
Evaluation of chemical constituents, antioxidant and anti-inflammatory properties of n-hexane extract of Viscum album L. (Mistletoe) leaves
Charles Nnanna Chukwu*, Uchechi Bliss Onyedikachi, and Emmanuel EjioforPublished Date : 2022-01-21
DOI : https://doi.org/10.12982/CMUJNS.2022.010
Journal Issues : Number 1, January-March 2022
Abstract Viscum album L. (Mistletoe) is used in ethnomedicine for the management of some ailments ranging from inflammation, pains and oxidative stress. The phytoconstituents, antioxidant and anti-inflammatory properties of n-hexane extract of mistletoe leaves (nHEML) were evaluated in this study. nHEML was obtained from fresh leaves of Viscum album L. using a Soxhlet extractor. Total phenol and flavonoid compositions were assayed using standard colourimetric methods. Gas chromatography-mass spectrometry (GC-MS) analysis was used to ascertain the presence of phytochemicals in the extract. The antioxidant property was determined using 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) assays, while the anti-inflammatory property was investigated using membrane stabilization (hypotonicity) and heat-induced hemolysis of human red blood cell (HRBC) assays. The results showed a high amount of total phenolic content (37.82 ± 0.22 mg GAE/g) and total flavonoid content (128.85 ± 3.85 mgQE/100mg). GC-MS analysis showed the presence of essential phytoconstituents including phytosterols, vitamin C, fatty acids etc., with known potent biological activities. In vitro, the antioxidant assay showed that DPPH scavenging activity of nHEML was only detected at 400μg/ml with 13.46%, while there was a dose-dependent increase in FRAP activity of nHEML from 50 to 400μg/ml compared to the standard. For the in vitro anti-inflammatory assay, there was a dose-dependent increase in HRBC membrane stabilization and anti-hemolytic activities, which were higher than those of the standards at 200 and 400µg/mL. nHEML contains a significant amount of flavonoids which improved the anti-inflammatory activities against hypotonic and heat-induced inflammation, hence justifying its potential as a possible anti-inflammatory agent.
Keywords: Anti-inflammatory activity; antioxidant; DPPH scavenging activity; GC-MS; hemolysis; oxidative stress
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Citation: Chukwu, C.N., Onyedikachi, U.B., and Ejiofor, E. 2022. Evaluation of chemical constituents, antioxidant and anti-inflammatory properties of n-hexane extract of Viscum album L. (Mistletoe) leaves. CMU J. Nat. Sci. 21(1): e2022010.
INTRODUCTION
Oxidative stress is established when there is excessive production of reactive oxygen species (ROS) which overwhelms the body’s ability to combat them effectively (Hussain et al., 2016). This condition is known to be a prominent promoter of inflammation (Oluwafemi, 2019). With increased ROS generation, cellular functions and biomolecules in living systems are affected. Phytotherapy is an ancient practice of healthcare recognized by humankind whereby bioactive substances existent in plants are exploited for medicinal benefits (Kamaraj et al., 2020). Extracts obtained from plant materials have been reported to contain numerous chemical compounds such as flavonoids, saponins, tannins, alkaloids, steroids, cardiac glycosides and phenol compounds, which in general are termed phytochemicals (Parekh et al., 2005; Kaur and Arora, 2009). These compounds exhibit different pharmacological properties such as antioxidant, anti-inflammatory, hepatoprotective, antimicrobial, antidiabetic activities e.t.c (Veiga et al., 2020).
Viscum album L., (Family: Viscaceae, previously Loranthaceae) popularly known as "Mistletoe", is considered a semi-parasitic plant that grows on several host trees mostly in Africa, Madagascar and southern Asia, while few species are native to Europe, temperate Asia, Malaysia and eastern Australia (APweb, 2013; Valle et al., 2021). V. album L. has found wide application as a therapeutic agent in folk medicine against a wide range of ailments. Several studies have reported usage of V. album in management of cardiovascular diseases, bone and joint ailments, headache, immune and nervous system disorders (Wichtl and Bisset, 1994; Bartram,1995; Murray, 1995; Newall et al., 1996). V. album has also been shown to exert anti-tumor activity by selective cytotoxicity (Valle et al., 2020), induce apoptosis (Han et al., 2015), and inhibit angiogenesis (Elluru et al., 2009). Furthermore, both in vitro and in vivo, V. album has been shown to possess anticancer (Valle et al., 2021; Skidmore-Roth, 2006), anti-diabetic (Gray and Flatt, 1999; Simsek et al., 2004; Ohiri et al., 2003), vasodilating, sedative, cardiac-depressant, diuretic, anti-inflammatory and immune-stimulant (Yesilada et al., 1998) effects. These properties stem from the presence of vital phytochemical components including alkaloids, phenolics, viscotoxins, glycosides, flavonoids, phenylpropanoids, tannins, lignans found in Viscum album leaves (Ergun and Deliorman, 1995; Nazaruk and Orlikowski, 2016).
Studies have established the antioxidant potential of ethanolic extract of V. album leaves in uncooked pork patties (Suk-Nam, 2016) and the methanol extract of leaves of
V. album growing on cocoa and cashew trees (Ademiliyu and Oboh, 2008). Also, the anti-inflammatory potential of 0.9% sodium chloride (NaCl2) extract of V. album has been established via inhibition of cytokine expression (Pushpa et al., 2011). Furthermore, phenolic compounds such as catechin, epicatechin, rutin and quercetin have been found to partly contribute to the antioxidant effect of mistletoe extracts obtained by high-temperature batch extraction (Rahmawati et al., 2014). However, limited information is available on the in vitro antioxidant and anti-inflammatory potential of n-hexane extract of V. album, especially via its protection of red blood cell membrane integrity – thus, warranting this study. Therefore, the present study was aimed at evaluating the in vitro antioxidant and anti-inflammatory potentials of nHEML. The total phenolics, flavonoids and GC-MS analysis of phytochemical compounds were also determined.
MATERIALS AND METHODS
Collection and preparation of plant sample
Fresh young leaves of V. album used in this study were collected from the humid forest in the Michael Okpara University of Agriculture, Umudike (MOUAU), Ikwuano Local Government Area of Abia State, Nigeria, in June 2019 during the rainy season. They were identified and authenticated by a Taxonomist (Dr. Ibe K. Ndukwe) in the Herbarium section of the Department of Forestry and Environmental Management, MOUAU (Specimen voucher number = FHI 41321). The fresh leaves were washed under running tap water and dried under shade for twelve days at room temperature (25 ± 2 °C). The dried leaves were ground into powder with an electric blender and stored in a tight-lid container pending its use.
Extraction of plant material
The extraction was carried out using Soxhlet apparatus at 40°C with n-hexane as the solvent under reflux for 6 hours. The solvent was evaporated using a rotary evaporator. Thereafter, the extracted sample was put in a sterilized container and stored at 4°C in a refrigerator until further analysis.
Determination of total phenolic content
The total phenolic content (TPC) was determined colourimetrically using Folin–Ciocalteu reagent, as described by Paśko et al. (2009).
Determination of total flavonoid content (TFC)
The total flavonoid content (TFC) was determined by a colourimetric method as described by Gorinstein et al. (2007).
