
Effects of Dissolved O2 and Fe Availability on Growth, Morphology, Aerenchyma Formation and Radial Oxygen Loss of Canna indica L. and Heliconia psittacorum L.f.
Tanapong Suriyakaew and Arunothai Jampeetong*Published Date : 2021-09-13
DOI : https://doi.org/10.12982/CMUJNS.2021.086
Journal Issues : Number 4, October-December 2021
Abstract In constructed wetlands (CWs), plants are usually affected by low O2 levels. Under such conditions, most soluble iron is reduced to ferrous (Fe2+) which is highly soluble, and toxic to plants as well. As a consequence of excessive ferrous iron with low O2 supply, plant growth is reduced, leading to declining nutrient removal efficiency. This study was conducted to determine the effects of different dissolved oxygen levels (normoxia and hypoxia) with Fe supplied on growth, morphology, and root anatomy of two wetland plants (Canna indica and Heliconia psittacorum). The plants were grown on a nutrient solution modified from Smart and Barko (1985) under normoxic and hypoxic conditions. All plants were grown in greenhouse conditions for 42 days. Plant growth rates and biomass accumulation were drastically reduced under hypoxia while leaf number was not affected. Under hypoxia, root diameter and root porosity also increased in C. indica, whereas H. psittacorum had greater aerenchyma formation. Moreover, C. indica showed adaptive traits to cope with hypoxia and Fe stress by increasing radial oxygen loss (ROL), releasing O2 to the rhizosphere to resist toxic effects of ferrous iron under hypoxia. In contrast, H. psittacorum had no ROL under hypoxia. Moreover, the plants showed leaf chlorosis, leaf roll, and root rotting. Hence, it is suggested that C. indica could have better performance than H. psittacorum to treat wastewater in CWs as this species can adapt to hypoxic conditions and releases O2 into rhizosphere which improves dissolved oxygen (DO) in the wastewater.
Keywords: Aerenchyma, Dissolved oxygen, Iron, Root porosity, Wetland emergent plant
Funding: The authors are appreciated for the research funding partly provided by the Chiang Mai University, Thailand.
Citation: Suriyakaew, T. and Jampeetong, A. 2021. Effects of dissolved O2 and Fe availability on growth, morphology, aerenchyma formation and radial oxygen loss of Canna indica L. and Heliconia psittacorum L.f. CMU J. Nat. Sci. 20(4): e2021086.
INTRODUCTION
Water pollution is a widespread global problem which is continuously increasing with increasing human population. The wastewater mostly has low dissolved oxygen which is a result of organic matter degradation by microorganisms. In addition, eutrophication from excessive nutrients (nitrogen and phosphorous) promotes the growth of phytoplankton and macrophytes. Subsequently, dissolved oxygen in water is consumed drastically. Moreover, domestic and agricultural wastewater may contain iron from household products, detergents, fertilizers, mine water drainage, and groundwater irrigation. Iron in water has an unfavorable impact on humans, microorganisms, and plants (Lesley et al., 2008; Tjandraatmadja et al., 2008; Jia et al., 2018; Marin-Rivera et al., 2019).
In an effort to reduce pollutants in the wastewater run into water bodies, many treatment systems have been developed such as physical aeration, iron exchange, reverse osmosis, chemical neutralization, and chemical precipitation (Lesley et al., 2008). Most wastewater treatment methods are expensive and require electrical power and are complicated to manage. Constructed wetlands (CWs) are an economical, eco-friendly, effective, and aesthetic method to improve water quality (Inamori et al., 2008; Marin-Rivera et al., 2019). In CWs, plants have essential roles in nutrient absorption and turning nutrients from the wastewater into plant biomass. Besides plant roots perform biofiltration by promoting suspended solid precipitation. The roots of plants provide areas for microbial growth and also release oxygen into the rhizosphere, facilitating nutrient removal by microbial degradation and plant uptake. Moreover, the secretion of root exudates, which are organic carbon compounds stimulates microbial activity and promotes the denitrification process (Sandoval et al., 2019; Zhai et al., 2013). However, plants in CWs tend to suffer from oxygen insufficiency, especially when high-strength wastewater is drained into CWs. As a result, plants decrease growth and lower their nutrient requirement, with consequent impact on water treatment efficiency. Moreover, under hypoxic conditions, most ferric iron (Fe3+) in wastewater is reduced into ferrous iron (Fe2+), which is more soluble and is easily taken up by plant roots. According to Sidek et al. (2020), when using Eleocharis dulcis (Burm.f) Trin. ex Hensch to treat Fe-contaminated wastewater (Fe = 2.20 mg L-1), the plants expressed leaf chlorosis and some plants were dead after 14 days. Treating acid mine drainage with Phragmites australis (Cav.) Trin. ex Steud., it was found that iron concentration higher than 75 mg L-1 can affect plant growth, and then decrease water treating efficiency (Wu et al., 2019).
