human sperm immotility after compared with 100 μg/mL (37 μM) of maximin 1 and 250 μg/mL (101 μM) of Magainin 2-amide which possessed their efficacy at 80 and 50%, respectively (Chen et al., 1988; Lai et al., 2002). Faster immobilization time of bacteriocin B84/7 F2 nearly 30 seconds while LL-37, and magainin A completely inhibits human sperm motility within five, and seven minutes, respectively (Chen et al., 1988). The concentrations of magainin A, dermaseptin S4, and sarcotoxin Pd to immobilize human sperm were 300 μg/mL, 100 μg/mL, and 80 μg/mL, respectively (Chen et al., 1988; Zairi et al., 2005; Zare-Zardini et al., 2016). Although these peptides exerted the immediate immobilization of human sperm, lower EC100 of bacteriocin B84/7 F2 was detected in the present study. To compare with other bacteriocins, higher EC100 for human sperm than our bacteriocin were observed in the study of nisin A, pediocin CP2 and subtilosin are 300–400 μg/mL, 250 μg/mL and 110 μg/mL, respectively (Sutyak et al., 2008; Gupta, 2009; Kumar, 2012). Only dermaseptin S4 derivative possessed the lower ED100 than our bacteriocin to induce complete sperm immotility effects at a dose of 20 μg/mL, respectively (Zairi et al., 2005). Only two reports regarding the mechanism of action of bacteriocin toward the spermatozoa. Bacteriocin-like peptides of Weissella confusa MBF8-1 was the potential agent to affect the sperm motility (Sartono et al., 2019). Whereas the action on coiling, clumping, and agglutination of sperms was detected in the exposure to higher concentrations of the fermenticin HV6b. This bacteriocin also affected on the forward progression of human sperms and their aggregation was observed to be a dose-dependent interaction (Kaur et al., 2013)
Ion exchange-purified bacteriocin B84/7 F2 revealed 2 significant protein bands on tricine SDS-PAGE which are 7.08 and 8.81 kDa. Therefore, the bacteriocin was more likely to be a peptide or small protein molecule. These selected bacteriocins need to be further analyzed for their MALDI-TOF MS/MS fingerprints to elucidate their amino acid sequences, and study their modes of action. Some questions including, an in vivo condition to confirm our results about its contraceptive, cytotoxicity and antibacterial effects, an impairment of in vivo vaginal flora, and an increase of purification yield and its stability are also required to be study in the next part of study. Based on the present results of antimicrobial and spermicidal activities, bacteriocin B84/7 F2 may be applied to be developed as the novel effective and safe vaginal contraceptive or spermicidal agent to protect the sperm migration into the vagina, prevent unplanned pregnancy, prevent the healthy vaginal condition from any infections, and the acquisition of STI.
We shall also further evaluate the spermicidal activity of this bacteriocin on the increasing numbers of normozoospermic volunteers for determining the more accuracy and sensitivity of sperm immobilization time and percent spermicidal activity and also investigate the mechanism of action. In the present study, the ratio of volume between the bacteriocin and semen used to determine the sperm immobilization time was 1:2. It was impossible to apply as the formulation. Therefore, the effect of the ratio between protein and semen should be determined.
CONCLUSION
In conclusion, the present study found considerable antimicrobial and spermicidal activities of bacteriocins produced by vaginal lactobacillus on selected bacterial indicators and some clinical strains of N. gonorrhoeae, and also affected on sperm immobilization and sperm viability. Possession of bacteriocin by vaginal lactobacillus is an indication that the bacteria can be used as a probiotic and as an antimicrobial producer. It has an impact to further investigate the broad-spectrum antibacterial activity and dual spectrums of bacteriocins. It may be strong candidates for prevention or treatment of the healthy vaginal condition from any infections and to protect the imbalance vaginal microecology of the women patients and unwanted pregnancy. The selected bacteriocin would be further analyzed their MALDI-TOF MS/MS fingerprints, elucidated its amino acid sequence, and studied its mode of action.
ACKNOWLEDGEMENTS
The authors are thankful for the research funding provided by the Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand. The authors sincerely thank our technicians and nurses at Maharaj Nakorn Chiang Mai Hospital, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand for providing the bacterial samples and laboratory diagnosis. We would like to acknowledge the Research Ethic Committee of the Faculty of Medicine, CMU for expediting the approval of this project. Last, we appreciate our volunteers for their kindness to provide semen specimens.
AUTHOR CONTRIBUTIONS
Siriwoot Sookkhee collected the bacteria, did the experiment, wrote the manuscript. Sarawuth Manonuek performed the experiment. Chatchawann Apichartpiyakul provided techniques and wrote the proposal. Choompone Sakonwasun provided research funding, recruited volunteers, performed the statistical analysis. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Abts, A., Mavaro, A., Stindt, J., Bakkes, P.J., Metzger, S., Driessen, A.J., et al. 2011. Easy and rapid purification of highly active nisin. International Journal of Peptides, 2011: 175145.