Gas chromatography-mass spectrometry analysis
An Agilent 6890N gas chromatography equipped with an autosampler connected to an Agilent Mass Spectrophotometric Detector was used. One (1) microliter of the sample was injected in the pulsed spitless mode onto a 30 m x 0.25 mm ID DB5 MS coated fused silica column with a film thickness of 0.15 micrometer (mm). Helium gas was used as a carrier gas and the column head pressure was maintained at 20 psi to give a constant flow rate of 1 ml/min. Other operating conditions were preset. The column temperature was initially held at 55°C for 0.4 min, increased to 200°C at a rate of 25°C/minutes, then to 280°C at a rate of 8°C/minutes and to a final temperature of 300°C at a rate of 25°C/minutes, held for 2 minutes. The identification was based on relative retention times and mass spectra compared with the library data of the GC-MS system, literature data and standards of the main constituents. Experimental retention indices were compared with known retention indices from NIST Chemistry Web Book and Wiley libraries. The match percentage of the peaks with reference library was >90%.
2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) radical scavenging assay
The free radical scavenging activity of the extract was investigated by the DPPH assay (Mensor et al., 2001) using a spectrophotometer. The experiment was carried out in triplicate. The percentage antioxidant activities were calculated as follows:
% antioxidant activity (AA) = 100 -[{(ABS sample—ABS blank) ×100}/ABS control]
Ascorbic acid (vitamin C) was used as reference standard (Iwalewa et al., 2008).
Ferric reducing antioxidant power
The ferric reducing antioxidant power was carried out as described by Benzie and Strain (1999).
FRAP value = abs 4 minutes – abs 0 minute
Ascorbic acid (vitamin C) was used as the reference standard
Determination of anti-inflammatory activity
Hypotonicity induced haemolysis assay
The effect of nHEML on the haemolysis of human red blood cell (HRBC) in hypotonic saline solution was evaluated as described by Anosike et al. (2012). A blood sample (5 mL) was collected from a healthy male donor (that has not received an anti-inflammatory drug in the past 14 days) into an EDTA sample bottle. The HRBC was repeatedly washed with normal saline by centrifugation until the supernatant was clear. Thereafter, 0.5 mL of 10% suspension of the HRBC was added to test tubes containing different concentrations (25 – 400 µg/mL) of nHEML dissolved in hypotonic solution in triplicate. The mixtures were incubated for 30 min at 37°C and later centrifuged at 3,000 rpm for 5 min. The absorbance of the supernatants was recorded at 560 nm with a spectrophotometer. A hypotonic solution was used as control while diclofenac (200 µg/mL) was used as the reference standard.
Inhibition (%) = (AA - BB) x 100
AA 1
Where: AA = absorbance of control, BB = absorbance of test substance
Heat-induced haemolysis assay
The effect of nHEML on heat-induced hemolysis of HRBC was evaluated as described by Anosike et al. (2012). The blood collection and preparation were as stated in the previous section. Thereafter, 0.5 mL of 10% suspension of the HRBC was added to test tubes containing different concentrations (25 – 400 µg/mL) of nHEML dissolved in phosphate buffer saline in triplicate. The mixtures were incubated for 30 minutes at 54°C and later centrifuged at 3,000 rpm for 5 minutes. The absorbance (ABS) of the supernatants was determined at 560 nm with a spectrophotometer. A hypotonic solution was used as control while diclofenac (200 µg/mL) was used as the reference standard.
Inhibition (%) = (AA - BB) x 100
AA 1
Where: Abo = absorbance of the control, Abu = absorbance of the test.
RESULTS
Total phenolic and flavonoid contents of nHEML
The total phenolic and flavonoid contents of nHEML are shown in Table 1. The total phenolic content was 37.82 ± 0.22 mg GAE/g, while the total flavonoid was 128.85 ± 3.85 mg QE/100mg. The TPC and TFC obtained in this study are higher than those of methanol extracts from mistletoe berries harvested from various host trees (Pietrzak et al., 2017), but lower than those reported by Rahmawati et al. (2014) for six mistletoe extracts obtained by high-temperature batch extraction method.
Table 1. Total phenolic and flavonoid contents of n-hexane extract of V. album (Mistletoe) leaves.
|
Sample |
TPC (GAE.mg/g) |
TFC (mgQE/100mg) |
|
nHEML |
37.82 ± 0.22 |
128.85 ± 3.85 |
Note: nHEML = n-hexane extract of V. album (Mistletoe) leaves; GAE = gallic acid equivalent; QE = quercetin equivalent. TPC = total phenolic content; TFC = total flavonoid content.
GC-MS analysis of phytocompounds in nHEML
The GC-MS total ion content (TIC) chromatograms of the 23 peaks of the compounds detected are shown in Figure 1. The GC-MS details of the chemical composition of nHEML are shown in Table 2. The mass spectrums of some of the major compounds are shown in Figures 2 – 12, while the stuctures are shown in Figures 13 – 35. The structures of the compounds are The major compounds detected were Cyclohexanol, 2-methyl-3-(1-methylethenyl)-, (1α,2α,3α)- (20.490 %), vitamin C (27.583 %), 2,4- decadienal (6.970 %), 1,2-Naphthalenediol, 1,2,3,4-tetrahydro-3,3-dimethyl-, cis (6.690 %), 1H-Inden-1-one, 2,3-dihydro-3,3,5,6-tetramethyl (6.010 %) and stigmasterol (4.495 %).
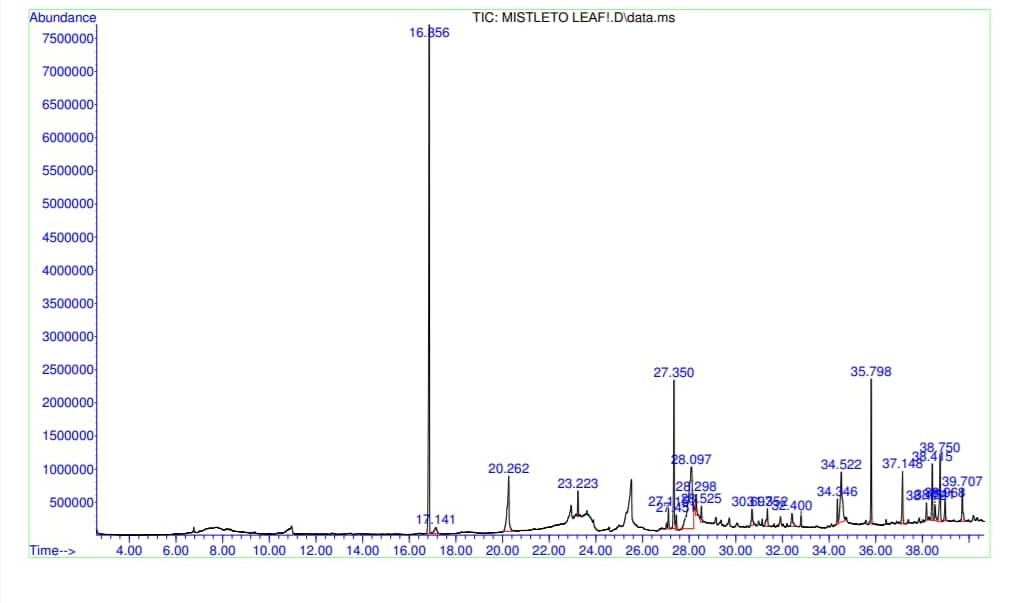
Figure 1: Gas chromatography-mass spectrometry (GC-MS) total ion content (TIC) chromatogram of n-hexane extract of V. album (Mistletoe) leaves
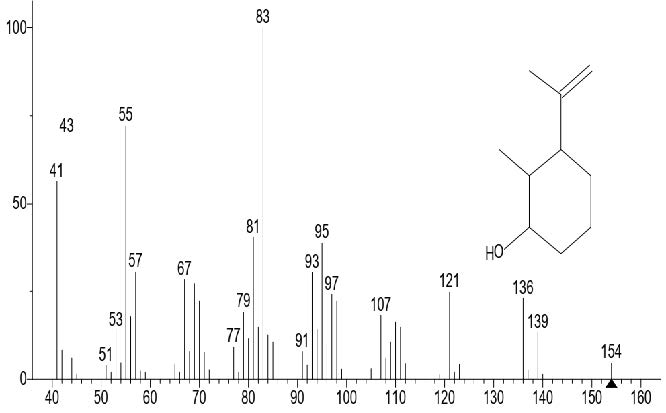
Figure 2. Cyclohexanol, 2-methyl-3-(1-methylethenyl)-, (1α,2α,3α)-
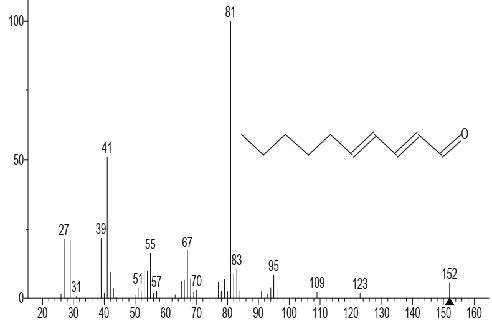
Figure 3. 2,4-Decadienal
Table 2. Chemical composition (GC-MS) of the n-hexane extract of V. album (Mistletoe).
