
Neutrophil-Lymphocyte Ratio (NLR) is Positively Associated with Impaired Cognitive Performance inPatients with Metabolic Syndrome
Noppamas Pipatpiboon, Jirapas Sripetchwandee*, Piangkwan Sa-nguanmoo, Chiraporn Tachaudomdach, Tanyarat Jomgeow, Arintaya Phrommintikul, Nipon Chattipakorn, and Siriporn C. ChattipakornPublished Date : 2022-01-21
DOI : https://doi.org/10.12982/CMUJNS.2022.013
Journal Issues : Number 1, January-March 2022
Abstract Metabolic syndrome (MetS) is known to be related to mild cognitive impairment (MCI). A prognostic biomarker for the MCI condition in these patients has not been thoroughly determined. A neutrophil-lymphocyte ratio (NLR) has been widely used as a biomarker for the progression of cancers and cardiovascular diseases. However, its association with the MCI condition in patients with MetS is not known. The present study aimed to investigate the correlation between NLR and cognitive function in patients with MetS. A total of sixty patients with MetS (45-65 years old) were enrolled in the present study, and their metabolic parameters, including plasma levels of glucose, insulin, lipid profiles, inflammatory markers, and the complete blood count, were determined. The NLR level was calculated by the ratio of neutrophils to lymphocytes derived from the complete blood count. The Montreal Cognitive Assessment (MoCA) test was used to determine the cognitive performance in patients with MetS. Most patients with MetS have the possibility of an MCI condition. Moreover, glycated hemoglobin (HbA1C), fasting plasma glucose (FPG), and NLR were negatively correlated with the MoCA scores of these patients. Interestingly, NLR was the strongest independent factor which correlated with the MoCA score. Collectively, poor glycemic control and increased NLR levels may be used as possible predictors for poorer cognitive performance outcomes in patients with MetS.
Keywords: Metabolic syndrome; Mild cognitive impairment; Neutrophil-lymphocyte ratio; Prognostic marker; Montreal cognitive assessment
Citation: Pipatpiboon, N., Sripetchwandee, J., Sa-nguanmoo, P., Tachaudomdach, C., Jomgeow, T., Phrommintikul, A., Chattipakorn, N., and Chattipakorn, S.C. 2022. Neutrophil-Lymphocyte Ratio (NLR) is positively associated with impaired cognitive performance in patients with metabolic syndrome. CMU J. Nat. Sci. 21(1): e2022013.
INTRODUCTION
Keywords: Metabolic syndrome (MetS) has become a major worldwide health problem (Kaur, 2014) since patients with MetS have an increased risk for Type 2 diabetes mellitus and cardiovascular diseases (Alberti et al., 2009; Salekzamani et al., 2016). Several studies have demonstrated the progression of cognitive decline in the MetS condition (Panza et al., 2010; Niu et al., 2016). Recent clinical studies have shown that a high glucose level, in both subjects with or without diabetes, is correlated with a risk of cognitive impairment (Crane et al., 2013; Kerti et al., 2013). Furthermore, numerous studies demonstrated a positive relationship among high-levels of lipid profiles and an incidence of dementia (Hokanson and Austin, 1996; Wanamaker et al., 2015). Those findings suggest that the MetS condition may lead to the development of cognitive decline.
Mild cognitive impairment (MCI) is known as a state of cognitive dysfunction in which the severity of the cognitive decline is insufficient to cause dementia (Petersen, 2016). Several cognitive tests including the Quick Mild Cognitive Impairment screen and the Montreal Cognitive Assessment (MoCA) were used for MCI screening (O'Caoimh et al., 2016; Clarnette et al., 2017). MCI has been reported as a possible risk factor for Alzheimer’s disease (AD) (Albert et al., 2011). A positive correlation between MetS and MCI has been reported (Yao et al., 2016). Several factors in patients with MetS, including the duration of diabetes, degree of glycemic control, use of glucose-lowering drugs, high blood pressure, and biochemical parameters, can be predictors for MCI in those patients (Gao et al., 2015; Liu et al., 2015; Ng et al., 2016; Yao et al., 2016).
Systemic inflammation is commonly found in several pathological conditions including cancer, heart disease, and cognitive decline (Bhat et al., 2013; Halazun et al., 2014; Xiao et al., 2014). During systemic inflammation, white blood cells, particularly neutrophil and lymphocyte, play an important role by releasing pro-inflammatory cytokines and chemokines (Mantovani et al., 2010; Vendramini-Costa and Carvalho, 2012). Recently, It has been known that a neutrophil to lymphocyte ratio (NLR) can be a prognostic marker to predict several pathological conditions, including sepsis and infectious pathologies (de Jager et al., 2010), cancers (Templeton et al., 2014), and major cardiac events (Dong et al., 2018), since it is a non-invasive, inexpensive and applicable method to detect systemic inflammation. Moreover, NLR can also be used as a predictor in brain pathologies which included ischemic stroke (Switonska et al., 2020) and cerebral hemorrhage (Lattanzi et al., 2017; Lattanzi et al., 2018). Taken together, these findings suggest the broad clinical utility of NLR as a predictor of inflammation-related pathological conditions.
In the brain, neuronal inflammation also contributes to neurodegenerative diseases, particularly AD (De Felice and Ferreira, 2014; Jayaraj et al., 2014). Plasma inflammatory cytokines have been reported to be correlated with the development of cognitive impairment (Bermejo et al., 2008; Zheng et al., 2016). Recently, the NLR Family Pyrin Domain Containing 3 inflammasome (NLRP3), a biomarker for chronic inflammation, has been reported to be related to cognitive decline (Zhuang et al., 2017).
Those findings suggest that NLR, inflammatory cytokines and the NLRP3 may be possible biomarkers for memory deficit in patients with MetS. However, the association between NLR and cognitive decline in patients with MetS is not known. Therefore, the present study was aimed to investigate the association between NLR and cognitive performance in patients with MetS.
