
Biology and Life-Table Parameters of Fall Armyworm, Spodoptera frugiperda on Three Maize Cultivars Grown in Thailand
Sothearith Hong, Manas Titayavan, Suphannika Intanon and Panisara Thepkusol*Published Date : 2022-01-21
DOI : https://doi.org/10.12982/CMUJNS.2022.001
Journal Issues : Number 1, January-March 2022
Abstract Fall armyworm, Spodoptera frugiperda has become a new invasive species in Thailand that mainly affects maize. There has been limited research on the baseline biological aspects of this insect under controlled conditions. Our objective was to detail the biological parameters of S. frugiperda reared on maize (Zea mays L.) cultivars, field maize, sweet maize and waxy maize under controlled conditions at 30 ± 2°C, 55 ± 5% RH and a 12-hour photoperiod. Results suggest that larvae develop through six instars on all the maize cultivars. Significant effects (P < 0.05) of the host plant were found in the duration of larval stage 10.83 ± 0.14, 11.15 ± 0.15 and 11.28 ± 0.05 days when fed with sweet maize, waxy maize and field maize, respectively. More than 70.5% of them transformed into the pupal stage. The life cycle duration lasted 28.11 ± 0.40 days (field maize), 27.16 ± 0.37 days (sweet maize) and 28.41 ± 0.34 days (waxy maize). Significant differences among host plants were not observed for the different development durations. S. frugiperda exhibited a type I survivorship curve. The highest values of net reproductive rate, R0 (220.41 ± 5.88), innate capacity of increase, rc (0.23 ± 0.001) and finite rate of increase, λ (1.25 ± 0.002) obtained on sweet maize, were not statistically different from other cultivars. The mean generation time (Tc) was significantly different among the 3 maize cultivars, ranging from 26.36 ± 0.43 days on waxy maize to 23.80 ± 0.24 days on sweet maize. Information presented here should greatly expand the understanding of S. frugiperda biology; this understanding can be used to improve the efficiency of management techniques for this critical crop pest.
Keywords: Invasive species, Maize pest, Biological cycle, Reproductive rate, Population growth
Citation: Hong,S., Titayavan, M., Intanon, S., and Thepkusol, P. 2022. Biology and life table parameters of fall armyworm, Spodoptera frugiperda on three maize cultivars grown in Thailand. CMU J. Nat. Sci. 21(1): e2022001.
INTRODUCTION
The fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), is a polyphagous insect and pest native to tropical and subtropical regions of the United States (Luginbill, 1928); it created a huge infestation throughout the Southeast and along the Atlantic coast during the 1970s (Sparks, 1979). In recent years, S. frugiperda has been reported for the first time in Southern India (CABI, 2018) and has continued to spread across Asia, including Sri Lanka, Bangladesh, Myanmar and Thailand (CABI, 2019). In Thailand, the presence of S. frugiperda has been reported in more than 50 of Thailand's 76 provinces, and is concentrated in 6 western provinces with large maize areas (Isranews, 2020). The larvae of S. frugiperda feed on a wide host range consisting of over 353 species, but have preferences for maize, sorghum, bermudagrass, and cotton (Hardke et al., 2015; Montezano et al., 2018; Capinera, 2001). It has been calculated that S. frugiperda has caused damage up to 75,800 hectares of maize (30.60% of total production in 41 provinces) in Thailand since the start of the infestation (Wareerat, 2019). In addition to its feeding characteristics, S. frugiperda has great mobility, is widespread on several crop species, and has high reproductive potential, which has caused a serious impact not only on the economy and food security, but also makes the pest particularly hard to control (Prasanna et al., 2018).
The development of an adequate management strategy with minimum pesticide use requires basic knowledge of the population dynamics of insect pests. Life tables are an important tool for understanding the changes in insect populations during different developmental stages throughout their life cycle, and for determining key factors of mortality under various environmental conditions (Price et al., 2011; Kakde et al., 2014). Life tables are constructed by a combination of 4 classical parameters: fertility, longevity, the birth rate, and the death rate (Caswell, 1982; Carey, 2001). The net reproductive rate (R0) shows the multiplication rate per generation obtained by the summing of the multiplication between the age-specific survival (lx) and the age-specific fertility (mx) (Deevey, 1947; Southwood and Henderson, 2000). Furthermore, the innate capacity of increase (rc) is defined as the intrinsic rate of natural increase, which is the most useful life table parameter for predicting the potential for population growth under a given environmental condition (Birch, 1948; Laughlin, 1965; Southwood and Henderson, 2000). From a pest management viewpoint, life tables are very important to identifying the most susceptible stage of the pest’s lifecycle and the most opportune periods for controlling them.
To our knowledge, several studies of S. frugiperda biology on different food sources have been carried out (Da Silva et al., 2017; Murúa and Virla, 2004), but there have been no reports in Thailand so far. Therefore, in an effect to contribute useful information for planning and management, this study aimed to detail the effects of three different maize cultivars on S. frugiperda biological characteristics and to identify the parameters of the fertility life table of this species under laboratory conditions with unlimited food supply.
