
Characterization of Polylactic/Polyethylene glycol/Bone Decellularized Extracellular Matrix Biodegradable Composite for Tissue Regeneration
Wassanai Wattanutchariya, and Kullapop Suttiat*Published Date : 2022-01-21
DOI : https://doi.org/10.12982/CMUJNS.2022.008
Journal Issues : Number 1, January-March 2022
Abstract This study focused on evaluating the Polylactic acid /Polyethylene glycol (PLA/PEG) combining with bone decellularized extracellular matrix (bone dECM) as the alternative biodegradable material for tissue regenerative purposes. The casting membranes of pure PLA, PLA/PEG blend, and PLA/PEG combining with 5, 10 and 20 wt% bone dECM particles were fabricated and the characteristics of surface morphology, surface contact angle, thermal properties, cell viability, in vitro osteogenesis, and in vitro biodegradative behaviors were investigated. The improvement in hydrophilic characteristic was found in the developing composite. Following the in vitro degradation test in PBS/lysozyme for 7, 30, 60 and 90 days, the composite with higher ratio of bone dECM particles showed the higher percentage of material weight loss. However, the statistically significant of material weight alteration was observed only on the PLA/PEG/20wt% bone dECM after degradation test for 90 days (P ≤0.05). All specimens showed the physically intact at the end of the 90-day in vitro hydrolytic degradation test. The metabolic activities of L929 cells were significantly enhanced by the presence of PLA/PEG/bone dECM composites comparing to pure PLA (P ≤ 0.05). The result from Alizarin red S staining confirmed the osteo-inductive property of developing composite. The present study addressed the promising potential of PLA/PEG/bone dECM composites for applying as an alternative biodegradable material in tissue regenerative purpose.
Keywords: Biomaterial, Biodegradable Composite, Bone Decellularized Extracellular Matrix, Polylactic Acid
Citation: Wattanutchariya, W. and Suttiat, K. 2022. Characterization of polylactic/polyethylene glycol/bone decellularized extracellular matrix biodegradable composite for tissue regeneration. CMU J. Nat. Sci. 21(1): e2022008.
INTRODUCTION
The progression in tissue regenerative technology as well as the advance in molecular biology significantly affect to the treatment options in the modern world. The tissue engineering approach that utilizes specific biodegradable scaffolds, molecular signaling, and patients’ own cells to restore the defective structures has been increasingly interested as the treatment modality for the future. This disruption attracted the researchers as well as the clinicians to focus on developing the biomaterials that met their requirements (Chen & Liu, 2016; Tatullo, Codispoti, Paduano, Nuzzolese, & Makeeva, 2019).
During the last few decades, the extensive effort had been spent on applying the biodegradable Polylactic acid (PLA) as biomaterial for medical and dental applications in many aspects (Nair & Laurencin, 2006; Song et al., 2018). For tissue engineering approach, PLA is the most attracted polyester for fabricating the imitate extracellular matrix due to its excellence biocompatibility and good mechanical properties (Saini, Arora, & Kumar, 2016; Singhvi, Zinjarde, & Gokhale, 2019). However, its bioinert property is the significant inherent disadvantage that restricted the PLA application (DeStefano, Khan, & Tabada, 2020).
To overcome this limitation, many techniques had been recommended for tailoring the materials properties. The composite formation by mixing with the different polymers and bioactive particles is one of the simple and cost-effective approach for modifying the mechanical, thermal, and biological property of PLA (Saini et al., 2016; Nofar, Sacligil, Carreau, Kamal, & Heuzey, 2019).
The previous studies mentioned that the employing of lower molecular weight Polyethylene glycol (PEG) into PLA resulted in increasing material ductility and improving material processability by means of the plasticizing effect (Chieng, Ibrahim, Yunus, & Hussein, 2013; Ozdemir & Hacaloglu, 2017). Furthermore, the mixing of PEG into PLA also caused the alteration in physico-chemical characteristics of the resulting blend. The presence of PEG provided the enhancing effect to the living cell, altered in material hydrophilicity as well as modified the material degradative behavior (Serra, Ortiz-Hernandez, Engel, Planell, & Navarro, 2014; Bhaskar et al., 2018).
The incorporation of calcium phosphate particle such as hydroxyapatite (HA) or tricalcium phosphate (TCP) into bioinert polymer matrix has been widely recommended for long time as the usual and effective approach for improving the bioactivity of bio-inert material (He et al., 2011; Ling et al., 2015; Pitjamit et al., 2020). Recently, the enhancing effect of bone decellularized extracellular matrix (bone dECM) on bone cell migration and proliferation had been addressed and proved (Mansour, Mezour, Badran, & Tamimi, 2017). The similarity of Ca/P ratio and the collagen remnant as well as other remaining minor natural bone components in bone dECM particles were claimed as the reason for this advantage (Papadimitropoulos, Scotti, Bourgine, Scherberich, & Martin, 2015).
According to this information, it seems that the composite made from combination of PLA, PEG, and bone dECM particle should express the possibility as the alternative biodegradable material for applying in tissue regenerative purposes. Furthermore, there are few studies focused on applying the PLA/PEG as biodegradable material for regenerative medicine. In addition, there is no publication regarding the potential of applying PLA/PEG/bone dECM composite as the alternative material for tissue engineering. Therefore, this present study aims to investigate the material morphology, surface energy, thermal properties, cell viability, the osteo-regenerative characteristic as well as its hydrolytic degradative behavior for initially evaluate the possibility in utilizing this developing material for tissue regenerative treatment.