MATERIALS AND METHODS
Study design and setting
This cross-sectional study was carried out at Maharaj Nakorn Chiang Mai Hospital, Sriphum district, Chiang Mai, Thailand.
Population
The study protocol was approved by the Institutional Ethics Committee of the Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand (463/2557). All participants read a patient information sheet and signed an informed consent form for research participation.
Participants of both genders (n = 60, 45-65 years old), who met 3 out of 5 criteria for MetS (Alberti et al., 2009), from Maharaj Nakorn Hospital, Sriphum district, Chiang Mai, Thailand, were invited to participate in the present study.
Patients with any of following conditions were excluded from the study: depressed mental status, alcoholism or substance abuse, history of head trauma, and severe neurological or psychiatric illness. A summarized experimental protocol is shown in Figure 1.
Figure 1. The summarized experimental protocols.
Determination of MCI
A Thai version of the Montreal Cognitive Assessment (MoCA) test was used to evaluate mild cognitive impairment (Julayanont et al., 2015). A score of 26 or above demonstrated normal cognitive function as previously reported (Banjongrewadee et al., 2018). In contrast, patients who had an individual MoCA lower than 26 points were categorized as having a possible MCI condition.
Determination of biochemical markers
Blood samples were collected via venipuncture to investigate the metabolic profiles, including fasting plasma glucose (FPG), fasting plasma insulin (FINS), lipid profiles, total cholesterol (TC), triglycerides (TG), very low-density cholesterol (VLDL-C), low-density cholesterol (LDL-C), and high-density cholesterol (HDL-C), plasma HbA1C and WBC counts. Colorimetric assay was used for determining levels of FPG (ERBA diagnostic, Mannheim, Germany), while FINS levels were detected by a sandwich ELISA assay kit (Millipore, MI, USA) (Pipatpiboon et al., 2013).
Determination of insulin resistance
Homeostasis model assessment-estimated insulin resistance (HOMA-IR) was used to determine the degree of insulin resistance. A higher HOMA-IR indicated a higher degree of insulin resistance (Phrommintikul et al., 2018).
NLR determination
Absolute counts of neutrophil and lymphocyte counts from WBC were used to calculate the NLR.
Blood sample collection for peripheral blood mononuclear cell (PBMC) isolation
Blood samples were kept in heparinized tubes and then centrifuged at 2,500 rpm (4˚C, 5 minutes). Then, the buffy-coated section was collected, washed with 2-ml phosphate buffer saline (PBS) and centrifuged at 2,500 rpm (4˚C, 5 minutes); this process was repeated for twice. The isolated PBMC were overlaid on ficoll-hypaque (1.077g/L) at a 1:1 ratio, and subsequently centrifuged at 2,500 rpm (4˚C, 10 minutes). Then, isolated PBMC were washed with PBS (1:1) and centrifuged twice at 2,500 rpm (4˚C, 5 minutes).
Determination of the NLRP3 gene
Total RNA from the isolated PBMC was prepared using the Superscript III Kit (Invitrogen, CA, USA) according to the manufacturer's protocol. The NLRP3 gene expression was expressed and reported as a ratio between NLRP3 and GAPDH.
Plasma inflammatory markers
The inflammatory markers IL-18 and IL-1β from the plasma were determined using a homemade ELISA kit. Absorbance was determined at 450 nm using a microplate reader (Metertech-Inc, Nankang, Taipei, Taiwan). Data were expressed as mean ng/mL ± SEM.
Data analysis
Demographic and metabolic parameter data were presented as mean ± a standard error of mean (SEM). The independent t-test was used for comparison between two groups. The Pearson correlation method was used to determine the possible factors which correlated with an individual MoCA score. Univariate analysis was performed for the metabolic parameters that had a significant correlation with an individual MoCA score. Moreover, the significant parameters from the univariate analysis in these patients were further collected into multivariate models and a multivariate analysis was performed. A P-value of <0.05 was considered statistically significant.
RESULTS
Demographic data and clinical parameters in all patients with MetS
The demographic data from patients with MetS were shown in Table 1, while the clinical characteristics of metabolic parameters from these patients were demonstrated in Table 2. Regarding the evaluation of cognitive performance by a MoCA test, we found that the average MoCA score from these patients was approximately 19 points (Table 2). Since an individual MoCA score of less than 26 points is considered as a cut-off for a possible MCI condition, the prevalence of MCI was about 90% in patients with MetS (54 out of a total of 60 patients). Taken together, these findings indicated that most patients with MetS might be affected by an MCI condition. Therefore, the metabolic profiles of these patients with MetS who have MCI were further categorized as being a possible MCI group and analyzed to determine the correlation between these parameters and the MoCA score.
Table 1. The demographic data of MetS patients.
|
Demographic parameters |
Patients with MetS (n = 60) |
|
Male, n (%) |
28 (47%) |
|
Average age (years) |
57.47 ± 0.59 |
|
Years of education (years) |
7.10 ± 0.52 |
|
Duration of T2DM (years) |
11.12 ± 1.09 |
|
Duration of HTN (years) |
11.11 ± 1.05 |
|
Duration of dyslipidemia (years) |
9.34 ± 1.18 |
|
MoCA score (points) |
19.77 ± 0.58 |
Note: Data were presented as N (%) or mean ± SE. HTN; hypertension, MetS; metabolic syndrome, MCI; mild-cognitive impairment, T2DM; type 2 diabetes mellitus.