MATERIALS AND METHODS
Description of the study area
Late larval instar of S. frugiperda were originally collected from unsprayed maize fields in the Phitsanulok area during March 2020. Larvae were placed in a 20.5cm (L) x 15cm (W) x 6.5 cm (H) plastic rearing container with 10-20 per container. The containers were kept in a temperature-controlled room, maintained at 30 ± 2°C and 55 ± 5% relative humidity, with a light-dark 12L:12D of artificial photoperiod at the National Biological Control Research Center (NBCRC) (16044'10.5''N 100011'37.0''E). The center is located at Naresuan University in Phitsanulok Province, in northern Thailand. Larvae were reared to the pupal stage on fresh green maize leaves. The pupae were then transferred onto a paper napkin which was placed in a container, and water was then added daily to moisten the paper until eclosion. At eclosion, adult moths were sorted by sex and released into insect breeding cages (30 x 20 x 30 cm) provided with nylon wire screen. These cages contained pieces of paper that allowed the females to rest and to lay eggs. Food was provided via a cotton plug saturated with honey and water mixture (1:9 vol/vol). All trials in the study were conducted at NBCRC.
Biology and life table of S. frugiperda on three maize cultivars
The experiment was installed in a completely randomized design (CRD) to investigate the effects on insect biology of 3 plant resources, field maize (Nakhonsawan-3), sweet maize (Insee-2) and waxy maize (Pacific-1), which are common cultivars commonly grown in Thailand. Each different plant host was directly planted in a planting bag, and on different dates in order to obtain the same growth stages during the experiment. The cultivated plant hosts were maintained in a greenhouse, and were fertilized 10 days after planting with granular commercial chemical fertilizers 46-0-0 (urea fertilizer) at the rate of 174 kg ha-1. Plants were manually hand watered with hoses, and no pesticides were applied. Three weeks after planting (at vegetative stage), the young tender leaves were cut and kept in moist plastic bags for feeding. A single 1-day-old S. frugiperda egg was placed individually in a clear plastic cup (7.9cm (W) x 7.1cm (D) x 5.8cm (H)). For aeration, each cap of the experimental plastic cup was cut and a circular hole of 3 cm in diameter was made at the center and closed with a nylon mesh cloth. Upon hatching, the larva was fed fresh maize leaves. The food was changed daily. Developmental stages were checked daily and the developing insect was observed at each larval ecdysis. Four replicates were run sequentially for a total of 100 eggs tested at each maize cultivar. Mortality of eggs, larvae, pupae, adults, sex ratio, and longevity of each adult were recorded. The experiment was continued until the death of all individual members of each cohort. To obtain sex ratio, a cohort life table was constructed with the headings proposed by Southwood and Henderson (2000). The initial lx was based on the total number of 100 eggs. Statistical analysis of the data from this experiment was by analysis of variance and the F-test. It was followed by the Duncan Multiple Range Test (DMRT) to evaluate all possible pairs of treatment means. All data were analyzed by R-statistics (version 3.6.1) (R Core Team, 2019).
Reproduction and population growth parameters of S. frugiperda
Three pairs of newly emerged male and female adults of S. frugiperda were used to collect innate capacity and finite rate of increase data on field maize, sweet maize and waxy maize cultivars with 3 replications on each maize cultivar respectively. Adult moths were less than 24 hours old. An oviposition cage (50 x 30 x 60 cm) covered with fine nylon mesh for ventilation was used to confine the insects. A clean cotton wick containing a 10% honey solution was placed in the cage to provide food for adults. A 3-week-old maize plant was added to the cage on which the females could lay their eggs. Moths were introduced into the cage and left for 24 hours. The plant provided for oviposition was replaced daily and the number of eggs laid by each female on subsequent days recorded. The observation on fecundity was made daily from the day after emergence up to the death of the last female. As the sex ratio was 1:1, the number of eggs obtained per female was divided by two to get the number of female birth (mx). To obtain the fertility rate, deposited eggs produced on each maize cultivar were maintained until the larvae emerged and the fertility rate of emerged larvae was determined. In this experiment it was assumed that the time interval (t) equals the cohort generation (Tc), and the net reproductive rate (R0) = Σlxmx equals the finite (geometric) rate of increase (λ) = erc. The mean cohort generation, or the mean period elapsing between the birth of parents and the offspring’s birth, was estimated by dividing the log to the base e of R0 by the innate capacity of population increase (rc) = loge R0/ Tc, and Tc = Σlxmx.x/Σlxmx, where x is the age of S. frugiperda in days (Carey, 1993; Southwood and Henderson, 2000).
RESULTS
Biology and life table of S. frugiperda on three maize cultivars
In the laboratory and under the established ambient conditions, the life cycle (egg to adult) of S. frugiperda lasted a mean of 28.11 ± 0.40 days on field maize, 27.16 ± 0.37 days on sweet maize, and 28.41 ± 0.34 days on waxy maize (Table 1). However, there were no statistically significant differences among these means. Egg incubation periods lasted 2.00 ± 0.00, 2.03 ± 0.02, and 2.02 ± 0.01 days on field maize, sweet maize, and waxy maize respectively. Significant effects (P < 0.05) of the host plant were found in the duration of larval stage 11.28 ± 0.05, 10.83 ± 0.14 and 11.15 ± 0.15 days when fed with field maize, sweet maize, and waxy maize, respectively. Significant differences (P < 0.05) were also found in the mean durations of pupal stages: 7.93±0.09 when fed on field maize, 7.57 ± 0.09 on sweet maize and 8.24 ± 0.09 days on waxy maize. The shortest duration both in the larval and pupal stages was observed with sweet maize. There was a slight difference between the sexes in development time of S. frugiperda fed on 3 different maize cultivars. Significant differences (P < 0.05) were found only in the adult male life cycle. The shortest duration of male life cycle was observed with sweet maize.