|
Peak No. |
Name of compound |
RT (Mins) |
Exact mass (g) |
RI Exp |
RI Lit.* |
Conc. (%) |
Formula |
Class of Compound |
|
1 |
Cyclohexanol, 2-methyl-3-(1-methylethenyl)-, (1α,2α,3α)- |
16.855 |
154.14 |
- |
1202 |
20.490 |
C10H18O |
Secondary alcohol |
|
2 |
2(1H)-Naphthalenone, 3,4,4a,5,6,7-hexahydro-1,1,4a-trimethyl |
17.140 |
192.15 |
- |
1491 |
1.060 |
C13H20O |
Naphthalene derivative |
|
3 |
2,4-Decadienal |
20.261 |
152.12 |
1317 |
1317 |
6.970 |
C10H16O |
Aldehyde |
|
4 |
Phenol, 2,6-dimethoxy |
23.220 |
154.06 |
1355 |
1357 |
1.200 |
C8H10O3 |
Phenol |
|
5 |
Naphthalene, 1,2-dihydro-1,1,6-trimethyl |
27.114 |
172.12 |
1354 |
1355 |
0.999 |
C13H16 |
Naphthalene derivative |
|
6 |
2-Butanone, 4-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)- |
27.350 |
192.15 |
1424 |
1424 |
7.570 |
C13H20O |
Sesquiterpenoid |
|
7 |
4,4,5,8-Tetramethylchroman-2-ol |
27.450 |
206.13 |
- |
- |
0.830 |
C13H18O2 |
Vitamin E Analog |
|
8 |
Vitamin C |
28.095 |
176.03 |
- |
- |
27.583 |
C6H8O6 |
Organic acid |
|
9 |
1,2,4-Cyclopentaetrione |
28.295 |
112.02 |
1014 |
1113 |
1.053 |
C5H4O3 |
Ketone |
|
10 |
Trans-calamenene |
28.524 |
202.17 |
1529 |
1529 |
1.001 |
C15H22 |
Phytosterol |
|
11 |
4-[4-(Benzyloxy)-3-methoxyphenyl]-3H,4H,5H,6H,-imidazo[4,5-C]pyridine] |
30.697 |
335.16 |
- |
- |
1.459 |
C20H21N3O2 |
Carboxylic acid |
|
12 |
16-Heptadecen-2,5,8-trione |
31.352 |
280.20 |
- |
- |
1.451 |
C17H28O3 |
Ketone |
|
13 |
3-Butanone,1-(2,3,6-trimethylphenyl)- |
32.400 |
190.14 |
1445 |
1445 |
1.222 |
C13H18O |
Ketone |
|
14 |
4-(2,6,6-Trimethyl-cyclohexa-1,3-dienyl)-but-3-en-2-one |
34.346 |
190.14 |
1423 |
1485 |
1.235 |
C13H18O |
Ketone |
|
15 |
1,2-Naphthalenediol, 1,2,3,4-tetrahydro-3,3-dimethyl-, cis |