Table 2. Clinical characteristics of metabolic parameters in patients with MetS.
|
Clinical parameters |
Patients with MetS (n = 60) |
Non-MCI |
Possible MCI |
P-Value |
|
BMI (kg/m2) |
25.96 ± 0.39 |
26.02 ± 0.79 |
25.95 ± 0.42 |
0.615 |
|
WHR |
0.90 ± 0.01 |
0.94 ± 0.03 |
0.90±0.01 |
0.253 |
|
FPG (mmol/L) |
135.78 ± 9.57 |
116.64 ± 29.57 |
137.25±10.09 |
0.584 |
|
HbA1C (%) |
7.47 ± 0.24 |
7.36 ± 0.46 |
7.50 ± 0.27 |
0.866 |
|
FINS (mIU/L) |
9.12 ± 0.86 |
9.97 ± 3.65 |
9.05 ± 0.90 |
0.781 |
|
HOMA-index |
2.84 ± 0.28 |
3.62 ± 2.21 |
2.78 ± 0.26 |
0.436 |
|
SBP (mmHg) |
155.77 ± 18.22 |
135.17 ± 9.52 |
158.06 ± 20.22 |
0.710 |
|
DBP (mmHg) |
76.43 ± 1.31 |
76.17 ± 3.40 |
76.46 ± 1.42 |
0.947 |
|
TG (mmol/L) |
128.14 ± 9.57 |
112.40 ± 25.75 |
130.15 ± 10.35 |
0.562 |
|
TC (mmol/L) |
161.89 ± 5.11 |
163.00 ± 13.20 |
161.76 ± 5.54 |
0.940 |
|
HDL-C (mmol/L) |
48.49 ± 2.14 |
53.40 ± 9.05 |
47.96 ± 2.19 |
0.456 |
|
LDL-C (mmol/L) |
99.35 ± 4.51 |
95.00 ± 18.38 |
99.83 ± 4.66 |
0.754 |
|
VLDL-C (mmol/L) |
25.43 ± 2.04 |
22.50 ± 6.76 |
25.81 ± 2.16 |
0.613 |
|
Plasma IL-1β (ng/ml) |
530.99 ± 45.31 |
325.04 ± 116.16 |
553.89 ± 47.95* |
0.043 |
|
Plasma IL-18 (ng/ml) |
393.84 ± 27.57 |
347.48 ± 79.90 |
398.99 ± 29.47 |
0.760 |
|
NLRP3/GAPDH ratio |
1.48 ± 0.06 |
1.45 ± 0.21 |
1.48 ± 0.06 |
0.224 |
|
NLR |
1.83 ± 0.10 |
1.61 ± 0.06 |
1.84 ± 0.11* |
0.012 |
Note: Data presented as mean ± SE. BMI; body mass index, DBP; diastolic blood pressure, FINS; fasting insulin, FPG; fasting plasma glucose, HbA1C; glycated hemoglobin, HDL-C; high-density cholesterol, IL-1β; interleukin-1β, IL-18; interleukin-18, LDL-C; low-density cholesterol, MetS; metabolic syndrome, MCI; mild-cognitive impairment, NLRP3; NLR family pyrin domain containing 3, NLR; neutrophil-lymphocyte ratio, TC; total cholesterol, TG; triglyceride, SBP; systolic blood pressure, VLDL; very low-density cholesterol, WHR; waist-hip ratio.
Negative correlation of glycemic parameters and/or NLR with individual MoCA scores in patients with a possible MCI condition.
A correlation between the metabolic parameters and the MoCA scores of patients with MetS and possible MCI conditions was demonstrated (Table 3). The associative correlation between the individual MoCA scores of these patients and their metabolic parameters demonstrated that individual MoCA scores were positively correlated with BMI (Figure 2A), while a negative correlation of MoCA with blood glycemic profiles, HbA1C (Figure 2B) and FPG (Figure 2C) was observed.
Table 3. Correlation of metabolic data and MoCA scores from patients with possible MCI conditions
|
Metabolic parameters |
r |
P-Value (univariate) |
|
|
|
BMI (kg/m2) |
0.358 |
0.008 |
|
|
WHR |
-0.085 |
0.540 |
|
|
FPG (mmol/L) |
-0.314 |
0.023 |
|
|
HbA1C (%) |
-0.369 |
0.011 |
|
|
FINS (mIU/L) |
0.094 |
0.521 |
|
|
HOMA-index |
-0.059 |
0.687 |
|
|
SBP (mmHg) |
-0.030 |
0.827 |
|
|
DBP (mmHg) |
-0.085 |
0.541 |
|
|
TG (mmol/L) |
-0.241 |
0.095 |
|
|
TC (mmol/L) |
-0.114 |
0.489 |
|
|
HDL-C (mmol/L) |
0.020 |
0.896 |
|
|
LDL-C (mmol/L) |
-0.215 |
0.151 |
|
|
VLDL-C (mmol/L) |
-0.204 |
0.272 |
|
|
Plasma IL-1β (ng/ml) |
-0.258 |
0.060 |
|
|
Plasma IL-18 (ng/ml) |
0.060 |
0.668 |
|
|
NLRP3/GAPDH ratio |
-0.252 |
0.066 |
|
|
NLR |
-0.497 |
<0.001 |
Note: Data presented as mean ± SE. BMI; body mass index, DBP; diastolic blood pressure, FINS; fasting insulin, FPG; fasting plasma glucose, HbA1C; glycated hemoglobin, HDL-C; high-density cholesterol, IL-1β; interleukin-1β, IL-18; interleukin-18, LDL-C; low-density cholesterol, MetS; metabolic syndrome, MCI; mild-cognitive impairment, NLRP3; NLR family pyrin domain containing 3, NLR; neutrophil-lymphocyte ratio, TC; total cholesterol, TG; triglyceride, SBP; systolic blood pressure, VLDL; very low-density cholesterol, WHR; waist-hip ratio.