Many wetland plants have strategies to cope with low O2 availability and high external ferric iron. Some species, for example, Cyperus flabelliformis Rottb., Myriophyllum spicatum L., Vallisneria spiralis L. and Juncus effusus L. produce aerenchyma for air preservation and release oxygen from roots into the rhizosphere. Therefore, hazardous dissolved substances (Fe2+, Mn2+, NH4+) turn into less toxic, insoluble, or unabsorbed forms (Fe3+, FeOOH, Mn3+, NO3-) (Lemonie et al., 2012; Liu et al., 2004; Deng et al., 2009).
Nowadays, a great number of wetland plant species have potential for use in treating many kinds of wastewater due to their fast growth, high nutrient uptake, robustness to wastewater stress conditions, and ease of management (Latune et al., 2017). Previous studies showed that the efficiency of CWs varied with different plant species. Wetland plants in tropical areas, that are exposed to higher temperatures and sunlight, tend to be more efficient than those in sub-tropical areas (Tran et al., 2019). Not only should the plants have good potential performance in CWs, but they should be easily available in their regions and have resistance to environmental conditions and diseases. Thus, we focused on naturalized species as a good choice for wastewater treatment in CWs. Among a large number of plant species, Canna indica L. and Heliconia psittacorum L.f. were selected. C. indica has high-efficiency nitrogen and phosphorous removal in CWs, and immense tolerance to high nutrient concentrations, chemicals, and metal ions. Its high stem and broad leaves block sunlight from penetrating the water column, limiting the growth of phytoplankton (Huang et al., 2017; Jiang et al., 2020). While H. psittacorum is tolerant of high nutrients and drought, and maintains normal growth when CW’s are drained (Latune et al., 2017). Besides, Canna and Heliconia are ornamental plants, bear long-lasting large colorful raceme inflorescences, attract pollinators, and increase esthetics of CWs (Konnerup et al., 2009). Even though, these two species have been considered for use in CWs, there was little known on their ability to grow and cope with iron particularly at low O2 availability. Hence, this study aims to assess plant growth, morphology, aerenchyma formation, root porosity, and radial oxygen loss of C. indica and H. psittacorum when the plants grow under low O2 with an iron supply. The new insights will provide useful information for plant selection in CWs.
MATERIALS AND METHODS
Plant preparation
C. indica were collected from water-saturated clay in Chiang Mai Province and H. psittacorum were collected from well-drained sandy soil in Lamphun Province, Thailand. Approximately 100 mm rhizome sections with mature plants were washed by hand to remove clay and soil, then grown in water-saturated sand until new plants emerged. Afterward the new plants (approximately 300 mm shoot length) were selected and gently cleaned by hand to remove sand. Then the plants were placed in 5 L buckets containing nutrient solution modified from Smart and Barko (1985) to which 0.25 mM NH4-N and 0.25 mM NO3-N prepared from (NH4)2SO4 and KNO3 and a plant micronutrient solution (Tropica, Denmark) were added. The pH of the nutrient solution was 6.8 ± 0.2 adjusted using 1.0 M NaOH and HCl. The plants were acclimatized under greenhouse conditions at the Department of Biology, Chiang Mai University for one week.