34.522 |
192.12 |
- |
- |
6.690 |
C12H16O2 |
Naphthalene derivative |
|
16 |
1H-Inden-1-one, 2,3-dihydro-3,3,5,6-tetramethyl |
35.798 |
188.12 |
1555 |
1555 |
6.010 |
C13H16O |
Indanone |
|
17 |
Oleic Acid |
37.148 |
282.26 |
2141 |
2140 |
3.021 |
C18H34O2 |
Fatty acid |
|
18 |
Palmitic anhydride |
38.172 |
494.47 |
- |
- |
0.889 |
C32H62O3 |
Fatty acid |
|
19 |
Estra-1,3,5,(10)-trien-17-ol |
38.415 |
256.18 |
2259 |
2300 |
4.189 |
C18H24O |
Steroid lipid |
|
20 |
Behenic alchohol |
38.541 |
326.35 |
2493 |
2470 |
1.818 |
C22H46O |
Fatty alcohol |
|
21 |
Stigmasterol |
38.750 |
412.37 |
3170 |
3170 |
4.495 |
C29H48O |
Phytosterol |
|
22 |
β-Sitosterol |
38.967 |
414.39 |
3321 |
3351 |
1.189 |
C29H50O |
Phytosterol |
|
23 |
2H,8H-Benzo[1,2-b:3,4-b']dipyran-2-one, 8,8-dimethyl |
39.706 |
228.08 |
2085 |
2085 |
2.258 |
C14H12O3 |
Pyran |
Note: RT: Retention time, RI Exp: Experimental retention indices, RI Lit: Retention Index from literature (*Source: NIST Chemistry Web Book and Wiley libraries)
Figure 4. 2-Butanone, 4-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)-
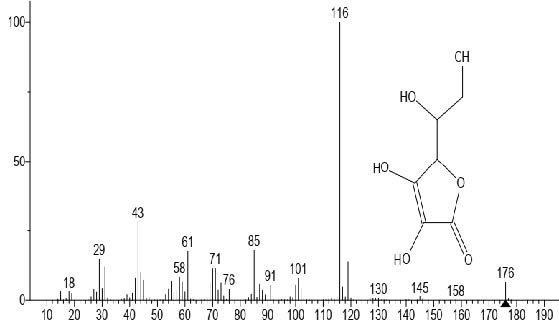
Figure 5. Vitamin C
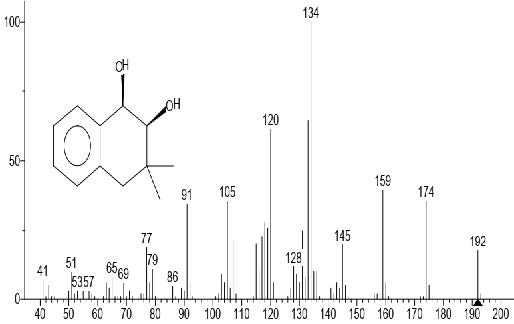
Figure 6. 1,2-Naphthalenediol, 1,2,3,4-tetrahydro-3,3-dimethyl-, cis
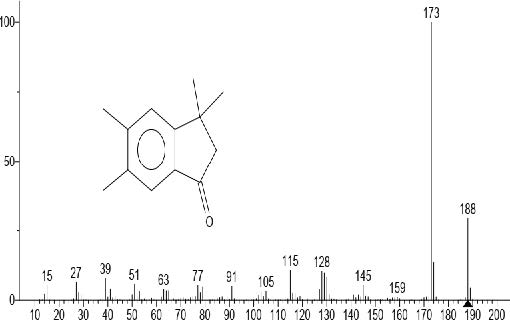
Figure 7. 1H-Inden-1-one, 2,3-dihydro-3,3,5,6-tetramethyl
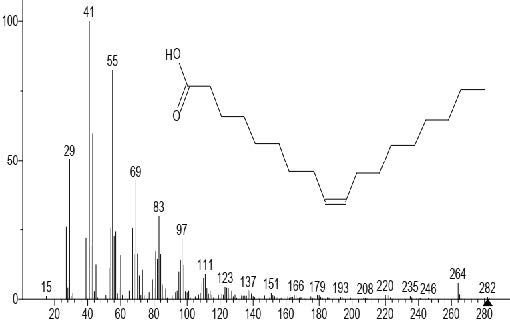
Figure 8. Oleic acid
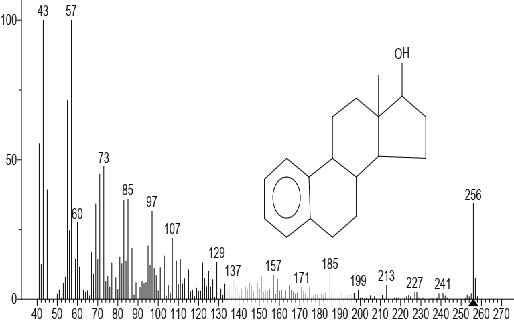
Figure 9. Estra-1,3,5,(10)-trien-17-ol
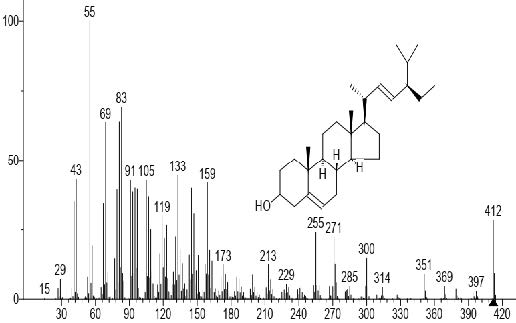
Figure 10. Stigmasterol
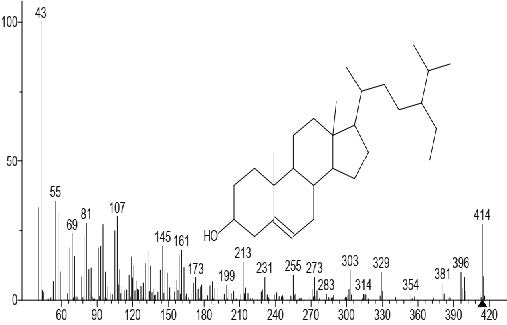
Figure 11. β-Sitosterol
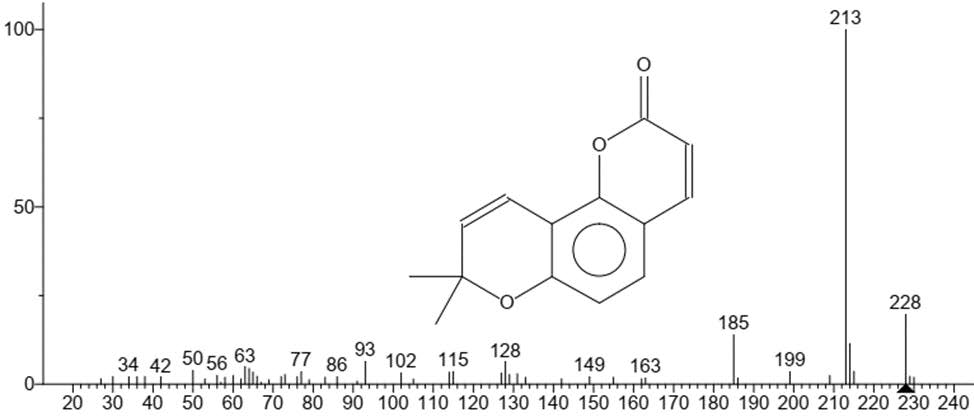
Figure 12. 2H,8H-Benzo[1,2-b:3,4-b']dipyran-2-one, 8,8-dimethyl.