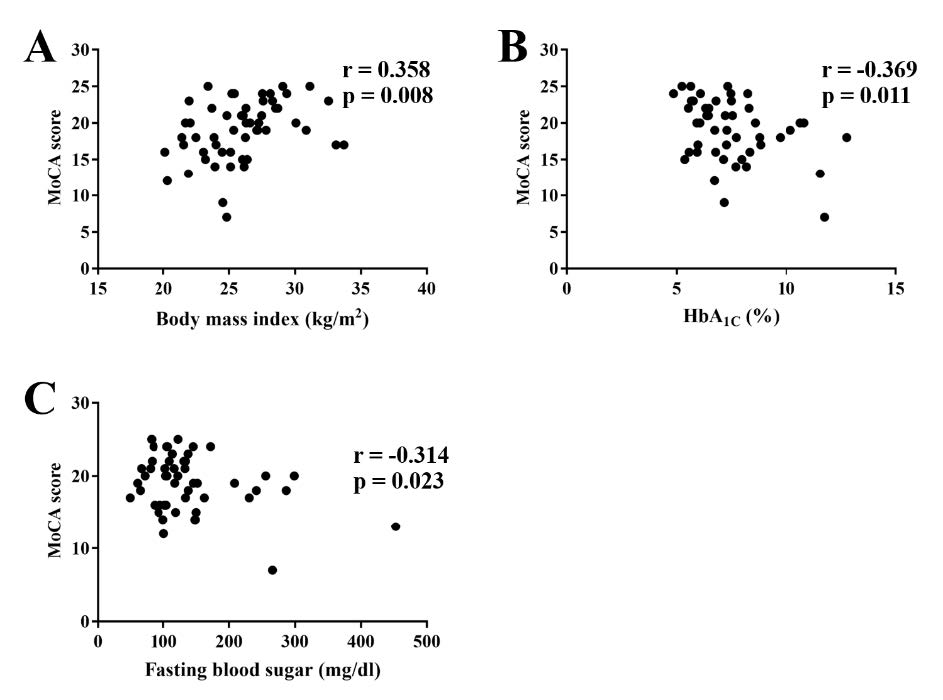
Figure 2. Correlative scatter plots between an individual MoCA score and BMI (a), HbA1C (b), and fasting plasma glucose level (c). MoCA; BMI; body mass index, Montreal cognitive assessment, HbA1C; glycated hemoglobin.
Surprisingly, an individual MoCA score was negatively correlated with neutrophil-lymphocyte ratio (NLR) (Figure 2A). However, other inflammatory markers including plasma IL-1β, IL-18 level and NLRP3 gene expression were not correlated with the MoCA scores (Figure 3B-3D).
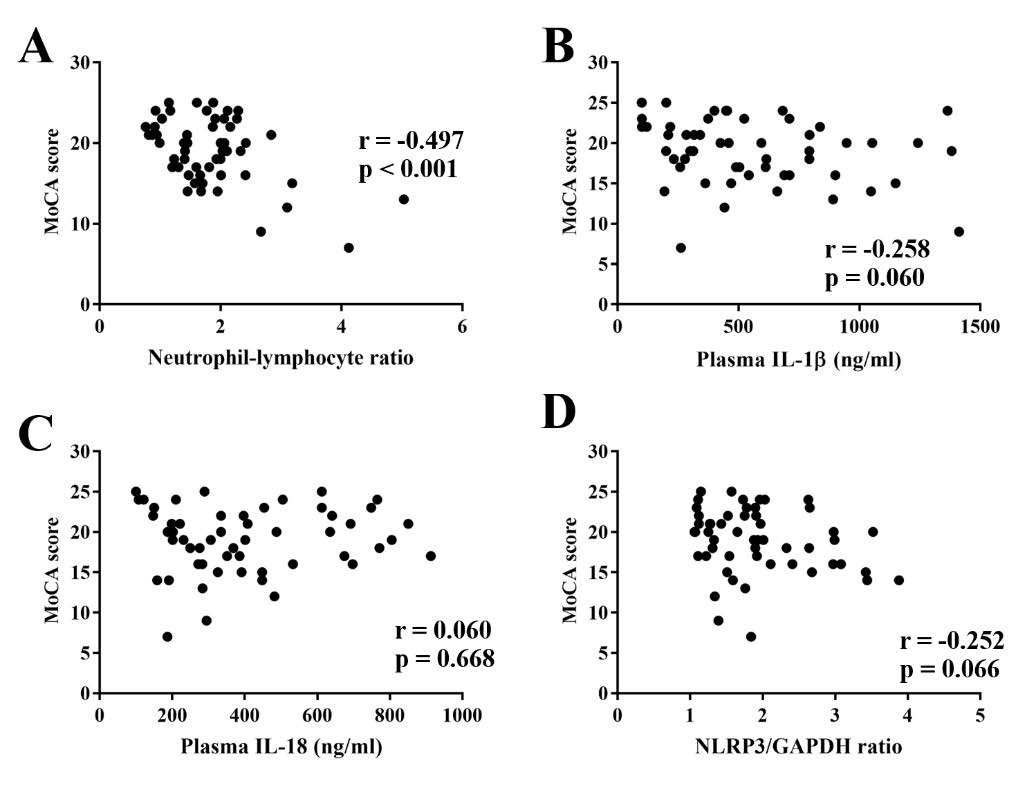
Figure 3. Correlative scatter plots between an individual MoCA score and systemic inflammatory markers including neutrophil-lymphocyte ratio (NLR) (a), plasma IL-1β level (b), plasma IL-18 (c), and NLRP3/GAPDH ratio (d). IL-1β; interleukin-1β, IL-18; interleukin-18, MoCA; Montreal cognitive assessment, NLRP3; NLR family pyrin domain containing 3.
Multivariate analysis for the association between MoCA scores with various parameters of patients with possible MCI conditions.
Significantly correlated factors such as BMI, plasma HbA1C, FPG, and the NLR were used for univariate analysis and the MoCA score was set as a dependent variable (Table 4). Multivariate analyses of the correlated metabolic parameters and MoCA scores were also determined in order to consider the influence of multiple independent variables on the variability of dependent variables (Table 4). After multivariate analysis was performed, the results demonstrated that only NLR showed a negative correlation with the individual MoCA score (Table 4), suggesting that NLR might be a prognostic indicator of poor cognitive performance in patients with MetS.