MATERIALS AND METHODS
Materials
The PLA pellets (PLA 4032D) was purchased from NatureWorks (Minnetonka, MN, USA). The PEG with molecular weight of 1,500 g/mol was supplied by Sigma-Aldrich (St. Louis, MO, USA). Bone dECM particle was prepared following the protocol reported in previous studies (Gardin et al., 2015). Briefly, the porcine trabecular bones were collected, cleaned, and cut into small pieces. Four cycles of thermal shock (121˚C for 20 min followed by immersed in liquid nitrogen for 16 h) were carried out. The sequential chemical soaking in 1% Triton X-100 for 8 h, second wash in 0.1% Triton X-100 for 16h. Rinsing two times with distilled water for 24h to remove the residual detergent. All steps were conducted under continuous shaking at room temperature. The decellularized bone were lyophilized and ground to obtained particle form. The bone dECM particles was kept in desiccator until use. Other chemicals and reagents were analytical grade and used as received.
Preparation for film casting
The PLA and PEG pellets were dried in oven at 80˚C for 4 h and 45˚C for 8h respectively to minimize the residual humidity. The 10 wt/v% solution of PLA, PLA mixing with PEG (90/10 wt/wt) and PLA mixing with PEG (90/10 wt/wt) combining with 5, 10 and 20%wt bone dECM particle dissolved in chloroform were prepared. The homogeneous polymeric solutions were poured into the glass plates and 24 h left in the fume hood at room temperature for solvent evaporation. The casting films were dried overnight in an oven at 45˚C to remove any residual solvent and moisture. All prepared membranes were kept in desiccator until use.
Scanning electron microscopy (SEM)
The membrane surface morphology of each group was characterized by SEM (JEOL-JSM 5910 LV, JEOL Ltd., Tokyo, Japan). The specimens were sputtered with gold and the SEM imaging was performed.
Thermal properties investigation
The glass transitional temperature (Tg), melting temperature (Tm), and enthalpy of melting (∆Hm) were studied by differential scanning calorimeter (DSC) (Star®, Mettler Toledo, Schwerzenbach, Switzerland). The approximately 5 mg of specimen was collected and heated by scanning temperature range from 0˚C to 200˚C at a rate of 10˚C/min under a nitrogen atmosphere with the flow rate of 20 ml/min. The degree of crystallinity (Xc) for samples was calculated according to the following equation (1) as described in the previous study (Abdelwahab et al., 2012).
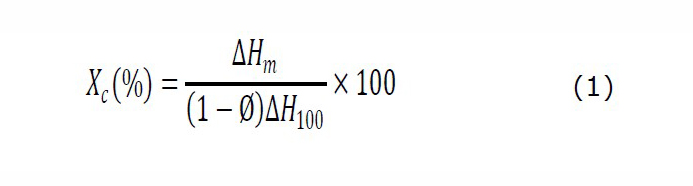
where ∆Hm is the melting enthalpy (J/g). Ø is the weight fraction of the disperse phase in the blends. ∆H100 is the melting enthalpy of 100% crystalline PLA (93.7 J/g).
In vitro degradation study
After 2 h UV irradiation for specimen sterilization, twenty-four specimens of known weight from each group were transferred to 24-well plate that filled each well with 2 ml of a phosphate-buffered saline (PBS) mixed with lysozyme (1.6 µg/mL). The specimens were incubated at 37˚C for 7, 30, 60 and 90 days. At each time interval, six samples from each group were removed and thoroughly washed with distilled water then freeze-dried for 48h. The corresponding dried weight of each specimen was measured. The percentage of weight alteration for each specimen was calculated following equation (2) as the previous study (Wattanutchariya & Thunsiri, 2015).
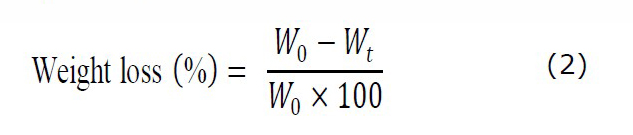
Where W0 refer to the initial weight of specimen. Wt is the dried weight of specimen after in vitro degradation test.
Contact angle assessment
The specimens (n = 4) from each group were evaluated by Contact Angle Goniometer (CMU-PHYS: CMU-PHYS-nanolabs, CMU, Chiang Mai, Thailand). A droplet of distilled water at 25 ± 1.0°C was dispensed onto the air contact surface of each specimen as recommended by previous studies (Zeng et al., 2011). The images of the droplets were captured immediately. The angle at the point of air-water-specimen intersection from left and right boundaries of the magnified picture were measured. The average value was determined for each group.
In vitro cellular response
The metabolic activity of fibroblast cell line (L929 cells) was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT assay) to evaluate the influence of PLA/PEG/bone dECM composites to the living cells. The 2 x 104 cells of L929 were seeded into 24-well culture plate containing Dulbecco’s modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml Penicillin and 100 µg/ml Streptomycin. On each group, three wells with 5-mm disc shaped membraned were assigned as test group and the remaining three wells without specimens were assigned as controlled. The culture plates were incubated at 37°C in 5% CO2 atmosphere. The metabolic activity of L929 cells were investigated at 1 and 7 days after incubation. The culture medium was changed every two days.