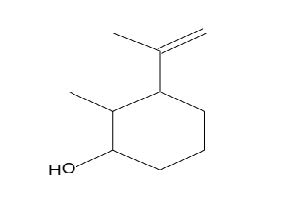
Figure 13. Cyclohexanol, 2-methyl-3-(1-methylethenyl)-, (1α,2α,3α)-
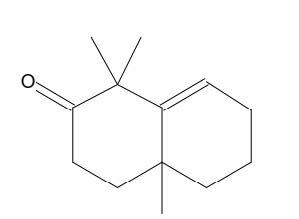
Figure 14. 2(1H)-Naphthalenone, 3,4,4a,5,6,7-hexahydro-1,1,4a-trimethyl
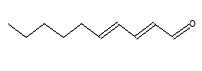
Figure 15. 2,4-Decadienal
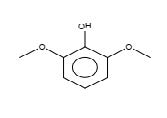
Figure 16. Phenol, 2,6-dimethoxy
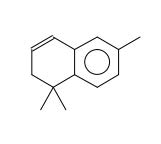
Figure 17. Naphthalene, 1,2-dihydro-1,1,6-trimethyl
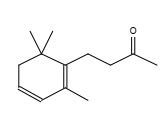
Figure 18. 2-Butanone, 4-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)-
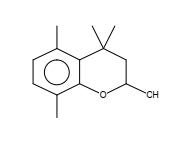
Figure 19. 4,4,5,8-Tetramethylchroman-2-ol
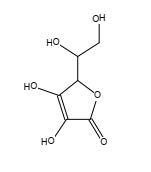
Figure 20. Vitamin C
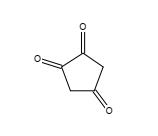
Figure 21. 1,2,4-Cyclopentaetrione
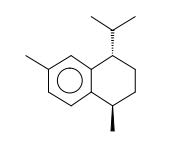
Figure 22. Trans-calamenene

Figure 23. 4-[4-(Benzyloxy)-3-methoxyphenyl]-3H,4H,5H,6H,-imidazo[4,5-C]pyridine]
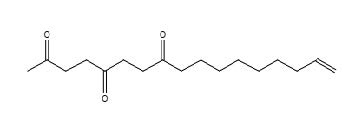
Figure 24. 16-Heptadecen-2,5,8-trione
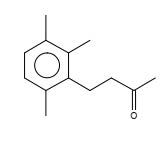
Figure 25. 3-Butanone,1-(2,3,6-trimethylphenyl)-
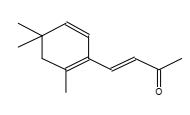
Figure 26. 4-(2,6,6-Trimethyl-cyclohexa-1,3-dienyl)-but-3-en-2-one
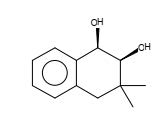
Figure 27. 1,2-Naphthalenediol, 1,2,3,4-tetrahydro-3,3-dimethyl-, cis
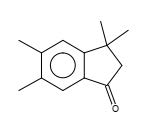
Figure 28. 1H-Inden-1-one, 2,3-dihydro-3,3,5,6-tetramethyl
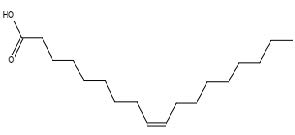
Figure 29. Oleic acid
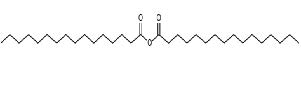
Figure 30. Palmitic anhydride
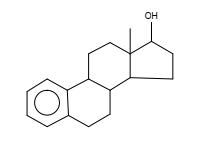
Figure 31. Estra-1,3,5,(10)-trien-17-ol
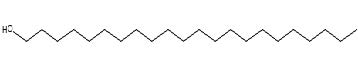
Figure 32. Behenic alchohol
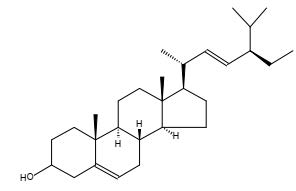
Figure 33. Stigmasterol
Figure 34. β-Sitosterol
Figure 35. 2H,8H-Benzo[1,2-b:3,4-b']dipyran-2-one, 8,8-dimethyl
In vitro antioxidant activities of nHEML
Table 3 shows the DPPH scavenging activity of nHEML using ascorbic acid as the reference standard. Scavenging activity of nHEML was only detected at 400 μg/ml with 13.46% inhibition and significantly (P < 0.05) low when compared with the standard (66.42 – 84.95%).
Table 3. DPPH scavenging effect of n-hexane extract of V. album (Mistletoe) leaves.
|
Concentration of samples (μg/mL) |
%DPPH Inhibition |
|
|
nHEML |
Ascorbic acid |
|
|
25 |
NA |
66.42 ± 2.99 |
|
50 |
NA |
90.41 ± 0.09 |
|
100 |
NA |
90.26 ± 0.23 |
|
200 |
NA |
88.28 ± 1.49 |
|
400 |
13.46 ± 1.73* |
84.95 ± 1.94 |
Note: Values are means ± standard deviations of triplicate determinations. Values with asterisk (*) are significantly different across the rows. nHEML = n-hexane extract of V. album (Mistletoe) leaves. NA = No activity
Table 4 shows the reducing power (FRAP) of nHEML using ascorbic acid as the reference standard. There was a dose-dependent increase in FRAP of nHEML from 25 to 400 μg/ml compared to the standard.
Table 4. FRAP reducing power of n-hexane extract of V. album (Mistletoe) leaves.
|
Concentration of samples(μg/mL) |
FRAP |
|
|
nHEML (µM) |
Ascorbic acid (µM) |
|
|
25 |
NA |
0.029 ± 0.002 |
|
50 |
0.009 ± 0.001* |
0.035 ± 0.006 |
|
100 |
0.011 ± 0.001* |
0.049 ± 0.004 |
|
200 |
0.014 ± 0.001* |
0.067 ± 0.002 |
|
400 |
0.031 ± 0.002* |
0.106 ± 0.011 |
Note: Values are means ± standard deviations of triplicate determinations. Values with asterisk (*) are significantly different across the rows.
nHEML = n-hexane extract of V. album (Mistletoe) leaves. NA = No activity
In vitro anti-inflammatory activities of nHEML
Human red blood cell (HRBC) membrane stabilization assay
The result of the HRBC membrane stabilization activity (Table 5) indicates that there was a concentration-dependent increase in activity from 50 to 400 µg/mL. The lowest anti-inflammatory activity was detected at 50 µg/mL with 3.46% inhibition, while the highest anti-inflammatory activity was detected at a concentration of 400 µg/mL with 42.66% inhibition and much higher than that of the standard (13.09% at 200 µg/mL).
Table 5. Human red blood cell (HRBC) membrane stabilization activity of nHEML.
|
Concentration (µg/mL) |
% inhibition |
|
|
nHEML |
Diclofenac |
|
|
25 |
4.93 ± 2.66 |
|
|
50 |
3.46 ± 1.70 |
|
|
100 |
6.22 ± 1.28 |
|
|
200 |
21.42 ± 1.12 |
13.09 ± 2.06 |
|
400 |
42.66 ± 1.17 |
|
Note: Values are means ± standard deviations of triplicate determinations. nHEML = n-hexane extract of V. album (Mistletoe) leaves.
Heat-induced hemolysis assay
From the result of the heat-induced hemolysis assay (Table 6), there was a dose-dependent increase in anti-inflammatory response by nHEML. The lowest anti-inflammatory activity was detected at 25 µg/mL with 9.91% inhibition, while the highest anti-inflammatory activity was detected at a concentration of 400 µg/mL with 45.50% inhibition compared to the standard (25.49%). The inhibitory response of nHEML at 100 µg/mL (25.79%) was comparable to that of the reference (25.49%) albeit at 200 µg/mL.
Table 6. Heat-induced hemolysis assay of nHEML.
|
Concentration (µg/mL) |
% inhibition |
|
|
nHEML |
Diclofenac |
|
|
25 |
9.91 ± 5.14 |
|
|
50 |
17.64 ± 5.06 |
|
|
100 |
25.79 ± 7.03 |
|
|
200 |
29.68 ± 8.80 |
25.49 ± 0.70 |
|
400 |
45.50 ± 1.72 |
|
Note: Values are means ± standard deviations of triplicate determinations. nHEML = n-hexane extract of V. album (Mistletoe) leaves.
DISCUSSION
Inflammatory responses in several disease conditions are commonly managed with traditional therapies; therefore, the study of the potential of herbal drugs as novel and potent anti-inflammatory agents becomes necessary. This necessitated the investigation of antioxidant and anti-inflammatory properties of nHEML including the total phenolic and flavonoid contents. The potency of phenols and flavonoids as antioxidants in different plant parts for therapeutic purposes has been severally reported (Rahmawati et al., 2014)
Total phenolic and flavonoid concentrations in nHEML were 37.82 ± 0.22 GAE.mg/g and 12.85 ± 3.85 mgQE/100 mg respectively. The values reported for total flavonoids in this study was greater than the values reported by Carla et al. (2020) in V. album ethanolic extracts growing on three host trees (6.30, 9.67, 4.67 mg/g FW). However, studies by Wioleta et al. (2013) reported similar values of total phenolics in V. album methanol leaves extract obtained using different extraction techniques (18.39 to 57.67 GAE.mg/g). They further reported that the extraction method could affect the concentration of phytocompounds in plant extracts. Phenolics and flavonoids have been reported to possess antioxidant and anti-inflammatory properties (Diaz et al., 2012). Phenolic compounds are capable of stabilizing and scavenging free radicals because they possess the ability to donate hydrogen to the free radical thus making them stable. Flavonoids are active hydrophilic antioxidants and free radical scavengers capable of thwarting oxidative cell damage and avert the development of malignant tumors (Donnarumma et al., 2011). The remarkably high amounts of total phenol and flavonoids in the nHEML as shown by this study indicate that they can contribute significantly to the antioxidant and anti-inflammatory potentials of the extract. This is in agreement with the report of Nazaruk and Orlikowski (2016) who investigated the phytochemical profile and therapeutic potentials of V. album L.
