
Effects of Indian Gooseberry Fruit on Anxiety-Related Behaviors and Memory Performance in High-fat Diet-induced Obese Mice
Sirikran Juntapremjit* and Yoottana JanthakhinPublished Date : 2021-09-13
DOI : https://doi.org/10.12982/CMUJNS.2021.094
Journal Issues : Number 4, October-December 2021
Abstract Indian gooseberry (Phyllanthus emblica L.) is widely used in Ayurvedic medicine, traditional Chinese medicine, as well as traditional medicine to treat health complications including disorders of diabetes and obesity. The aim of this study was to investigate the effects of Indian gooseberry fruit on anxiety-related behaviors and memory performance in high-fat diet-induced obese mice. C57BL/6 mice were randomly divided into four groups (n = 11 per group); group 1: normal diet control, 2: normal diet treated with Indian gooseberry fruit juice, 3: high-fat diet control, and 4: high-fat diet treated with Indian gooseberry fruit juice. Each mouse was orally and daily administrated with 5mL/kg of Indian gooseberry fruit juice. After six weeks, all groups were tested for blood glucose levels, anxiety and memory performances, and the level of interleukin 6 (IL-6) in the hippocampus. The results revealed that the treatment with Indian gooseberry juice for six weeks produced a significant decrease in blood glucose levels (P <0.05). In anxiety-related behaviors, Indian gooseberry juice showed a remarkable decrease in self-grooming behavior (P <0.001). In addition, there was a significant increase in memory performance in the high-fat diet treated with Indian gooseberry fruit juice compared to the high-fat diet control (P <0.05). Furthermore, the level of inflammatory cytokine IL-6 in the hippocampus was significantly decreased after oral administration of Indian gooseberry fruit juice (P <0.05). These findings suggest that Indian gooseberry fruit can serve as a natural nutritional treatment for preventing high-fat diet-induced cognitive impairment.
Keywords: Anxiety-related behaviors, High-fat diet, Indian gooseberry, Memory performance, Obesity
Funding: This work was supported by a research grant from the College of Research Methodology and Cognitive Science, Burapha University, Chonburi, Thailand
Citation: Juntapremjit, S. and Janthakhin, Y. 2021. Effects of indian gooseberry fruit on anxiety-related behaviors and memory performance in high-fat diet-induced obese mice. CMU J. Nat. Sci. 20(4): e2021094.
INTRODUCTION
Obesity caused by environmental factors such as a high-fat diet is becoming a prevalent problem worldwide. It is associated with various diseases such as cardiovascular disease, type 2 diabetes, hyperlipidemia, hypertension and many types of cancer (Rossner, 2002). In addition, it is linked to cognitive impairment due to local neuroinflammation causing the affections of the hippocampus, hypothalamus, cortex, brainstem and amygdala. Particularly, obesity-derived neuroinflammation has been shown to result in synaptic remodeling and neurodegeneration within the hypothalamus and hippocampus (Miller and Spencer, 2014; Guillemot-Legris and Muccioli, 2017). Moreover, obesity has been shown to increase the risk of Alzheimer’s disease (AD) and anxiety- and depressive-like behaviors (Zemdegs et al., 2016; Ruanpang et al., 2018; Lloret et al., 2019).
Evidence has previously confirmed that fruits are effective in the prevention of obesity, and diabetes via reduction in energy intake, enhancing metabolic promoters, and decreasing fat absorption (Boeing et al., 2012; Payab et al., 2020). Particularly, antioxidant properties of fruits help in lowering oxidative damage through free radicals and reactive oxygen species (ROS) (Firuzi et al., 2005). Additionally, anti-inflammatory properties of phytochemical compounds have been shown to reduce pro-inflammatory cytokine such as IL-6, TNF-α and IL-1β, and enhance anti-inflammatory IL-10 production (Shal et al., 2018).
Indian gooseberry (Phyllanthus emblica L.) is one of the most common medical herbs used in many traditional medicines. Different parts of the plant have been used to defend against diseases (Variya et al., 2016). Particularly, its fruit contains the highest source of vitamin C and also various bioactive substances including tannin, gallic acid, quercetin, ellagic acid, corilagin, apigenin and luteolin (Habib-ur-Rehman et al., 2007). Furthermore, previous studies have shown the anti-hyperglycemic, anti-diabetic, anti-hyperlipidemic, anti-inflammatory, anti-oxidant, anti-cancer and immune-enhancer properties in Indian gooseberry fruits (Variya et al., 2016; Yadav et al., 2017; Srinivasan et al., 2018; Variya et al., 2018). Moreover, vitamin c is a vital antioxidant in the brain, linked to cognitive performances such as memory and learning (Travica et al., 2017).
However, few studies have been conducted so far to demonstrate such an effect of Indian gooseberry on high-fat diet-induced cognitive impairment model. Therefore, the purpose of this study was to investigate the effects of Indian gooseberry fruit on anxiety-related behaviors and memory performance in high-fat diet-induced obese mice.
MATERIALS AND METHODS
Fresh Juice preparation
The fresh fruits of Indian gooseberry were purchased from farmers' orchards in Nakhon Pathom Province. Fresh fruits were cut into small pieces and seeds were removed. Fruits pieces were weighed and an equal volume of water added and ground in mixer grinder. After that, Indian gooseberry juice was filtered and stored at -30°C (Tirgar et al., 2010).
