
Antioxidant and anti-inflammatory properties of essential oils from three Eucalyptus species
Orapin Insuan, Benchaluk Thongchuai, Rujirek Chaiwongsa, Supaporn Khamchun, and Wimonrut Insuan*Published Date : 2021-09-13
DOI : https://doi.org/10.12982/CMUJNS.2021.091
Journal Issues : Number 4, October-December 2021
Abstract Eucalyptus essential oils are used as traditional medicines in many countries. The objective of this study was to evaluate the antioxidant and anti-inflammatory activities of leaf essential oils extracted from three different Eucalyptus species on HepG2 and RAW264.7 cells. Essential oils were distilled from fresh leaf samples, and the chemical constituents were analyzed using gas chromatography–mass spectrometry. The antioxidant activities of essential oils were determined using ABTS and hydroxyl radical scavenging assays, and hydrogen peroxide (H2O2)-induced oxidative stress in HepG2 cells. Additionally, lipopolysaccharide (LPS)-activated RAW264.7 macrophages were used to evaluate the anti-inflammatory properties. The results revealed that Eucalyptus citriodora and Eucalyptus urophylla leaf essential oils had a high content of oxygenated monoterpenes, whereas Eucalyptus deglupta contained a high amount of monoterpene hydrocarbons. Essential oils extracted from the three Eucalyptus species showed antioxidant and anti-inflammatory activities. E. citriodora and E. urophylla leaf essential oils had strong antioxidant activity against H2O2-induced oxidative stress in human HepG2 cells. Additionally, E. citriodora leaf essential oil, which contains a high amount of citronellal, exhibited the most potent anti-inflammatory activity in LPS-activated RAW264.7 macrophages. The antioxidant and anti-inflammatory effects of essential oils depended on their chemical composition. A principal component analysis explained 100% of the variance was performed to construct three groups based on the chemical components and antioxidant and anti-inflammatory activities. This study suggests that E. citriodora leaf essential oil, which represents a good source of oxygenated monoterpenes, could be considered a potential phytochemical agent for the prevention of oxidative stress and inflammation.
Keywords: Antioxidant, Anti-inflammatory effect, Eucalyptus essential oils, Gas chromatography–mass spectrometry, Principal component analysis
Funding: This work was supported by the grant from the University of Phayao, Thailand, under the National Research Council of Thailand (NRCT), Thailand (Grant number RD62070).
Citation: Insuan, O., Thongchuai, B., Chaiwongsa, R., Khamchun,S., and Insuan, W. 2021. Antioxidant and anti-inflammatory properties of essential oils from three Eucalyptus species. CMU J. Nat. Sci. 20(4): e2021091.
INTRODUCTION
The genus Eucalyptus (part of the family Myrtaceae) comprises about 900 species and shows a worldwide distribution (Döll-Boscardin et al., 2012). Eucalyptus urophylla, Eucalyptus deglupta, and Eucalyptus camaldulensis are commercial Eucalyptus species that are cultivated in Thailand (Siramon et al., 2007; Dlamini et al., 2017; Chahomchuen et al., 2020). These species represent the major sources of pulpwood for paper production, fuelwood, and construction (Dhakad et al., 2018; Salehi et al., 2019). On the other hand, E. citriodora, which has a slower growth rate than the other three commercial species, is usually cultivated for essential oil production (used in fragrances) (Chahomchuen et al., 2020). Paper production and fuelwood capacities have consistently increased; however, the large amounts of leaf waste from these processes are currently not utilized. Thus, possible uses for Eucalyptus leaves should be investigated (Siramon et al., 2007). Traditionally, Eucalyptus leaves are used for wound healing and the treatment of fungal infections (Döll-Boscardin et al., 2012; Bardaweel et al., 2014). Several studies have demonstrated that essential oils and extracts from some Eucalyptus species possess biological effects, including antibacterial, antifungal, anti-inflammatory, and antioxidant activities (Silva et al., 2003; Bardaweel et al., 2014; Ghaffar et al., 2015; Vuong et al., 2015). In addition, cytotoxic and antitumor properties of Eucalyptus leaf extracts have been reported (Ashour, 2008; Döll-Boscardin et al., 2012; Bardaweel et al., 2014; Vuong et al., 2015). Essential oils from the leaves of E. sideroxylon have been shown to have a cytotoxic effect against MCF7 cells (Ashour, 2008). Furthermore, an E. robusta (Sm.) leaf aqueous extract showed marked cytotoxicity against a pancreatic cancer cell line (Vuong et al., 2015). In addition, essential oils from young and adult leaves of E. benthamii presented cytotoxic effects against Jurkat and HeLa cell lines (Döll-Boscardin et al., 2012).
In recent years, the preventive effect of bioactive phytochemicals on oxidative stress and inflammation has been studied. Oxidative stress and chronic inflammation are considered crucial factors in the development of several diseases, including cardiovascular disease, diabetes mellitus, metabolic syndrome, and cancer (Reuter et al., 2010; Rea et al., 2018). Oxidative stress, which results from an imbalance between the excessive production of reactive oxygen species (ROS) and limitations of antioxidant defense systems, can activate several transcription factors that promote the expression of genes involved in inflammatory pathways (Hussain et al., 2016). A previous study showed that leaf essential oils from E. deglupta, E. urophylla, E. citriodora, and E. camaldulensis exhibited radical scavenging activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH) and antibacterial properties (Chahomchuen et al., 2020). While a large amount of work has focused on the biological activity of Eucalyptus essential oils, studies on whether extracts from Eucalyptus species can protect against intracellular oxidative damage and inflammation are limited. Therefore, the present study aimed to investigate the in vitro antioxidant activity of essential oils from the leaves of three Eucalyptus species, including E. citriodora, E. urophylla, and E. deglupta, against hydrogen peroxide (H2O2)-induced oxidative stress in HepG2 (human hepatocellular carcinoma) cells, as well as the 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical (ABTS•+) and hydroxyl radical (•OH) scavenging activity assays were also performed. To study the anti-inflammatory potential of Eucalyptus leaf essential oils, lipopolysaccharide (LPS)-induced RAW264.7 (mouse macrophage) cell model was used. Additionally, principal component analysis (PCA) was performed to examine correlations between the chemical composition of leaf essential oils and their antioxidant and anti-inflammatory effects.
MATERIALS AND METHODS
Plant materials
Leaves of E. citriodora, E. urophylla, and E. deglupta were collected in August 2019 from a Eucalyptus plantation located in Lampang Province, Thailand (18°22.188’N, 99°32.320’E; 250 m altitude). The species were identified by the voucher numbers QBG 118494, 118495, and 118497, respectively. The voucher specimens were deposited at Queen Sirikit Botanic Garden Herbarium, Chiang Mai, Thailand.
Chemicals and reagents
Lipopolysaccharide (LPS) from Escherichia coli serotype O111:B4 and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum, streptomycin, and penicillin were obtained from Invitrogen Gibco (Grand Island, NY, USA). The Griess reagent was purchased from Invitrogen, Thermo Fisher Scientific, Inc. (Eugene, OR, USA). Superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA) assay kits were obtained from Cayman Chemicals (Ann Arbor, MI, USA). Tumor necrosis factor (TNF)-α and interleukin (IL)-6 enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems, Inc. (Minneapolis, MN, USA). All other chemicals were analytical grade.