Table 1. The duration of developmental stages (days) and sexes obtained of Spodoptera frugiperda fed on three maize cultivars at 30 ± 2°C, 55 ± 5 RH and a 12-hour photoperiod.
|
Developmental stage
|
Field maize1/ |
Sweet maize1/ |
Waxy maize1/ |
|||
|
Mean ± SE |
Range |
Mean ± SE |
Range |
Mean ± SE |
Range |
|
|
Egg |
2.00 ± 0.00a |
2 |
2.03 ± 0.02a |
2-3 |
2.02 ± 0.01a |
2-3 |
|
Larval instar: |
|
|
|
|
|
|
|
Instar I |
2.08 ± 0.05a |
2-3 |
2.23 ± 0.18a |
2-3 |
2.03 ± 0.03a |
2-3 |
|
Instar II |
1.64 ± 0.22a |
1-3 |
1.31 ± 0.09a |
1-3 |
1.61 ± 0.20a |
1-4 |
|
Instar III |
1.52 ± 0.13a |
1-2 |
1.40 ± 0.23a |
1-5 |
1.41 ± 0.19a |
1-3 |
|
Instar IV |
1.57 ± 0.12a |
1-3 |
1.51 ± 0.17a |
1-3 |
1.64 ± 0.28a |
1-4 |
|
Instar V |
2.34 ± 0.05a |
1-4 |
2.31 ± 0.15a |
1-3 |
2.11 ± 0.13a |
1-3 |
|
Instar VI |
2.14 ± 0.06a |
2-3 |
2.07 ± 0.01a |
2-4 |
2.35 ± 0.07b |
1-4 |
|
Total larval duration |
11.28 ± 0.05b |
10-16 |
10.83 ± 0.14a |
9-14 |
11.15 ± 0.15ab |
10-14 |
|
Pre-pupae |
1.03 ± 0.02a |
1-2 |
1.04 ± 0.02a |
1-2 |
1.09 ± 0.04a |
1-2 |
|
Pupae |
7.93 ± 0.09b |
7-9 |
7.57 ± 0.09a |
7-9 |
8.24 ± 0.09c |
7-9 |
|
Adult: |
|
|
|
|
|
|
|
Male life cycle |
28.26 ± 0.39b |
24-32 |
26.90 ± 0.37a |
25-31 |
28.61 ± 0.30b |
25-32 |
|
Female life cycle |
27.96 ± 0.41a |
24-32 |
27.42 ± 0.36a |
24-31 |
28.21 ± 0.38a |
22-32 |
|
Sexes |
|
|
|
|
|
|
|
Male |
7.25 ± 2.02a |
|
7.25 ± 2.66a |
|
8.00 ± 1.08a |
|
|
Female |
6.50 ± 2.33a |
|
7.75 ± 1.25a |
|
9.75 ± 2.21a |
|
Note: 1/ Treatment means within a row not followed by the same letter are significantly different (P < 0.05, DMRT)
Reproduction and population growth parameters
Sequential examination of the development of the individuals revealed that the mortality of S. frugiperda occurred sequentially in the successive development stages on each of the host plant cultivars. A relatively high mortality rate was experienced during the egg and the early stages of S. frugiperda, but no mortality occurred on the fourth larval instar. After completing six molts, more than 70.5% of the larvae were transformed into the pupal stage and adult emergence percentages of 89.06%, 85.71% and 92.31% were obtained from field maize, sweet maize and waxy maize, respectively. Sex ratio as the larvae reached the adult stage varied between 0.83:1 (Female:Male) when reared on field maize to 1.18:1 (F:M) for those reared on waxy maize. Larvae reared on sweet maize yielded a sex ratio of 1.06:1 (F:M). These results are shown in Table 2.
Survivorship curve (lx) analysis of S. frugiperda showed maximum mortality at older ages, having a type I age-specific survivorship curve (Deevey, 1947). The emergence of the first females was on the 20th day when larvae were reared on sweet maize, and on the 21st day for those reared on the field and waxy maizes. First egg-lay occurred on the third day after the emergence of females under all treatments tested. The females began to lay eggs at the age of 23.67 ± 0.67, 24.67 ± 0.33 and 25.00 ± 0.58 days on sweet maize, field maize, and waxy maize cultivars, respectively. The life cycle (egg to adult) of S. frugiperda lasts 26 days on sweet maize, and 27 days in both the field and waxy maize. It was noted that females of all the treatments died approximately 5.5 days after their last oviposition (Figure 1).
Table 2. Partial ecological life table of Spodoptera frugiperda fed on three maize cultivars under controlled laboratory conditions.