At each predetermined incubation time, the culture medium was removed from each well, washed with 500 μl PBS, and replaced with 300 μl of DMEM containing 10% MTT (MTT solution) then incubated at 37°C at 95% humidity and 5% CO2 for 1 hour. The excessive MTT solution was removed and 500 μL of dimethyl sulfoxide (DMSO) was added into each well to dissolve the formazan crystals. The optical density (OD) at 540 nm, as the indicator for cell viability, was measured by Titertek Multiskan M340 multiplate reader (ICN Flow, Costa Mesa, CA, USA). The percentage of cell viability was calculated following equation (3) as the previous study (Marra, Szem, Kumta, DiMilla, & Weiss, 1999).

Determination of osteo-inductive property
The in vitro osteo-inductive property was performed by incubating the human osteosarcoma cell line (MG-63) with non-osteogenic medium (DMEM medium supplemented with 10% SBF and 1% antibiotic), non-osteogenic medium with PLA/PEG/bone dECM extraction and Osteo-inductive medium (complete DMEM supplemented with β-glycerophosphate, ascorbic acid, and dexamethasone) for 7 days and 14 days then the osteogenic differentiation was investigated by Alizarin red S staining method.
The PLA/PEG/bone dECM composite was sterilized under UV light for 1h on each side. The composite extraction mediums were prepared by soaking the sterile specimen (0.2g/ml) in complete DMEM and osteo-inductive medium for 24h at 37˚C with 5% CO2 and 98% humidity. The 24-well plate with 80% MG-63 cell confluent in each well was divided into 3 groups according to the culturing medium, non-osteogenic medium (n=3), non-osteogenic medium with material extraction (n = 3), and Osteo-inductive medium (n = 3). The culturing medium was changed every two days. After incubation for 7 days and 14 days, the samples were fixed with cold methanol for 10 min at 4˚C and then washed with deionized water. Subsequently, the samples were stained with Alizarin red S at 37˚C for 3 min then washed three times with deionized water to remove the excessive staining medium. The calcification on each specimen was observed and imaged using a light microscope (10x). The Alizarin red S stained on calcified substrate was dissolved and the optical density of the solution from each group was measured at 568 nm by multiplate reader.
Statistical analysis
The data of contact angle measurement and optical density of Alizarin red S staining were expressed as mean ± standard deviation. The percentage of material weight loss and cell viability as well as the optical density of Alizarin Red S dye were analyzed by the one-way ANOVA with Tukey’s multiple comparison test (IBM SPSS Statistics 24, IBM corporation, New York, USA). The P-values less than 0.05 were considered statistically significant.
RESULTS
Surface morphology
The SEM images exhibited the uniform formation of microcracks on outer surface of casting membrane (Figure1). The increasing of surface irregularity and roughness were observed when the amount of incorporated bone dECM particles was increased. The bone particles agglomeration was observed in the composite with 20wt% bone dECM particle (Figure 1e).
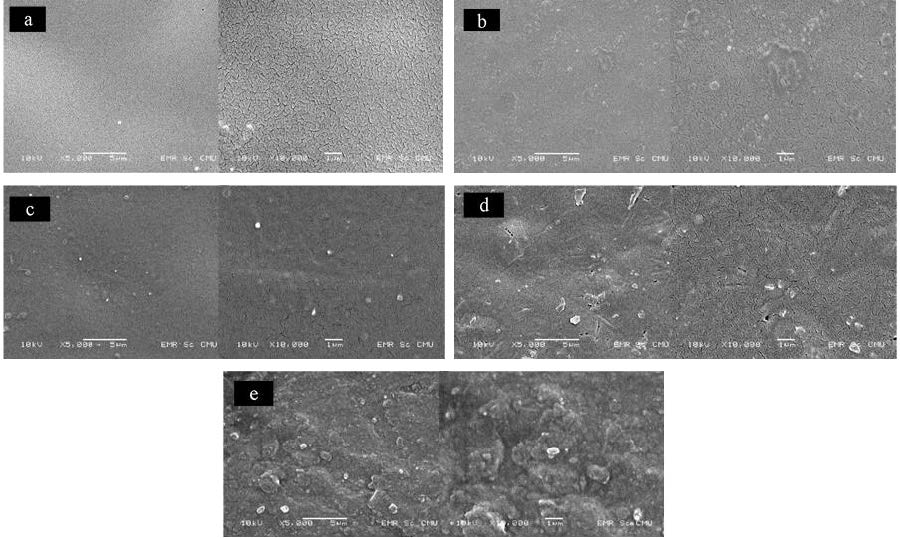
Figure 1. SEM photography of prepared membranes at magnification of 5,000x and 10,000x. a: pure PLA, b: PLA/PEG, c: PLA/PEG/5%bone dECM, d: PLA/PEG/10% bone dECM, e: PLA/PEG/20% bone dECM.
Thermal properties
The DSC thermograms of developing composites are demonstrated in Figure 2. The thermal properties data was summarized in Table 1. A little bit reduction of the glass transitional temperature (Tg) were observed when PEG was mixed into PLA base. The addition of bone dECM particles with different ratio did not affect to the Tg of PLA/PEG/bone dECM composite. The reduction of the percentage of PLA crystallinity was observed when the bone dECM particles reached to 20 wt%.