Extraction of essential oils and gas chromatography-mass spectrometry (GC-MS) analysis
The essential oils of fresh leaves from E. citriodora, E. urophylla, and E. deglupta were separated by water distillation. The Eucalyptus leaf essential oils (EOs) were dried with anhydrous sodium sulphate and stored in glass vials at -20°C and protected from light until use. The identification of volatile constituents was performed using a GCMS-QP2010 apparatus (Shimadzu, Kyoto, Japan) and a ZB-WAX plus capillary column (30 m x 0.25 mm, 0.25 µm film thickness; Zebron, CA, USA) and a method was modified from Insuan and Chahomchuen (2020). GC-MS was carried out using split injection (split ratio 1:50) for a 0.2 µL oil sample, with the following temperature settings: injector set at 230 °C, column set at 60°C, heating ramp of 5°C/min, final temperature of 230°C, and detector set at 250°C. Helium was used as the carrier gas at a flow rate of 1.2 mL/min. Fragmentation was performed by electron impact (70 eV), and the mass range was between 40 and 500 amu. Identification of compounds was based on comparisons of the mass spectra with those of the NIST (NIST14) mass spectral library data standard of the GC/MS system.
2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical (ABTS•+) scavenging activity assay
The radical cation ABTS•+ scavenging activities of EOs were determined according to Re et al. (1999) with some modifications. An ABTS•+ stock solution was prepared by dissolving 7 mM ABTS and 2.45 mM potassium persulfate in deionized water.
After 12–16 h, the ABTS•+ stock solution was diluted with ethanol to an absorbance of 0.70 ± 0.02 at 734 nm. Subsequently, 10 μL of Trolox (standard reference compound) or sample was mixed with 1000 μL of the ABTS•+ solution, and the absorbance at 734 nm was measured after 6 min. The percentage of ABTS•+ inhibition was calculated and plotted as a function of concentration of antioxidants and of Trolox for the standard reference data. To calculate the Trolox equivalent antioxidant capacity (TEAC), the slope of the plot of the percentage inhibition vs. concentration plot for the sample was divided by the slope of the plot for Trolox, expressed as the millimolar TEAC.
Hydroxyl radical (•OH) scavenging activity assay
The •OH scavenging activity assay was performed according to the Fenton method (Chung et al., 1997). A 200 μL aliquot of each EO sample was incubated with a mixture containing FeSO4•7H2O (10 mM), ethylenediaminetetraacetic acid (EDTA, 10 mM), and 2-deoxyribose solution (10 mM). Then, phosphate buffer (pH 7.4) was added to increase the volume to 1.8 mL. H2O2 solution (200 μL, 10 mM) was added, and the mixture was incubated at 37°C for 4 h. After incubation, 1 mL each of a trichloroacetic acid solution (2.8%) and thiobarbituric acid solution (1.0%) was added, and the mixture was boiled for 5 min and then cooled on ice. The absorbance was measured at 520 nm. The percentage •OH scavenging activity was calculated using the following equation, where A indicates absorbance:
- OH scavenging activity (%) = [(Acontrol – Asample)/Acontrol] × 100
The inhibition concentration of the EOs that reduced 50% of OH radical formation (IC50) was calculated using a calibration curve in the linear range by plotting concentration of the EOs vs. percentage of radical scavenging activity.
Evaluation of the antioxidant effect of essential oils on H2O2-induced oxidative stress in HepG2 cells
Cell culture and essential oil preparation
HepG2 cell line was obtained from American Type Culture Collection (ATCC) and cultured in DMEM containing 10% fetal bovine serum, 100 units/mL penicillin, and 100 µg/mL streptomycin at 37°C under a humidified (95%) atmosphere containing 5% CO2. Dimethyl sulfoxide (DMSO) was used to prepare sample solutions of the EOs (final concentration of 0.1%).
Cell viability test
The MTT assay was used to determine cell viability, as previously described (Insuan et al., 2021). Briefly, cells were seeded in 96-well plates for 24 h and then treated with various concentrations of EOs for 24 h. Next, cells were treated with or without H2O2 for another 2 h. The medium was removed and incubated with MTT solution (final concentration of 0.5 mg/mL) for 4 h. After incubation, the supernatant was removed, formazan crystals were dissolved in 100 µL of DMSO, and the absorbance was determined at 540 nm using a microplate spectrophotometer (Berthold Technologies GmbH & Co. KG, Bad Wildbad, Germany). Untreated cells (no EOs or H2O2) were used as a control, considered to have a cell viability of 100%. Cell viability was calculated as the percentage of viable cells in treated wells compared to control wells.
Measurement of antioxidant parameters
HepG2 cells were cultured in 6-well plates (1 x 106 cells/mL) and pretreated with different concentrations of EOs for 24 h. Then, cells were exposed to H2O2 for 2 h. The supernatant was removed, and cells were lysed by the repeated freeze-thaw method. The MDA level and catalase activity were measured using commercial assay kits (Cayman Chemicals, Ann Arbor, MI, USA) following the manufacturer’s instructions. The MDA level is expressed as µM/mg protein, and the activities of SOD and CAT are expressed as U/mg protein.
Evaluation of anti-inflammatory effect of essential oils on LPS-induced inflammation in RAW264.7 cells
Cell viability test
RAW264.7 cell line was obtained from ATCC and cultured in DMEM containing 10% fetal bovine serum, 100 units/mL penicillin, and 100 µg/mL streptomycin at 37°C under a humidified (95%) atmosphere containing 5% CO2. Cells were seeded in 96-well plates for 24 h and then treated with various concentrations of EOs for 2 h. Next, cells were treated with LPS (100 ng/mL) for another 22 h. The medium was removed and the MTT assay was used to measure cell viability, as described above.
Measurement of nitric oxide (NO) production
RAW264.7 cells (2 x 105 cells/mL) were pretreated with various concentrations of EOs (50, 100, and 200 µg/mL) for 2 h and then treated with 100 ng/mL LPS for 22 h (Insuan et al., 2021). Next, the cell culture medium was analyzed for NO by the Griess reaction. To determine the NO concentration, the Griess reagent was mixed with cell culture supernatant at room temperature for 30 min. The NO concentration was determined by measuring the absorbance at 542 nm with a microplate reader. Sodium nitrite (NaNO2) was used to generate a standard curve.
Determination of pro-inflammatory cytokine levels
RAW264.7 cells were seeded into 6-well plates (2 x 106 cells/mL) and incubated in the presence of different concentrations of EOs for 2 h, followed by stimulation with LPS for 22 h (Insuan et al., 2021). Culture supernatants were harvested, and IL-6 and TNF-α concentrations were measured using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Statistical analysis
All results are expressed as the mean ± standard deviation (SD) from triplicate samples of three independent experiments. Differences between the treatments were determined by one-way analysis of variance (ANOVA), with P < 0.05 considered statistically significant. The PCA was used to comprehend the similarity between the essential oils in relation to the contents of their chemical components, antioxidant and anti-inflammatory properties.).