Table 4. Multivariate analysis of significant variables from univariate analysis of MoCA scores in patients with possible MCI conditions.
|
Parameters |
B |
P-Value |
VIF |
|
BMI |
0.273 |
0.056 |
1.196 |
|
FPG |
0.017 |
0.936 |
2.701 |
|
HbA1C |
-0.197 |
0.339 |
2.554 |
|
NLR |
-0.326 |
0.043 |
1.497 |
Note: BMI; body mass index, FPG; fasting plasma glucose, HbA1C; glycated hemoglobin, MetS; metabolic syndrome, NLR; neutrophil-lymphocyte ratio.
DISCUSSION
The major findings of this study are as follows: 1) patients with MetS had a possible MCI condition; 2) several metabolic parameters, including BMI, HbA1C¬, FPG, and NLR, were shown to be correlated with MoCA scores; and 3) NLR level had the strongest negative correlation with MoCA scores in patients with MetS.
Cognitive impairment can be categorized as ranging from mild to severe. Mild cognitive impairment is characterized by small changes in individuals’ cognitive functions that do not disrupt daily-life activities (Brooke and Ojo, 2014). In contrast, a severe degree of cognitive impairment leads to a loss of an individual’s ability or skill to understand the meaning or importance of their surroundings; hence, they are incapable of live independently. Moreover, a severe degree of cognitive impairment can lead to the development of AD. Furthermore, patients with cognitive impairment have a higher risk of being hospitalized compared with individuals who are hospitalized for other conditions.
Several clinical studies reported an association between MetS and cognitive impairment (Collinson et al., 2014; Frisardi, 2014), although there are few studies measuring the factors associated with MCI in patients with MetS (Roberts et al., 2010; Yan et al., 2011). Therefore, the known correlation between what factors that have been studied and the development of MCI in MetS is still controversial. These findings imply that early detection of cognitive impairment in patients with MetS may be more useful for prevention rather than treatment, and further investigations supporting the association of MCI with MetS are needed.
In patients with MetS, peripheral inflammation was observed (Lumeng and Saltiel, 2011). This is thought to be caused by the production and release of pro-inflammatory adipokines, cytokines, and chemokines. Previous studies have reported that inflammation can lead to the progression of cognitive dysfunction and AD (Lee et al., 2008; Sell and Eckel, 2010). Moreover, a high level of plasma adiponectin has been reported to be a risk factor for AD (van Himbergen et al., 2012). Brain inflammation is also responsible for causing neurodegenerative diseases (De Felice and Ferreira, 2014; Spielman et al., 2014; Zhou et al., 2014). Currently, NLR has been used as a pathogenic marker for predicting several pathological conditions, particularly cancer and cardiovascular disease (Bhat et al., 2013; Xiao et al., 2014; Xue et al., 2014), since it is a novel and non-invasive method of assessing these conditions. In terms of neurodegenerative diseases, several clinical reports demonstrated the association of NLR with the neurological complications of multiple sclerosis and early stages of AD(Hemond et al., 2019; Kalelioglu et al., 2017). Furthermore, the role of NLR as a good predictor for cognitive dysfunction in Carotid Endarterectomy patients has been reported (Halazun et al., 2014). Collectively, these findings imply that NLR may also be a prognostic marker for cognitive impairment in patients with MetS, although there is no study reporting this relationship.
In the present study, we found several factors that were correlated with the possible development of an MCI condition in patients with MetS, which include HbA1C and FPG. Interestingly, we found that systemic inflammation was also found to be correlated with a possible MCI condition, as indicated by the significant correlation of NLR with MoCA scores. Notably, the strongest correlation found among other significant parameters was between NLR and the MoCA cognitive score. Our findings supported a recent study in subjects with early AD and MCI by Kalelioglu and colleagues in that NLR levels from both groups had higher than the subjective cognitive decline (SCD) group, suggesting a new potential inflammatory marker for MCI condition (Kalelioglu et al., 2017). Several studies have reported that NLR may be a good predictor of cognitive deficit from several pathological conditions including carotid endarterectomy, AD, and even diabetes mellitus (Halazun et al., 2014; Rembach et al., 2014; Velayudhan et al., 2010). Interestingly, although NLR demonstrated its significant correlation with the MoCA score of MetS patients with possible MCI conditions, the association of plasma inflammatory markers including IL-1β and IL-18 did not significantly correlate. This might be due to the fact that NLR reflected the balance between the innate inflammatory response (as indicated by the neutrophil count) and adaptive immunity as indicated by lymphocyte count (Izaks et al., 2003; Silvestre-Roig et al., 2020; Song et al., 2021). Taken together, NLR might be involved with the poor cognitive performance of MetS patients in terms of host immunity rather than an inflammatory aspect.
In the present study, a MoCA test has been used to interpret the cognitive performance. However, lower MoCA scores alone were insufficient for indicating an MCI condition. An MCI condition has been diagnosed when patients met the Petersen criteria for an MCI condition (Petersen, 2004) including: 1) a subjective memory complaint, 2) an objective cognitive decline, 3) normal cognitive performance as indicated by a Mini-Mental State Examination (MMSE) score of more than 23 (Folstein et al., 1975), 4) independence in activity daily life (ADL), and 5) absence of clinical criteria for dementia. Therefore, further investigation is needed to validate that NLR can be a potential diagnostic marker for an MCI condition.
Collectively, this finding suggests that peripheral systemic inflammation could be one of the possible mechanisms for the induction of cognitive impairment in patients with MetS, and NLR may be a novel prognostic marker for these patients, although further studies to support this finding are still required.
CONCLUSION
In conclusion, the present study showed that most patients with MetS demonstrated the possibility of an MCI condition. Additionally, several peripheral parameters correlated with a possible MCI condition displayed in patients with MetS. Notably, we found that NLR was closely correlated with a possible MCI condition. This suggests that NLR could be one of the prognostic markers for the evaluation of poor cognitive performance in the MetS condition before the development of other severe complications.
