
Chemical Constituents from Leaves of Gardenia sootepensis and Pseudomussaenda flava Biological Activity and Antioxidant Activity
Nichthima Warinthip, Boonsom Liawruangrath, Surapol Natakankitkul, Teeraboon Pojanakaroon, Narabhats Rannurags, Stephen G. Pyne, and Saisunee Liawruangrath*Published Date : 2022-01-21
DOI : https://doi.org/10.12982/CMUJNS.2022.004
Journal Issues : Number 1, January-March 2022
Abstract The chemical constituents of hexane and ethyl acetate extracts of Gardenia sootepensis (G. sootepensis) and ethyl acetate extract of Pseudomussaenda flava (P. flava) were analyzed for the first time using GC and GC-MS. For the hexane extract of G. sootepensis: Nineteen compounds were identified constituting of 74.70% of the total chromatographical fraction components. The principle compounds were 9, 12, 15-octadecatrienoic acid (23.10%) and squalene (16.50%). For the ethyl acetate extract of G. sootepensis: Sixteen compounds were identified. The main compounds were octadecane (15.40%) and eicosane (14.50%). The ethyl acetate extract of P. flava: Nine compounds were identified. The founded compounds were squalene (21.20%) and 9, 12, 15-octadecatrienoic acid (17.40%). The in vitro antibacterial activity of the leave extracts in various solvents against four bacterial strains were investigated. The hexane and ethyl acetate extracts of G. sootepensis were found to possess antibacterial activity against S. aureus and S. pyogenes with the MIC value of 10 mg/mL respectively. The hexane and ethyl acetate extracts of G. sootepensis showed significant cytotoxicity against NCI-H187 cell lines with the IC50 values of 2.25 and 2.21 µg/mL respectively. But the extracts were non-cytotoxic to MCF-7 cell line. The results revealed that all the medicinal plant extracts possessed antioxidant activity. The ethyl acetate extract of G. sootepensis and P. flava exhibited the highest antioxidant activity with the IC50 value of 6.36 ± 0.02 mg/mL. and 9.74 ± 0.09 mg/mL.
Keywords: Antioxidant activity, Biological activity, Chemical constituents, Gardenia sootepensis, Pseudomussaenda flava, Leaves extracts
Citation: Warinthip, N., Liawruangrath, B., Natakankitkul, S., Pojanakaroon, T., Rannurags, N., Pyne, S.G., and Liawruangrath, S. 2022. Chemical Constituents from Leaves of Gardenia sootepensis and Pseudomussaenda flava Biological Activity and Antioxidant Activity. CMU J. Nat. Sci. 21(1): e2022004.
INTRODUCTION
The use of medicinal plants is important throughout the world, especially in traditional or alternative medicine. In underdeveloped or developing countries, medicines derived from plants are important weapons against serious diseases. Traditional medicine has enabled the treatment of common illnesses in approximately 60 to 80% of the world population. The Rubiaceae family is a large diversity of substances such as iridoids, indole alkaloids, anthraquinones, terpenoids, flavonoids and other phenolic derivatives.
In this research, Gardenia sootepensis Hutchinson and Pseudomussaenda flava Verdc were used for this study, because these two plants are used for traditional medicine. They also possessed many biological activities.
Gardenia sootepensis Hutchinson, grows only in the northern part of Thailand. It is 7-10 m tall and often with gelatinous secretion, branches with both developed and shortened internodes. The leaves are opposite; petiole 0.6-1.2 cm and puberulent or tomentulose. The flowers are pseudoaxillary usually near branch apices solitary; peduncle 1-1.5 cm puberulent. The corolla is yellow or white, salverform. The fruits are ellipsoid or ellipsoid-oblong, puberulent, smooth or with 5 or 6 longitudinal lines or very weak ridges, leathery to hard. Gardenia sootepensis have been used in folk medicine for treatment of blood congestion and swelling [Tao, 1753].
The biological activities from an ethyl acetate of the apical buds extract of Gardenia sootepensis were found to be cytotoxic. The isolation and identification of five new 3,4-secocycloartane triterpenes, sootepins and four known compounds were evaluated for cytotoxic activity against human breast (BT474), lung (CHAGO), liver (Hep G2), gastric (KATO-3), and colon (SW-620) cancer cell lines [Nuanyai, et al. 2009]. The biological activities of an ethyl acetate from leaves and twigs extract of Gardenia sootepensis. were isolated two new 3,4-seco-cycloartane triterpenes. The 3, 4-seco-cycloartane triterpenes have biological activities, such as cytotoxic and anti-HIV-1 effects [Song, et al. 2016].
Pseudomussaenda flava Verdc is a fabulous evergreen tropical shrub and large white floral sepals in palest yellow-white and small yellow corollas. The flowers are creamy yellow with white bracts and also produce a faint perfume. The height is 4-8 feet, it grows partial to full sun, moisture and freely draining soil. It is tolerating more dryness and cooler temperatures. This plant blooms all year round but especially well during the warm summer months [Arno, 2013].
The biological activities from the methanol extracts of the leaves, flowers and stems/barks of P. flava showed more inhibition activity against Bacillus subtilis with the zone of inhibition of 15, 20.0 and 9.0 mm, respectively. The ethyl acetate extracts of leaves and stems/barks showed more inhibition activity against Bacillus subtilis with the zone of inhibition of 10.0 and 7.0 mm, respectively [Abdullah, et al., 2011].