RESULTS
Chemical characteristics of Eucalyptus leaf essential oils
The percentage yield of three EOs extracted using hydro distillation from E.citriodora, E.urophylla and E.deglupta were 0.8, 0.6 and 0.2 (v/w), respectively. The major chemical components of the three EOs evaluated in the current study are presented in Table 1. Only compounds with concentrations above 1.00% were registered. Leaf oils of E. citriodora and E. urophylla had a high content of oxygenated monoterpenes, whereas E. deglupta leaf oil contained a high amount of monoterpene hydrocarbons. The major component of E. citriodora leaf essential oil was citronellal (64.1%), and the major component of E. urophylla leaf essential oil was eucalyptol (40.8%). E. deglupta leaf essential oil was found to be rich in eucalyptol (15.7%) and α-phellandrene (15.1%). PCA was performed to detect the degree of similarity among the compositions of EOs analyzed (Figure 1). Different groups could be identified in the loading plots of PC1 and PC2. Furthermore, PC1 and PC2 accounted for 100% of the total variance. The near-far distance represented the difference in chemical composition of different areas, where the closet chemical components were separated as groups I and II (Figure 1A). As seen in Figure 1B, the score plot of samples is obviously scattered according to the different quadrants. The samples from E. citriodora were far away from the other samples, indicating a large difference in chemical composition. Thus, our data suggest a possible relationship between essential oil chemical components and species.
Table 1. Chemical constituents and their percentages for the three Eucalyptus leaf essential oils.
|
No. |
RT (min) |
Volatile Compound |
Chemical Formula |
Peak Area (%) |
||
|
E.citriodora |
E.urophylla |
E. deglupta |
||||
|
1 |
2.95 |
β-Pinene |
C10H16 |
2.14 ± 0.00 |
- |
- |
|
2 |
3.81 |
a-Phellandrene |
C10H16 |
* |
1.85 ± 0.01 |
15.10 ± 0.56 |
|
3 |
4.11 |
α-Terpinene |
C10H16 |
- |
* |
2.04 ± 0.24 |
|
4 |
4.45 |
D-Limonene |
C10H16 |
* |
- |
4.09 ± 0.34 |
|
5 |
4.58 |
Eucalyptol |
C10H18O |
1.64 ± 0.01 |
40.78 ± 0.09 |
15.72 ± 0.35 |
|
6 |
5.30 |
α-Pinene |
C10H16 |
- |
5.83 ± 0.05 |
3.12 ± 0.12 |
|
7 |
5.38 |
g-Terpinene |
C10H16 |
2.21 ± 0.01 |
23.83 ± 0.05 |
6.19 ± 0.15 |
|
8 |
5.86 |
β -Cymene |
C10H14 |
* |
7.43 ± 0.14 |
12.61 ± 0.30 |
|
9 |
6.11 |
Cis-4-Carene |
C10H16 |
- |
1.78 ± 0.01 |
1.51 ± 0.04 |
|
10 |
9.57 |
Citronellal |
C10H18O |
64.06 ± 0.13 |
- |
- |
|
11 |
10.36 |
Linalool |
C10H18O |
* |
- |
1.83 ± 0.03 |
|
12 |
10.52 |
dl-Isopulegol |
C10H18O |
4.03 ± 0.02 |
- |
* |
|
13 |
10.64 |
dl-Isopulegol |
C10H18O |
7.58 ± 0.02 |
- |
- |
|
14 |
10.88 |
Caryophyllene |
C15H24 |
1.08 ± 0.00 |
- |
- |
|
15 |
11.04 |
Terpinen-4-ol |
C10H18O |
- |
3.59 ± 0.02 |
4.48 ± 0.12 |
|
16 |
11.33 |
p-Menth-2-en-1-ol |
C10H18O |
- |
- |
1.05 ± 0.09 |
|
17 |
11.93 |
p-Menth-6-en-2-one |
C10H16O2 |
- |
- |
1.13 ± 0.07 |
|
18 |
12.09 |
Terpinyl acetate |
C12H20O2 |
- |
6.14 ± 0.05 |
* |
|
19 |
12.22 |
α -Terpineol |
C10H18O |
- |
- |
4.46 ± 0.11 |
|
20 |
12.33 |
Bisabolene |
C15H24 |
- |
2.11 ± 0.05 |
- |
|
21 |
13.07 |
Citronellol |
C10H20O |
10.06 ± 0.18 |
- |
- |
|
22 |
13.50 |
Sabinol |
C10H16O |
- |
- |
1.87 ± 0.04 |
|
23 |
14.24 |
Safrole |
C10H10O2 |
- |
- |
1.84 ± 0.11 |
|
24 |
15.67 |
Methyleugenol |
C11H14O2 |
1.70 ± 0.01 |
- |
- |
|
25 |
15.92 |
Nerolidol |
C15H26O |
- |
- |
5.04 ± 0.18 |
|
26 |
16.50 |
Cis-p-menthane-3,8-diol |
C10H20O2 |
1.22 ± 0.01 |
- |
- |
|
27 |
17.64 |
Carvacrol |
C10H14O |
- |
- |
1.34 ± 0.04 |
|
Compounds identified |
|
|
|
|
||
|
Monoterpene hydrocarbons |
|
4.35 |
40.72 |
44.66 |
||
|
Sesquiterpene hydrocarbons |
|
1.08 |
2.11 |
- |
||
|
Oxygenated monoterpenes |
|
90.29 |
50.51 |
36.92 |
||
|
Other |
|
- |
- |
1.84 |
||
Note: Each value was expressed as mean ± SD of three measurements. RT: Retention time; (*): peaks lower than 1.0% were not documented; (-): not detected.
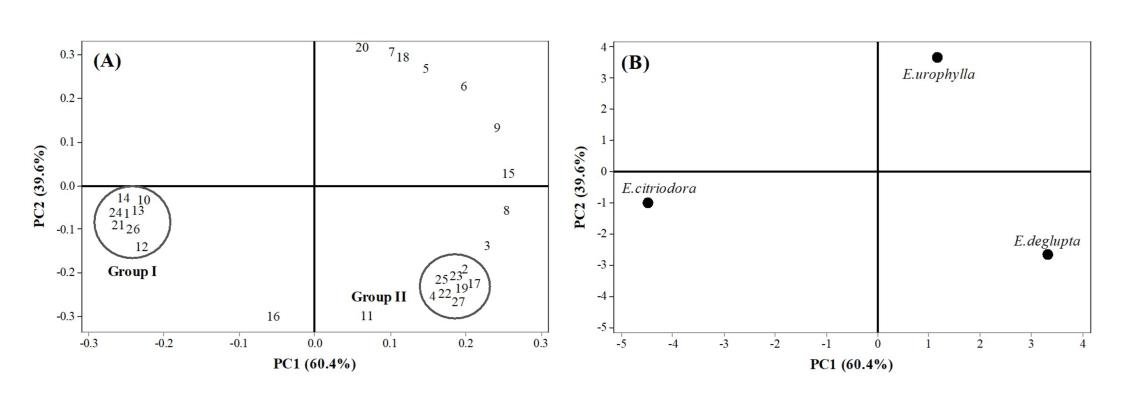
Figure 1. The loading plot of sample from the principal component analysis PCA (A) and the score plot of 3 EO samples (B). The chemical components are present by number (Table1)
Radical scavenging activity of Eucalyptus leaf essential oils
The antioxidant activities of E. citriodora, E. urophylla, and E. deglupta leaf essential oils were evaluated in vitro using ABTS•+ and •OH radical scavenging assays. The essential oils extracted from E. citriodora, E. deglupta and E. urophylla exhibited the ABTS•+ reducing capacity of 5.725 ± 0.061, 5.683 ± 0.181, and 4.959 ± 0.048 mM Trolox/g extract, respectively. (Table 2). E. urophylla, E. deglupta, and E. citriodora leaf oils showed the •OH scavenging power with half maximal inhibitory concentrations (IC50) of 0.838 ± 0.025, 0.835 ± 0.035, and 0.962 ± 0.001 mg/mL, respectively.