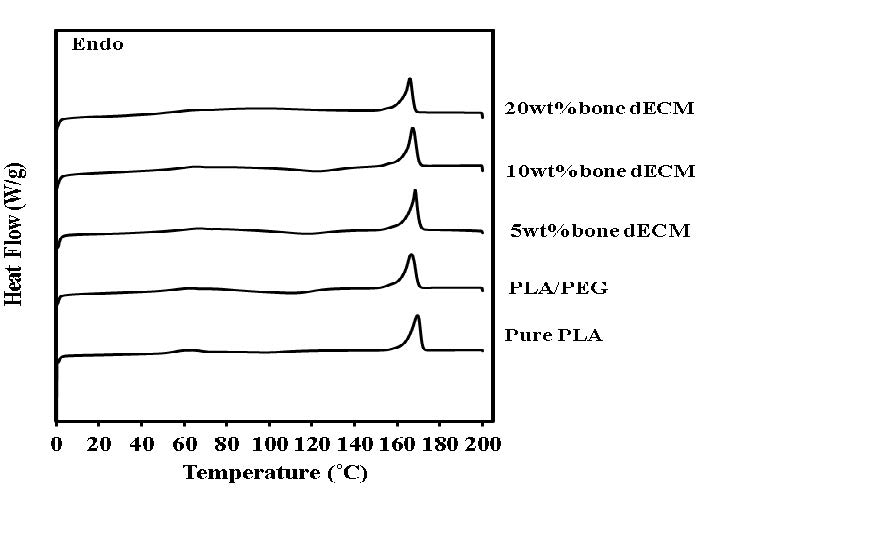
Figure 2. DSC thermograms of testing materials.
Table 1. Thermal properties from the DSC thermograph.
|
Sample |
Tg (˚C) |
Tm (˚C) |
∆Hm (J/g) |
Crystallinity of PLA (%) |
|
Pure PLA |
61.11 |
169.17 |
30.6 |
32.66 |
|
PLA/PEG |
57.43 |
166.50 |
31.17 |
33.27 |
|
PLA/PEG/5 wt% bone dECM |
62.30 |
168.17 |
31.25 |
33.35 |
|
PLA/PEG/10 wt% bone dECM |
60.87 |
167.00 |
30.39 |
32.43 |
|
PLA/PEG/20 wt% bone dECM |
56.13 |
165.67 |
24.50 |
26.15 |
Hydrolysis degradation
The physically intact was found in all specimens following the in vitro degradation test in PBS/lysozyme solution for 90 days. The percentage of the material weight alteration at 7, 30, 60 and 90 days of the in vitro degradation test were presented in Figure 3. At each observing period, all groups showed the significant higher percentage of weight alteration compared to pure PLA. After incubation for 90 days, the composite with 5 wt% bone dECM exhibited the significant lower percentage of weight alteration compared to composite with 10wt% and 20wt% (P ≤ 0.05).
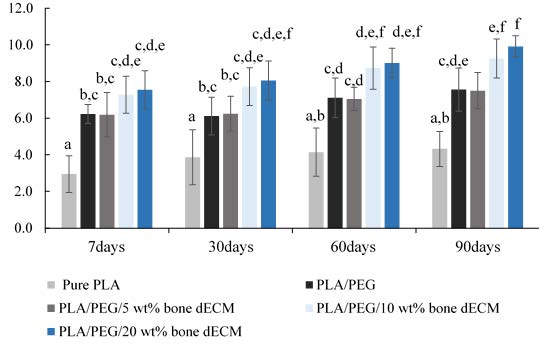
Figure 3. The percentage of material weight loss at 7, 30, 60 and 90 days of in vitro degradation test in PBS/Lysozyme solution. The statistically significant differences (P ≤ 0.05) are indicated by different superscript letter.
Contact angle
The average angle of water drop test on the film of pure PLA, PLA/PEG blend, and PLA/PEG/bone dECM composites were given in Figure 4. The materials with PEG (PLA/PEG blends and PLA/PEG/bone dECM composites) showed the decreasing of the degree of contact angle compared to the pure PLA.
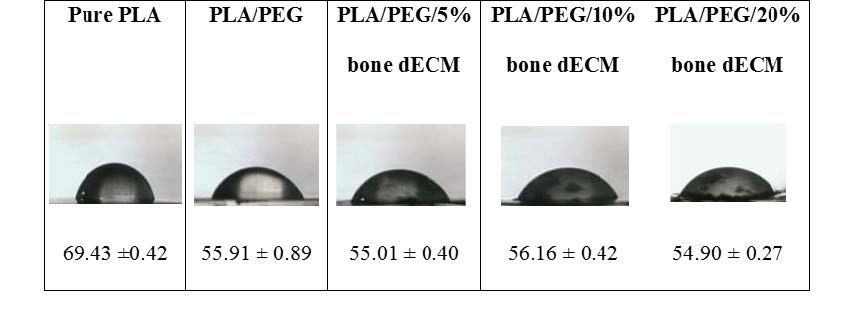
Figure 4. Contact angle (Mean ± SD degree) of composite membranes.