A new cycloartane-type saponin and two new monoterpenoid glucoindole alkaloids were isolated from the methanol extracts of an aerial parts of P. flava. All isolated compounds were evaluated for antiprotozoal activities against Leishmania donovani Promastigote, L. donovani Amastigote, L. donovani Amastigote/THP1 cells and Trypanosoma brucei brucei. 5(S)-5-carboxystrictisidine showed good antitrypasonomal activity with IC50 and IC90 values of 13.7 and 16.6 µM compared to IC50 and IC90 values of 13.06 and 28.99 µM. using the difluoromethylornithine (DFMO) as a positive control [Mohamed, et al., 2016].
Five new triterpenoid saponins were isolated from the methanol extracts of an aerial parts of P. flava. Compounds 1–5 were evaluated for their antiprotozoal activities and cannabinoid and opioid receptor binding affinities. Heinsiagenin A 3-O-[α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranosyl-(1→2)]-β-D-glucopyranoside showed potent antitrypanosomal activity with an IC50 value of 8.80 μM. Compounds 2–4 showed highly potent antitrypanosomal activity with IC50 values of 2.57, 2.61 and 2.84 μM, respectively and IC90 values of 3.56, 3.36, and 4.35 μM, respectively while compound 5 showed no antitrypanosomal activity within the tested concentrations range (0.32–8.23 μM) compared to IC50 and IC90 values of 13.06 and 28.99 μM, respectively, using DFMO as the positive control [Mohamed, et al., 2015].
In present study is focused towards analysis of chemical constituents, biological activity and antioxidant activity of the leaves extract from Gardenia sootepensis and Pseudomussaenda flava. (Figure 1, Figure 2)

Figure 1. Gardenia sootepensis Hutchinson.

Figure 2. Pseudomussaenda flava Verdc.
MATERIALS AND METHODS
Chemicals and reagents
All chemicals are analytical reagent grade (AR grade). Ethyl acetate, n-hexane, dichloromethane, ethyl alcohol, methyl alcohol, sulfuric acid, petroleum ether, acetic anhydride and hydrochloric acid are purchased from RCI Labscan Limited (Bangkok, Thailand). Sodium hydroxide, sodium carbonate, anhydrous sodium sulphate, potassium hydroxide, potassium iodide, aluminium chloride, and ferric chloride are purchased from Merck (Darmstadt, Germany). Chloroform and ammonia are purchased from BDH, England.
Plant materials
Two species of Rubiaceae family plants were selected for this study. The fresh leaves of Gardenia sootepensis Hutchinson and Pseudomussaenda flava Verdc. were collected from the medicinal plants garden, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand, in February, 2017.
The plant materials were identified by a botanist as to ascertain the correct identity of the material. The voucher specimen numbers of G. sootepensis (N. Warinthip 05) and P. flava (N. Warinthip 03) were deposited at the Herbarium of the Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand.
Samples preparation
The fresh leaves of all medicinal plants were washed with distilled water and dried in a hot air oven at 45°C for 24 h. then the dried leaves were ground and 500 g of each plant powder were macerated in 1,000 mL of 95% ethanol for one week at room temperature with continuous shaking. The extract was filtered through Whatmann filter paper No.1. The filtrate was evaporated to dryness using a rotary evaporator and weighed. The percentage yield of the extract was obtained. Then ten grams of the ethanolic extract was partitioned with hexane, ethyl acetate or dichloromethane respectively. The liquid-liquid extraction are shown in Figure 3. The hexane and ethyl acetate extracts of the leaves from G. sootepensis and P. flava were taken for analysis.
Analysis of the chemical constituents from leaves of G. sootepensis and P. flava by using gas chromatography (GC-FID) and gas chromatography-mass spectrometry (GC-MS)
The hexane and ethyl acetate extracts of G. sootepensis and the ethyl acetate extract of P. flava were analyzed on a GC 6890N Agilent Technology gas chromatography (FID) equipped with a HP5-MS 30 m × 0.25 mm ID × 0.25 µL thickness column. The analytical conditions were the oven temperature was programmed from 50°C (0 min) →5°C/min→300°C (10 min). Injection volume was 1.0 µL. Sample were injected automatically by splitting and the split ratio was 2:1. Injector temperature was 250°C. Helium was used as carrier gas with constant flow rate of 20.0 mL/min. Detector temperature was 280°C. Total runtime was 60 min.
The GC-MS analysis was performed on an Agilent MSD 5975C mass selective detector under the same condition as for GC. Significant quadrupole MS operating parameter: interface temperature 230°C; electron impact ionization at 70 eV with scan mass rang of 50-550 m/z at sampling rate of 1.0 scan/s, carrier gas He with constant flow rate of 1.0 mL/min and the split ratio was 2:1.
The identification of the sample components was accomplished by comparative of their mass spectra (NIST and NISTREP) with corresponding data of authentic compound or published spectra.