ACKNOWLEDGEMENTS
This work was supported by the Senior Research Scholar grant from the National Research Council of Thailand (SCC), Thailand Research Fund: MRG5980200 (NP), RSA5780040 (AP), Chiang Mai University (CMU) Junior Research Fellowship Program (NP), Chiang Mai University (CMU) Research Grant (CT), a NSTDA Research Chair Grant from the National Science and Technology Development Agency (NC) and a Chiang Mai University Center of Excellence Award (NC).
AUTHOR CONTRIBUTIONS
Noppamas Pipatpiboon: Formal analysis, Investigation, Writing – Original draft, Writing – Review & Editing, Visualization, Project administration.
Jirapas Sripetchwandee: Formal analysis, Investigation, Writing - Original draft, Writing – Review & Editing, Visualization, Project administration
Piangkwan Sa-nguanmoo: Investigation
Chiraporn Tachaudomdach: Funding acquisition
Tanyarat Jomgeow: Investigation Resources
Arintaya Phrommintikul: Formal analysis, Funding acquisition
Nipon Chattipakorn: Conceptualization, Methodology, Writing – Original Draft, Writing – Review & Editing, Supervision, Funding acquisition
Siriporn C. Chattipakorn: Conceptualization, Methodology, Formal analysis, Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision, Project administration, Funding acquisition. All authors have read and approved the final draft of this manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
Albert, M.S., DeKosky, S.T., Dickson, D., Dubois, B., Feldman, H.H., Fox, N.C., Gamst, A., Holtzman, D.M., Jagust, W.J., Petersen, R.C. et al. 2011. The diagnosis of mild cognitive impairment due to alzheimer's disease: Recommendations from the national institute on aging-alzheimer's association workgroups on diagnostic guidelines for alzheimer's disease. Alzheimers Dement. 7: 270-279.
Alberti, K.G., Eckel, R.H., Grundy, S.M., Zimmet, P.Z., Cleeman, J.I., Donato, K.A., Fruchart, J.C., James, W.P., Loria, C.M., Smith, S.C., Jr. et al. 2009. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 120: 1640-1645.
Banjongrewadee, M., Wongpakaran, N., Wongpakaran, T., Pipanmekaporn, T., Punjasawadwong, Y., and Mueankwan, S. 2018. Role of perceived stress in postoperative delirium: An investigation among elderly patients. Aging and Mental Health.1-7.
Bermejo, P., Martin-Aragon, S., Benedi, J., Susin, C., Felici, E., Gil, P., Ribera, J.M., and Villar, A.M. 2008. Differences of peripheral inflammatory markers between mild cognitive impairment and alzheimer's disease. Immunology Letters. 117: 198-202.
Bhat, T., Teli, S., Rijal, J., Bhat, H., Raza, M., Khoueiry, G., Meghani, M., Akhtar, M., and Costantino, T. 2013. Neutrophil to lymphocyte ratio and cardiovascular diseases: A review. Expert Review of Cardiovascular Therapy. 11: 55-59.
Brooke, J., and Ojo, O. 2014. Cognitive screening in patients with diabetes in primary care. British Journal of Community Nursing. 19: 401-406.
Clarnette, R., O'Caoimh, R., Antony, D.N., Svendrovski, A., and Molloy, D.W. 2017. Comparison of the quick mild cognitive impairment (qmci) screen to the montreal cognitive assessment (moca) in an australian geriatrics clinic. International Journal of Geriatric Psychiatry. 32: 643-649.
Collinson, S.L., Tong, S.J., Loh, S.S., Chionh, S.B., and Merchant, R.A. 2014. Midlife metabolic syndrome and neurocognitive function in a mixed asian sample. International Psychogeriatrics. 26: 1305-1316.
Crane, P.K., Walker, R., Hubbard, R.A., Li, G., Nathan, D.M., Zheng, H., Haneuse, S., Craft, S., Montine, T.J., Kahn, S.E. et al. 2013. Glucose levels and risk of dementia. New England Journal of Medicine. 369: 540-548.
De Felice, F.G., and Ferreira, S.T. 2014. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to alzheimer disease. Diabetes. 63: 2262-2272.
de Jager, C.P., van Wijk, P.T., Mathoera, R.B., de Jongh-Leuvenink, J., van der Poll, T., and Wever, P.C. 2010. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Critical Care. 14: R192.
Dong, C.H., Wang, Z.M., and Chen, S.Y. 2018. Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: A systematic review and meta-analysis. Clinical Biochemistry. 52: 131-136.
Folstein, M.F., Folstein, S.E., and McHugh, P.R. 1975. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 12: 189-198.
Frisardi, V. 2014. Impact of metabolic syndrome on cognitive decline in older age: Protective or harmful, where is the pitfall? Journal of Alzheimer's Disease. 41: 163-167.
Gao, Y., Xiao, Y., Miao, R., Zhao, J., Zhang, W., Huang, G., and Ma, F. 2015. The characteristic of cognitive function in type 2 diabetes mellitus. Diabetes Research and Clinical Practice. 109: 299-305.
Halazun, H.J., Mergeche, J.L., Mallon, K.A., Connolly, E.S., and Heyer, E.J. 2014. Neutrophil-lymphocyte ratio as a predictor of cognitive dysfunction in carotid endarterectomy patients. Journal of Vascular Surgery. 59: 768-773.
Hemond, C.C., Glanz, B.I., Bakshi, R., Chitnis, T., and Healy, B.C. 2019. The neutrophil-to-lymphocyte and monocyte-to-lymphocyte ratios are independently associated with neurological disability and brain atrophy in multiple sclerosis. BMC Neurol. 19: 23.
Hokanson, J.E., and Austin, M.A. 1996. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies. Journal of Cardiovascular Risk. 3: 213-219.
Izaks, G.J., Remarque, E.J., Becker, S.V., and Westendorp, R.G. 2003. Lymphocyte count and mortality risk in older persons. The leiden 85-plus study. Journal of the American Geriatrics Society. 51: 1461-1465.