Animals
All experiments were approved by Burapha University-Institutional Animal Care and Use Committee (BUU-IACUC) (approval no. IACUC 027/2561). Three-week-old C57BL/6 male mice were purchased from Nomura Siam International Co., Ltd. (Bangkok, Thailand) and accessed water and food ad libitum. During the experiment, mice were housed in an ambient temperature of 22 ± 2°C and 30-60% relative humidity within a 12-hour light/dark cycle (lights on at 8 a.m. and off at 8 p.m.).
Experimental design
After one week acclimation, the mice were fed with a normal diet (19.77% energy from fat) from the National Laboratory Animal Center of Mahidol University, Nakorn-Pathom, Thailand) or a high-fat diet (59.28% energy from fat) from Nomura Siam International Co., Ltd. (Bangkok, Thailand). After 12 weeks of feeding, the mice were randomly divided into four groups (11 mice/group): group I (ND) were fed with a normal diet and treated with distilled water throughout the experiment, group II (ND-IG) were fed with a normal diet and treated with fresh juice of Indian gooseberry (5mL/kg/day, p.o.), group III (HFD) were fed with a high-fat diet and treated with distilled water throughout the experiment, and group IV (HFD-IG) were fed with a high-fat diet and treated with fresh juice of Indian gooseberry (5mL/kg/day, p.o.). Mouse body weights were monitored every week for six weeks of treatment. Caloric intake was calculated based on the kcal/g of each diet.
Fasting blood glucose and glucose tolerance tests
Fasting blood glucose levels and glucose tolerance were measured after six weeks of treatment. After a 4-hour fast, blood samples were collected from a small incision at the end of the mice's tails. A drop of blood was placed onto a glucose strip and blood glucose measured with a glucose meter according to the user instructions guideline (ACCU-CHEK performa, Bangkok, Thailand). Animals were then administered a 2g of glucose/kg body mass by intra-peritoneal (IP) injection. The blood glucose levels were measured at 30, 60, 90, and 120 minutes after the glucose load.
Open field test
OFT was used to measure the anxiety-related behaviors. Briefly, each mouse was placed in the center of the open square box (40x40x25cm) made from white and non-porous plastic and allowed to explore freely for 10 minutes. Time spent in the center zone (the reflection of anxiety state), time spent with grooming and grooming latency (time spent until the first grooming) were recorded (Rusznák et al., 2018).
Novel object recognition test
NORT was used to investigate learning and memory in mice. The test consisted of three phases: habituation phase, training phase and test phase (Wang et al., 2018). During the habituation phase, each mouse was exposed to the open field box without objects for 10 minutes. On the following day (training phase), the mouse was placed in the open field box with two identical objects made from plastic building blocks that were sufficiently heavy to prevent being moved by the animal. After 24 hours of the training phase, the test phase was performed by each mouse being allowed to explore the open field box with one original and one novel object for 10 minutes. During the experiment, the open field box and objects were cleaned using 70% alcohol. Time spent exploring each object was collected in the test phase in which the mouse’s nose was within 2 cm of the object. Furthermore, the recognition index (time spent with novel object/time spent with both objects x 100) and the discrimination index [(time spent with novel object - time spent with familiar object)/time spent with both objects] were measured.
Sample collections and preparation of the brain homogenate
After the behavior experiments, the mice were euthanized by isoflurane overdose (VIRBAC, France). Following transcardial perfusion with heparinized saline, the hippocampus was collected, frozen in liquid nitrogen and then stored at -80°C until lysate preparation. Total protein was extracted from the hippocampus tissue, and they were homogenized in lysis buffer (50mM Tris, 2% SDS, 5M urea) with phosphatase/protease inhibitor cocktail (Thermo Fisher Scientific, USA). After centrifugation (12,000g, 5 min, 4°C), the supernatant was collected and the protein concentration was determined by Bradford-based protein microassay using bovine serum albumin (Bio-Rad Laboratories, USA) as a standard.
Western blot analysis
Briefly, 50μg of protein lysates separated on SDS-PAGE gels were transferred to a nitrocellulose membrane blocked with 5% skim milk-PBS (120mM NaCl, 16mM Na2HPO4, 4 mM NaH2PO4, pH 7.4) and probed with antibody directed against interleukin 6 (IL-6) (rabbit, 1:1,000, Cell Signaling Technology, USA). Then, the membrane was probed
with secondary antibody (anti-rabbit IgG, 1:2,000, Cell Signaling Technology, USA). Detection was achieved using ECL Detection Reagent (Bio-Rad Laboratories, USA) under chemiluminescence technique using ChemiDoc Image System (Bio-Rad Laboratories, USA). The expression ratios were normalized to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Cell Signaling Technology, USA).
Statistical analysis
All data are presented as mean ± standard error for the 11 mice. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test.
RESULTS
Effect of Indian gooseberry on general features of animals during the study
Treatment with Indian gooseberry juice (5mL/kg/day, p.o.) for six weeks produced a decrease in body weight and visceral fat of high-fat diet-induced obese mice compared with the control group, whereas there was no significant difference on food- and water intake among all groups. However, the caloric intake of mice in the high-fat diet groups was significantly higher than the control group, as shown in Table 1.