Cell viability
The percentage of cell viability following one- and seven-day incubation were presented in Figure 5. Following one day incubation, the PLA/PEG with 20 wt% bone dECM provided the highest cell viability followed by composites with 10 wt% and 5 wt% while the pure PLA and PLA/PEG blend showed the significant lowest values (P ≤ 0.05). After incubation for seven days, the viability of L929 cells cultured with the PLA/PEG blend and all regimens of PLA/PEG/bone dECM exhibited the significantly higher percentage compared to pure PLA (P ≤ 0.05).
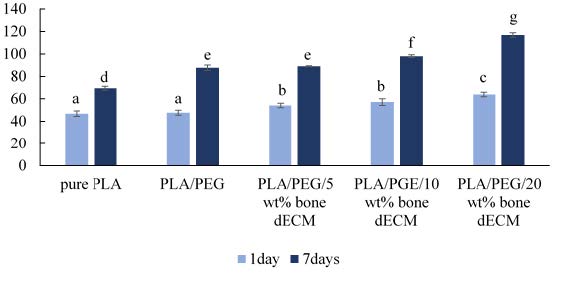
Figure 5. The percentage of cell viability of L929 cells when cultured with pure PLA, PLA/PEG, and PLA/PEG combined with different ratio of bone dECM particles (n = 3). The statistically significant differences (P ≤ 0.05) are indicated by difference letters.
In vitro osteogenesis
In this study, the osteogenic differentiation of human osteosarcoma cell line (MG-63) cultured in non-osteogenic, non-osteogenic medium with material extraction and osteogenic mediums was evaluated. The Alizarin red S staining was utilized for investigating the calcium formation. Figure 6(a). Showed the mineralization stained following the application of Alizarin red S staining. After 14-day incubation, the obviously intense stain was observed in MG-63 cells cultured in non-osteogenic medium with material extraction comparing to non-osteogenic medium without material extraction. The quantitative of Alizarin red S dye stained in each group following the 14-day incubation was demonstrated in Figure 6(b).
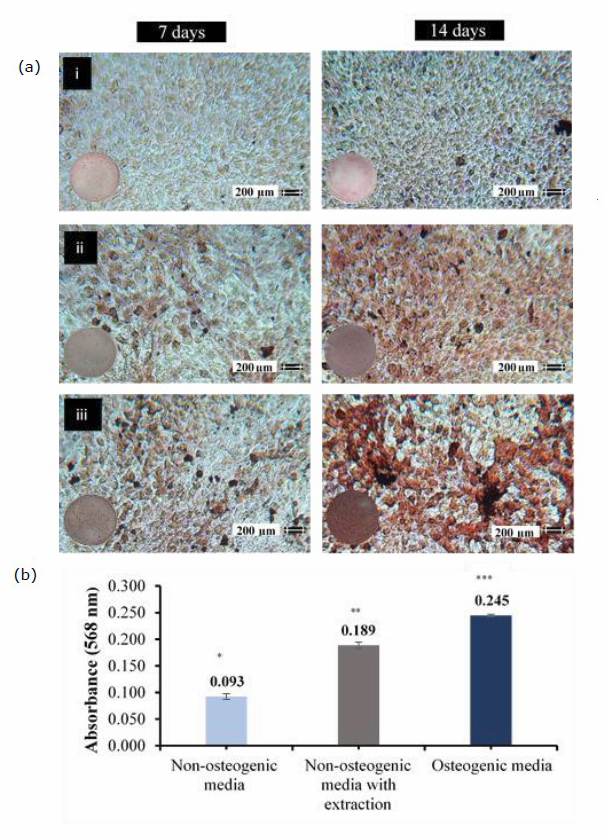
Figure 6. a: The light microscope images (10x) of Alizarin Red S staining (ARS) on mineralized nodules of MG-63 cells after 7 days and 14 days incubation in non-osteogenic medium (i), non-osteogenic medium with PLA/PEG/bond dECM extraction (ii), and osteogenic medium (iii). b: The absorbance at 568 nm of Alizarin Red S dye stained on mineralized nodules of MG-63 cells following 14-day incubation in different culture mediums (n = 3). The statistically significant differences (P ≤ 0.05) are indicated by different superscript marker.
DISCUSSION
In this present study, a series of biodegradable composite made from the PLA/PEG based combining with varying amount of bone dECM particle were developed and investigated. The characteristics of developing composite had been studied for evaluating the possibility for applying as the alternative biomaterial in tissue regenerative aspect. From our study, the developing composites expressed the biocompatibility and bio enhancement on fibroblast cells (L929) as well as the osteo-inductive property on human osteosarcoma cell line (MG63). The improvement on surface hydrophilicity of developing composite compared to pure PLA was found. Moreover, this material also exhibited the durability along the 90-day simulated hydrolytic degradation test. According to these data, PLA/PEG/bone dECM composite provided the promising possibility for applying as the alternative biomaterial for tissue regenerative purpose.