Table 2. ABTS and hydroxyl radical scavenging activities for the Eucalyptus leaf essential oils.
|
Eucalyptus species/compounds |
TEAC (mM Trolox/g extract) |
·OH (IC50, mg/mL) |
|
|
E. citriodora |
5.725 ± 0.061 |
0.962 ± 0.001 |
|
|
E. urophylla |
4.959 ± 0.048 |
0.838 ± 0.025 |
|
|
E. deglupta |
5.683 ± 0.181 |
0.835 ± 0.035 |
|
|
Gallic acid |
- |
0.694 ± 0.054 |
|
Note: All activities were expressed as mean ± SD of three measurements. TEAC: Trolox equivalent antioxidant capacity; (-): not detected.
Antioxidant effect of Eucalyptus leaf essential oils on H2O2-induced oxidative stress in HepG2 cells
Hydrogen peroxide is an important active oxygen molecule that is often used in in vitro models of oxidative stress (Chiu et al., 2013). To establish an oxidative damage model, HepG2 cells were treated with different concentrations of H2O2 (0-2 mM), then the viability of HepG2 cells was determined by MTT assay. As shown in Figure 2A, HepG2 cell viability significantly decreased with increasing H2O2 concentrations (P < 0.05). The viability of HepG2 cells was reduced to 54.92 ± 1.40% after treatment with 2 mM H2O2 for 2 h. Therefore, this concentration was used in the following experiments. The cytotoxicity of EOs on HepG2 cells is shown in Figure 2B. Our results show that treatment with EOs (50–200 µg/mL) extracted from the three Eucalyptus species had no toxic effect on HepG2 cells, whereas treatment with EOs at a concentration of 400 µg/mL significantly inhibited cell growth compared to untreated control cells (P <0.05). Consequently, EOs extracted from all three Eucalyptus species at concentrations of 50, 100, and 200 µg/mL were used in subsequent experiments.
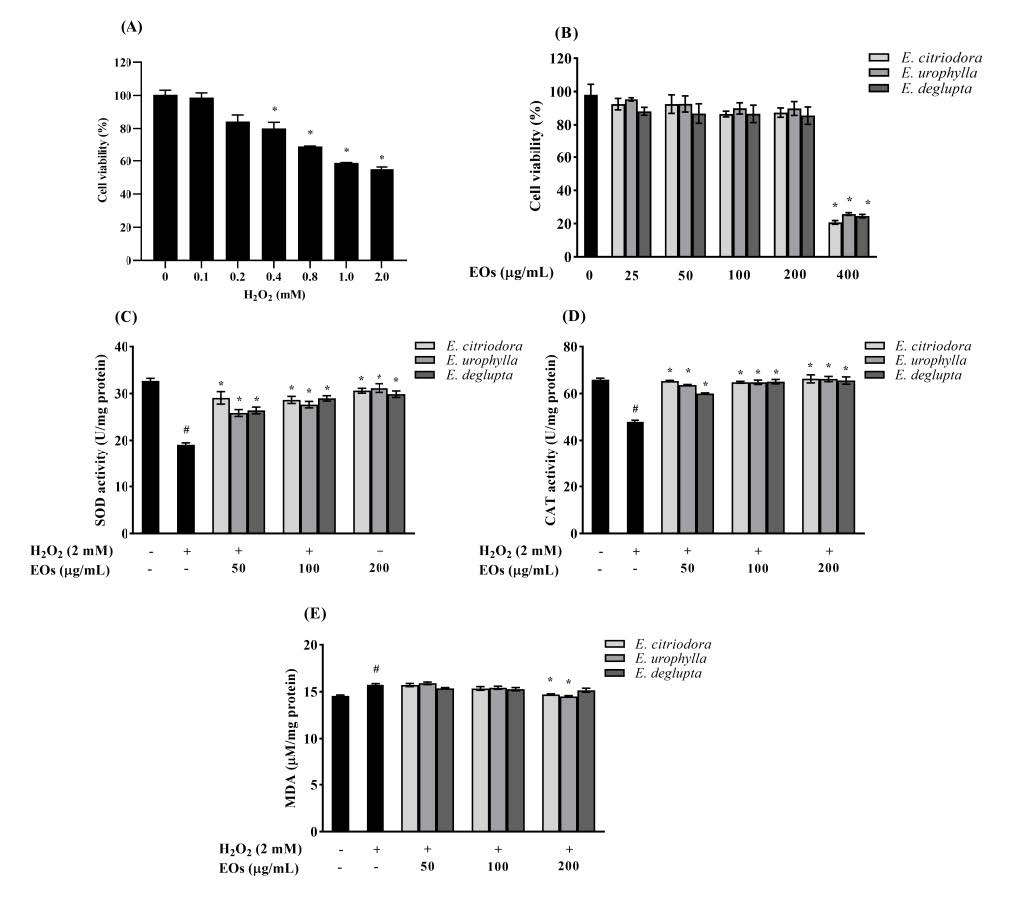
Figure 2. The effect of Eucalyptus leaf essential oils (EOs) on antioxidant system: (A) HepG2 cells were treated with different concentrations of H2O2 (0-2 mM) for 2 h; (B) the viability of HepG2 cells treated with various concentrations of EOs for 24 h; The effect of EOs on the antioxidant enzyme activity and lipid peroxidation in H2O2-stimulated HepG2 cells: (C) Superoxide dismutase (SOD) activity; (D) catalase (CAT) activity; (E) malondialdehyde (MDA) level. The results are expressed as the mean ± standard deviation (n = 3). #P < 0.05 compared to untreated control cells; *P < 0.05 compared with the H2O2-treated cells.
To understand the antioxidant effect of EOs, we evaluated the activity of intracellular antioxidant enzymes and measured lipid peroxidation under oxidative stress. Our results demonstrate that the activities of SOD and CAT were significantly reduced in H2O2-treated HepG2 cells compared to the negative control group (P < 0.05; Figure. 2C and 2D). However, SOD and CAT activities were significantly increased in HepG2 cells treated with each of the tested EOs compared to the H2O2-treated group (P < 0.05). As shown in Figure 2E, there was a marked increase in the MDA level of H2O2-treated HepG2 cells. Pretreatment with E. citriodora and E. urophylla leaf essential oils significantly reduced the MDA levels, especially when applied at a concentration of 200 µg/mL (P < 0.005). These results suggest that pretreatment with EOs extracted from E. citriodora and E. urophylla helped to normalize the activity of intracellular antioxidant enzymes and the MDA level compared to H2O2-treated cells.