The GC-MS analysis showed the presence of six (6) major compounds; Cyclohexanol, 2-methyl-3-(1-methylethenyl)-, (1α,2α,3α)- (20.490 %), vitamin C (27.583 %), 2,4- decadienal (6.970 %), 1,2-Naphthalenediol, 1,2,3,4-tetrahydro-3,3-dimethyl-, cis (6.690 %), 1H-Inden-1-one, 2,3-dihydro-3,3,5,6-tetramethyl (6.010 %) and stigmasterol (4.495 %). In addition, small quantities of Oleic acid (3.021%) and β-sitosterol (1.189) were shown by the GC-MS chromatogram. Result obtained for GC-MS analysis of nHEML showed similar compounds as those detected by Daliborca et al. (2016) in ethanol extract of leaves of V. album but with different concentration (stigmasterol 3.14%, sitosterol 8.14% and 2,4-decadienal 0.17%). Stigmasterol possesses a stabilizing effect on phospholipids bilayer and a protective effect against cardiovascular diseases, colon and breast cancer (Mu et al., 2007). Stigmasterol also possesses anti-osteoarthritic, anti-hypercholesterolemic, cytotoxic, anti-tumour, hypoglycemic, antioxidant, antimutagenic and anti-inflammatory activities (Navarro et al., 2001; Lim et al., 2005; Panda et al., 2009; Kaur et al., 2011). β-sitosterol is a powerful antioxidant (Choudhary and Tran, 2011) as well as possessing antihepatotoxic, anti-inflammatory, antinociceptive, antiophidic, antiviral, artemicide, cancer-preventive, estrogenic, hypocholesterolemic, ovulant, sedative, thyroid inhibitory, antiperoxidative and hypoglycemic activities Singh and Patra, 2018). The nHEML contains vitamin C, which is a potent natural antioxidant with immunomodulatory, antimicrobial, antibacterial, antiviral, antiparasitic and antifungal activities (Mousavi et al., 2019). Other constituents include: 2,4-Decadienal - nematicidal and oxidative activities (Caboni et al., 2012), naphthalene derivatives- cataractogenic activity (Singh and Patra, 2018), trans-calamenene - anticancer and cytotoxic activities (Fajriah et al., 2017), phenol - antimicrobial and antioxidant activities (Bahri-Sahloul et al., 2014), 4-(2,4,4-Trimethyl-cyclohexa-1,5-dienyl)-but-3-en-2-one - antibacterial activity (Idan et al., 2015), oleic acid - antifungal activity (Singh and Patra, 2018) etc.
The DPPH activity of nHEML was only detected at 400 μg/mL which gave a DPPH inhibition of 13.46%. Compared to standard vitamin C used at 400 μg/mL (84.95%), nHEML can be considered a poor DPPH scavenger, but can be improved as the concentration of nHEML is increased. Onay-Ucar et al. (2006) reported that seasonal changes and the nature of the host plant affect the antioxidant activity of V. album. Since the antioxidant capability of phenolic compounds is largely determined by their chemical nature (position and number of hydroxyl group, presence of a carbohydrate moiety, molecular weight, etc.) and the chemical matrix in which it is found, a high phenolic content does not always imply a high antioxidant power. Also, the intricacy of the oxidation process and the diverse nature of phenolic compounds, which contain both hydrophilic and hydrophobic elements, may make it difficult to quantify their antioxidant activity. Hence, antioxidant assay could have altered the phenolic compound's structure during the experiment thereby affecting its antioxidant activity (Khokhar and Apenten, 2003). On the other hand, concentration-dependent scavenging activity of nHEML against FRAP radicals was observed. Therefore, nHEML can contribute to counteracting oxidative effects on biomolecules in the body. This can be due to the effect of the total flavonoid content, which was very high and can improve the overall antioxidant and anti-inflammatory properties of the nHEML. This is in agreement with the report of Orhan et al. (2006), Pelzer et al. (1998) and Hsieh et al. (1998) who investigated the anti-inflammatory effects of isolated flavonoids both in vitro and in vivo. Antioxidant activity as highlighted by a review (Dhakad et al., 2018) entails inhibition of initiator radical production and cessation of radicals in the development stage or by boosting and/or inducing enzymes’ activities against reactive species (Demirci and Studer, 2012; Amorati et al., 2013).
Human red blood cells (HRBCs) are prone to hemolysis and membrane destabilization when exposed to a hypotonic solution or heat (Ferrali et al., 1992) as a result of the attack by reactive oxygen species released by free radical-induced lipid peroxidation (Halliwell and Whiteman, 2004). This leads to inflammatory effects, which can be attenuated by using anti-inflammatory agents to stabilize the lysosomal membranes and/or inhibit the release of lysosomal enzymes. Inhibition of hypotonicity and heat-induced hemolysis in HRBC has been used as a model to study the anti-inflammatory effect of exogenous substances on lysosomal membrane components (Mounnissamy et al., 2007; Anosike et al., 2012). Likewise, this was utilized in this study with n-hexane extract of V. album leaves because of the similarity of HBRC to the lysosomal membrane. Within the range of 50 to 400 µg/mL, nHEML dose-dependently stabilized the HBRC membrane and prevented its hemolysis induced by the hypotonic solution and heat. The inhibitory effects at 200 and 400 µg/mL for both hypotonic and heat-induced models were higher than the standard non-steroidal anti-inflammatory drug (Diclofenac), suggesting a more potent anti-inflammatory effect of nHEML. According to Chaitanya et al. (2011) and Anosike et al. (2012), the anti-inflammatory effect could have been caused by blocking serum protein, lytic enzymes and active mediators of inflammation from leaking into the tissues. The anti-inflammatory activity of nHEML is correlated with the high flavonoid content obtained in this study and agrees with the report of Orhan et al. (2006) who reported a potent and dose-dependent anti-inflammatory effect of flavonoids isolated from V. album in a Carrageenan-induced inflammation model, and Pelzer et al. (1998) who used flavonoid derivatives. The anti-inflammatory effect could be linked to inhibition of cyclooxygenase and phospholipases involved in acute and chronic inflammation as suggested by Mounnissamy et al. (2007) and Aitadafoun et al. (1996).
CONCLUSION
The outcome of this study clearly shows that nHEML contains numerous phytochemicals as revealed by GC-MS analysis. Furthermore, phenolics and flavonoids in partnership with major compounds identified are responsible for the anti-inflammatory activities observed. This supports several reports of the local usage of V. album and other flavonoid derivatives in the management of inflammatory ailments. Significant antioxidant activity was not observed at the particular concentrations used in this study. Further research on the specific mechanism of the anti-inflammatory effect of nHEML and its major constituents in both in vitro and in vivo systems is required.