Experimental design
After acclimation, 20 plants (each individual plant was approximately 350 mm height and 30 g fresh weight) were selected. Each individual plant was grown in a tall cylinder-shaped glass container (Ø = 10 cm and height 20 cm) containing 1 L nutrient solution modified from Smart and Barko (1985) supplied with 0.25 mM NH4-N, 0.25 mM NO3-N and 80 mg L–1 Fe-EDDHA (Fe3+)(pH = 6.8). The plants were grown in the following treatments (n = 5): (i) normoxic condition (DO = 6-8 mg L-1), the nutrient solution was continually supplied with air pumped by an aerator and (ii) hypoxic condition (DO = 2-3 mg L-1), agar was added to the nutrient solution (0.5g L–1) and the water surface was covered with a polyethylene sheet to prevent air from penetrating the water.
The plants were grown in greenhouse conditions at the Department of Biology, Chiang Mai University. The temperature and light regimes during the experiment were 33-36˚C: 15-23˚C day: night and approximately 13 h light/ 11 h dark. The nutrient solutions were changed every week to ensure adequate nutrient, iron concentration, pH, and dissolved oxygen levels. The treatments were placed in a randomized arrangement.
Growth and morphological study
After 42 days, plant growth and morphology were determined by measuring total plant height, root length, leaf number, lateral root density, and average leaf area. Then, the plants were harvested and separated into 3 parts: leaves, rhizomes, and roots. The relative growth rate (RGR, d⁻¹) was calculated according to Evans (1972) using the formula: (lnW2-lnW1)/ (d2-d1) where W1 and W2 are initial and final dry mass (g) and d1 and d2 are initial and final time (days).The shoot elongation rate (SER, mm d–1) was calculated from the increase of plant height (mm) divided by number of days of experiment.
Root radial oxygen loss (ROL) and porosity
A day before harvest, the ROL from roots was observed according to Armstrong and Armstrong (1988). The plants (n = 5) were cleaned and placed in glass vials containing 2 L agar solution. The solutions were prepared by dissolving methylene blue 0.012 g L–1 in agar-water medium (0.5 g L–1) and O2 was reduced by addition of 0.12 g L–1 sodium dithionite (Na2S2O4). The solution is colorless after O2 is reduced. Then, a blue area develops around roots as methylene blue reacts with O2 released from the roots. Approximately 300 mg fresh mature roots were used for root porosity measurement by the pycnometer method according to Sojka (1988). The roots were cut, then dried with tissue paper. The fresh weight was recorded as Wr. Then, the pycnometer was filled with distilled water and weighed as Ww. The roots were put into the pycnometer containing water and weighed as Ww+r. To remove air collected in the roots, the roots were crushed using mortar and pestle and placed back into the pycnometer filled with water and weighed as Wh. The root porosity was calculated following by the formula:
% root porosity = 100 (Wh-Ww+r)/(Ww+Wr-Ww+r)
Root anatomy
Three parts (basal, middle, and apical) of the mature roots (n = 10) were selected. Root cross-sections were made by free-hand section techniques (Lux et al., 2005). The sections were stained with 0.05% safranin O. Then, the sections were observed and photographed under a 40x light microscope (Olympus, Japan). Root diameters and cortical air spaces were calculated using the program Image J (National Institutes of Health, USA).
Statistical analysis
All results were analyzed using SPSS statistics, version 17.0 (SPSS Inc., USA). The results were tested for variance homogeneity using Levene’s test. Then, one-way analysis of variance (ANOVA) was performed followed by Tukey’s HSD test at a 5% significance level to determine treatment differences. All results were presented as means with standard errors (Mean ± SE).