Anti-inflammatory effect of Eucalyptus leaf essential oils on LPS-induced RAW264.7 cells
We initially investigated the cytotoxic effects of EOs in RAW264.7 cells using the MTT assay. As shown in Figure 3, no cytotoxicity was observed (cell viability >80%) when RAW264.7 cells were treated with EOs with or without LPS. Therefore, we investigated the effects of EOs extracted from all three Eucalyptus species at concentrations of 50, 100, and 200 µg/mL on NO and pro-inflammatory cytokine production in these cells. Figure 4A shows that NO release was significantly and dose-dependently inhibited by EOs (P < 0.05). At the highest concentration of 200 µg/mL, E. citriodora, E. urophylla, and E. deglupta leaf essential oil extracts significantly decreased NO production by 53%, 50%, and 45%, respectively. Moreover, the IL-6 and TNF-α levels of LPS-treated cells were significantly increased compared to untreated control cells, and these changes were dose-dependently reversed by treatment with EOs (P < 0.05; Figure 4B and 4C). The E. citriodora leaf essential oils exerted the strongest anti-inflammatory activity in LPS-induced RAW264.7 cells compared to the other examined species.
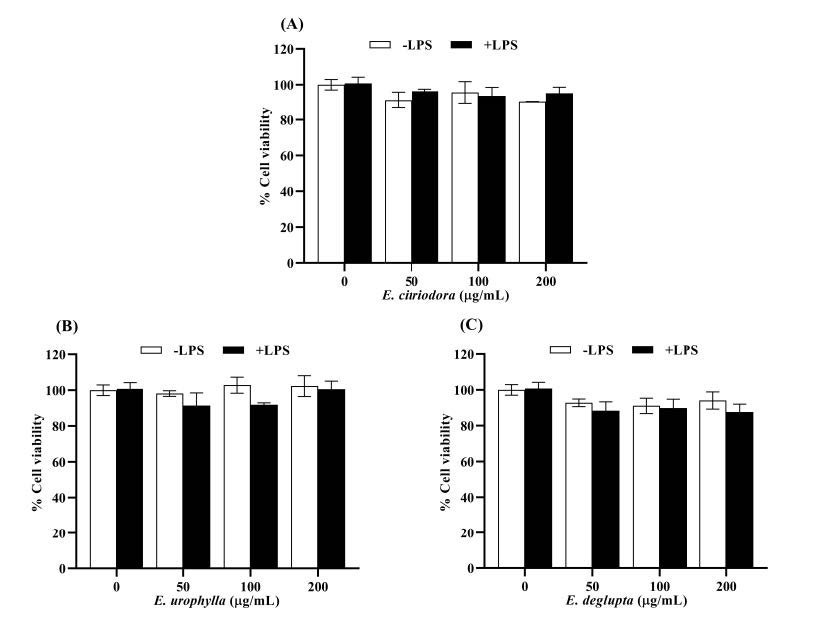
Figure 3. The effect of Eucalyptus leaf essential oils (EOs) on cell viability of RAW264.7 cells treated with or without LPS: (A) E. citriodora; (B) E. urophylla; (C) E. deglupta. The results are expressed as the mean ± standard deviation (n = 3).
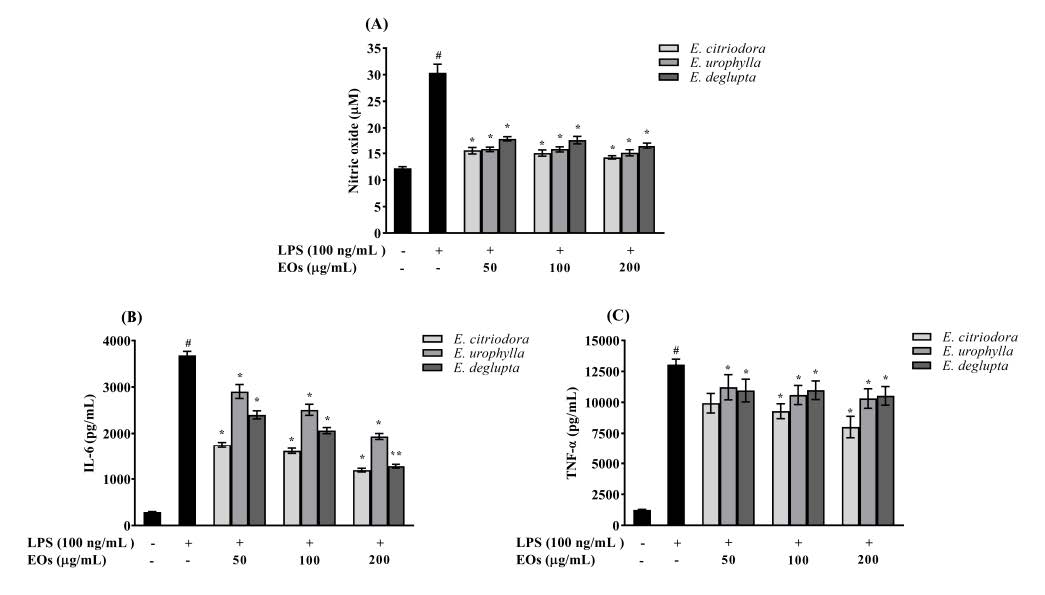
Figure 4. The effect of Eucalyptus leaf essential oils (EOs) on the production of NO and pro-inflammatory cytokines in LPS-induced RAW264.7 cells: (A) the level of nitric oxide (NO); (B) the levels of interleukin 6 (IL-6); (C) the level of tumor necrosis factor α (TNF-α) in cell culture supernatant. The results are expressed as the mean ± standard deviation (n = 3). #P < 0.05 compared to untreated control cells, *P < 0.05 compared with the LPS-treated cells.
The correlation between the chemical composition of Eucalyptus leaf essential oils and their antioxidant and anti-inflammatory effects
PCA is a tool that is used to minimize the multidimension from the facts and generate a two-dimensional map to explain the determined variance. In the PCA, all major parameters, including groups of chemical components and antioxidant and anti-inflammatory activity measurements, were introduced to categorize the genotypes. The first two principal components, PC1 and PC2, explained 100% of the variance (PC1 = 57.8% and PC2 = 42.2%) of the studied parameters. The loading plot of the first two PCs demonstrates the main dependencies between variables. PC1 correlated mainly with the contents of oxygenated compounds and monoterpene hydrocarbon, •OH scavenging activity, CAT activity, TNF-α, and NO, whereas PC2 correlated with sesquiterpene hydrocarbon, ABTS•+ scavenging activity, SOD activity, MDA, and IL-6. The score plot for PC1 versus PC2 for each EO sample is presented in Figure 5. The plot group for E. citriodora essential oil was located in the upper left of the plot, which differed considerably from all other species based on their phytochemical, antioxidant, and anti-inflammatory activities. E. citriodora had the highest content of oxygenated monoterpenes and showed the best antioxidant and anti-inflammatory activities, whereas E. urophylla was characterized by different properties, indicating that the composition in terms of some measured in addition to its antioxidant and anti-inflammatory activity. The other group, E. deglupta had rather low values in composition. By applying PCA, the three EOs were successfully distinguished into three groups based on their chemical profile and antioxidant and anti-inflammatory activities.