Jayaraj, R.L., Elangovan, N., Dhanalakshmi, C., Manivasagam, T., and Essa, M.M. 2014. Cnb-001, a novel pyrazole derivative mitigates motor impairments associated with neurodegeneration via suppression of neuroinflammatory and apoptotic response in experimental parkinson's disease mice. Chemico-Biological Interactions. 220: 149-157.
Julayanont, P., Tangwongchai, S., Hemrungrojn, S., Tunvirachaisakul, C., Phanthumchinda, K., Hongsawat, J., Suwichanarakul, P., Thanasirorat, S., and Nasreddine, Z.S. 2015. The montreal cognitive assessment-basic: A screening tool for mild cognitive impairment in illiterate and low-educated elderly adults. Journal of the American Geriatrics Society. 63: 2550-2554
Kalelioglu, T., Yuruyen, M., Gultekin, G., Yavuzer, H., Ozturk, Y., Kurt, M., Topcu, Y., Doventas, A., and Emul, M. 2017. Neutrophil and platelet to lymphocyte ratios in people with subjective, mild cognitive impairment and early alzheimer's disease. Psychogeriatrics. 17: 506-508.
Kaur, J. 2014. A comprehensive review on metabolic syndrome. Cardiology Research and Practice. 2014:943162.
Kerti, L., Witte, A.V., Winkler, A., Grittner, U., Rujescu, D., and Floel, A. 2013. Higher glucose levels associated with lower memory and reduced hippocampal microstructure. Neurology. 81: 1746-1752.
Lattanzi, S., Cagnetti, C., Provinciali, L., and Silvestrini, M. 2017. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. 8: 57489-57494.
Lattanzi, S., Cagnetti, C., Rinaldi, C., Angelocola, S., Provinciali, L., and Silvestrini, M. 2018. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. Journal of the Neurological Sciences. 387: 98-102.
Lee, Y.H., Tharp, W.G., Maple, R.L., Nair, S., Permana, P.A., and Pratley, R.E. 2008. Amyloid precursor protein expression is upregulated in adipocytes in obesity. Obesity (Silver Spring). 16: 1493-1500.
Liu, M., He, Y., Jiang, B., Wu, L., Wang, J., Yang, S., and Wang, Y. 2015. Association between metabolic syndrome and mild cognitive impairment and its age difference in a chinese community elderly population. Clinical Endocrinology (Oxf). 82: 844-853.
Lumeng, C.N., and Saltiel, A.R. 2011. Inflammatory links between obesity and metabolic disease. Journal of Clinical Investigation. 121: 2111-2117.
Mantovani, A., Savino, B., Locati, M., Zammataro, L., Allavena, P., and Bonecchi, R. 2010. The chemokine system in cancer biology and therapy. Cytokine & Growth Factor Reviews. 21: 27-39.
Ng, T.P., Feng, L., Nyunt, M.S., Feng, L., Gao, Q., Lim, M.L., Collinson, S.L., Chong, M.S., Lim, W.S., Lee, T.S. et al. 2016. Metabolic syndrome and the risk of mild cognitive impairment and progression to dementia: Follow-up of the singapore longitudinal ageing study cohort. JAMA Neurology. 73: 456-463.
Niu, L., Han, D.W., Xu, R.L., Han, B., Zhou, X., Wu, H.W., Li, S.H., Qu, C.X., and Liu, M. 2016. A high-sugar high-fat diet induced metabolic syndrome shows some symptoms of alzheimer's disease in rats. Journal of Nutrition, Health and Aging. 20: 509-513.
O'Caoimh, R., Timmons, S., and Molloy, D.W. 2016. Screening for mild cognitive impairment: Comparison of "mci specific" screening instruments. Journal of Alzheimer's Disease. 51: 619-629.
Panza, F., Frisardi, V., Capurso, C., Imbimbo, B.P., Vendemiale, G., Santamato, A., D'Onofrio, G., Seripa, D., Sancarlo, D., Pilotto, A. et al. 2010. Metabolic syndrome and cognitive impairment: Current epidemiology and possible underlying mechanisms. Journal of Alzheimer's Disease. 21: 691-724.
Petersen, R.C. 2004. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 256: 183-194.
Petersen, R.C. 2016. Mild cognitive impairment. Continuum (Minneap Minn). 22(2 Dementia): 404-418.
Phrommintikul, A., Sa-Nguanmoo, P., Sripetchwandee, J., Vathesatogkit, P., Chattipakorn, N., and Chattipakorn, S.C. 2018. Factors associated with cognitive impairment in elderly versus nonelderly patients with metabolic syndrome: The different roles of fgf21. Scientific Reports. 8: 5174.
Pipatpiboon, N., Pintana, H., Pratchayasakul, W., Chattipakorn, N., and Chattipakorn, S.C. 2013. Dpp4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. European Journal of Neuroscience. 37: 839-849.
Rembach, A., Watt, A.D., Wilson, W.J., Rainey-Smith, S., Ellis, K.A., Rowe, C.C., Villemagne, V.L., Macaulay, S.L., Bush, A.I., Martins, R.N. et al. 2014. An increased neutrophil-lymphocyte ratio in alzheimer's disease is a function of age and is weakly correlated with neocortical amyloid accumulation. Journal of Neuroimmunology. 273: 65-71.
Roberts, R.O., Geda, Y.E., Knopman, D.S., Cha, R.H., Boeve, B.F., Ivnik, R.J., Pankratz, V.S., Tangalos, E.G., and Petersen, R.C. 2010. Metabolic syndrome, inflammation, and nonamnestic mild cognitive impairment in older persons: A population-based study. Alzheimer Disease and Associated Disorders. 24: 11-18.