Antonio, M.A., Hawes, S.E., and Hillier, S.L. 1999. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. Journal of Infectious Diseases, 180: 1950-1956.
Aranha, C., Gupta, S., and Reddy, K.V. 2004. Contraceptive efficacy of antimicrobial peptide Nisin: in vitro and in vivo studies. Contraception, 69: 333-338.
Aroutcheva, A.A., Simoes, J.A., and Faro, S. 2001. Antimicrobial protein produced by vaginal Lactobacillus acidophilus that inhibits Gardnerella vaginalis. Infectious Diseases in Obstetrics and Gynecology, 9: 33-39.
Chatterjee, C., Paul, M., Xie, L., and van der Donk, W.A. 2005. Biosynthesis and mode of action of lantibiotics. Chemical Reviews, 105: 633-684.
Chen, H.C., Brown, J.H., Morell, J.L., and Huang, C.M. 1988. Synthetic magainin analogues with improved antimicrobial activity. FEBS Letters, 236: 462-466.
Fichorova, R.N., Tucker, L.D., and Anderson, D.J. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. Journal of Infectious Diseases, 184: 418-428.
Gaspar, C., Donders, G.G., Palmeira-de-Oliveira, R., Queiroz, J.A., Tomaz, C., Martinez-de-Oliveira, J., et al. 2018. Bacteriocin production of the probiotic Lactobacillus acidophilus KS400. AMB Express, 8: 153-153.
Gordon, M.A., Lapa, E.W., Fitter, M.S., and Lindsay, M. 1980. Susceptibility of zoopathogenic fungi to phytoalexins. Antimicrob Agents Chemother, 17: 120-123.
Graver, M.A., and Wade, J.J. 2011. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Annals of Clinical Microbiology and Antimicrobials, 10: 8.
Gupta, S.M., Aranha, C.C., Bellare, J.R., and Reddy, K.V. 2009. Interaction of contraceptive antimicrobial peptide nisin with target cell membranes: implications for use as vaginal microbicide. Contraception, 80: 299-307.
Halvaei, I., Khalili, M.A., Soleimani, M., and Razi, M.H. 2011. Evaluating the role of first polar body morphology on rates of fertilization and embryo development in ICSI Cycles. International Journal of Fertility and Sterility, 5: 110-115.
Inácio Â, S., Mesquita, K.A., Baptista, M., Ramalho-Santos, J., Vaz, W.L., and Vieira, O.V. 2011. In vitro surfactant structure-toxicity relationships: implications for surfactant use in sexually transmitted infection prophylaxis and contraception. PLoS One, 6: e19850.
Jozala, A.F., de Lencastre Novaes, L.C., Cholewa, O., Moraes, D., and Penna, T.C 2005. Increase of nisin production by Lactococcus lactis in different media. African Journal of Biotechnology, 4: 262-265.
Kaewsrichan, J., Peeyananjarassri, K., and Kongprasertkit, J. 2006. Selection and identification of anaerobic lactobacilli producing inhibitory compounds against vaginal pathogens. FEMS Immunology and Medical Microbiology, 48: 75-83.
Kaur, B., Balgir, P.P., Mittu, B., Kumar, B., and Garg, N. 2013. Biomedical Applications of Fermenticin HV6b Isolated from Lactobacillus fermentum HV6b MTCC10770. BioMed Research International, 2013: 168438.
Kaur, S., and Sharma, P. 2013. In vitro synergistic antimicrobial activity of bacteriocin of vaginal lactobacilli and antibiotics against Pseudomonas aeruginosa. International Journal of Biotechnology and Bioengineering Research, 4: 573-574.
Klebanoff, S.J. 1992. Effects of the spermicidal agent nonoxynol-9 on vaginal microbial flora. Journal of Infectious Diseases, 165: 19-25.
Kumar, B., Balgir, P.P., Kaur, B., Mittu, B., and Garg, N. 2012. Antimicrobial and spermicidal activity of native and recombinant pediocin CP2: a comparative evaluation. Archives of Clinical Microbiology, 3: 1-12.
Lai, R., Zheng, Y.T., Shen, J.H., Liu, G.J., Liu, H., Lee, W.H., et al. 2002. Antimicrobial peptides from skin secretions of Chinese red belly toad Bombina maxima. Peptides, 23: 427-435.
López-Teijón, M., Elbaile, M., and Alvarez, J. G. 2008. Geographical differences in semen quality in a population of young healthy volunteers from the different regions of Spain. Andrologia, 40: 318-328.
Maldonado-Barragán, A., Caballero-Guerrero, B., Martín, V., Ruiz-Barba, J.L., and Rodríguez, J.M. 2016. Purification and genetic characterization of gassericin E, a novel co-culture inducible bacteriocin from Lactobacillus gasseri EV1461 isolated from the vagina of a healthy woman. BMC Microbiology, 16: 37.
Martin, H.L., Richardson, B.A., Nyange, P.M., Lavreys, L., Hillier, S.L., Chohan, B., et al. 1999. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis, 180: 1863-1868.