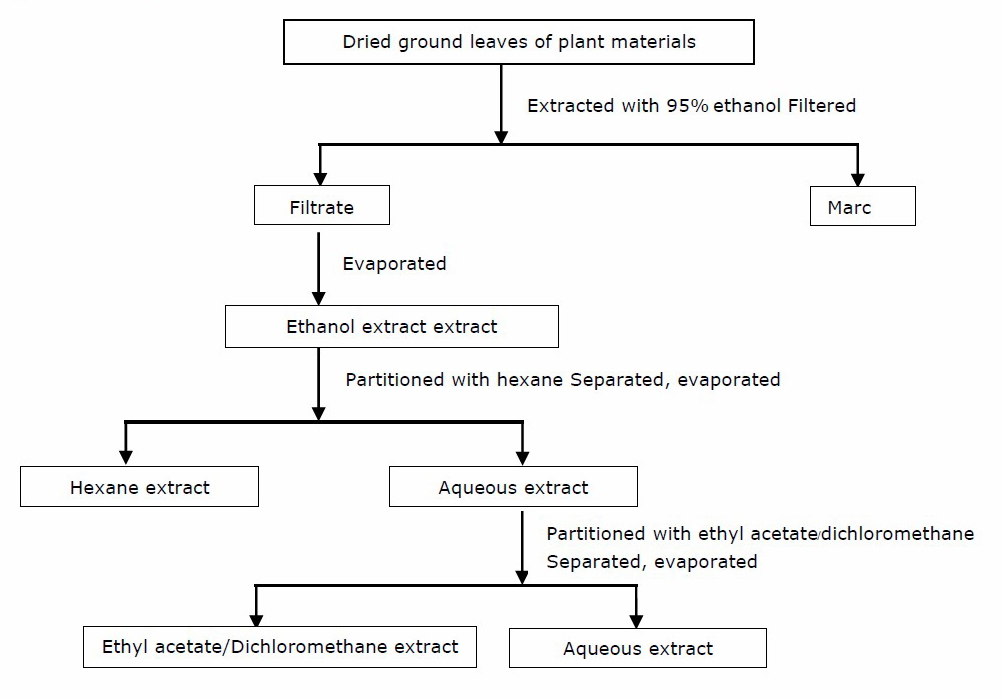
Figure 3. Liquid-liquid extraction of the plant materials
Evaluation of antibacterial activities
Agar disc diffusion and blood agar disc diffusion were used as screening tests to evaluate antibacterial property of crude extracts of Rubiaceae plants based on standard protocol [Zaidan et al., 2005]. Four bacteria was used as bacteria strains and obtained from the Central Diagnostic Laboratory, Faculty of Medicine, Chiang Mai University. Staphylococcus aureus (ATCC 25923), Streptococcus pyogenes (gr.A) (blood agar), Pseudomonas aeruginosa (ATCC 27853) and Escherichia coli (ATCC 25922) were tested. The bacterial was suspended to obtain turbidity visually comparable which has been adjusted to 0.5 McFarland. The bacteria was spread using a sterile cotton bud on a nutrient agar. The sterile paper discs size 5 mm. (Whatman no.1) were placed on the surface of agar plate. The crude extracts which serial dilutions (v/v) were prepared 50 µL of each dilution was poured on each paper disc in order. And then, agar plates were incubated at 37°C for 24 h under cultivation conditions. The diameter of inhibition zone was measured and reported in the scale of millimeter (mm). The MIC value was determined as the lowest concentration of the sample which inhibits the growth of bacteria. Norfloxacin was used as positive control and solvent extracts were used as negative control.
Cytotoxic activities
The cytotoxicities of the extracts of Rubiaceae plants were determined against the human breast cancer (MCF-7) and small cell lung cancer (NCI-H187) cell lines using the Resazurin microplate assay (REMA) [O’Brien et al., 2000]. In 384-well plates total volume was 50 μL which was consisted of 5μL of the crude extracts serially diluted in 5% DMSO and 45 μL of cell suspension. The plate was incubated at 37°C, 5% CO2 in an incubator. After the incubation, 3 days of MCF-7 and 5 days of NCI-H187, the resazurin solution was added and the plates were incubated at 37°C for 4 hours. The fluorescence was measured using a multi-detection microplate reader (Molecular Devices, USA) at the excitation 530 nm and emission 590 nm. Tamoxifen, doxorubicin and ellipticine were used as positive controls and 0.5% DMSO was used as negative control. IC50 indicated the concentration effected 50% reduction in cancer cell line growth.
Antioxidant activity
DPPH radical scavenging assay
The free radical scavenging activity of the extract of plant materials was determined according to the DPPH method with some modifications. All fractions were prepared in the concentration of 2.0, 5.0, 10.0, 15.0, 20.0, 25.0 and 30.0 mg/mL. Twenty microliters of each sample was added to 180 µL of DPPH in EtOH in a 96-well microtiter plate. After incubation for 30 min in the dark, the absorbance of each well was measured at 520 nm spectrophotometrically (spectrophotometer: Multimode detector, Beckman Coulter DTX 880, USA). The DPPH solution was used as a negative control. Trolox and ascorbic acid were used as reference standards in the concentration range 0.01-0.5 mg/mL. Triplicate determinations were performed. The percentage of DPPH scavenging activity was using the follower equation:
[(Ac-As)/Ac] × 100
Where Ac is the absorbance of the control and As is the absorbance of the sample. The IC50 values denote the concentration of the sample which is required to scavenging 50% of DPPH free radicals [Keawsa-ard et al., 2005].
RESULTS AND DISCUSSION
Analysis of the chemical constituents from the leaves extract of G. sootepensis and P. flava by using GC (FID) and GC-MS
The composition of the hexane and ethyl acetate extracts of G. sootepensis and P. flava were analyzed by GC (FID) and GC-MS. Nineteen compounds of the hexane extract of G. sootepensis were identified, constituting 74.70% of the total chromatographical fraction components. Sixteen compounds of the ethyl acetate extract of G. sootepensis were identified, collectively accounting for 99.40% of the gas chromatographical components. Nine compounds of the ethyl acetate extract of P. flava were identified accounting for 67.20% of the total fraction components. The compounds of each fraction were identified by comparing their retention indices (RI) relative to n-hexane indices on HP-5 column and by a comparison of mass spectra from libraries (Wiley 7n.l, NIST and NISTREP). Results are shown in Table 1-3.