Table 1. General features of control and high-fat diet groups.
|
Parameters |
Groups |
|||
|
ND |
ND-IG |
HFD |
HFD-IG |
|
|
Body weight after treatment (g) |
32.29 ± 0.62 |
28.28 ± 0.73a |
40.42 ± 0.86b |
34.73 ± 1.37c |
|
Visceral fat after treatment (g) |
2.01 ± 0.43 |
1.45 ± 0.16 |
4.37 ± 0.70b |
2.99 ± 1.09d |
|
Food intake (g/day) |
2.85 ± 0.15 |
2.78 ± 0.19 |
2.75 ± 0.10 |
2.81 ± 0.27 |
|
Water intake (mL/day) |
4.89 ± 0.11 |
5.25 ± 0.23 |
4.79 ± 0.15 |
5.10 ± 0.21 |
|
Caloric intake (kcal/day) |
11.45 ± 0.60 |
11.17 ± 0.77 |
14.90 ± 0.54a |
15.03 ± 1.44a |
Note: Data are presented as mean ± SEM (n=11).
Statistical significance: a P < 0.05, b P < 0.001 when compared with the normal diet control group; c P < 0.01, d P < 0.001 when compared with the high-fat diet control group.
Effects of Indian gooseberry on fasting blood glucose and glucose tolerance test
To examine the effect of Indian gooseberry fruit juice on glucose homeostasis in obese mice, fasting blood glucose and glucose tolerance tests were measured after six weeks of treatment. The high-fat control group was found to significantly increase in fasting blood glucose level compared to the normal diet control group (P < 0.001). Additionally, the high-fat diet treated with Indian gooseberry juice group showed significantly lower blood glucose levels compared to that of the high-fat diet control (P < 0.05), as indicated in Figure 1A.
Results of the glucose tolerance test revealed that the blood glucose level in the high-fat diet treated with Indian gooseberry juice group had decreased significantly by 30 min (P < 0.01) and this was maintained until 60 minutes (P < 0.05) compared to the high-fat diet control group. However, there was no significant decreased in blood glucose level between the normal diet treated with Indian gooseberry juice group and the normal diet control group from 0 minutes to ensuing measurement point set at 30, 60, 90 and 120 minutes, as shown in Figure 1B.
As illustrated in Figure 1C, the high-fat diet treated with Indian gooseberry juice group displayed a significantly lower area under the curve (AUC) than the high-fat diet control group (P < 0.05), whereas the normal diet treated with Indian gooseberry juice group had no significant difference in area under the curve when compared to the normal diet control group.
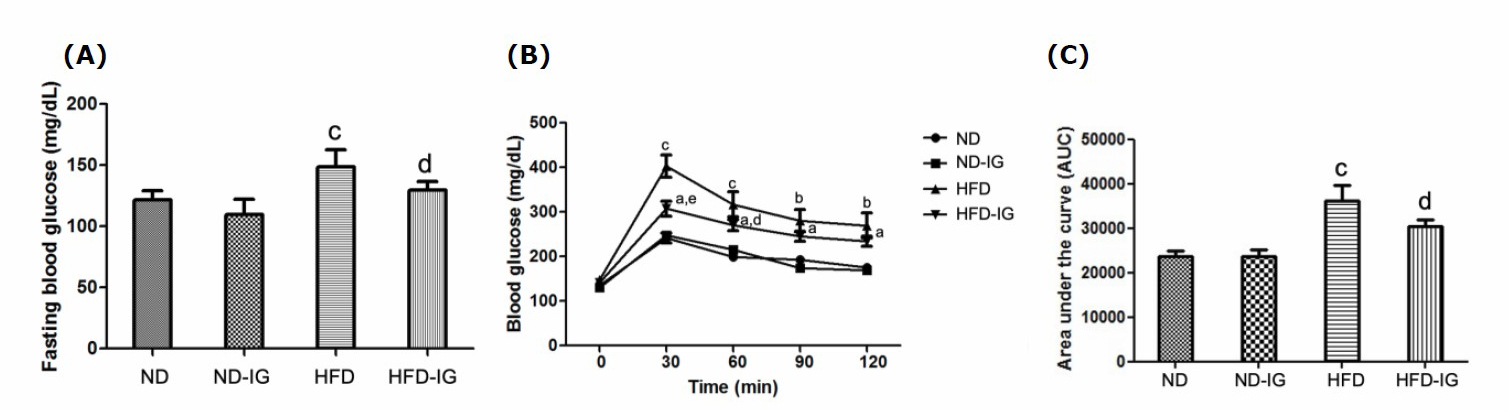
Figure 1. Effects of Indian gooseberry on fasting blood glucose (A), glucose tolerance test (B) and area under the curve (C). Data are presented as means ± SEM. Number of animals in each group (n) = 11. aP <0.05, bP <0.01, cP dP <0.05, eP
Effect of Indian gooseberry on anxiety-related behaviors using open field test
The open field test was performed to assess the anxiety-related behaviors. Animals with an increased level of anxiety spent less times in the central area of the open arena and showed an increase in excessive grooming.
As illustrated in Figure 2A, there was no significant difference on the time spent in the center between the normal diet control group and the high-fat diet control group. In addition, Indian gooseberry juice treatment had no effect on this parameter, although animals tended to explore more time in the center of the arena.