|
Developmental |
Field maize |
Sweet maize |
Waxy maize |
|||||||||
|
lx |
dx |
100qx |
Sx |
lx |
dx |
100qx |
Sx |
lx |
dx |
100qx |
Sx |
|
|
Egg |
100 |
15 |
15.00 |
85.00 |
100 |
19 |
19.00 |
81.00 |
100 |
7 |
7.00 |
93.00 |
|
Larva: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Instar I |
85 |
7 |
8.24 |
91.76 |
81 |
6 |
7.41 |
92.59 |
93 |
6 |
6.45 |
93.55 |
|
Instar II |
78 |
3 |
3.85 |
96.15 |
75 |
2 |
2.67 |
97.33 |
87 |
2 |
2.30 |
97.70 |
|
Instar III |
75 |
10 |
13.33 |
86.67 |
73 |
0 |
0.00 |
100.00 |
85 |
4 |
4.71 |
95.29 |
|
Instar IV |
65 |
0 |
0.00 |
100.00 |
73 |
0 |
0.00 |
100.00 |
81 |
0 |
0.00 |
100.00 |
|
Instar V |
65 |
1 |
1.54 |
98.46 |
73 |
1 |
1.37 |
98.63 |
81 |
0 |
0.00 |
100.00 |
|
Instar VI |
64 |
0 |
0.00 |
100.00 |
72 |
1 |
1.39 |
98.61 |
81 |
1 |
1.23 |
98.77 |
|
Pre-pupa |
64 |
0 |
0.00 |
100.00 |
71 |
1 |
1.41 |
98.59 |
80 |
2 |
2.50 |
97.50 |
|
Pupa |
64 |
7 |
10.94 |
89.06 |
70 |
10 |
14.29 |
85.71 |
78 |
6 |
7.69 |
92.31 |
|
Adult: |
57 |
|
|
|
60 |
|
|
|
72 |
|
|
|
|
Male |
31 |
|
|
|
29 |
|
|
|
33 |
|
|
|
|
Female |
26 |
|
|
|
31 |
|
|
|
39 |
|
|
|
Note: lx: Number of surviving at the beginning of X, dx: Number of dying in stage, 100qx: Mortality rate, Sx: Survival rate in stage
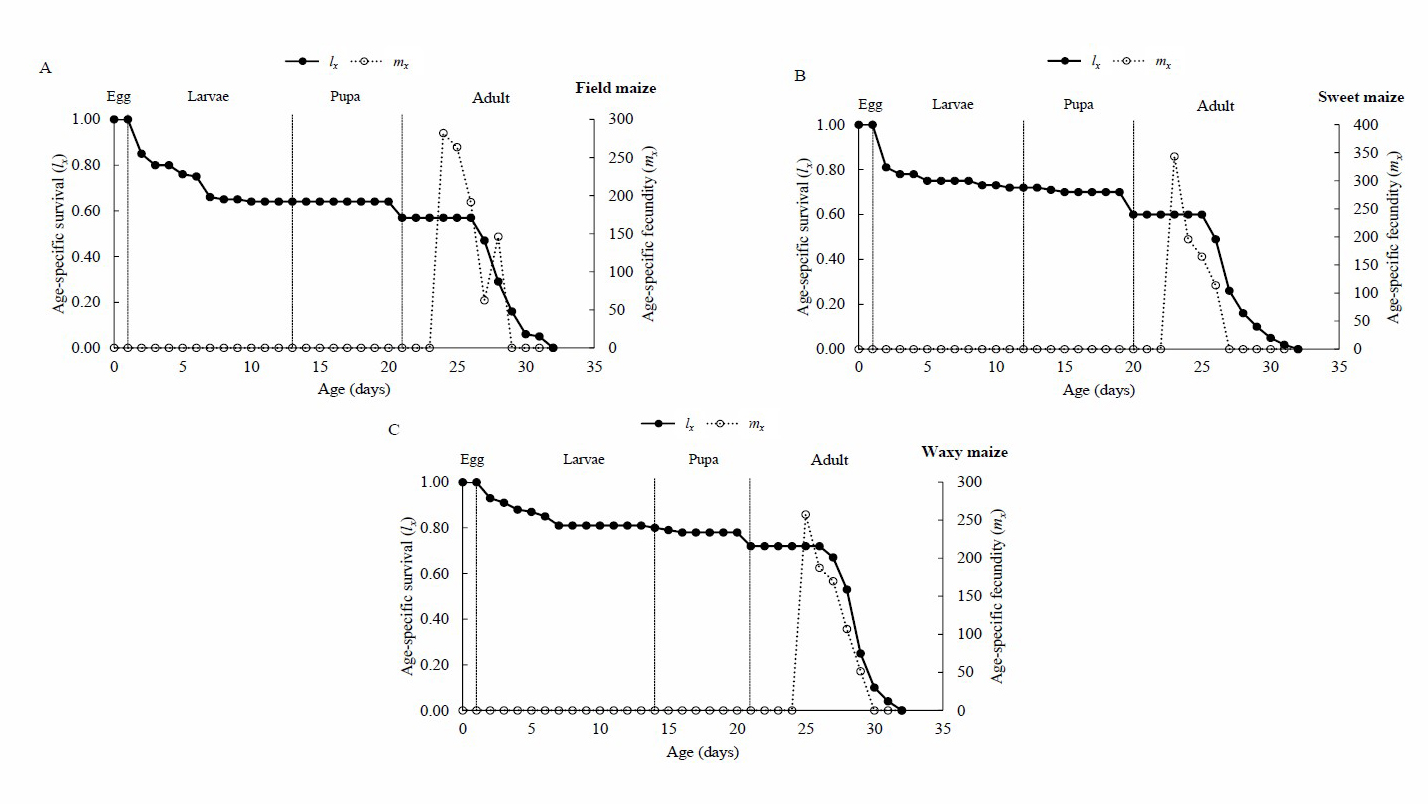
Figure 1. Age-specific survival (lx) and age-specific fecundity (mx) of Spodoptera frugiperda as larvae reared on field maize (A), sweet maize (B) and waxy maize (C) at 30 ± 2°C, 55 ± 5 RH and a 12-hour photoperiod
In all treatments, adult females lived slightly longer than males, but the differences were not statistically significant. The longest pre-oviposition period was found on field maize (3.33 ± 0.33 days), while the longest oviposition period (4.00 ± 0.00 days) was found on waxy maize. There was a significant effect (P < 0.05) of host plant in the fecundity rate: 1523.00 ± 56.58 when fed on sweet maize, 1400.50 ± 41.28 on field maize, and 1238.00 ± 69.28 on waxy maize, respectively. Significant differences (P < 0.05) were also found in the oviposition rate. The highest number of eggs laid per female per day was observed with sweet maize. In addition, nearly 90% of egg hatching was collected from all maize cultivars (Table 3).