The presence of bone dECM particles in PLA/PEG matrix altered the response of material to the living cells. The metabolic activities of L929 cells were enhanced when increased the ratio of bone dECM particles in developing composites. The osteogenic inductive property of developing PLA/PEG/bone dECM was observed in this present study. The presence of PLA/PEG/bone dECM extraction in non-osteogenic medium caused the significant difference in optical density of Alizarin Red S dye that represented the formation of calcium on pre-osteoblastic cell line after 14-days incubation comparing to control group. The remaining chemical compositions from bone dECM particle could be responded for this biological enhancement. The significant role of bone hydroxyapatite as well as the remaining bone extracellular matrix components found on bone dECM particles on bioactive property of polymeric biomaterial had been confirmed by the previous study (Gardin et al., 2015). The presence of tissue specific extracellular matrix environment on artificial tissue scaffold was critical in cellular regulation that influenced to the maintenance and renewal of living tissues (Gothard et al., 2015). The previous studies found that the bone dECM particle provided the bioactive effects in many ways such as the enhancement on osteogenic differentiation of rat MSCs (Hashimoto et al., 2011), the stimulating effect on collagen type I expression (Marcos-Campos et al., 2012), including the promoting effect on cell infiltration and angiogenesis upon subcutaneous implantation in rats (Hashimoto et al., 2011). The remaining collagens type I, IV and X, and non-collagenous proteins, as well as growth factors such as bone morphogenetic proteins (BMPs) and transforming growth factor-beta 1, 2, and 3 remaining in bone decellularized particles may response for these properties (Buckwalter, Glimcher, Cooper, & Recker, 1996).
Besides the bioactive property, the degradative behavior under biological environment of developing composite is the one of crucial aspects that should be concerned. In this present study, the in vitro hydrolytic degradation test in PBS/lysozyme solution at 37˚C exhibited the higher degradation rate of PLA/PEG/bone dECM composites comparing to pure PLA which showed the very small alteration following the degradation test. The damage or physically alteration of the specimens due to the degradation test were not found in all groups. Normally, the degradation mechanism of pure PLA film in aqueous environment takes place through the random cleavage of ester bonds in amorphous regions.(Elsawy, Kim, Park, & Deep, 2017b) As the hydrolytic chain scission progressed, the number of carboxylic acid chain ends known as the autocatalyze ester hydrolysis tend to increase (Grizzi, Garreau, Li, & Vert, 1995; Schliecker, Schmidt, Fuchs, & Kissel, 2003). This condition enhances the breaking of ester bond in PLA specimen and speed up the material degradation. Moreover, the escape of the aqueous soluble oligomer fragments closer to the surface of PLA film also accelerates the degradation rate of the PLA membrane over the time (Grizzi et al., 1995; Göpferich, 1996; Sheth, Kumar, Davé, Gross, & McCarthy, 1997; Elsawy, Kim, Park, & Deep, 2017a;). For our PLA/PEG/bone dECM composite, the dissolving of aggregated PEG from the surface of composite could play a significant role in higher degradation behavior at the initial stage of investigation as mentioned in the previous study (Zhu, Zhong, Huang, & Wan, 2015) Moreover, the oxygen atoms in the PEG molecules that act as the bonding sites for hydrogen atoms from water molecule also leaded to the proper affinity of PEG to water molecules (Kiani, Mousavi, Shahtahmassebi, & Saljoughi, 2015). These phenomena caused the higher water adsorption and water penetration into the PLA/PEG/bone dECM composite which leaded to the higher material degradation comparing to pure PLA. Furthermore, the increasing of water penetration into polymer matrix due to the polymer immiscibility and subsequent phase separation found in polymer mixing could be classified as the significant accelerating factor for the progression of material hydrolytic degradation process (López-Rodríguez, López-Arraiza, Meaurio, & Sarasua, 2006). The formation of crystalline phases of a major components (PLA) dispersed in an amorphous matrix which created by the minor constituent (PEG) and the amorphous phase of the major component (PLA) was expected for PLA/PEG polymer blend (Younes & Cohn, 1988). This phenomenon could be happened in our composite that used the PLA/PEG polymer blend as polymer matrix.
The characteristic of differential scanning calorimetry (DSC) thermograph was used for initially investigated the molecular structure of the developing composite. The reducing of Tg in PLA/PEG blend comparing to pure PLA was observed. This change can be explained by increasing of the segmental mobility of the PLA chains in polymer matrix due to the presence of PEG plasticizing molecules (Serra et al., 2014). For PLA/PEG/bone dECM composites, the adding of bone dECM particle did not cause the alteration of Tg comparing to PLA/PEG blend. The increasing of bone dECM particle reached to 20wt% caused the reduction of the percentage of PLA crystallinity. It could be assumed that the higher amount of bone dECM particles in PLA/PEG matrix may cause the interference in PLA chains arrangement. This hypothesis was supported by the previous study that reported the disruption on the bonding between the PLA chains following the incorporation of inorganic filler such as hydroxyapatite (HA) or other calcium salt into PLA matrix. This alteration led to the reduction of PLA crystalline array and different polymer chain arrangement during the solvent evaporation (Liu, Wang, Chow, Yang, & Mitchell, 2014). This explanation corresponded to the smallest value of percentage of material crystallinity found in PLA/PEG with 20 wt% bone dECM in the present study.
CONCLUSION
According to the data from our study, the PLA/PEG/bone dECM composite provided the biocompatibility and bioactive property to both fibroblast cell line (L929) and human osteosarcoma cell line (MG63). The presence of bone dECM particles responded for the osteo-regenerative enhancement of the composite. These composites can be withstanding to the 90-day in vitro degradation test.