The typical compounds of the hexane extract of G. sootepensis were 9,12,15-octadecatrienoic acid (23.10%), squalene (16.50%), 3,7,11,15-tetramethyl-2-hexadecen-1-ol (8.80%), linoleic acid: ethyl ester (6.50%), n-hexadecanoic acid (6.20%), Octadecanoic acid (2.9%) and eicosane (2.1%). The minor compounds were octadecane (1.6%), docosane (1.6%) and 1-methylbicyclo (321) octane (1.2%).
The principle compounds of the ethyl acetate extract of G. sootepensis were octadecane (15.4%), eicosane (14.5%), octacosane (11.0%), hexadecane (8.5%), tetracosane (7.4%), n-hexadecanoic acid (5.0%), nonadecane (4.6%), phenol (4.5%), 2-methyl-7-nonadecane (4.1%), 2-tetradecanol (2.5%) and 2-chroropropionic acid (2.4%).
The founded compounds of the ethyl acetate extract of P. flava were squalene (21.2%), 9,12,15-octadecatrienoic acid (17.4%), 5-mrthyl-2-phenylinololizine (9.5%), hexadecenoic acid (7.0%), linoleic acid: ethyl ester (3.6%), 5-eicosane (2.6%), hexadecane (2.3%), pentadecane (2.1%) and α-tocopherol (1.5%).
Table 1. Chemical constituents of the hexane extract of G. sootepensis.
|
|
Compound |
RT |
Area |
RI |
RI |
ID |
References |
|
|
(min) |
(%) |
(exp) |
(lit) |
|
|
|
|
1 |
Phenol |
19.87 |
0.7 |
980 |
980 |
RI, MS |
Adams, 2001 |
|
2 |
Hexadecane |
21.89 |
0.7 |
1599 |
1600 |
RI, MS |
Adams, 2001 |
|
3 |
Cyclohexane |
26.08 |
0.3 |
- |
- |
MS |
- |
|
4 |
Octadecane |
26.22 |
1.6 |
1801 |
1800 |
RI, MS |
Adams, 2001 |
|
5 |
Isopropyl myristate |
26.78 |
0.5 |
- |
- |
MS |
- |
|
6 |
4-hexen-1-ol |
27.03 |
0.6 |
- |
- |
MS |
- |
|
7 |
n-Hexadecanoic acid |
29.49 |
6.2 |
1960 |
1960 |
RI, MS |
Kahriman, et al, 2012 |
|
8 |
8-Cyclohexadecen-1-one |
29.97 |
0.4 |
- |
- |
MS |
- |
|
9 |
Eicosane |
30.17 |
2.1 |
2000 |
2000 |
RI, MS |
Adams, 2001 |
|
10 |
3,7,11,15-tetramethyl-2-hexadecen-1-ol¢ |
32.26 |
8.8 |
- |
- |
MS |
- |
|
11 |
9,12-Octadecadienoic acid |
32.62 |
0.9 |
- |
- |
MS |
- |
|
12 |
1-Methylbicyclo (321) octane |
32.98 |
1.2 |
- |
- |
MS |
- |
|
13 |
Linoleic acid, ethyl ester (ethyl linolenate) |
32.13 |
6.5 |
2163 |
2162 |
RI, MS |
Kahriman, et al, 2012 |
|
14 |
9,12,15-Octadecatrienoic acid |
33.25 |
23.1 |
- |
- |
MS |
- |
|
15 |
Octadecanoic acid |
33.71 |
2.9 |
- |
- |
MS |
- |
|
16 |
Docosane |
33.78 |
1.6 |
2201 |
2200 |
RI, MS |
Adams, 2001 |
|
17 |
Nonadecanoic acid |
37.05 |
0.7 |
- |
- |
MS |
- |
|
18 |
Unknown |
40.19 |
0.4 |
- |
- |
- |
- |
|
19 |
Squalene |
43.49 |
16.5 |
2790 |
2790 |
RI, MS |
Zito, et al, 2010 |
|
|
|
Total |
74.7 |
|
|
|
|
Note: RT: retention time, RI (exp): retention indices on DB-5 MS column; relative to n-alkane, RI (lit): values from literature data, ID: methods of identification MS; comparison of the mass spectrum with MS libraries; RI of literature
Table 2. Chemical constituents of the ethyl acetate extract of G. sootepensis.