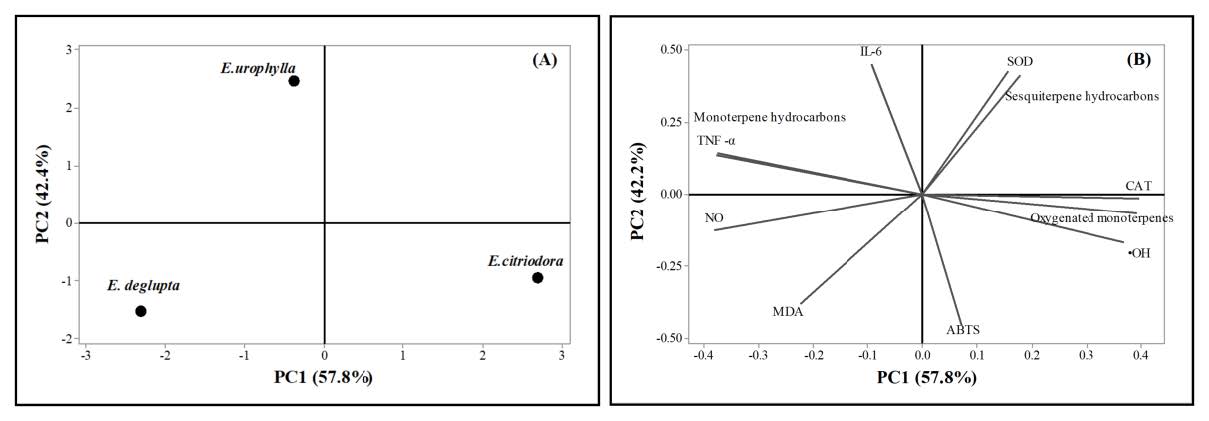
Figure 5. Principal component analysis (PCA) plots: (A) The score plot for different 3 EOs of E. citriodora, E. urophylla, and E. deglupta; (B) the loading plot for different study parameters on PC1 and PC2.
DISCUSSION
The leaves of Eucalyptus species contain many secondary metabolite essential oils that possess various biological functions, including antioxidant, antibacterial, antifungal, antitumor, and anti-inflammatory properties (Ho et al., 2020). Previous studies have reported that eucalyptol (1,8-cineole) is the major compound found in EOs (Elaissi et al., 2012; Lingan, 2018; Salem et al., 2018; Chahomchuen et al., 2020). As shown in this study, E. citriodora leaf extracts had high contents of oxygenated monoterpenes, mainly citronellal, isopulegol, and citronellol. Eucalyptol was the main compounds in EOs from E. urophylla and E. deglupta. PCA confirmed a large difference in chemical composition of E. citriodora from the other species. Thus, our data suggest a possible relationship between essential oil chemical components and species. The chemical composition of EOs can vary depending on the species, leaf age, harvesting period, geographical location, and extraction procedures (Siramon et al., 2013; Sebei et al., 2015; Barbosa et al., 2016; Lingan, 2018; Salem et al., 2018; Almasa et al., 2019; Insuan and Chahomchuen, 2020). The difference in chemical constituents of EOs is related to a difference in biological activity. We found that the E. urophylla, E. deglupta and E. citriodora leaf essential oils exhibited scavenging activity against ABTS•+ and •OH. The differences in antioxidant properties of the EOs may be due to differences in chemical compounds containing oxygenated monoterpene and monoterpene hydrocarbon groups (Warsito et al., 2018). Oxygenated monoterpenes, especially thymol and carvacrol, have high antioxidant activity (Zengin and Baysal, 2014), whereas eucalyptol and borneol present weak antioxidant activity against DPPH and •OH radicals (Zengin and Baysal, 2014; Horvathova et al., 2014). Furthermore, the E. citriodora leaf essential oil extract, specifically the three major constituent monoterpenes (citronellal, β-citronellol, and isopulegol), possessed moderate to strong DPPH and H2O2 scavenging activity (Singh et al., 2012). However, combinations of oxygenated monoterpenes (e.g., eucalyptol, chrysanthenone, camphor, borneol, and verbenone) and monoterpenoid phenols (e.g., thymol and p-cymene) play a vital role in antioxidant activity (Benyoucef et al., 2018). Our results confirm that EOs extracted from the three tested Eucalyptus species exhibit antioxidant activity. E. citriodora leaf oil presented the most potent radical scavenging activity against ABTS•+, which can be attributed to the high content of oxygenated monoterpenes.
Excessive ROS production has potent oxidative effects on DNA, leading to alterations in DNA structure and, consequently, mutations (Klaunig et al., 2010). The superoxide anion radical (O2•-) is generally found within the mitochondria, where O2•- is converted to H2O2 by superoxide dismutase (SOD), and then H2O2 is converted into H2O by either CAT or glutathione peroxidase (GPx) (Valko et al., 2006; Rolo et al., 2012). On the other hand, •OH, produced by the Fenton reaction, can cause lipid peroxidation (Valko et al., 2006), which is one of the main causes of peroxidative tissue damage related to inflammation and carcinogenicity (Chiu et al., 2013; Vipin et al., 2017). However, exposure to ROS causes the body to develop defense mechanisms against oxidative stress through the synthesis of endogenous antioxidant enzymes, including SOD, CAT, GPx, and glutathione reductase (GR), as well as non-enzymatic systems such as glutathione (GSH) (Valko et al., 2006). H2O2 is considered a major intermediary of oxidative-stress-induced cytotoxicity (Chiu et al., 2013). Similarly, our results demonstrated that H2O2 exposure led to decreased HepG2 cell viability. Pretreatment with EOs from all three Eucalyptus species, especially E. citriodora and E. urophylla, resulted in the normalization of SOD and CAT activities, and MDA level compared with H2O2 treatment. It can be hypothesized that the combination of oxygenated monoterpenes present in EOs is responsible for the observed antioxidant effects.