RESULTS
Effect of O2 levels under Fe supplied on plant growth and morphology
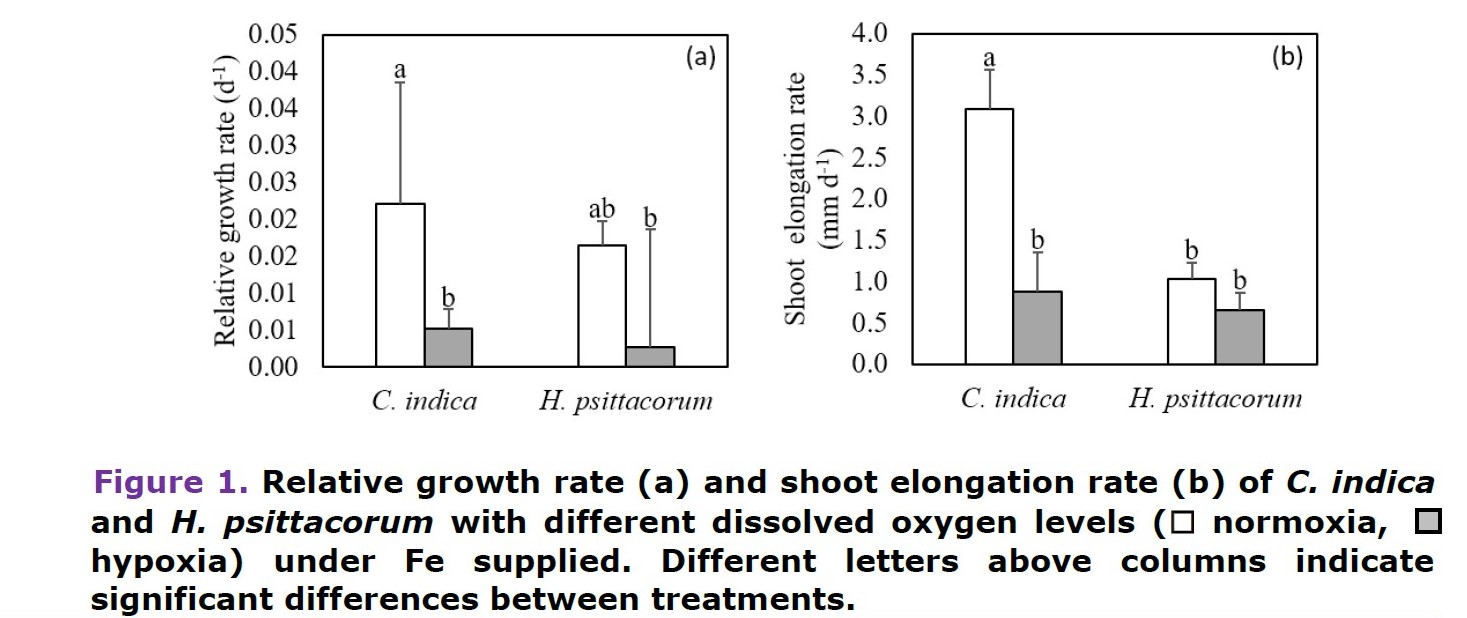
Generally, C. indica had higher growth rates and total biomass than H. psittacorum. Under hypoxic condition, C. indica decreased relative growth rates and shoot elongation rates (Figure 1a, b). Total biomass, particularly leaf dry mass of each species, significantly decreased when the plants grew under hypoxia, resulting in low shoot:root ratios (Table 1).
C. indica had no symptoms on leaves under low O2 supply, while H. psittacorum showed leaf chlorosis, leaf curling (epinasty), withered leaves and defoliation under hypoxia.
O2 levels did not affect root biomass, but they affected root morphology in both species. Under hypoxia, H. psittacorum root length significantly increased. New roots of C. indica had a slightly red-brown color. In H. psittacorum under hypoxia, it had longer but fewer number of roots. Moreover, it had decreased apical lateral root density (Table 1, Figure 3).
Table 1. Biomass, growth and root parameters of C. indica and H. psittacorum (mean ± SE) grown at different dissolved oxygen levels under Fe supplied. Different letters designate significant differences between treatments (P < 0.05).