|
|
Compound |
RT |
Area |
RI |
RI |
ID |
References |
|
|
(min) |
(%) |
(exp) |
(lit) |
|
|
|
|
1 |
Phenol |
19.87 |
4.5 |
980 |
980 |
RI, MS |
Adams, 2001 |
|
2 |
Hexadecane |
21.88 |
8.5 |
1600 |
1600 |
RI, MS |
Adams, 2001 |
|
3 |
2-Tetradecanol |
26.07 |
2.5 |
- |
- |
MS |
Adams, 2001 |
|
4 |
Octadecane |
26.22 |
15.4 |
- |
- |
MS |
- |
|
5 |
n-Hexadecanoic acid |
29.44 |
5.0 |
1969 |
1968 |
RI, MS |
Kahriman, et al, 2012 |
|
6 |
2-Methyl-7-nonadecene |
30.05 |
4.1 |
- |
- |
MS |
- |
|
7 |
Eicosane |
30.17 |
14.5 |
2000 |
2000 |
RI, MS |
Adams, 2001 |
|
8 |
Unknown |
32.69 |
1.0 |
- |
- |
- |
- |
|
9 |
2-Chloropropionic acid |
33.68 |
2.4 |
- |
- |
MS |
- |
|
10 |
Octacosane |
33.78 |
11.0 |
2801 |
2800 |
RI, MS |
Adams, 2001 |
|
11 |
Unknown |
34.07 |
10.7 |
- |
- |
- |
- |
|
12 |
Tetracosane |
37.11 |
7.4 |
- |
- |
MS |
- |
|
13 |
Unknown |
39.48 |
2.0 |
- |
- |
- |
- |
|
14 |
Nonadecane |
40.19 |
4.6 |
- |
- |
MS |
- |
|
15 |
Unknown |
43.06 |
3.3 |
- |
- |
- |
- |
|
16 |
Unknown |
43.49 |
2.5 |
- |
- |
- |
- |
|
|
|
Total |
99.4 |
|
|
|
|
Note: RT: retention time, RI (exp): retention indices on DB-5 MS column; relative to n-alkane, RI (lit): values from literature data, ID: methods of identification MS; comparison of the mass spectrum with MS libraries; RI of literature
Table 3. Chemical constituents of the ethyl acetate extract of P. flava.
|
|
Compound |
RT |
Area |
RI |
RI |
ID |
References |
|
|
(min) |
(%) |
(exp) |
(lit) |
|
|
|
|
1 |
Hexadecane |
26.22 |
2.30 |
1600 |
1600 |
RI, MS |
Adams, 2001 |
|
2 |
Hexadecanoic acid |
30.08 |
7.00 |
1969 |
1968 |
RI, MS |
Kahriman, et al, 2012 |
|
3 |
5-Eicosane |
30.17 |
2.60 |
2000 |
2000 |
RI, MS |
Adams, 2001 |
|
4 |
Linoleic acid, ethyl ester |
33.13 |
3.60 |
2162 |
2161 |
RI, MS |
Kahriman, et al, 2012 |
|
5 |
9,12,15-Octadecatrienoic acid |
33.25 |
17.40 |
- |
- |
- |
- |
|
6 |
Pentadecane |
33.78 |
2.10 |
2500 |
2501 |
RI, MS |
Adams, 2001 |
|
7 |
Squalene |
43.48 |
21.20 |
2790 |
2792 |
RI, MS |
Zito et al, 2010 |
|
8 |
α-Tocophenol |
47.47 |
1.50 |
3149 |
3149 |
RI, MS |
NIST |
|
9 |
5-Methyl-2-phenylindodizine |
49.68 |
9.50 |
- |
- |
MS |
- |
|
|
|
Total |
67.20 |
|
|
|
|
Note: RT: retention time, RI (exp): retention indices on DB-5 MS column; relative to n-alkane, RI (lit): values from literature data, ID: methods of identification MS; comparison of the mass spectrum with MS libraries; RI of literature
NIST: http://www.nist.gov/srd/nist1a.cfm
Squalene [Azalia Lorano-Grande et al. 2018] was the main constituent in the hexane extract of G. sootepensis (16.5%) and the ethyl acetate extract of P. flava (21.2%). Squalene is a linear triterpene. The bioactive property of squalene are cardioprotector, antioxidant, antibacterial, anticancer and detoxifying. The application of squalene are: (I) intravenous injection, oral consumption to cholesterol control, (II) topical emulsions, oral administration cream topical, oral medication, (III) preventive and chemotherapeutic substance: drugs and vaccines (emulsion, conjugates) and (IV) nutritional supplement.
Hexadecenoic acid and linoleic acid, ethyl ester are present in the hexane extract of G. sootepensis (Hex. 6.2%, Lin. 6.5%) and the ethyl acetate extract of P. flava (Hex. 7.0%, Lin. 3.6%). Hexadecenoic acid and linoleic acid, ethyl ester are lipidic derivatives, which are used as flavor and fragrance agents, essential ingredient for making soap and shampoo. They are antioxidants and cancer preventive agent [Shoh et al., 2015].
α-Tocopherol (vitamin E) is found only in the ethyl acetate extract of P. flava (15%). This compound is used for cosmetic production such as moisturizing face cream, body lotion etc. It is also used as vitamin for health supplement.
On the other significant compounds are found in the leave fractions of G. sootepensis and P. flava such as docosane (1.6%), octacosane (11.0%), tetracosane (7.4%), eicosane (14.5%).
The chemical structures of some important compounds: squalene, hexadecenoic acid, linoleic acid (ethyl ester) and α-tocopherol are presented in Figure 4.