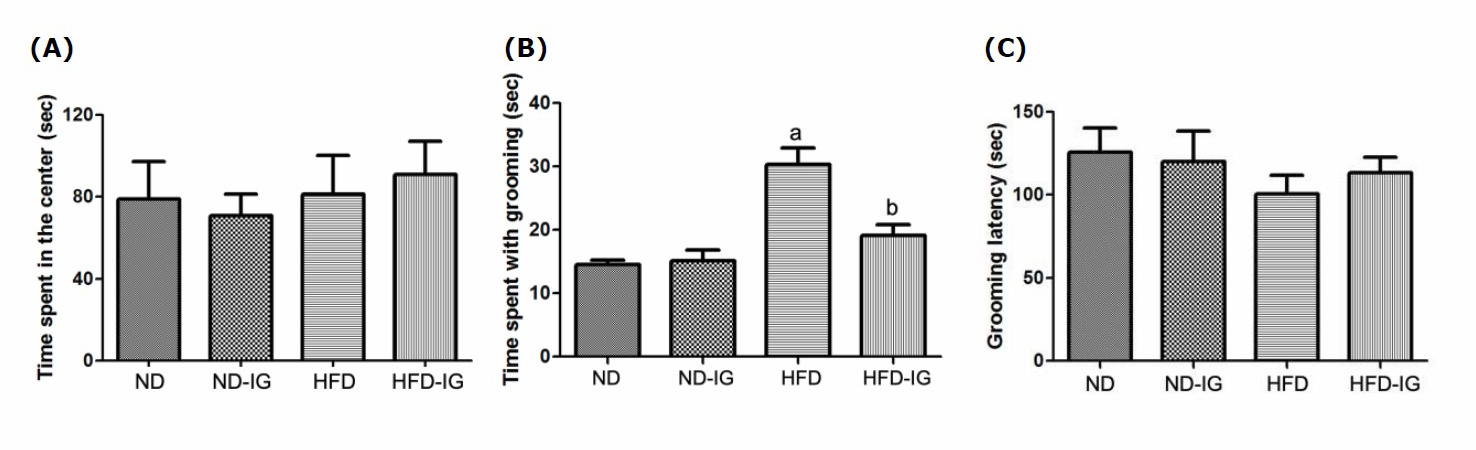
Figure 2. Effects of Indian gooseberry on the time spent in the center (A), the spent with grooming (B) and the grooming latency (C). Data are presented as means ± SEM. Number of animals in each group (n) = 11. aP bP
The analysis of grooming behavior revealed that the high-fat diet control group exhibited significantly higher time spent with grooming than the normal diet control group (P <0.001). Additionally, the high-fat diet treated with Indian gooseberry juice group exhibited a significant reduction of grooming time compared to that of the high-fat diet control group (P <0.001), as indicated in Figure 2B. However, no effects of treatment on the grooming latency were detected among groups, as shown in Figure 2C.
Effect of Indian gooseberry on memory using novel object recognition test
The memory performance of the animals was evaluated using novel object recognition test. Results of the recognition index (the time spent investigating the novel object relative to the total object investigation) and the discrimination index (the difference between time spent exploring novel and familiar objects) were presented in Figure 3. As indicated in Figure 3A, the high-fat diet control group exhibited significantly lower on the recognition index than the normal diet control group (P <0.05). Interestingly, six-week oral administration of Indian gooseberry fruit juice group showed significantly higher on the recognition index than the high-fat diet control group (P <0.05).
Moreover, the high-fat diet control group was found to significantly decrease in the discrimination index when compared to the normal diet control group (P <0.05). A significant increase in the discrimination index was observed in the high-fat diet treated with Indian gooseberry juice group compared to the high-fat diet control group (P < 0.05), as shown in Figure 3B.
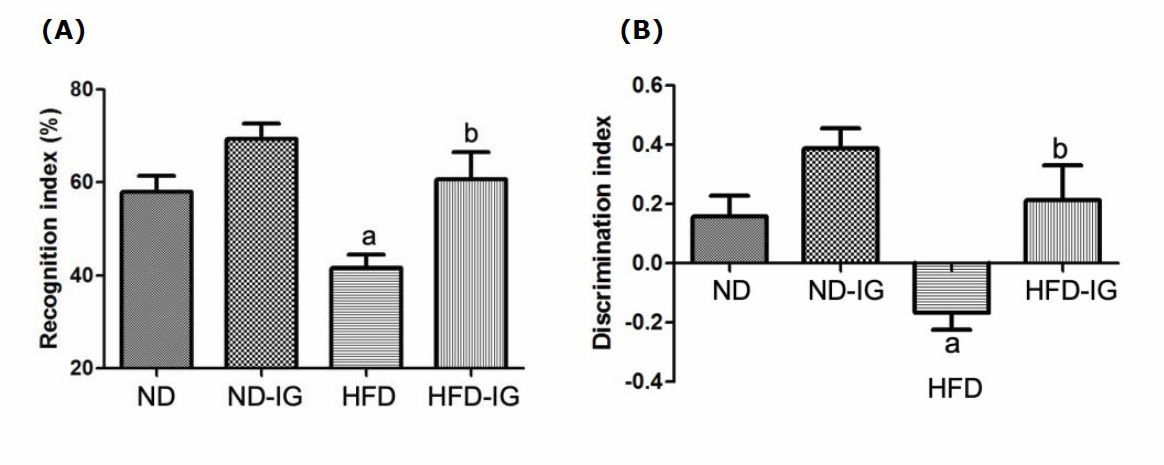
Figure 3. Effects of Indian gooseberry on the recognition index (A) and the discrimination index (B). Data are presented as means ± SEM. Number of animals in each group (n) = 11. aP bP
Effect of Indian gooseberry on IL-6 level in the hippocampus
The inflammatory cytokine in the hippocampus such as IL-6 was associated with memory impairment (Donzis and Tronson, 2014). As shown in Figure 4, a significant increase in the IL-6 level was observed in the high-fat diet control group compared to those of the normal diet control group (P <0.05). Additionally, the high-fat diet treated with Indian gooseberry juice group exhibited a significantly lower the level of IL-6 in the hippocampus than the high-fat diet control group (P <0.05). No significant difference was observed in IL-6 level between the normal diet treated with Indian gooseberry juice group and the normal diet control group.