Table 3. Reproduction parameters of Spodoptera frugiperda reared on three maize cultivars under controlled laboratory conditions.
|
Parameters |
Maize cultivars |
||
|
Field maize1/ |
Sweet maize1/ |
Waxy maize1/ |
|
|
Male longevity (days) |
4.82 ± 0.46a |
4.71 ± 0.42a |
4.53 ± 0.52a |
|
Female longevity (days) |
5.64 ± 0.44a |
6.13 ± 0.35a |
5.35 ± 0.43a |
|
Pre-oviposition period (days) |
3.33 ± 0.33a |
2.67 ± 0.67a |
2.67 ± 0.33a |
|
Oviposition period (days) |
3.33 ± 0.33a |
3.00 ± 0.58a |
4.00 ± 0.00a |
|
Fecundity (eggs/female) |
1400.50 ± 41.28ab |
1523.00 ± 56.58a |
1238.00 ± 69.28b |
|
Oviposition rate (egg/female/day) |
405.50 ± 21.65a |
440.13 ± 20.14a |
309.50 ± 17.32b |
|
Hatching rate (%) |
91.06 ± 3.47a |
95.13 ± 1.92a |
89.71 ± 1.50a |
Note: 1/ Treatment means within a row not followed by the same letter are significantly different (P < 0.05, DMRT)
The combined results of life table parameters of S. frugiperda mature females whose larvae were fed with field maize, sweet maize, and waxy maize were tabulated and are shown in Table 4. The highest net reproductive rate, R0 (220.41 ± 5.88), innate capacity of increase, rc (0.23 ± 0.001), and finite rate of increase, λ (1.25 ± 0.002) were obtained on sweet maize. However, there was no significant difference between the values of these parameters on other maize cultivars. The mean age of females in a female offspring at birth or the mean cohort generation time, Tc (26.36 ± 0.43 days) was significantly higher on waxy maize compared to field maize (25.25 ± 0.49 days) and sweet maize (23.80 ± 0.24 days). The net innate reproductive rate varied from 156.61 to 220.41; it was highest on sweet maize (220.41 ± 5.88), but there was no significant difference compared to field maize (156.61 ± 6.08) and waxy maize (189.18 ± 35.81), respectively. The values of finite rate of increase (λ) 1.22 ± 0.007, 1.25 ± 0.002, and 1.22 ± 0.013 obtained on field maize, sweet maize, and waxy maize, respectively, were not found to be significantly different on these maize cultivars.
Table 4. Population growth parameters of Spodoptera frugiperda reared on three maize cultivars under controlled laboratory conditions.
|
Parameters |
Maize cultivars |
||
|
Field maize1/ |
Sweet maize1/ |
Waxy maize1/ |
|
|
Net reproductive rate (R0) |
156.61 ± 6.08a |
220.41 ± 5.88a |
189.18 ± 35.81a |
|
Cohort generation time (Tc) |
25.25 ± 0.49ab |
23.80 ± 0.24a |
26.36 ± 0.43b |
|
Innate capacity of increase (rc) |
0.20 ± 0.005a |
0.23 ± 0.001a |
0.20 ± 0.011a |
|
Finite rate of increase (λ) |
1.22 ± 0.007a |
1.25 ± 0.002a |
1.22 ± 0.013a |
Note: 1/ Treatment means within a row not followed by the same letter are significantly different (P < 0.05, DMRT)\
DISCUSSION
Host plant availability and quality play an important role in the population dynamics of herbivorous insects by affecting immature development as well as adult performance. Shorter duration of immaturity and higher fecundity of adult females of herbivore insects on a host indicate greater suitability of a host crop. Based on the findings of this study, the type of maize cultivar had direct consequences on the development of immature stages (or instars) and some physiological fitness of S. frugiperda adult females. Our results represent the number of S. frugiperda developed through 6 instars when fed on all 3 maize cultivars (field maize, sweet maize, and waxy maize). The shortest developmental duration was recorded on those larvae reared on sweet maize (10.83 days). Previous laboratory studies illustrated that the additional (seventh) larval instar was more common on female Spodoptera spp. i.e. S. cosmioides, S. albula, S. eridania and S. dolichos reared on an artificial diet (Montezano et al., 2013; Montezano et al., 2014; Montezano et al., 2016; Specht and Roque-Specht, 2016). However, there are some reports showing that S. frugiperda has highly variable larval development due to different host plants, surviving for 5 larval instars when reared on sweet and field maize (Santos et al., 2003), 6 larval instars on maize leaves and artificial diet (Luginbill, 1928; Montezano et al., 2019), and from 7 up to 10 larval instars when reared on various wild grasses (Pencoe and Martin, 1981; 1982; Murúa and Virla, 2004). The variation of larval development could be due to unsuitable host-plants and their biological flexibility from different geographic areas, which causes longer development (Esperk et al., 2007). The larval duration of S. frugiperda in our tests ranged between 10.83 and 11.28 days, similar to data reported by Da Silva et al. (2017) who studied S. frugiperda reared on maize and oat, and Du Plessis et al. (2020), who studied S. frugiperda reared on sweet maize stems and leaves. Some studies have suggested that the larvae that feed on artificial diets have slightly longer the duration of larval development than those that feed on a natural diet (Montezano et al., 2019; Pinto et al., 2019).