REFERENCES
Ademiliyu A.O. and Oboh G. 2008. Antioxidant properties of methanol extracts of mistletoe (Viscum album) from cocoa and cashew trees in Nigeria. African Journal of Biotechnology. 7: 3138- 3142
Aitdafoun, M., Mounier, C., Heymans, F., Binisti, C., Bon, C., and Godfroid, J.J. 1996. 4-Alkoxybenzamidines as new potent phospholipase A2 inhibitors. Biochemical Pharmacology. 51: 737-742.
Amorati, R., Foti, M.C., and Valgimigli, L. 2013. Antioxidant activity of essential oils. Journal of Agricultural and Food Chemistry. 61: 10835-10847.
Anosike, C.A., Obidoa, O., and Ezeanyika, L.U. 2012. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). Daru, Journal of Pharmaceutical Sciences. 20: 76.
APweb – Angiosperm Phylogeny Website, version 13. 2013. Santalales; Available from: http://www.mobot.org/MOBOT/research/APweb/
Bahri-Sahloul, R., Fredj, R.B., Boughalleb, N., Shriaa, J., Saguem, S., Hilbert, J.L., ... and Harzallah-Skhiri, F. 2014. Phenolic Composition and Antioxidant and Antimicrobial Activities of Extracts Obtained from Crataegus azarolus L. var. aronia (Willd.) Batt. Ovaries Calli. Journal of Botany. Article ID 623651, 11 pages.
Bartram T. 1995, Encyclopedia of Herbal Medicine. Grace, Dorsett, p. 296.
Benzie, I.F. and Strain, J.J. 1999. [2] Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods in Enzymology. 299: 15-27.
Caboni, P., Ntalli, N.G., Aissani, N., Cavoski, I., and Angioni, A. 2012. Nematicidal activity of (E, E)-2, 4-decadienal and (E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. Journal of Agricultural and Food Chemistry. 60: 1146-1151.
Carla H., Michelle N.O., Adriana P.O., Joao V., Marcia A.M., Rafael G., Mirio G., Hartmut R., Claudia D., Gerhard S., Konrad U., Ulrike W. and Stephan B. 2020. Phytochemical analysis and in vitro antiproliferative activity of Viscum album ethanolic extracts. BMC Complementary Med. Ther., 20: 215
Chaitanya, R.S., Sandhya, S., David, B., Vinod, K.R., and Murali, S. 2011. HRBC membrane-stabilizing property of root, stem and leaf of Glochidion velutinum. International Journal of Research in Pharmaceutical and Biomedical Sciences. 2: 256-259.
Choudhary, S., and S Tran, L. 2011. Phytosterols: perspectives in human nutrition and clinical therapy. Curr. Med. Chem., 18: 4557-4567.
Daliborca C.V., Roxana P., Victor D., Adinela C., Cristian S., Csaba V., Judith K., Cristina D. and Florin G. 2016. Phytocomponents identification in mistletoe (Viscum album) young leaves and branches, by GC-MS and antiproliferative effect on HEPG2 and McF7 cell lines. Farmacia, 64: 1
Demirci, C. and Studer, A. 2012. Encyclopedia of Radicals in Chemistry, Biology, and Materials, Vol. 2, John Wiley and Sons, Chichester, UK.
Dhakad, A.K., Pandey, V.V., Beg, S., Rawat, J.M., and Singh, A. 2018. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: a review. Journal of the Science of Food and Agriculture. 98: 833-848.
Diaz, P., Jeong, S.C., Lee, S., Khoo, C. and Koyyalamudi, S.R. 2012. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chinese Medicine. 7: 1-9.
Donnarumma, G., Paoletti, I., Buommino, E., Fusco, A., Baudouin, C., Msika, P., ... and Baroni, A. 2011. AV119, a natural sugar from avocado gratissima, modulates the LPS-induced pro-inflammatory response in human keratinocytes. Inflammation, 34: 568-575.
Elluru, S.R., Van Huyen, J.P.D., Delignat, S., Prost, F., Heudes, D., Kazatchkine, M.D., ... and Kaveri, S.V. 2009. Antiangiogenic properties of Viscum album extracts are associated with endothelial cytotoxicity. Anticancer Res., 29: 2945-2950.
Ergun, F., and Deliorman, D. 1995. Chemical constituents of Viscum album L. Ankara Univ. Eczacilik Fak. Derg., 24: 95-107.
Fajriah, S., Megawati, Hudiyono, S., Kosela, S., and Hanafi, M. 2017. Chemical constituents and potential cytotoxic activity of n-hexane fraction from Myristica fatua Houtt leaves. In AIP Conference Proceedings (Vol. 1862, No. 1, p. 030087). AIP Publishing LLC.
Ferrali, M., Signorini, C., Ciccoli, L., and Comporti, M. 1992. Iron release and membrane damage in erythrocytes exposed to oxidizing agents, phenylhydrazine, divicine and isouramil. Biochemical Journal. 285: 295-301.
Gorinstein, S., Vargas, O.J.M., Jaramillo, N.O., Salas, I.A., Ayala, A.L.M., Arancibia-Avila, P., ... and Trakhtenberg, S. 2007. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. European Food Research and Technology. 225: 321-328.
Gray, A.M., and Flatt, P.R. 1999. Insulin-secreting activity of the traditional antidiabetic plant Viscum album (mistletoe). Journal of Endocrinology and Metabolism. 160: 409-414.
Halliwell, B., and Whiteman, M. 2004. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? British Journal of Pharmacology. 142: 231-255.
Han, S.Y., Hong, C.E., Kim, H.G., and Lyu, S.Y. 2015. Anti-cancer effects of enteric-coated polymers containing mistletoe lectin in murine melanoma cells in vitro and in vivo. Molecular and Cellular Biochemistry. 408: 73-87.
Hsieh, H.K., Lee T.H., Wang J.P., Wang J.J. and Lin, C.N. 1998. Synthesis and anti-inflammatory effect of chalcones and related compounds. Pharm. Res., 15:39-46.
Hussain, T., Tan, B., Yin, Y., Blachier, F., Tossou, M.C., and Rahu, N. 2016. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Medicine and Cellular Longevity. 2016: 7432797.
Idan, S.A., Al-Marzoqi, A.H., and Hameed, I.H. 2015. Spectral analysis and antibacterial activity of methanolic fruit extract of Citrullus colocynthis using gas chromatography-mass spectrometry. African Journal of Biotechnology. 14: 3131-3158.
Iwalewa, E.O., Adewale, I.O., Taiwo, B.J., Arogundade, T., Osinowo, A., Daniyan, O.M., and Adetogun, G.E. 2008. Effects of Harungana madagascariensis stem bark extract on the antioxidant markers in alloxan-induced diabetic and carrageenan-induced inflammatory disorders in rats. Journal of Complementary and Integrative Medicine. 5.
Kamaraj, M., Kumar, V.D.R., Nithya, T.G., and Danya, U. 2020. Assessment of antioxidant, antibacterial activity and phytoactive compounds of aqueous extracts of avocado fruit peel from Ethiopia. International Journal of Peptide Research and Therapeutics, 26: 1549-1557.