McGroarty, J.A., and Reid, G. 1988. Detection of a Lactobacillus substance that inhibits Escherichia coli. Canadian Journal of Microbiology, 34: 974-978.
McGroarty, J.A., Tomeczek, L., Pond, D.G., Reid, G., and Bruce, A.W. 1992. Hydrogen peroxide production by Lactobacillus species: correlation with susceptibility to the spermicidal compound nonoxynol-9. Journal of Infectious Diseases, 165: 1142-1144. Retrieved from http://www.jstor.org/stable/30112205
Méndez, F., Castro, A., and Ortega, A. 1986. Use effectiveness of a spermicidal suppository containing benzalkonium chloride. Contraception, 34: 353-362.
Ocaña, V.S., Pesce De Ruiz Holgado, A.A., and Nader-Macías, M.E. 1999. Characterization of a bacteriocin-like substance produced by a vaginal Lactobacillus salivarius strain. Applied and Environmental Microbiology, 65: 5631-5635.
Sabia, C., Anacarso, I., Bergonzini, A., Gargiulo, R., Sarti, M., Condò, C., et al. 2014. Detection and partial characterization of a bacteriocin-like substance produced by Lactobacillus fermentum CS57 isolated from human vaginal secretions. Anaerobe, 26: 41-45.
Sartono, G., Rizqiyah, I., Asmarinah, Heng, N.C.K., and Malik, A. 2019. Three Bacteriocin Peptides from a Lactic Acid Bacterium Weissella confusa MBF8-1 with Spermicidal Activity. Current Pharmaceutical Biotechnology, 20: 766-771.
Silkin, L., Hamza, S., Kaufman, S., Cobb, S.L., and Vederas, J.C. 2008. Spermicidal bacteriocins: lacticin 3147 and subtilosin A. Bioorg Med Chem Lett, 18: 3103-3106.
Sookkhee, S., Chulasiri, M., and Prachyabrued, W. 2001. Lactic acid bacteria from healthy oral cavity of Thai volunteers: inhibition of oral pathogens. Journal of Applied Microbiology, 90: 172-179.
Spurbeck, R.R. and Arvidson, C.G. 2008. Inhibition of Neisseria gonorrhoeae epithelial cell interactions by vaginal Lactobacillus species. Infection and Immunity, 76: 3124-3130.
Srakaew, N., Young, C.D., Sae-wu, A., Xu, H., Quesnel, K.L., di Brisco, R., et al. 2014. Antimicrobial host defence peptide, LL-37, as a potential vaginal contraceptive. Human Reproduction, 29: 683-696.
St Amant, D.C., Valentin-Bon, I.E., and Jerse, A.E. 2002. Inhibition of Neisseria gonorrhoeae by Lactobacillus species that are commonly isolated from the female genital tract. Infection and Immunity, 70: 7169-7171.
Starke, K. and Visser, A.P. 1994. Sexuality, sexual behaviour and contraception in East Germany. Patient Education and Counseling, 23: 217-226.
Stephenson, J. 2000. Widely used spermicide may increase, not decrease, risk of HIV transmission. Jama, 284: 949.
Sutyak, K.E., Anderson, R.A., Dover, S.E., Feathergill, K.A., Aroutcheva, A.A., Faro, S., et al. 2008. Spermicidal activity of the safe natural antimicrobial peptide subtilosin. Infectious Diseases in Obstetrics and Gynecology, 2008: 540758.
Wiesenfeld, H.C., Hillier, S.L., Krohn, M.A., Landers, D.V., and Sweet, R.L. 2003. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clinical Infectious Diseases, 36: 663-668.
World Health Organization. 2010. Laboratory Manual for the Examination of Human Semen (5th ed. Vol. 4). Cambridge: Cambridge University Press.
Zairi, A., Belaïd, A., Gahbiche, A., and Hani, K. 2005. Spermicidal activity of dermaseptins. Contraception, 72: 447-453.
Zare-Zardini, H., Fesahat, F., Anbari, F., Halvaei, I., and Ebrahimi, L. 2016. Assessment of spermicidal activity of the antimicrobial peptide sarcotoxin Pd: A potent contraceptive agent. European Journal of Contraception & Reproductive Health Care, 21: 15-21.
Zheng, H.Y., Alcorn, T.M., and Cohen, M.S. 1994. Effects of H2O2-producing lactobacilli on Neisseria gonorrhoeae growth and catalase activity. Journal of Infectious Diseases, 170: 1209-1215.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Siriwoot Sookkhee*, Sarawuth Manonuek, Chatchawann Apichartpiyakul, and Choompone Sakonwasun
Department of Microbiology, Faculty of Medicine, Chiang Mai University, Chiang Mai, 50200 Thailand
Corresponding author: Siriwoot Sookkhee, E-mail: siriwoot.s@cmu.ac.th
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: october 7, 2021;
Revised: November 2, 2021;
Accepted: November 9, 2021