The survivorship curve of S. frugiperda on all 3 maize cultivars in our study was considered to be type I, in which most adults died in the older ages. A similar result has been reported for beet armyworm, S. exigua reared on 4 commercial sugar beet cultivars (Karimi-Malati et al., 2012). Although, most lepidopteran species have a high mortality rate in the early larval stage, and tend to exhibit a type III survivorship curve (Zalucki et al., 2002), a well-fed insect in the laboratory probably demonstrates a high survival rate until the end of its maximum life span, resulting in a type I curve (Hutchinson, 1978). The adult longevity from our study ranged between 5.42 and 4.94 days, which was shorter than the study conducted by Bailey and Chada (1968), who observed longevity of 7.5 days when fed on sorghum and artificial (wheat germ) diet. Pencoe and Martin (1982) reported S. frugiperda adult longevity was slightly longer, at 8.70, 9.10, 9.85, 10.45 and 10.70 days on bahiagrass, yellow nutsedge, large crabgrass, coastal bermudagrass and goose grass, respectively. The pre-oviposition period in our study ranged from 2.67 and 3.33 days, with the oviposition beginning after 3 to 4 days (Johnson, 1987; Sparks, 1979; Capinera, 2001). S. frugiperda females have shown an attraction to oviposit on maize plant rather than potato and tobacco (Guo et al., 2020). The oviposition period ranged from 3.00 to 4.00 days, whereas, in the study conducted by Murúa and Virla (2004), the oviposition period was 8.5 to 11 days. The short period of oviposition was probably due to high reproductive output in the early life stage, which shows the cost of reproduction. The highest values for fecundity and oviposition rate in the current study were recorded on sweet maize. Murúa and Virla (2004) also reported similar values for fecundity while fed on maize. Likewise, Barros et al. (2010) showed the fecundity of S. frugiperda ranged between 1604.2 and 1144.7 eggs per female when fed on maize and cotton plants. However, the fecundity of S. frugiperda was simply higher when fed on artificial diets (Pinto et al., 2019; Silva and Parra, 2013). Regarding the level of egg hatching in our study, it seemed to be higher than the study by Murúa et al. (2008), which found 76.81, 46.53, 49.04, 93.84 and 77.12 percent of egg hatched on maize, alfalfa, soybean, wheat and weeds, respectively.
The innate capacity of increase (rc), the finite rate of increase (λ) and cohort generation time (Tc) were used to describe the population growth rate under the given growing conditions (Carey, 1993; Southwood and Henderson, 2000). rc provided the potential of reproduction and was used similarly to rm, the intrinsic rate of natural increase (Laughlin, 1965). A high value of rm indicates the susceptibility of a host plant to insect feeding (Naseri et al., 2009). According to our results, the highest values of rc (0.23), λ (1.25) and the lowest Tc (23.80 days) were observed on sweet maize, suggesting it as being the most suitable for S. frugiperda population growth. On the other hand, waxy maize provided lower values of rc, λ and the highest Tc (26.36 days), shown in the S. frugiperda population decline. Pinto et al. (2019) reported the values for rc (0.23) and λ (1.26) similar to the results that we obtained in this study, but observed in S. frugiperda fed on an artificial diet. However, Guo et al. (2020) reported the rc values varied, reporting 0.16, 0.07 and 0.03 on maize, potato and tobacco, respectively. The current R0 value observed on 3 maize cultivars was similar to that reported by Guo et al. (2020), with a value of 248.35 on maize. Santos-Amaya et al. (2017) demonstrated the value of R0 ranged between 178.6 and 183.0 obtained from S. frugiperda resistant and susceptible strain derived by the field collection while fed on non-Bt corn. The variation of R0 value was likely influenced by the insect life history (established colonies or difference population) and the absence of natural enemies or other mortality factors (Birley, 1978; Murúa et al., 2008), which resulted in inflated R0 values. There are many other factors affecting S. frugiperda development including the nutrients provided to the host plant (Nascimento et al., 2018), the secondary substance of the host, and the capability of digestion as well as absorption by the insect (Holtof et al., 2019), and opportunistic pathogens and predators. Moreover, the survival rate and fecundity of S. frugiperda can be also affected by many environmental conditions, including temperature and humidity (Simmons and Marti, 1992; Dias et al., 2016; Da Silva et al., 2017; Du Plessis et al., 2020). To better understand the insect-plant interaction, basic biochemical studies on the isolation and identification of phytochemicals, which adversely affect the build-up of S. frugiperda population as well as obtaining more accurate results by implementing in field conditions, are required.
CONCLUSION
S. frugiperda larvae developed through 6 larval instars on all maize cultivars. The life table shows that most S. frugiperda successfully developed from the egg through adult. S. frugiperda has a high rate of survival until the end of its life span, which is considered a type I survivorship curve. The shortest immature developmental period and the highest values of reproduction were recorded on sweet maize, showing the possible high risk to this maize cultivar. Therefore, the current results are a useful tool for tracking S. frugiperda populations across crop systems, as well as for planning an efficient integrated pest management strategies.
ACKNOWLEDGEMENTS
The authors gratefully appreciates the Her Royal Highness Princess Maha Chakri Sirindhorn education scholarship, and would like to thank the staff worker at NBCRC for their kind supports of our fieldwork.
AUTHOR CONTRIBUTIONS
Sothearith Hong conducted the experiments, data assembled, performed statistical analysis and wrote the manuscript. Manas Titayavan data interpreted, critical revision and final approval. Suphannika Intanon data interpreted and critical revision. Panisara Thepkusol designed research concept, critical revision and final approval. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Bailey, D.L., and Chada, H.L. 1968. Effects of natural (Sorghum) and artificial (Wheat Germ) diets on development of the corn earworm, fall armyworm, and southwestern corn borer. Journal of Economic Entomology. 61: 257–260.