|
|
C. indica |
H. psittacorum |
F-ratio |
|
||||
|
normoxia |
hypoxia |
normoxia |
hypoxia |
|
|
|||
|
Total leaf dry mass (g) |
3.6 ± 0.2a |
2.3 ± 0.2b |
2.4 ± 0.2b |
1.2 ± 0.4c |
14.4*** |
|
||
|
Total rhizome dry mass(g) |
1.8 ± 0.5a |
1.1 ± 0.1ab |
0.7 ± 0.1ab |
0.8 ± 0.2b |
3.6* |
|
||
|
Total root dry mass (g) |
0.8 ± 0.2 |
0.9 ± 0.1 |
0.6 ± 0.1 |
0.5 ± 0.1 |
2.7 |
|
||
|
Total dry biomass (g) |
6.3 ± 0.7a |
4.3 ± 0.1b |
3.6 ± 0.4bc |
2.4 ± 0.4c |
13.1*** |
|
||
|
Shoot:root ratio |
3.9 ± 0.7 |
2.7 ± 0.2 |
3.9 ± 0.9 |
2.5 ± 0.6 |
1.8 |
|
||
|
Plant height (mm) |
427.7 ± 58.3 |
404.6 ± 16.2 |
394.0 ± 15.5 |
403.0 ± 14.8 |
0.2 |
|
||
|
Average leaf area (cm2) |
82.80 ± 8.41a |
58.46 ± 3.15ab |
56.04 ± 10.00ab |
48.58 ± 2.09b |
4.7* |
|
||
|
Number of leaves |
8.2 ± 0.4a |
8.8 ± 0.9a |
5.4 ± 0.07b |
2.8 ± 0.7b |
16.3*** |
|
||
|
Root length (mm) |
160.4 ± 12.0ab |
127.6 ± 13.8b |
135.6 ± 13.8b |
199.2 ± 6.0a |
5.9** |
|
||
|
Lateral root density (LRs/cm) |
|
|
|
|
||||
|
Basal |
7.5 ± 0.7 |
9.0 ± 1.2 |
8.8 ± 1.3 |
5.2 ± 1.1 |
2.6 |
|
||
|
Middle |
12.8 ± 1.0ab |
17.8 ± 3.8a |
8.9 ± 1.3b |
10.8 ± 2.8b |
8.3** |
|
||
|
Apical |
12.4 ± 1.6ab |
17.8 ± 3.9a |
6.9 ± 2.6bc |
4.7 ± 2.2c |
15.2*** |
|
||
|
Root diameter (µm) |
|
|
|
|
||||
|
Basal |
2,324.3 ± 130.2a |
2,390.8 ± 161.8a |
594.6 ± 48.1b |
546.8 ± 43.9b |
90.0*** |
|
||
|
Middle |
2,003.0 ± 56.9b |
2,441.9 ± 121.9a |
452.4 ± 35.2c |
476.2 ± 24.9c |
212.9*** |
|
||
|
Apical |
1,533.6 ± 71.5ab |
1,717.8 ± 141.4a |
401.8 ± 23.8bc |
361.8 ± 40.1c |
76.5*** |
|
||
|
Aerenchyma formation (%) |
|
|
|
|
||||
|
Basal |
0.5 ± 0.2b |
2.8 ± 1.2ab |
3.8 ± 1.6ab |
6.1 ± 1.0a |
4.5* |
|
||
|
Middle |
2.7 ± 0.8b |
3.2 ± 0.8b |
7.0 ± 1.8ab |
12.9 ± 2.2a |
8.5** |
|
||
|
Apical |
1.8 ± 0.6b |
1.4 ± 0.3b |
3.6 ± 1.8b |
10.0 ± 2.0a |
6.6** |
|
||
|
Internal air space (µm2) |
|
|
|
|
||||
|
Basal |
25,790.0 ± 3,800.5b |
87,254.4 ± 10,572.5a |
25,492.2 ± 10,008.2b |
22,055.4 ± 2,415.8b |
16.2* |
|
||
|
Middle |
63,235.3 ± 10,729.4b |
108,903.4 ± 19,035.0a |
21,022.6 ± 3,595.6b |
24,522.5 ± 6,122.9b |
12.4** |
|
||
|
Apical |
23,164.7 ± 6,035.0 |
28,735.6 ± 10,740.5 |
6,830.1 ± 2,966.0 |
15,238.5 ± 838.9 |
2.0 |
|
||
*P <0.05, **P <0.01, ***P <0.001
Effect of O2 levels under Fe supplied on root anatomy, aerenchyma formation, root porosity and radial oxygen loss
Under hypoxia, C. indica significantly increased root diameter and internal air space in the basal and middle parts of the roots. While H. psitacorum tended to increase internal air space under hypoxia but there was no significantly different from the normoxic conditions. Similarly, both species tended to increase aerenchyma under hypoxia. However, significantly increased in aerenchyma formation in apical root zone of H. psitacorum was observed (Table 1, Figure 4).