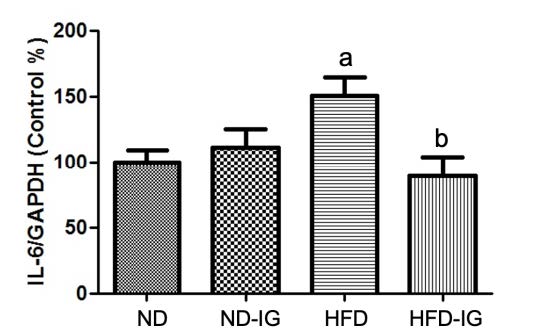
Figure 4. Effects of Indian gooseberry on IL-6/GAPDH level in the hippocampus. Data are presented as means ± SEM. Number of animals in each group (n) = 11. aP bP
DISCUSSION
The principal aim of the present study was to investigate the effects of Indian gooseberry fruit on anxiety-related behaviors and memory performance in a high-fat diet-induced obesity model. The results revealed a significant loss of body weight was found in high-fat diet-induced obese mice over the period of six-week oral administration of Indian gooseberry fruit juice. The loss of body weight of obese mice could be due to the effect of gallic acid in Indian gooseberry fruit on lipid and cholesterol metabolism through the increase of peroxisome proliferator-activated receptors-α (PPARα) expression (Sato et al., 2010, Variya et al., 2018). High-fat diet produced a significant increase in blood glucose levels compared to that of the control group. The obesity upon high-fat diet consumption develops hyperlipidemia, hyperglycemia and insulin resistance (Pratchayasakul et al., 2011; Czech, 2017). Many studies confirmed that the significant increase in blood glucose levels was observed in animals exposed to high-fat diet (Wang and Liao, 2012). Treatment with Indian gooseberry fruit juice showed a significant decrease in fasting blood glucose levels as compared to the high-fat diet control group which was near to the normal diet control group. Additionally, glucose tolerance test which directly measures the action of insulin in response to a glucose stimulus showed a significant decrease in area under the curve (AUC) in the high-fat diet treated with Indian gooseberry group compared to the high-fat diet control group. These findings suggested that the oral administration of Indian gooseberry fruit appeared to be promising for controlling blood glucose level in the high-fat diet-induced obesity. The possible mechanism of glycemic control might be due to the important constituents of Indian gooseberry fruit such as gallic acid, ellagic acid and quercetin. Mechanistic studies showed that its phytochemicals could maintain glucose homeostasis and improve glucose utilization (D'souza et al., 2014). Interestingly, Srinivasan et al. (2018) presented that quercetin extracted from Indian gooseberry fruit exhibited remarkable antihyperglycemic action mediated by changes in the levels of glucose. Moreover, Thongra-ar et al. (2021) revealed that bioactive phytochemicals including gallic acid, chlorogenic acid, and isoquercetin showed the inhibition effects on alpha-glucosidase and alpha-amylase related to reduce glucose absorption and suppress starch digestion in the gastrointestinal tract, leading to the control of blood glucose level.
Previous work by Gainey et al. (2016) showed a short-term exposure to a high-fat diet- induced anxiety-like behaviors and memory impairment in mice. The behavioral tests evaluated the anxiety-related behaviors and memory performance of the animal after six weeks of exposure. The results of the open field test showed administration of Indian gooseberry induced no change in the time spent in the center and the grooming latency, although animals tended to explore more time in the center of the arena and start to groom themselves much later than the high-fat diet control group. Interestingly, Indian gooseberry-treated obese mice spent significantly less time with grooming when compared to control. These results suggested that Indian gooseberry fruit might be a potential action for mental disorders associated with stress. Due to the high content of flavonoids in Indian gooseberry fruit, Girish et al. (2013) demonstrated an involvement of GABAergic system in the anxiolytic action of the bioflavonoid ellagic acid. Moreover, Merzoug et al. (2014) demonstrated that quercetin, the potential bioflavonols found in Indian gooseberry fruit might be a neuroprotective agent to reduce anxiety- and depression-like behaviors.
The novel object recognition test was used to assess memory performance. The present study evaluated the six-week oral administration of Indian gooseberry for its memory enhancing effect against high-fat diet-induced memory impairment in mice.
The fresh juice of Indian gooseberry-treated obese mice spent significantly more time exploring the novel object than the familiar object. This was also confirmed by the discrimination index indices which showed a significant difference as compared to the high-fat diet control group. These findings indicated that the increase in the recognition index and the discrimination index suggested memory enhancing performance in Indian gooseberry-treated obese mice.