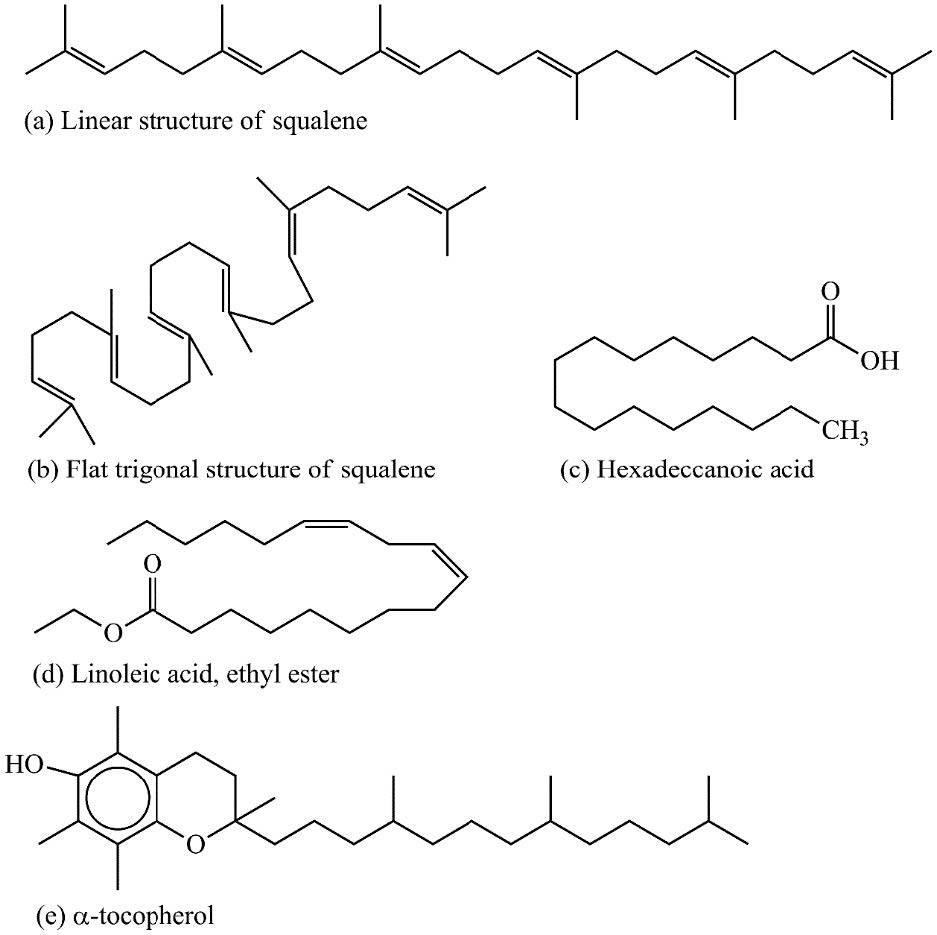
Figure 4. Interaction results using STITCH DB.
Antibacterial activity
The in vitro antibacterial activity of the extracts against four bacterial strains were investigated. The results are shown in Figure 5. The hexane extract of G. sootepensis [GSH] was found to possess antibacterial activity against Gram-positive S. aureus and S. pyogenes with inhibition zones of 8.6 mm, 6.2 mm with the MIC value of 10 mg/mL. The ethyl acetate extract of G. sootepensis [GSEa] was found to possess antibacterial activity against S. aureus and S. pyogenes with inhibition zones of 6.4 mm, 7.8 mm respectively with the MIC value of 10 mg/mL. The ethyl acetate and dichloromethane extracts of P. flava [PFEa] and [PFD] did not inhibit antibacterial activity against S. aureus, S. pyogenes, P. aeruginosa and E. coli. The [GSH] and [GSEa] did not possess antibacterial activity against Gram-negative E. coli and P. aeruginosa.
The methanol extracts of the leaves, flowers and stem or barks of P. flava had been reported to possess antibacterial activity against Bacillus subtilis with the zone of inhibition of 15.0, 20.0 and 9.0 mm respectively. But the ethyl acetate extracts of leaves and stem or barks showed more inhibition activity against Bacillus subtilis with the diameter of inhibition zone of 10.0 and 7.0 mm respectively. [Abdullah, et al., 2011].
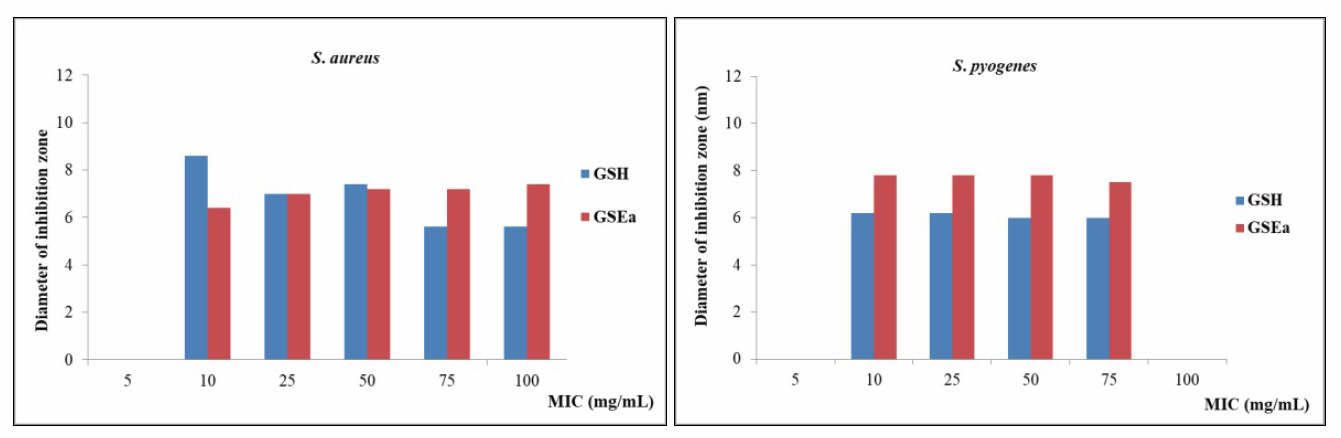
Figure 5. Antibacterial activity of the leaves extracts of [GSH] and [GSEa].
Anticancer activity
The cytotoxicity of the extracts of G. sootepensis against two cancerous MCF-7 (human breast cancer) and NCI-H187 (small cell lung cancer) were determined using the Resazurin microplate assay (REMA). The results are shown in Figure 6. The hexane and ethyl acetate extracts of G. sootepensis inhibited the growth of the NCI-H187 cell lines with IC50 values of 2.25 and 2.21 μg/mL. But both extracts were non-cytotoxic to MCF-7 cell lines respectively.