Barros, E.M., Torres, J.B., Ruberson, J.R., and Oliveira, M.D. 2010. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomologia Experimentalis et Applicata. 137: 237-245.
Birch, L.C. 1948. The intrinsic rate of natural increase of an insect population. Journal of Animal Ecology. 17: 15–26.
Birley, M.H. 1978. The estimation of the net reproductive rat (Ro) of multivoltine pest populations from census data. Journal of Animal Ecology. 47: 689–696.
CABI. 2018. CABI warns of rapid spread of crop-devastating fall armyworm across Asia. Centre for Agriculture and Bioscience International: CABI; [Accessed 04 October 2019]. Retrieved from https://blog.invasive-species.org/2018/08/02/cabi-warns-of-rapid-spread-of-crop-devastating-fall-armyworm-across-asia/.
CABI. 2019. Spodoptera frugiperda (fall armyworm). CABI: Invasive Species Compendium, CABI; [Accessed 24 May 2019]. Retrieved from https://www.cabi.org/isc/datasheet/29810.
Capinera, J.L. 2001. Handbook of Vegetable Pests. California, USA: Academic Press.
Carey, J.R. 1993. Applied Demography for Biologists: With special emphasis on insects. New York, USA: Oxford University Press.
Carey, J.R. 2001. Insect biodemography. Annual Review of Entomology. 46: 79–110.
Caswell, H. 1982. Stable population structure and reproductive value for populations with complex life cycles. Ecology. 63: 1223–1231.
Da Silva, D.M., Bueno, A.d.F., Andrade, K., Stecca, C.d.S., Neves, P.M.O.J., and De Oliveira, M.C.N. 2017. Biology and nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on different food sources. Scientia Agricola. 74: 18–31.
Deevey, E.S. 1947. Life tables for natural populations of animals. The Quarterly Review of Biology. 22: 283–314.
Dias, A.S., Marucci, R.C., Mendes, S.M., Moreira, S.G., Araújo, O.G., Santos, C.A., and Barbosa, T.A. 2016. Bioecology of Spodoptera frugiperda (Smith, 1757) in different cover crops. Bioscience Journal. 32: 337-345.
Du Plessis, H., Schlemmer, M.-L., and Van den Berg, J. 2020. The effect of temperature on the development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects. 11: 228.
Esperk, T., Tammaru, T., and Nylin, S. 2007. Intraspecific variability in number of larval instars in insects. Journal of Economic Entomology. 100(3): 627–645.
Guo, J.-F., Zhang, M.-D., Gao, Z.-P., Wang, D.-J., He, K.-L., and Wang, Z.-Y. 2020. Comparison of larval performance and oviposition preference of Spodoptera frugiperda among three host plants: Potential risks to potato and tobacco crops. Insect Science. 28: 602-610.
Hardke, J.T., Lorenz, G.M., and Leonard, B.R. 2015. Fall armyworm (Lepidoptera: Noctuidae) ecology in Southeastern cotton. Journal of Integrated Pest Management. 6: 10.
Holtof, M., Lenaerts, C., Cullen, D., and Vanden Broeck, J. 2019. Extracellular nutrient digestion and absorption in the insect gut. Cell and Tissue Research. 377: 397–414.
Hutchinson, G.E. 1978. An Introduction to Population Ecology. New Haven, Connecticut, USA: Yale University Press.
Isranews. 2020. Department of Agriculture revealed Thai maize worms suppressing FAO technology to lift Thailand as an ASEAN model. Bangkok 10300, Thailand: Isranews Agency; [Accessed 18 November 2020]. Retrieved from https://www.isranews.org/isranews-short-news/85057-isra-85057.html?fbclid=IwAR2ybAg3FT7W50n3I2GfjojwuKhEJi_BQy2hiZKPFR3dsttp1XT7n9Tauyc.
Johnson, S.J. 1987. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the western hemisphere. International Journal of Tropical Insect Science. 8: 543–549.
Kakde, A.M., Patel, K.G., and Shailesh, T. 2014. Role of life table in insect pest management. IOSR Journal of Agriculture and Veterinary Science. 7: 40–43.
Karimi-Malati, A., Fathipour, Y., Talebi, A.A., and Bazubandi, M. 2012. Comparative life table parameters of beet armyworm, Spodoptera exigua (Lep.: Noctuidae), on four commercial sugar beet cultivars. Journal of Entomological Society of Iran. 32: 109–124.
Laughlin, R. 1965. Capacity for increase: A useful population statistic. Journal of Animal Ecology. 34: 77–91.
Luginbill, P. 1928. The Fall Armyworm. Washington, D.C., USA: USDA.
Montezano, D.G., Sosa-Gómez, D.R., Paula-Moraes, S.V., Roque-Specht, V.F., Fronza, E., Barros, N.M., and Specht, A. 2016. Immature development of Spodoptera dolichos (Fabricius) (Lepidoptera: Noctuidae). Neotropical Entomology. 45: 22–27.
Montezano, D.G., Specht, A., Bortolin, T.M., Fronza, E., Sosa-Gómez, D.R., Roque-Specht, V., Pezzi, P., Luz, P.C., and Barros, N.M. 2013. Immature stages of Spodoptera albula (Walker) (Lepidoptera: Noctuidae): developmental parameters and host plants. Anais da Academia Brasileira de Ciências (Annals of the Brazilian Academy of Sciences). 85: 271–284.
Montezano, D.G., Specht, A., Sosa-Gómez, D., Roque-Specht, V., Paula-Moraes, S., Peterson, J., and Hunt, T. 2019. Developmental parameters of Spodoptera frugiperda (Lepidoptera: Noctuidae) immature stages under controlled and standardized conditions. Journal of Agricultural Science. 11: 76–89.