Previous studies demonstrated that high-fat diet consumption showed negative effects on metabolic parameters, neural processes, and brain pathologies interfering with cognitive performance in rodents (Gainey et al., 2016; Kothari et al., 2017; Chunchai et al., 2018; Saiyasit et al., 2020). Chunchai et al. (2018) revealed obese rats caused by prolonged high-fat diet consumption displayed gut and systemic inflammation, peripheral insulin resistance, brain and hippocampal oxidative stress, hippocampal dysplasticity, hippocampal apoptosis, and microglial dysfunction, leading to cognitive impairment. Moreover, high-fat diet exposure led to an imbalance of gut microbiota diversity resulted in the abnormal production of inflammatory cytokines (Stanisic et al., 2021). Chronic high-fat diet feeding impacted blood-brain barrier (BBB) permeability, and resulted in neuroinflammation via the increase in the exposure of the brain to various cytokines (de Aquino et al., 2019). Especially, neuroinflammatory events in the hippocampus led to memory deficits (Spencer et al., 2017; Nakandakari et al., 2019). One of the pro-inflammatory cytokines, interleukin-6 (IL-6) is commonly used as a marker for inflammation, particularly for obesity, insulin resistance and cardiovascular disease (Bastard et al., 2006). Previous studies revealed that high-fat diet increased hippocampus IL‐6 concentrations significantly when compared to normal diet (Dutheil et al., 2016, Farhangi et al., 2017). Additionally, Naneix et al. (2021) showed the important role of hippocampus in high-fat diet-induced recognition memory impairment.
Our results demonstrated that obese mice expressed the high level of pro-inflammatory cytokine IL-6 in the hippocampus region. A high-fat diet acted as an inflammatory insult to the body, triggering the secretion of pro-inflammatory cytokines and the imbalance between oxidants derivatives production and antioxidants defenses (Sakr et al., 2019). Moreover, Castanon et al. (2014) showed that obesity-related inflammatory processes, originating from adipose tissue, spread to the brain leading to impaired neuroendocrine activity and neurotransmitter function.
Our finding showed that Indian gooseberry revealed an anti-inflammatory effect in the hippocampus of high-fat feeding mice. The 100 mL of fresh Indian gooseberry juice contained ascorbic acid (1066.70 ± 0.68mg), ellagic acid (71.20 ± 0.12mg), gallic acid (37.95 ± 0.08mg), chlorogenic acid (17.43 ± 0.76mg) and quercetin (2.01 ± 0.43mg). Moreover, antioxidant capacity of fresh Indian gooseberry juice determined by calculating the inhibition of DPPH (2, 2-diphenyl-1-picrylhydrazyl) was found to be 92.16 ± 0.01% (Bansal et al., 2014). Due to the antioxidant enrich fraction, Indian gooseberry fruit had a beneficial role in neuroprotective properties. Particularly, polyphenol compounds were shown to cross the blood-brain barrier (BBB) and directly scavenge concentrations of reactive oxygen and nitrogen species resulting in protection from neuroinflammation (Figueira et al., 2017). Polyphenols such as quercetin and ellagic acid displayed the inhibition of enzymes and the activating signaling system involved in the process of inflammation (Hussain et al. 2016). Thus, the protective effect of Indian gooseberry fruit might be attributed to its antioxidant property by virtue of which brain cells get exposed to less oxidative stress leading to reduced brain damage and improved cognitive performance.
CONCLUSION
Findings from the present study demonstrated that Indian gooseberry fruit could prevent weight gain, improve hyperglycemia, and reduce anxiety-related behaviors and memory impairment in high-fat diet-induced obese mice. Nevertheless, further detailed investigation to find whether the possible mechanisms contribute toward the improvement of cognitive performance is of great interest.
ACKNOWLEDGEMENTS
The authors would like to thank Prof. Chanan Angsuthanasombat and Asst. Prof. Pratchaya Kaewkaen for supporting the instrumental facilities to complete this work. The authors are also thankful to Ms. Somsri Sakdee for technical assistance.
AUTHOR CONTRIBUTIONS
Sirikran Juntapremjit designed and performed experiments, analyzed data and wrote the manuscript. Yoottana Janthakhin performed experiment and wrote the manuscript.
All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
Bansal, V., Sharma, A., Ghanshyam, C., and Singla, M.L. 2014. Coupling of chromatographic analyses with pretreatment for the determination of bioactive compounds in Emblica officinalis juice. Analytical Methods. 6: 410-418.
Bastard, J.P., Maachi, M., Lagathu, C., Kim, M.J., Caron, M., Vidal, H., Capeau, J., and Feve, B. 2006. Recent advances in the relationship between obesity, inflammation, and insulin resistance. European Cytokine Network. 17: 4-12.
Boeing, H., Bechthold, A., Bub, A., Ellinger, S., Haller, D., Kroke, A., Leschik-Bonnet, E., Muller, M.J, Oberritter, H., Schulze, M., et al. 2012. Critical review: vegetables and fruit in the prevention of chronic diseases. European Journal of Nutrition. 51: 637-663.
Castanon, N., Lasselin, J., and Capuron, L. 2014. Neuropsychiatric comorbidity in obesity: role of inflammatory processes. Frontiers in Endocrinology. 5: 74.
Chunchai, T., Thunapong, W., Yasom, S., Wanchai, K., Eaimworawuthikul, S., Metzler, G., ... and Chattipakorn, S.C. 2018. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. Journal of Neuroinflammation. 15: 11.