Within the limitations of the present study, the PLA/PEG/bone dECM composite material provided the promising possibility for applying as an alternative biodegradable material for tissue regenerative purpose especially for bone regeneration. The presence of natural bone hydroxyapatite and other remaining natural bone components in bone dECM particle may provide the advantages in applying this composite as based material for bone scaffold fabrication. The releasing of chemical components which related to the pre-osteoblastic cell differentiation should be monitored in the further study. Moreover, the responses of this composite to the human bone cells as well as the in vivo testing in animal subjects should be performed. Moreover, the responses of this material to the immune system should be investigated before applying to the clinical situations.
ACKNOWLEDGEMENT
The authors would like to acknowledge the Biomedical Engineering Institute, Chiang Mai University, Advanced Manufacturing & Management Technology Research Center (AM2Tech), Department of Industrial Engineering, Faculty of Engineering, Chiang Mai University,as well as all staffs at both laboratory units for their helps and supports. This research was funded by the Faculty of dentistry, Chiang Mai University and The Research Assistant Scholarships from graduate school, Chiang Mai University, Chiang Mai, Thailand.
REFERENCES
Abdelwahab, M.A., Flynn, A., Chiou, B.-S., Imam, S., Orts, W., and Chiellini, E. 2012. Thermal, mechanical and morphological characterization of plasticized PLA–PHB blends. Polymer Degradation and Stability. 97: 1822-1828.
Bhaskar, B., Owen, R., Bahmaee, H., Wally, Z., Sreenivasa Rao, P., and Reilly, G. C. 2018. Composite porous scaffold of PEG/PLA support improved bone matrix deposition in vitro compared to PLA-only scaffolds. Journal of Biomedical Materials Research Part A. 106: 1334-1340.
Buckwalter, J.A., Glimcher, M.J., Cooper, R.R., and Recker, R. 1996. Bone biology. I: Structure, blood supply, cells, matrix, and mineralization. Instructional Course Lectures. 45: 371-386.
Chen, F.M. and Liu, X. 2016. Advancing biomaterials of human origin for tissue engineering. Progress in Polymer Science. 53: 86-168.
Chieng, B.W., Ibrahim, N.A., Yunus, W.M.Z.W., and Hussein, M.Z. 2013. Plasticized poly(lactic acid) with low molecular weight poly(ethylene glycol): Mechanical, thermal, and morphology properties. Journal of Applied Polymer Science. 130: 4576-4580.
DeStefano, V., Khan, S., and Tabada, A. 2020. Applications of PLA in modern medicine. Engineered Regeneration. 1: 76-87.
Elsawy, M. A., Kim, K.-H., Park, J.-W., & Deep, A. 2017. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renewable and Sustainable Energy Reviews. 79: 1346-1352.
Gardin, C., Ricci, S., Ferroni, L., Guazzo, R., Sbricoli, L., De Benedictis, G., and Zavan, B. 2015. Decellularization and delipidation protocols of bovine bone and pericardium for bone grafting and guided bone regeneration procedures. PLoS One. 10: e0132344.
Göpferich, A. 1996. Mechanisms of polymer degradation and erosion. Biomaterials. 17: 103-114.
Gothard, D., Smith, E. L., Kanczler, J.M., Black, C.R., Wells, J.A., Roberts, C.A., and Oreffo, R.O. 2015. In Vivo assessment of bone regeneration in alginate/bone ECM hydrogels with incorporated skeletal stem cells and single growth factors. PLoS One. 10: e0145080.
Grizzi, I., Garreau, H., Li, S., and Vert, M. 1995. Hydrolytic degradation of devices based on poly(DL-lactic acid) size-dependence. Biomaterials. 16: 305-311.
Hashimoto, Y., Funamoto, S., Kimura, T., Nam, K., Fujisato, T., and Kishida, A. 2011. The effect of decellularized bone/bone marrow produced by high-hydrostatic pressurization on the osteogenic differentiation of mesenchymal stem cells. Biomaterials. 32: 7060-7067.
He, H., Yu, J., Cao, J., E,L., Wang, D., Zhang, H., and Liu, H. 2011. Biocompatibility and Osteogenic Capacity of Periodontal Ligament Stem Cells on nHAC/PLA and HA/TCP Scaffolds. Journal of Biomaterials Science-Polymer Edition. 22: 179-194.
Kiani, S., Mousavi, S.M., Shahtahmassebi, N., and Saljoughi, E. 2015. Hydrophilicity improvement in polyphenylsulfone nanofibrous filtration membranes through addition of polyethylene glycol. Applied Surface Science. 359: 252-258.
Ling, L.E., Feng, L., Liu, H.C., Wang, D.S., Shi, Z.P., Wang, J.C., ... Lv, Y. 2015. The effect of calcium phosphate composite scaffolds on the osteogenic differentiation of rabbit dental pulp stem cells. Journal of Biomedical Materials Research Part A. 103: 1732-1745.
Liu, X., Wang, T., Chow, L.C., Yang, M., and Mitchell, J.W. 2014. Effects of Inorganic Fillers on the Thermal and Mechanical Properties of Poly (lactic acid). International journal of polymer science. 2014.
López-Rodríguez, N., López-Arraiza, A., Meaurio, E., and Sarasua, J.R. 2006. Crystallization, morphology, and mechanical behavior of polylactide/poly(ε-caprolactone) blends. Polymer Engineering and Science. 46: 1299-1308.