Currently, the link between oxidative stress-mediated chronic inflammation has been studied. During the inflammatory process, the excessive activation of macrophages can produce several kinds of inflammatory mediators and pro-inflammatory cytokines, such as nitric oxide (NO), prostaglandin E2 (PGE2), interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α), which can trigger detrimental effects in inflammatory diseases (Mosser and Gonçalves, 2015; Mcinnes and Schett, 2017; Wirtz and Von Känel, 2017). Lipopolysaccharides, which are an important component of the outer membrane of Gram-negative bacteria, are commonly used to establish in vitro macrophage inflammatory models and to assess the anti-inflammatory effects of natural phytochemicals (Dong et al., 2017). In this study, we examined the anti-inflammatory activities of EOs from three Eucalyptus species against LPS-induced RAW264.7 cells. The results demonstrated that EOs extracted from all three Eucalyptus species exhibited anti-inflammatory property. The E. citriodora leaf essential oils, which contains a high amount of citronellal, exerted the most potent anti-inflammatory effect in LPS-activated RAW264.7 cells compared to the other examined species, denoted by reduced levels of NO and pro-inflammatory cytokines IL-6 and TNF-α. It has been reported that E. citriodora essential oil, which contains citronellal, has anti-inflammatory and analgesic effects on formol-induced edema and acetic acid-induced abdominal cramps in Wistar rats (Gbenou et al., 2013). Citronellal exhibits anti-inflammatory and redox protective activities in carrageenan- and arachidonic acid-treated rats through the inhibition of enzymes in the arachidonic acid pathway (Melo et al., 2011). Citronellol is also effective as an analgesic in various pain models. Its action is thought to be mediated by the inhibition of TNF-α and NO synthesis, which could be associated with its strong antioxidant activity (Brito et al., 2012). In addition, 1,8-cineole, isolated from Eucalyptus essential oil extracts, inhibits the production of TNF-α and IL-1β in LPS-activated human blood monocytes (Juergens et al., 1998). Recently, Ho et al (2020) suggested that the inflammatory activity of E. citriodora essential oil might be due to a synergistic effect between essential oil components, which include 4-methylsyringol (41.3%), 4-hydroxyl-benzenemethanol (24.4%), citronellic acid (12.7%), trans, cis-iridolactone (7.6%), menthol (4.6%), 2-phenyl ethyl anthranilate (2.2%), manool (1.8%), citronellyl anthranilate (1.1%), and citronellyl tiglate (1.1%). The present study suggests that essential oils from Eucalyptus species, particularly E. citriodora, exert potent anti-inflammatory effects on LPS-induced inflammatory responses in RAW264.7 cells by inhibiting pro-inflammatory mediators and cytokines, including NO, IL-6, and TNF-α. Furthermore, the analysis by PCA separated the three EOs into three groups depended on their chemical profile and antioxidant and anti-inflammatory activities. The results suggested that E. citriodora had the highest content of oxygenated monoterpenes, mainly citronellal and exhibited the best antioxidant and anti-inflammatory activities compared to other oils.
CONCLUSION
Here, we investigated the in vitro antioxidant and anti-inflammatory activities of EOs from three Eucalyptus species, including E. citriodora, E. urophylla, and E. deglupta. The results demonstrated that EOs extracted from all three Eucalyptus species exhibited antioxidant and anti-inflammatory activities. The E. citriodora and E. urophylla leaf essential oils possessed strong antioxidant activity against H2O2-induced oxidative stress in human HepG2 cells. In addition, E. citriodora leaf essential oils, which contains a high concentration of citronellal, exerted the most potent anti-inflammatory effect in LPS-activated RAW264.7 macrophages. The antioxidant and anti-inflammatory effects of EOs are dependent on their chemical composition. The combination of oxygenated monoterpenes present in EOs might be responsible for these observed effects. This study suggests that among the three Eucalyptus species tested, E. citriodora leaf essential oils, which is a good source of oxygenated monoterpenes, could be considered as a potential phytochemical agent for the prevention of oxidative stress and inflammation.
ACKNOWLEDGMENTS
The authors are very thankful to Sahacogen Green, Co. Ltd. (Thailand), for providing the Eucalyptus leaf samples.
AUTHOR CONTRIBUTIONS
Orapin Insuan: Conceptualization, performed the experiments, analyzed the data, writing—original draft and editing, project administration, and funding acquisition. Benchaluk Thongchuai, Rujirek Chaiwongsa, and Supaporn Khamchun: Performed the experiments and analyzed the data. Wimonrut Insuan: Conceptualization, performed the experiments, analyzed the data, writing—original draft and editing. All co-authors revised and approved the final version of the manuscript.
CONFLICT OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
Almasa, I., Innocenta, E., Machumia, F.,and Kisinzab, W. 2019. Effect of Geographical location on yield and chemical composition of essential oils from three Eucalyptus species growing in Tanzania. Asian Journal of Traditional Medicines. 14: 1-12.
Ashour, H. M. 2008. Antibacterial, antifungal, and anticancer activities of volatile oils and extracts from stems, leaves, and flowers of Eucalyptus sideroxylon and Eucalyptus torquata. Cancer Biology & Therapy. 7: 399-403.
Barbosa, L.C.A., Filomeno, C.A., and Teixeira, R.R. 2016. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules. 21: 1671.
Bardaweel, S., Hudaib, M., and Tawaha, K. 2014. Evaluation of antibacterial, antifungal, and anticancer activities of essential oils from six species of Eucalyptus. Journal of Essential Oil Bearing Plants. 17: 1165-1174.
Benyoucef, F., Dib, M.E.A., Arrar, Z., Costa, J., and Muselli, A. 2018. Synergistic antioxidant activity and chemical composition of essential oils from Thymus fontanesii, Artemisia herba-alba and Rosmarinus officinalis. Journal of Applied Biotechnology Reports. 5: 151-156.
Brito, R.G., Guimarães, A.G., Quintans, J.S., Santos, M.R., De Sousa, D.P., Badaue-Passos, D. et al. 2012. Citronellol, a monoterpene alcohol, reduces nociceptive and inflammatory activities in rodents. Journal of Natural Medicines. 66: 637-644.
Chahomchuen, T., Insuan, O., and Insuan, W. 2020. Chemical profile of leaf essential oils from four Eucalyptus species from Thailand and their biological activities. Microchemical Journal. 158: 105248.
Chiu, Y.-W., Lo, H.-J., Huang, H.-Y., Chao, P.Y., Hwang, J.M., Huang, P.Y. et al. 2013. The antioxidant and cytoprotective activity of Ocimum gratissimum extracts against hydrogen peroxide-induced toxicity in human HepG2 cells. Journal of Food and Drug Analysis. 21: 253-260.
Chung, S.K., Osawa, T., and Kawakishi S. 1997. Hydroxyl radical-scavenging effects of spices and scavengers from brown mustard (Brassica nigra). Bioscience, Biotechnology, and Biochemistry. 61: 118-123.
Dhakad, A.K., Pandey, V.V., Beg, S., Rawat, J.M., and Singh A. 2018. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: a review. Journal of the Science of Food and Agriculture. 98: 833–848.
Dlamini, L.N., Pipatwattanakul, D., and Maelim, S. 2017. Growth variation and heritability in a second-generation Eucalyptus urophylla progeny test at Lad Krating Plantation, Chachoengsao province, Thailand. Agriculture and Natural Resources. 51: 158-162.
Döll-Boscardin, P.M., Sartoratto, A., Sales Maia, B.H.L.d.N., Padilha de Paula, J., Nakashima, T., Farago, P.V., et al. 2012. In vitro cytotoxic potential of essential oils of Eucalyptus benthamii and its related terpenes on tumor cell lines. Evidence-Based Complementary and Alternative Medicine. 2012: 342652.
Dong, L., Yin, L., Zhang, Y., Fu, X., and Lu, J. 2017. Anti-inflammatory effects of ononin on lipopolysaccharide-stimulated RAW 264.7 cells. Molecular Immunology. 83: 46-51.
Elaissi, A., Rouis, Z., Salem, N.A.B., Mabrouk, S., ben Salem Y., Salah, K.B.H., et al. 2012. Chemical composition of 8 eucalyptus species' essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complementary and Alternative Medicine. 12: 1-15.
Ghaffar, A., Yameen, M., Kiran, S., Kamal, S., Jalal, F., Munir, B., et al. 2015. Chemical composition an in vitro evaluation of the antimicrobial and antioxidant activities of essential oils extracted from seven Eucalyptus species. Molecules. 20: 20487-20498.