Czech, M.P. 2017. Insulin action and resistance in obesity and type 2 diabetes. Nature Medicine. 23: 804-814.
de Aquino, C.C., Leitão, R.A., Oliveira Alves, L.A., Coelho-Santos, V., Guerrant, R.L., Ribeiro, C.F., ... and Oriá, R.B. 2019. Effect of hypoproteic and high-fat diets on hippocampal blood-brain barrier permeability and oxidative stress. Frontiers in Nutrition. 5:131.
D'souza, J.J., D'souza, P.P., Fazal, F., Kumar, A., Bhat, H.P., and Baliga, M. S. 2014. Anti-diabetic effects of the Indian indigenous fruit Emblica officinalis Gaertn: active constituents and modes of action. Food & Function. 5: 635-644.
Donzis, E.J., and Tronson, N.C. 2014. Modulation of learning and memory by cytokines: signaling mechanisms and long term consequences. Neurobiology of Learning and Memory. 115: 68-77.
Dutheil, S., Ota, K.T., Wohleb, E.S., Rasmussen, K., and Duman, R.S. 2016. High-fat diet induced anxiety and anhedonia: impact on brain homeostasis and inflammation. Neuropsychopharmacology. 41: 1874-1887.
Farhangi, M.A., Mesgari-Abbasi, M., Nameni, G., Hajiluian, G., and Shahabi, P. 2017. The effects of vitamin D administration on brain inflammatory markers in high fat diet induced obese rats. BMC Neuroscience. 18: 81.
Figueira, I., Garcia, G., Pimpão, R.C., Terrasso, A.P., Costa, I., Almeida, A.F., ... and Santos, C.N. 2017. Polyphenols journey through blood-brain barrier towards neuronal protection. Scientific Reports. 7: 1-16.
Firuzi, O., Lacanna, A., Petrucci, R., Marrosu, G., and Saso, L. 2005. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochimica et Biophysica Acta (BBA)-General Subjects. 1721: 174-184.
Gainey, S.J., Kwakwa, K.A., Bray, J.K., Pillote, M.M., Tir, V.L., Towers, A.E., and Freund, G.G. 2016. Short-term high-fat diet (HFD) induced anxiety-like behaviors and cognitive impairment are improved with treatment by glyburide. Frontiers in Behavioral Neuroscience. 10: 156.
Girish, C., Raj, V., Arya, J., and Balakrishnan, S. 2013. Involvement of the GABAergic system in the anxiolytic-like effect of the flavonoid ellagic acid in mice. European Journal of Pharmacology. 710: 49-58.
Guillemot-Legris, O., and Muccioli, G.G. 2017. Obesity-induced neuroinflammation: beyond the hypothalamus. Trends in Neurosciences. 40: 237-253.
Habib-ur-Rehman, Yasin, K.A., Choudhary, M.A., Khaliq, N., Atta-ur-Rahman, Choudhary, M.I., and Malik, S. 2007. Studies on the chemical constituents of Phyllanthus emblica. Natural Product Research. 21: 775-781.
Hussain, T., Tan, B., Yin, Y., Blachier, F., Tossou, M.C., and Rahu, N. 2016. Oxidative stress and inflammation: what polyphenols can do for us?. Oxidative Medicine and Cellular Longevity, 2016.
Kothari, V., Luo, Y., Tornabene, T., O'Neill, A.M., Greene, M.W., Geetha, T., and Babu, J.R. 2017. High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 1863: 499-508.
Lloret, A., Monllor, P., Esteve, D., Cervera-Ferri, A., and Lloret, A. 2019. Obesity as a risk factor for Alzheimer’s disease: implication of leptin and glutamate. Frontiers in Neuroscience. 13: 508.
Merzoug, S., Toumi, M.L., and Tahraoui, A. 2014. Quercetin mitigates Adriamycin-induced anxiety-and depression-like behaviors, immune dysfunction, and brain oxidative stress in rats. Naunyn-Schmiedeberg's Archives of Pharmacology. 387: 921-933.
Miller, A.A., and Spencer, S.J. 2014. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain, Behavior, and Immunity. 42: 10-21.
Nakandakari, S.C.B.R., Munoz, V.R., Kuga, G.K., Gaspar, R.C., Sant'Ana, M.R., Pavan, I.C. B., ... and Pauli, J.R. 2019. Short-term high-fat diet modulates several inflammatory, ER stress, and apoptosis markers in the hippocampus of young mice. Brain, Behavior, and Immunity. 79: 284-293.
Naneix, F., Bakoyiannis, I., Santoyo-Zedillo, M., Bosch-Bouju, C., Pacheco-Lopez, G., Coutureau, E., ... and OBETEEN Consortium. 2021. Chemogenetic silencing of hippocampus and amygdala reveals a double dissociation in periadolescent obesogenic diet-induced memory alterations. Neurobiology of Learning and Memory. 178: 107354.
Payab, M., Hasani‐Ranjbar, S., Shahbal, N., Qorbani, M., Aletaha, A., Haghi‐Aminjan, H., Soltani, A., Khatami, F., Nikfar, S., Hassani, S., Abdollahi, M., and Larijani, B. 2020. Effect of the herbal medicines in obesity and metabolic syndrome: a systematic review and meta‐analysis of clinical trials. Phytotherapy Research. 34: 526-545.
Pratchayasakul, W., Kerdphoo, S., Petsophonsakul, P., Pongchaidecha, A., Chattipakorn, N., and Chattipakorn, S.C. 2011. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sciences. 88: 619-627.