Sootependial, a new compound which was isolated from the methanol apical buds extract of G. sootepensis and partition with ethyl acetate had been reported to show cytotoxicity against Hep-G2 cell lines with IC50 value of 1.47 μM. [Song, et al. 2016].
The biological activities, the methanol extracts of an aerial parts of P. flava were isolated five new triterpenoid saponins. Compound (1) exhibited strong activity of antitrypanosomal with an IC50 value of 8.80 μM. Compound (2) exhibited highest IC50 values 2.57, followed compound (3) showed IC50 values 2.61 and compound (4) presented IC50 values 2.84 μM of antitrypanosomal activity. [Mohamed, et al., 2015].
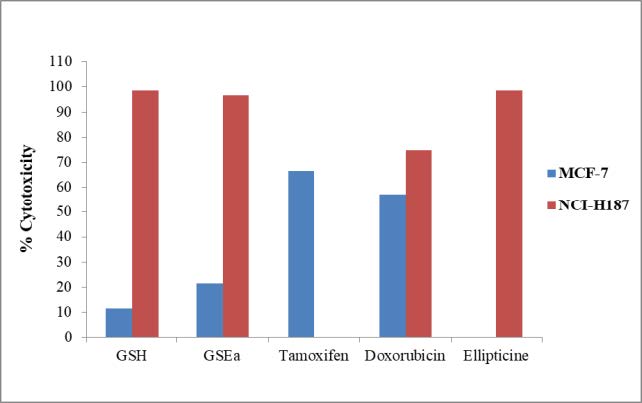
Figure 6. Cytotoxic activities of the leaves extracts of [GSH] and [GSEa].
Antioxidant activity
There is no previous report on the determination of antioxidant activity from G. sootepensis and P. flava extracts. In this work, their antioxidant activity were studied. The DPPH method is a stable free radical, due to the delocalization of the spare electron on the whole molecule. The delocalization on the DPPH• molecule determines the occurrence of a purple colour, with an absorbance band at 520 nm. The DPPH• reacts with a hydrogen donor, the reduced form DPPH(DPPH-H) is generated, the color changes from purple to yellow. Therefore, the absorbance depends on linearly of the antioxidant concentration [Pisoschi and Negulescu, 2011]. Trolox and vitamin C were used as standard antioxidant.
The antioxidant activities of the fractions of plant materials were evaluated using the DPPH assay. The series of standard solutions containing: 0.01-0.3 mg/mL of trolox and 0.01-0.2 mg/mL of vitamin C were prepared. The percentage inhibition of concentration of trolox and vitamin C are shown in Figure 7. The standard curves of Trolox and vitamin C were constructed by plotting the percentage inhibitions and the concentration of standard solutions linear equations (y = 420.88x + 13.26, R2 = 0.9964 and y = 496.16x + 12.375, R2 = 0.9925, respectively).
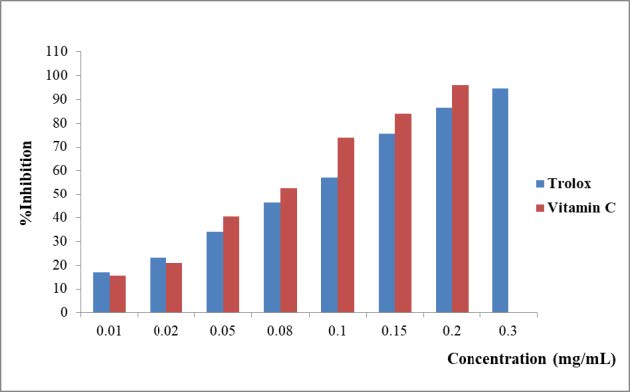
Figure 7. The percentage inhibition of Trolox and vitamin C solution by DPPH method.
G.sootepensis, the ethyl acetate extract [GSEa] showed the strong antioxidant activity with the IC50 values of 6.36 mg/mL while the hexane extract [GSH] showed weak antioxidant activity with the IC50 values of 32.08 mg/mL.
P. flava, the ethyl acetate extract [PFEa] showed the high antioxidant activity with the IC50 values 9.74 mg/mL and the dichloromethane extract [PFD] showed the moderate antioxidant with the IC50 values 20.50 mg/mL. The results are shown in Table 4.
Table 4. The antioxidant activities of G. sootepensis and P. flava by DPPH method.