Salekzamani, S., Mehralizadeh, H., Ghezel, A., Salekzamani, Y., Jafarabadi, M.A., Bavil, A.S., and Gargari, B.P. 2016. Effect of high-dose vitamin d supplementation on cardiometabolic risk factors in subjects with metabolic syndrome: A randomized controlled double-blind clinical trial. Journal of Endocrinological Investigation is interested. 39: 1303-1313.
Sell, H., and Eckel, J. 2010. Adipose tissue inflammation: Novel insight into the role of macrophages and lymphocytes. Current Opinion in Clinical Nutrition and Metabolic Care. 13: 366-370.
Silvestre-Roig, C., Braster, Q., Ortega-Gomez, A., and Soehnlein, O. 2020. Neutrophils as regulators of cardiovascular inflammation. Nature Reviews Cardiology. 17: 327-340.
Song, M., Graubard, B.I., Rabkin, C.S., and Engels, E.A. 2021. Neutrophil-to-lymphocyte ratio and mortality in the united states general population. Scientific Reports. 11: 464.
Spielman, L.J., Little, J.P., and Klegeris, A. 2014. Inflammation and insulin/igf-1 resistance as the possible link between obesity and neurodegeneration. Journal of Neuroimmunology. 273: 8-21.
Switonska, M., Piekus-Slomka, N., Slomka, A., Sokal, P., Zekanowska, E., and Lattanzi, S. 2020. Neutrophil-to-lymphocyte ratio and symptomatic hemorrhagic transformation in ischemic stroke patients undergoing revascularization. Brain Sciences. 10.
Templeton, A.J., McNamara, M.G., Seruga, B., Vera-Badillo, F.E., Aneja, P., Ocana, A., Leibowitz-Amit, R., Sonpavde, G., Knox, J.J., Tran, B. et al. 2014. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Journal of the National Cancer Institute. 106: dju124.
van Himbergen, T.M., Beiser, A.S., Ai, M., Seshadri, S., Otokozawa, S., Au, R., Thongtang, N., Wolf, P.A., and Schaefer, E.J. 2012. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and alzheimer disease: Results from the framingham heart study. Arch Neurol. 69: 594-600.
Velayudhan, L., Poppe, M., Archer, N., Proitsi, P., Brown, R.G., and Lovestone, S. 2010. Risk of developing dementia in people with diabetes and mild cognitive impairment. British Journal of Psychiatry. 196: 36-40.
Vendramini-Costa, D.B., and Carvalho, J.E. 2012. Molecular link mechanisms between inflammation and cancer. Current Pharmaceutical Design. 18: 3831-3852.
Wanamaker, B.L., Swiger, K.J., Blumenthal, R.S., and Martin, S.S. 2015. Cholesterol, statins, and dementia: What the cardiologist should know. Clinical Cardiology. 38: 243-250.
Xiao, W.K., Chen, D., Li, S.Q., Fu, S.J., Peng, B.G., and Liang, L.J. 2014. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: A meta-analysis. BMC Cancer. 14: 117.
Xue, T.C., Zhang, L., Xie, X.Y., Ge, N.L., Li, L.X., Zhang, B.H., Ye, S.L., and Ren, Z.G. 2014. Prognostic significance of the neutrophil-to-lymphocyte ratio in primary liver cancer: A meta-analysis. PLoS One. 9: e96072.
Yan, C.M., Deng, Q.N., and Zhong, W.Z. 2011. [correlations among metabolic syndrome and mild cognitive impairment]. Zhonghua Yi Xue Za Zhi. 91: 3193-3196.
Yao, Q., Jiang, G.X., Zhou, Z.M., Chen, J.M., and Cheng, Q. 2016. Metabolic syndrome and mild cognitive impairment: A case-control study among elderly in a shanghai suburb. Journal of Alzheimer's Disease 51: 1175-1182.
Zheng, T., Qin, L., Chen, B., Hu, X., Zhang, X., Liu, Y., Liu, H., Qin, S., Li, G., and Li, Q. 2016. Association of plasma dpp4 activity with mild cognitive impairment in elderly patients with type 2 diabetes: Results from the gdmd study in china. Diabetes care. 39: 1594-1601.
Zhou, W.W., Lu, S., Su, Y.J., Xue, D., Yu, X.L., Wang, S.W., Zhang, H., Xu, P.X., Xie, X.X., and Liu, R.T. 2014. Decreasing oxidative stress and neuroinflammation with a multifunctional peptide rescues memory deficits in mice with alzheimer's disease. Free Radical Biology and Medicine. 74: 50-63.
Zhuang, J., Wen, X., Zhang, Y.Q., Shan, Q., Zhang, Z.F., Zheng, G.H., Fan, S.H., Li, M.Q., Wu, D.M., Hu, B. et al. 2017. Tdp-43 upregulation mediated by the nlrp3 inflammasome induces cognitive impairment in 2 2', 4, 4'-tetrabromodiphenyl ether (bde-47)-treated mice. Brain, Behavior, and Immunity. 65: 99-110.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Noppamas Pipatpiboon1,2 , Jirapas Sripetchwandee 2, *, Piangkwan Sa-nguanmoo3, Chiraporn Tachaudomdach4, Tanyarat Jomgeow5, Arintaya Phrommintikul6, Nipon Chattipakorn2, and Siriporn C. Chattipakorn2,7
1 Department of Public Health, Faculty of Nursing, Chiang Mai University, Chiang Mai 50200, Thailand
2 Neurophysiology Unit, Cardiac Electrophysiology Research and Training Center, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand
3 Department of Physical Therapy, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
4 Department of Medical, Faculty of Nursing, , Chiang Mai 50200, Thailand
5 Division of Clinical Microscopy, Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
6 Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand
7 Department of Oral Biology and Diagnostic Sciences, Faculty of Dentistry, Chiang Mai University, Chiang Mai 50200, Thailand
Corresponding author: Jirapas Sripetchwandee, E-mail: jsripetchwandee@gmail.com, jirapas.sripetch@cmu.ac.th
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: August 13 2021;
Revised: September 27, 2021;
Accepted: October 21, 2021