Montezano, D.G., Specht, A., Sosa-Gómez, D.R., Roque-Specht, V.F., Sousa-Silva, J.C., Paula-Moraes, S.V.d., Peterson, J.A., and Hunt, T.E. 2018. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. African Entomology. 26: 286–300.
Montezano, D.G., Specht, A., Sosa–Gómez, D.R., Roque–Specht, V.F., and Barros, N.M. 2014. Immature stages of Spodoptera eridania (Lepidoptera: Noctuidae): developmental parameters and host plants. Journal of Insect Science. 14: 238.
Murúa, M.G., Vera, M.T., Abraham, S., Juarçz, M.L., Prieto, S., Head, G.P., and Willink, E. 2008. Fitness and mating compatibility of Spodoptera frugiperda (Lepidoptera: Noctuidae) populations from different host plant species and regions in Argentina. Annals of the Entomological Society of America. 101: 639–649.
Murúa, M.G., and Virla, E. 2004. Population parameters of Spodoptera frugiperda (Smith) (Lep.: Noctuidae) fed on corn and two predominant grasess in Tucuman (Argentina). Acta Zoologica Mexicana. 20: 199–210.
Nascimento, A.M., Assis, F.A., Moraes, J.C., and Souza, B.H.S. 2018. Silicon application promotes rice growth and negatively affects development of Spodoptera frugiperda (J. E. Smith). Journal of Applied Entomology. 142: 241–249.
Naseri, B., Fathipour, Y., Moharramipour, S., and Hosseininaveh, V. 2009. Life table parameters of the cotton bollworm, Helicoverpa armigera (Lep.: Noctuidae) on different soybean cultivars. Journal of Entomological Society of Iran. 29(1): 25–40.
Pencoe, N.L., and Martin, P.B. 1981. Development and reproduction of fall Armyworms on several wild grasses. Environmental Entomology. 10: 999–1002.
Pencoe, N.L., and Martin, P.B. 1982. Fall armyworm (Lepidoptera: Noctuidae) larval development and adult fecundity on five grass hosts. Environmental Entomology. 11: 720–723.
Pinto, J.R.L., Torres, A.F., Truzi, C.C., Vieira, N.F., Vacari, A.M., and De Bortoli, S.A. 2019. Artificial corn-based diet for rearing Spodoptera frugiperda (Lepidoptera: Noctuidae). Journal of Insect Science. 19: 1–8.
Prasanna, B.M., Huesing, J.E., Eddy, R., and Peschke, V.M. 2018. Fall Armyworm in Africa: A Guide for Integrated Pest Management, First Edition. Mexico: CDMX: CIMMYT.
Price, P.W., Denno, R.F., Eubanks, M.D., Finke, D.L., and Kaplan, I. 2011. Insect Ecology: Behavior, Populations and Communities. Cambridge, UK: Cambridge University Press.
R Core Team. 2019. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
Santos-Amaya, O.F., Tavares, C.S., Rodrigues, J.V.C., Campos, S.O., Guedes, R.N.C., Alves, A.P., and Pereira, E.J.G. 2017. Fitness costs and stability of Cry1Fa resistance in Brazilian populations of Spodoptera frugiperda. Pest Management Science. 73: 35-43.
Santos, L.M., Redaelli, L.R., Diefenbach, L.M.G., and Efrom, C.F.S. 2003. Larval and pupal stage of Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) in sweet and field corn genotypes. Brazilian Journal of Biology. 63: 627–633.
Silva, C.S.B.D., and Parra, J.R.P. 2013. New method for rearing Spodoptera frugiperda in laboratory shows that larval cannibalism is not obligatory. Revista Brasileira de Entomologia. 57: 347–349.
Simmons, A.M., and Marti, J.O.G. 1992. Mating by the Fall Armyworm (Lepidoptera: Noctuidae): frequency, duration, and effect of temperature. Environmental Entomology. 21: 371–375.
Southwood, T.R.E., and Henderson, P.A. 2000. Ecological Methods. Oxford, UK: Blackwell Science Ltd.
Sparks, A.N. 1979. A review of the biology of the fall armyworm. The Florida Entomologist. 62: 82–87.
Specht, A., and Roque-Specht, V.F. 2016. Immature stages of Spodoptera cosmioides (Lepidoptera: Noctuidae): developmental parameters and host plants. Zoologia (Curitiba). 33: e20160053.
Wareerat, S. 2019. Fall armyworm Spodoptera frugiperda (J.E. Smith). Chai Nat 17000, Thailand: Agricultural research and development, Chainat Province, Thailand; [Accessed 18 November 2020]. Retrieved from https://www.opsmoac.go.th/uthaithani-dwl-files-411491791831.
Zalucki, M.P., Clarke, A.R., and Malcolm, S.B. 2002. Ecology and behavior of first instar larval Lepidoptera. Annual Review of Entomology. 47: 361–393.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Sothearith Hong1, Manas Titayavan2, Suphannika Intanon1 and Panisara Thepkusol1,*
1 Department of Agricultural Science, Faculty of Agriculture, Natural Resources and Environment, Naresuan University, Phitsanulok 65000, Thailand
2 Division of Agriculture, School of Agriculture and Natural Resources, University of Phayao, Phayao 56000, Thailand
Corresponding author: Panisara Thepkusol, E-mail: panisarat@nu.ac.th
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: January 13, 2021;
Revised: September 7, 2021;
Accepted: September 8, 2021