Kaur, G.J. and Arora, D.S. 2009. Antibacterial and phytochemical screening of Anethem graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement Altern Med., 9: 30.
Khokhar, S. and Apenten, R.K.O. 2003. Iron binding characteristics of phenolic compounds: some tentative structure–activity relations. Food Chemistry, 81: 133-140.
Lim, J.C., Park, J.H., Budesinsky, M., Kasal, A., Han, Y.H., Koo, B.S., ... and Lee, D.U. 2005. Antimutagenic constituents from the thorns of Gleditsia sinensis. Chemical and Pharmaceutical Bulletin. 53: 561-564.
Mensor, L.L., Menezes, F.S., Leitão, G.G., Reis, A.S., Santos, T.C.D., Coube, C.S., and Leitão, S.G. 2001. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy Research. 15: 127-130.
Mounnissamy, V.M., Kavimani, S., Balu, V., and Quine, S.D. 2007. Evaluation of Anti-inflammatory and Membrane stabilizing property of Ethanol Extract of Cansjera rheedii J. Gmelin (Opiliaceae). Iranian Journal of Pharmacology and Therapeutics. 6: 235-0.
Mousavi, S., Bereswill, S., and Heimesaat, M.M. 2019. Immunomodulatory and antimicrobial effects of vitamin C. Eur. J. Microbiol. Immunol. 9: 73-79.
Mu, L., Kou, J., Zhu, D., and Yu, B. 2007. Comparison of neuroprotective effects of flavonoids, terpenoids, and their combinations from ginkgo biloba. On ischemia-reperfusion–injured mice. Pharmaceutical Biology. 45: 728-733.
Murray, M.T. 1995. The healing power of herbs: the enlightened person's guide to the wonders of medicinal plants, 2nd ed. Prima, Rocklin, CA, pp. 253-260.
Navarro, A., De Las Heras, B., and Villar, A. 2001. Anti-inflammatory and immunomodulating properties of a sterol fraction from Sideritis foetens Clem. Chem. Pharm. Bull., 24: 470-473.
Nazaruk, J., and Orlikowski, P. 2016. Phytochemical profile and therapeutic potential of Viscum album L. Nat. Prod. Res., 30: 373-385.
Newall C.A., Anderson L.A., and Phillipson J.D. 1996. Herbal Medicines. Pharmaceutical Press, London, pp. 193-196.
Ohiri, F.C., Esimone, C.O., Nwafor, S.V., Okoli, C.O., and Ndu, O.O. 2003. Hypoglycemic properties of Viscum album (Mistletoe) in alloxan-induced diabetic animals. Pharmaceutical Biology. 41: 184-187.
Oluwafemi O.O. 2019. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. International Journal of Physiology, Pathophysiology and Pharmacology. 11: 45-63.
Önay-Uçar, E., Karagöz, A., and Arda, N. 2006. Antioxidant activity of Viscum album ssp. album. Fitoterapia, 77: 556-560.
Orhan, D.D., Küpeli, E., Yesilada, E., and Ergun, F. 2006. Anti-inflammatory and antinociceptive activity of flavonoids isolated from Viscum album ssp. album. Zeitschrift für Naturforschung. C, Journal of biosciences. 61: 26-30.
Panda, S., Jafri, M., Kar, A., and Meheta, B.K. 2009. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia. 80: 123-126.
Parekh, J., Jadeja, D. and Chanda, S. 2005. Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk. J. Biol, 29(4), 203-210
Paśko, P., Bartoń, H., Zagrodzki, P., Gorinstein, S., Fołta, M., and Zachwieja, Z. 2009. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chemistry. 115: 994-998.
Pelzer, L.E., Guardia, T., Juarez, A.O., and Guerreiro, E. 1998. Acute and chronic anti-inflammatory effects of plant flavonoids. Farmaco, 53: 421-424.
Pietrzak, W., Nowak, R., Gawlik-Dziki, U., Lemieszek, M.K., and Rzeski, W. 2017. LC-ESI-MS/MS identification of biologically active phenolic compounds in mistletoe berry extracts from different host trees. Molecules, 22: 624.
Pushpa H., Mohan S.M., Alain F., Jagadeesh B. and Srini V.K. 2011. Viscum album exerts anti-inflammatory effect by selectively inhibiting cytokine-induced expression of cyclooxygenase-2. PLoS One 6: e26312
Rahmawati, S.I., Ishimaru, K., Hou, D.X., and Hayashi, N. 2014. Antioxidant activity and phenolic content of Mistletoe extracts following high-temperature batch extraction. Food Science and Technology Research. 20: 201-206.
Simsek, I., Aytekin, F., Yesilada, E., and Yildirimli, Ş. 2004. An ethnobotanical survey of the Beypazari, Ayas, and Güdül district towns of Ankara Province (Turkey). Economic Botany. 58: 705.
Singh, S.K. and Patra, A. 2018. Evaluation of phenolic composition, antioxidant, anti-inflammatory and anticancer activities of Polygonatum verticillatum (L.). Journal of Integrative Medicine. 16: 273-282.
Skidmore-Roth, L. 2006. Mosby's Handbook of Herbs and Natural Supplements. Mosby. p. 663
Suk-Nam K. 2016. Ethanol extracts from mistletoe (Viscum album L.) acts as antioxidants and antimicrobial agents in uncooked pork patties during refrigerated storage. Asian-Australas. Journal of Animal Science. 29: 109- 118
Valle, A.C.V., de Carvalho, A.C., and Andrade, R.V. 2021. Viscum Album-Literature Review. International Journal of Science and Research. 10: 63-71.
Valle, A.C., Andrade, R., Sibata, M., and Carvalho, A. 2020. Ultradiluted Viscum album in the Treatment of Melanoma in a Dog (Canis familiaris)–Case Report. Homeopathy, 109: P041.
Veiga, M., Costa, E.M., Silva, S., and Pintado, M. 2020. Impact of plant extracts upon human health: A review. Critical reviews in food science and nutrition, 60: 873–886.
Wichtl, M. and Bisset, N.G. 1994. Visci herba – Mistletoe herb. In: Herbal Drugs and Phyto-pharmaceuticals. CRC Press, Stuttgart, pp. 534-536.
Wioleta P., Renata N and Marta O. 2013. Effect of extraction method on phenolic content and antioxidant activity of mistletoe extracts from Viscum album subsp. abietis. Chemical Papers. 68: 976- 982.
Yesilada, E., Deliorman, D., Ergun, F., Takaishi, Y., and Ono, Y. 1998. Effects of the Turkish subspecies of Viscum album on macrophage-derived cytokines. Journal of Ethnopharmacology. 61: 195-200.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Charles Nnanna Chukwu1,*, Uchechi Bliss Onyedikachi1, and Emmanuel Ejiofor2
1 Department of Biochemistry, College of Natural Sciences, Michael Okpara University of Agriculture, Umudike, P. M. B. 7267, Umuahia, Abia State, Nigeria.
2 Department of Chemical Sciences, Faculty of Science, Clifford University, Owerrinta, Abia State, Nigeria
Corresponding author: Charles Nnanna Chukwu, E-mail: charles_chukwu@uniport.edu.ng; chukwu.charles@mouau.edu.ng
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: June 24, 2021;
Revised: September 28, 2021;
Accepted: October 4, 2021