Gbenou, J.D., Ahounou, J.F., Akakpo, H.B., Laleye, E., Yayi, F., Gbaguidi, F., et al. 2013. Phytochemical composition of Cymbopogon citratus and Eucalyptus citriodora essential oils and their anti-inflammatory and analgesic properties on Wistar rats. Molecular Biology Reports. 40: 1127-1134.
Ho, C.-L., Li, L.-H., Weng, Y.-C., Hua, K.F., and Ju, T.C. 2020. Eucalyptus essential oils inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through reducing MAPK and NF-κB pathways. BMC Complementary Medicine and Therapies. 20: 1-11.
Horvathova, E., Navarova, J., Galova, E., Sevcovicova, A., Chodakova, L., Snahnicanova, Z., et al. 2014. Assessment of antioxidative, chelating, and DNA-protective effects of selected essential oil components (eugenol, carvacrol, thymol, borneol, eucalyptol) of plants and intact Rosmarinus officinalis oil. Journal of Agricultural and Food Chemistry. 62: 6632-6639.
Hussain, T., Tan, B., Yin, Y., Blachier, F., Tossou, M.C., Rahu N. 2016. Oxidative stress and inflammation: what polyphenols can do for us?. Oxidative Medicine and Cellular Longevity. 2016: 7432797.
Insuan, O., Janchai, P., Thongchuai, B., Chaiwongsa, R., Khamchun, S., Saoin, S., et al. 2021. Anti-inflammatory effect of pineapple rhizome bromelain through downregulation of the NF-κB- and MAPKs-signaling pathways in lipopolysaccharide (LPS)-stimulated RAW264.7 cells. Current Issues in Molecular Biology. 43: 93-106.
Insuan, W., and Chahomchuen T. 2020. Chemical composition and antimicrobial activity of essential oil extracted from Eucalyptus citriodora leaf. Microbiology and Biotechnology Letters. 48: 148-157.
Juergens, U.R., Stöber, M., and Vetter, H. 1998. Inhibition of cytokine production and arachidonic acid metabolism by eucalyptol (1.8-cineole) in human blood monocytes in vitro. European Journal of Medical Research. 3: 508-510.
Klaunig, J.E., Kamendulis, L.M., and Hocevar, B.A. 2010. Oxidative stress and oxidative damage in carcinogenesis. Toxicologic Pathology. 38: 96-109.
Lingan, K. 2018. A review on major constituents of various essential oils and its application. Journal of Translational Medicine. 8: 2161-1025.1000201.
Mcinnes, I.B., and Schett, G. 2017. Pathogenetic insights from the treatment of rheumatoid arthritis. The Lancet. 389: 2328-2337.
Mosser, D.M., and Gonçalves, R. 2015. Activation of murine macrophages. Current Protocols in Immunology. 111: 14.12. 11-14.12. 10.
Melo, M.S., Guimarães, A.G., Santana, M.F., Siqueira, R.S., De Lima, A.D.C.B., Dias, A.S., et al. 2011. Anti-inflammatory and redox-protective activities of citronellal. Biological Research. 44: 363-368.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., Rice-Evans, C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 26: 1231-1237.
Rea, I.M., Gibson, D.S., Mcgilligan, V., Mcnerlan, S.E., Alexander, H.D., and Ross, O.A. 2018. Age and age-related diseases: role of inflammation triggers and cytokines. Frontiers in Immunology. 9: 586.
Reuter, S., Gupta, S.C., Chaturvedi, M.M., Aggarwal, B.B. 2010. Oxidative stress, inflammation, and cancer: how are they linked?. Free Radical Biology and Medicine. 49: 1603-1616.
Rolo, A.P., Teodoro, J.S., and Palmeira, C.M. 2012. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radical Biology and Medicine. 52: 59-69.
Salehi, B., Sharifi-Rad, J., Quispe, C., Llaique, H., Villalobos, M., Smeriglio, A., et al. 2019. Insights into Eucalyptus genus chemical constituents, biological activities and health-promoting effects. Trends in Food Science & Technology. 91: 609-624.
Salem, N., Kefi, S., Tabben, O., Ayed, A., Jallouli, S., Feres, N., et al. 2018. Variation in chemical composition of Eucalyptus globulus essential oil under phenological stages and evidence synergism with antimicrobial standards. Industrial Crops and Products. 124: 115-125.
Sebei, K., Sakouhi, F., Herchi, W., Khouja, M.L., and Boukhchina, S. 2015. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biological Research. 48: 1-5.
Silva, J., Abebe, W., Sousa, S., Duarte, V., Machado, M., Matos, F. 2003. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. Journal of ethnopharmacology. 89: 277-283.
Singh, H.P., Kaur, S., Negi, K., Kumari, S., Saini, V., Batish, D.R., et al. 2012. Assessment of in vitro antioxidant activity of essential oil of Eucalyptus citriodora (lemon-scented Eucalypt; Myrtaceae) and its major constituents. LWT-Food Science and Technology. 48: 237-241.
Siramon, P., and Ohtani, Y. 2007. Antioxidative and antiradical activities of Eucalyptus camaldulensis leaf oils from Thailand. Journal of Wood Science. 53: 498-504.
Siramon, P., Ohtani, Y., and Ichiura, H., 2013. Chemical composition and antifungal property of Eucalyptus camaldulensis leaf oils from Thailand. Records of Natural Products. 7: 49-53.
Valko, M., Rhodes, C., Moncol, J., Izakovic, M., and Mazur, M. 2006. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biological Interactions. 160: 1-40.
Vipin, A., Rao, R., Kurrey, N.K., KA, A., and Venkateswaran, G. 2017. Protective effects of phenolics rich extract of ginger against Aflatoxin B1-induced oxidative stress and hepatotoxicity. Biomedicine & Pharmacotherapy. 91: 415-424.
Vuong, Q.V., Hirun, S., Chuen, T.L., Goldsmith, C.D., Munro, B., Bowyer, M.C., et al. 2015. Physicochemical, antioxidant and anti-cancer activity of a Eucalyptus robusta (Sm.) leaf aqueous extract. Industrial Crops and Products. 64: 167-174.
Warsito, W., Noorhamdani, N., Sukardi, S., and Suratmo S. 2018. Assessment of antioxidant activity of citronellal extract and fractions of essential oils of Citrus hystrix DC. Tropical Journal of Pharmaceutical Research. 17: 1119-1125.
Wirtz, P.H., and Von Känel, R. 2017. Psychological stress, inflammation, and coronary heart disease. Current Cardiology Reports 19: 111.
Zengin, H., and Baysal, A. H. 2014. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules. 19: 17773-17798.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Orapin Insuan1, 2, Benchaluk Thongchuai1, 2, Rujirek Chaiwongsa3, Supaporn Khamchun1, 2, and Wimonrut Insuan4,*
1 Department of Medical Technology, School of Allied Health Sciences, University of Phayao, Phayao 56000, Thailand
2 Unit of Excellence in Integrative Molecular Biomedicine, School of Allied Health Sciences, University of Phayao, Phayao 56000, Thailand
3 Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
4 Department of Veterinary Technology, Faculty of Veterinary Technology, Kasetsart University, Bangkok 10900, Thailand
Corresponding author: Wimonrut Insuan, E-mail: cvtwri@ku.ac.th
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: May 2, 2021;
Revised: August 12, 2021;
Accepted: August 16, 2021;