Rossner, S. 2002. Obesity: the disease of the twenty-first century. International Journal of Obesity. 26: S2-S4.
Ruanpang, J., Pleumsamran, A., Pleumsamran, J., and Mingmalairak, S. 2018. Effect of a high-fat diet and cholesterol levels on depression-like behavior in mice. Chiang Mai University Journal of Natural Sciences. 17: 161-173.
Rusznák, K., Csekő, K., Varga, Z., Csabai, D., Bóna, Á., Mayer, M., Kozma, Z., Helyes, Z. and Czéh, B. 2018. Long-term stress and concomitant marijuana smoke exposure affect physiology, behavior and adult hippocampal neurogenesis. Frontiers in Pharmacology. 9: 786.
Saiyasit, N., Chunchai, T., Prus, D., Suparan, K., Pittayapong, P., Apaijai, N., ... and Chattipakorn, S.C. 2020. Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet–induced obese condition. Nutrition. 69: 110576.
Sakr, H.F., Abbas, A.M., Khalil, K., and Shata, A.M. 2019. Modulatory effect of concomitant administration of sitagliptin and vitamin E on inflammatory biomarkers in rats fed with high fat diet: role of adiponectin. Journal of Physiology and Pharmacology. 70: 955-967.
Sato, R., Buesa, L.M., and Nerurkar, P.V. 2010. Anti-obesity effects of Emblica officinalis (Amla) are associated with inhibition of nuclear transcription factor, peroxisome proliferator-activated receptor gamma (PPARγ). FASEB J. 24: 661-664
Shal, B., Ding, W., Ali, H., Kim, Y.S., and Khan, S. 2018. Anti-neuroinflammatory potential of natural products in attenuation of Alzheimer's disease. Frontiers in Pharmacology. 9: 548.
Spencer, S.J., D'Angelo, H., Soch, A., Watkins, L.R., Maier, S.F., and Barrientos, R.M. 2017. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal-and amygdalar-dependent memory. Neurobiology of Aging. 58: 88-101.
Srinivasan, P., Vijayakumar, S., Kothandaraman, S., and Palani, M. 2018. Anti-diabetic activity of quercetin extracted from Phyllanthus emblica L. fruit: in silico and in vivo approaches. Journal of Pharmaceutical Analysis. 8: 109-118.
Stanisic, D., Jeremic, N., Majumder, S., Pushpakumar, S., George, A., Singh, M., and Tyagi, S.C. 2021. High Fat Diet Dysbiotic Mechanism of Decreased Gingival Blood Flow. Frontiers in Physiology. 12: 229.
Thongra-ar, K., Rojsanga, P., Chewchinda, S., Mangmool, S., and Sithisarn, P. 2021. Antioxidant, α-glucosidases and α-amylase inhibitory activities of Persicaria odorata. Chiang Mai University Journal of Natural Sciences. 20: e2021051.
Tirgar, P.R., Shah, K.V., Patel, V.P., Desai, T.R., and Goyal, R.K. 2010. Investigation into mechanism of action of anti-diabetic activity of Emblica officinalis on streptozotocin induced type I diabetic rat. Research Journal Pharmaceutical Biological Chemical Science. 1: 672-682.
Travica, N., Ried, K., Sali, A., Scholey, A., Hudson, I., and Pipingas, A. 2017. Vitamin C status and cognitive function: a systematic review. Nutrients. 9: 960.
Variya, B.C., Bakrania, A.K., Chen, Y., Han, J., and Patel, S.S. 2018. Suppression of abdominal fat and anti-hyperlipidemic potential of Emblica officinalis: Upregulation of PPARs and identification of active moiety. Biomedicine & Pharmacotherapy. 108: 1274-1281.
Variya, B.C., Bakrania, A.K., and Patel, S.S. 2016. Emblica officinalis (Amla): a review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacological Research. 111: 180-200.
Wang, C.Y., and Liao, J.K. 2012. A mouse model of diet-induced obesity and insulin resistance. Methods in Molecular Biology. 821: 421-433.
Wang, Q., Yuan, J., Yu, Z., Lin, L., Jiang, Y., Cao, Z., and Li, X. 2018. FGF21 attenuates high-fat diet-induced cognitive impairment via metabolic regulation and anti-inflammation of obese mice. Molecular Neurobiology. 55: 4702-4717.
Yadav, S.S., Singh, M.K., Singh, P.K., and Kumar, V. 2017. Traditional knowledge to clinical trials: a review on therapeutic actions of Emblica officinalis. Biomedicine & Pharmacotherapy. 93: 1292-1302.
Zemdegs, J., Quesseveur, G., Jarriault, D., Pénicaud, L., Fioramonti, X., and Guiard, B.P. 2016. High‐fat diet‐induced metabolic disorders impairs 5‐HT function and anxiety‐like behavior in mice. British Journal of Pharmacology. 173: 2095-2110.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Sirikran Juntapremjit* and Yoottana Janthakhin
Cognitive Science and Innovation Research Unit (CSIRU), College of Research Methodology and Cognitive Science, Burapha University, Chonburi 20131, Thailand
Corresponding author: Sirikran Juntapremjit, E-mail: sirikran@go.buu.ac.th
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: April 10, 2021;
Revised: August 26, 2021;
Accepted: August 31, 2021;