|
Samples |
IC50 (mg/mL) |
|
(GSEa) (GSH) |
6.36 ± 0.02 32.08 ± 0.08 |
|
(PFEa) (PFD) |
9.74 ± 0.09 20.05 ± 0.33 |
|
Trolox |
0.08 ± 0.07 |
|
Vitamin C |
0.05 ± 0.03 |
CONCLUSION
The chemical constituents of hexane and ethyl acetate extracts of G. sootepensis and ethyl acetate extract of P. flava were analyzed for the first time using GC and GC-MS. The main compounds of hexane extract of G. sootepensis were squalene, n-hexadecanoic acid and ethyl linolenate. The founded compounds of ethyl acetate extract of G. sootepensis were n-hexadecanoic acid, phenol and some alkane. The typical compounds of P. flava were squalene, ethyl linolenate and α-Tocopherol. The important functions of squalene had been revealed the antioxidant agent in the skin and cancer inhibitor antitumor. The role of n-hexadecanoic acid, ethyl linolenate and α-Tocopherol had been reported to possess antioxidant and antibacterial activities. The G. sootepensis showed antibacterial activity against S. aureus and S. pyogenes. The methanol and ethyl acetate extracts of P. flava had been reported to possess antibacterial activity against Bacillus subtilis. The hexane and ethyl acetate extracts of G. sootepensis exhibited significant cytotoxicity against NCI-H187 cell line. Sootependial (a new compound which was isolated from apical buds extract of G. sootepensis) had been reported to show cytotoxicity against Hep-G2 cell lines. The ethyl acetate extract of G. sootepensis possessed the highest antioxidant activity with IC50 value of 6.36 ± 0.02 mg/mL. The ethyl acetate extract of P. flava showed high antioxidant activity with IC50 value of 9.74 ± 0.09 mg/mL. There is no previous report on antioxidant activity.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the Graduate School and Faculty of Pharmacy, Chiang Mai University for partial support and Central Diagnostic Laboratory, Faculty of Medicine, Chiang Mai University for their very kind partial support. Additionally, this research work was partially supported by Chiang Mai University.
REFERENCES
Abdullah E, Raus RA, Jamal P. 2011. Evaluation of antibacterial activity of flowering plants and optimization of process conditions for the extraction of antibacterial compounds from Spathiphyllum cannifolium leaves. African Journal of Biotechnology. 10: 18679-18689.
Adams R.P., 2001. Identification of essential oils components by gas chromatography/ quadrupole mass spectrometry. 3rd ed. Allured publishing Coporation, Carol Stream, Illinois.
Arno, K. 2013. Growing mussaenda. GardenDrum AU. Retrieved from http://gardendrum.com/2013/05/31/growing-mussaenda/
Azalia Lozano-Grande, M., Gorinstein, S., Espitia-Rangel, E., Darila-Ortiz, G. and Matinez-Ayala, A.L. 2018. Plant sources, extraction methods and uses of squalene. International Journal of Agronomy, 1-13.
Kahriman, N., Yayli, B., Yucel, M., Karaoglu, SA. and Yayli, N. 2012 Chemical constituent and antimicrobial activity of the essential oil from Vicia Dadianorum extracted hydro and microwave distillations, Records of Natural Products, 6: 49-56.
Keawsa-ards, S., Liawruangrath, B., Liawruangrath, S., Teerawutgulrag, A. and Pyne, S.G. 2012. Chemical constituents and antioxidant and biological activities of the essential oil from leaves of Solanum spirale. Natural Product Communication. 7: 955-958.
Mohamed, S.M., Backheet, E.Y., Bayoumi, S.A., Jain, S., Cutler, S.J, Tekwani, B.L., and Ross, S.A. 2015. Potent antitrypanosomal triterpenoid saponins from Mussaenda luteola. Fitoterapia. 107: 114-121.
Mohamed SM, Backheet EY, Bayoumi SA, Ross SA. 2016. Newcycloartane saponin and monoterpenoid glucoindole alkaloids from Mussaenda luteola. Fitoterapia. 110: 129-134.
Nuanyai, T., Sappapan, R., Teerawatananond, T., Muangsin, N. & Pudhom, K. 2009. Cytotoxic 3, 4-seco-Cycloartane Triterpenes from Gardenia sootepensis. Journal of Natural Products. 72: 1161-1164.
O'Brien, J., Wilson, I., Orton, T. and Pognan, F. 2000. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal of Biochemistry. 267: 5421-5426.
Pisoschi AM, Negulescu GP. 2011. Methods for Total Antioxidant activity determination: A Review. Biochemistry and Analytical Biochemistry. ISSN: 2161-1009.
Shoh, A., Singh, T., Vijayvengia, R. 2015. GC-MS analysis of bioactive phytoconstituents from Rumex vesicarius L. International Research Journal of Pharmacy. 6: 269-272.
Song, W.W., Wang XQ. & Li, B. 2016. Two New 3, 4-seco-Cycloartane Triterpenes from Gardenia sootepensis. Helvetica Chimica Acta. 99: 165-168.
Tao, C. 1753. Pavetta Linnaeus. Liantang, ShenZhen, Guangdong. China: 231-234.
Tao, C. 1761. Gardenia. Liantang, ShenZhen, Guangdong. China: 85-88.
Zaidan, M.R.S., Noor Rain, A., Badrul, A.R, Adlin, A., Norazah, A. and Zakiah, I. 2005. In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Tropical Biomedicine. 22: 165-170.
Zito, P., Sajeva, M., Bruno, M., Maggio, A., Rosselli, S., Formisano, C., Senatore, F. 2010. Essential oil composition of stem and fruits of Caralluma europaea N.E. Br. (Apocynaceae). Molecules. 15: 627-638.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Nichthima Warinthip1, Boonsom Liawruangrath1, Surapol Natakankitkul1, Teeraboon Pojanakaroon2, Narabhats Rannurags2, Stephen G. Pyne3, and Saisunee Liawruangrath2 ,4,*
1 Department of Pharmaceutical Sciences, Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand.
2 Department of Chemistry, Faculty of Sciences, Chiang Mai University, Chiang Mai 50200, Thailand.
3 School of Chemistry, Faculty of Sciences, University of Wollongong, Wollongong, NSW 2522, Australia.
4 Center of Excellent in Materials Science and Technology, Chiang Mai University, Chiang Mai 50200, Thailand.
Corresponding author: Saisunee Liawruangrath, E-mail: scislwrn@gmail.com
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: May 21, 2021;
Revised: September 5, 2021;
Accepted: September 9, 2021