Mansour, A., Mezour, M.A., Badran, Z., and Tamimi, F. 2017. Extracellular Matrices for Bone Regeneration: A Literature Review. Tissue Engineering Part A. 23: 1436-1451.
Marcos-Campos, I., Marolt, D., Petridis, P., Bhumiratana, S., Schmidt, D., and Vunjak-Novakovic, G. 2012. Bone scaffold architecture modulates the development of mineralized bone matrix by human embryonic stem cells. Biomaterials. 33: 8329-8342.
Marra, K.G., Szem, J.W., Kumta, P.N., DiMilla, P.A., and Weiss, L.E. 1999. In vitro analysis of biodegradable polymer blend/hydroxyapatite composites for bone tissue engineering. Journal of Biomedical Materials Research. 47: 324-335.
Nair, L.S. and Laurencin, C.T. 2006. Polymers as biomaterials for tissue engineering and controlled drug delivery. Advances in Biochemical Engineering / Biotechnology. 102: 47-90.
Nofar, M., Sacligil, D., Carreau, P.J., Kamal, M.R., and Heuzey, M.C. 2019. Poly (lactic acid) blends: Processing, properties and applications. International Journal of Biological Macromolecules. 125: 307-360.
Ozdemir, E. and Hacaloglu, J. 2017. Characterizations of PLA-PEG blends involving organically modified montmorillonite. Journal of Analytical and Applied Pyrolysis. 127: 343-349.
Papadimitropoulos, A., Scotti, C., Bourgine, P., Scherberich, A., and Martin, I. 2015. Engineered decellularized matrices to instruct bone regeneration processes. Bone. 70: 66-72.
Pitjamit, S., Thunsiri, K., Nakkiew, W., Wongwichai, T., Pothacharoen, P., and Wattanutchariya, W. 2020. The possibility of interlocking nail fabrication from FFF 3D printing PLA/PCL/HA composites coated by local silk fibroin for canine bone fracture treatment. Materials. 13: 1564.
Saini, P., Arora, M., and Kumar, M. 2016. Poly (lactic acid) blends in biomedical applications. Advanced Drug Delivery Reviews. 107: 47-59.
Schliecker, G., Schmidt, C., Fuchs, S., and Kissel, T. 2003. Characterization of a homologous series of D,L-lactic acid oligomers; a mechanistic study on the degradation kinetics in vitro. Biomaterials. 24: 3835-3844.
Serra, T., Ortiz-Hernandez, M., Engel, E., Planell, J.A., and Navarro, M. 2014. Relevance of PEG in PLA-based blends for tissue engineering 3D-printed scaffolds. Materials Science & Engineering C-Materials for Biological Applications. 38: 55-62.
Sheth, M., Kumar, R.A., Davé, V., Gross, R.A., and McCarthy, S.P. 1997. Biodegradable polymer blends of poly(lactic acid) and poly(ethylene glycol). Journal of Applied Polymer Science. 66: 1495-1505.
Singhvi, M.S., Zinjarde, S.S., and Gokhale, D.V. 2019. Poly lactic acid: synthesis and biomedical applications. Journal of Applied Microbiology. 127: 1612-1626.
Song, R., Murphy, M., Li, C., Ting, K., Soo, C., & Zheng, Z. 2018. Current development of biodegradable polymeric materials for biomedical applications. Drug Design, Development and Therapy. 12: 3117-3145.
Tatullo, M., Codispoti, B., Paduano, F., Nuzzolese, M., & Makeeva, I. 2019. Strategic Tools in Regenerative and Translational Dentistry. International Journal of Molecular Sciences. 20: 1879.
Wattanutchariya, W., & Thunsiri, K. 2015. Effects of Fibroin Treatments on Physical and Biological Properties of Chitosan/Hydroxyapatite/Fibroin Bone's Scaffold. Applied Mechanics and Materials. 799-800: 488-492.
Younes, H., & Cohn, D. 1988. Phase separation in poly (ethylene glycol)/poly (lactic acid) blends. European Polymer Journal. 24: 765-773.
Zeng, S., Fu, S., Guo, G., Liang, H., Qian, Z., Tang, X., & Luo, F. 2011. Preparation and characterization of nano-hydroxyapatite/poly (vinyl alcohol) composite membranes for guided bone regeneration. Journal of Biomedical Nanotechnology. 7: 549-557.
Zhu, X., Zhong, T., Huang, R., and Wan, A. 2015. Preparation of hydrophilic poly (lactic acid) tissue engineering scaffold via (PLA)-(PLA-b-PEG)-(PEG) solution casting and thermal-induced surface structural transformation. Journal of Biomaterials Science-Polymer Edition. 26:1286-1296.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Wassanai Wattanutchariya1, and Kullapop Suttiat2,3,4 *
1 Department of Industrial Engineering, Faculty of Engineering, Chiang Mai University, Chiang Mai, Thailand 50200
2 Biomedical Engineering Institute (BMEI), Chiang Mai University, Chaing Mai, Thailand 50200
3 Graduate School, Chiang Mai University, Chiang Mai, Thailand 50200
4 Department of Prosthodontics, Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand 50200
Corresponding author: Kullapop Suttiat, E-mail: kullapop@hotmail.com
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: May 14, 2021;
Revised: September 13, 2021;
Accepted: September 21, 2021