Under hypoxia, C. indica significantly increased in root porosity while there was no significant change in H. psittacorum (Figure 2a). Moreover, both species had slightly increased in root angle, and the roots tended to rise to the water surface when grown under low O2 supply. (Figure 2b).
Under normoxic conditions, the pattern of ROL was similar in both species, as the basal and the middle part of the roots stained light blue. After an hour, the lateral roots tended to blue-stain faster than the main roots, which took about twelve hours to appear blue stained. Interestingly, there were different ROL patterns between two plant species under hypoxia. C. indica had more O2 leaked from all parts of the roots, whereas H. psittacorum had no O2 leaked from the whole roots (Figure 3).
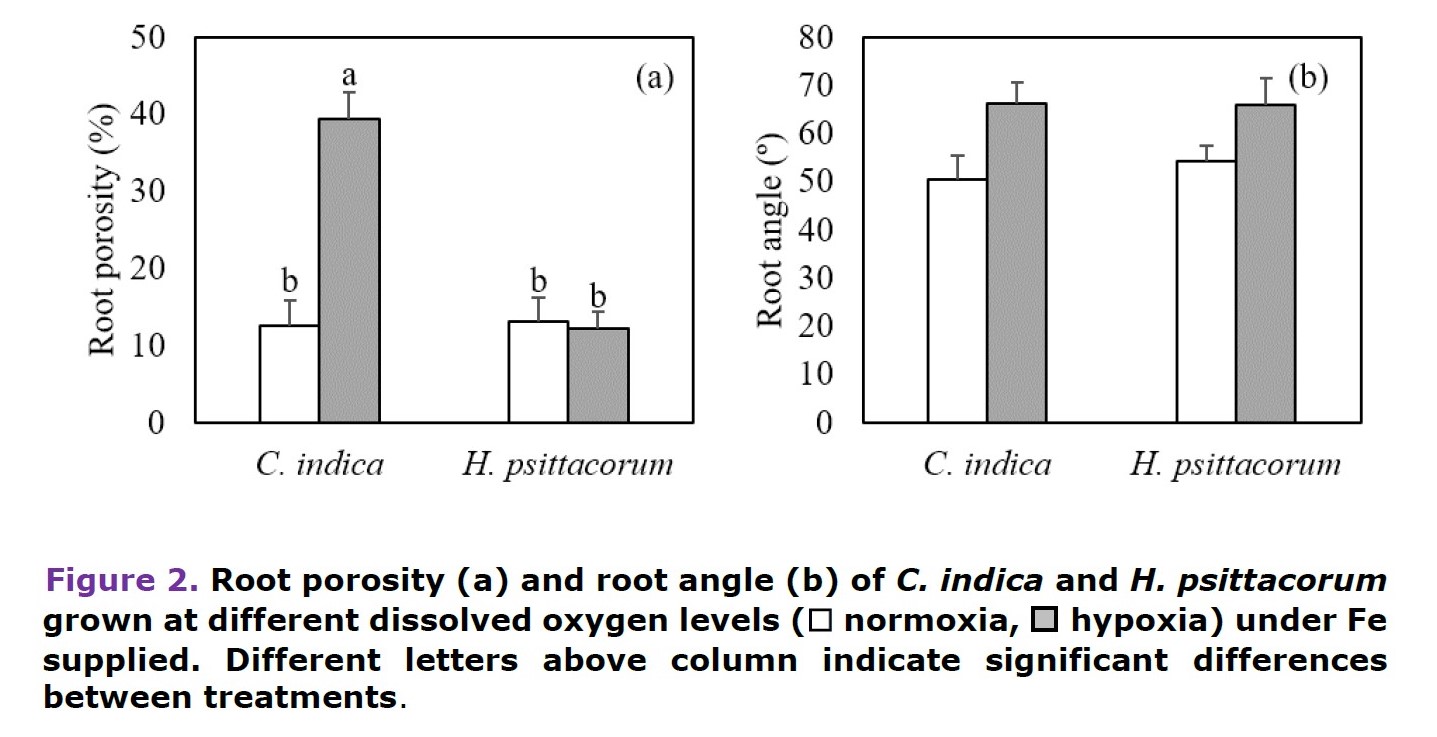
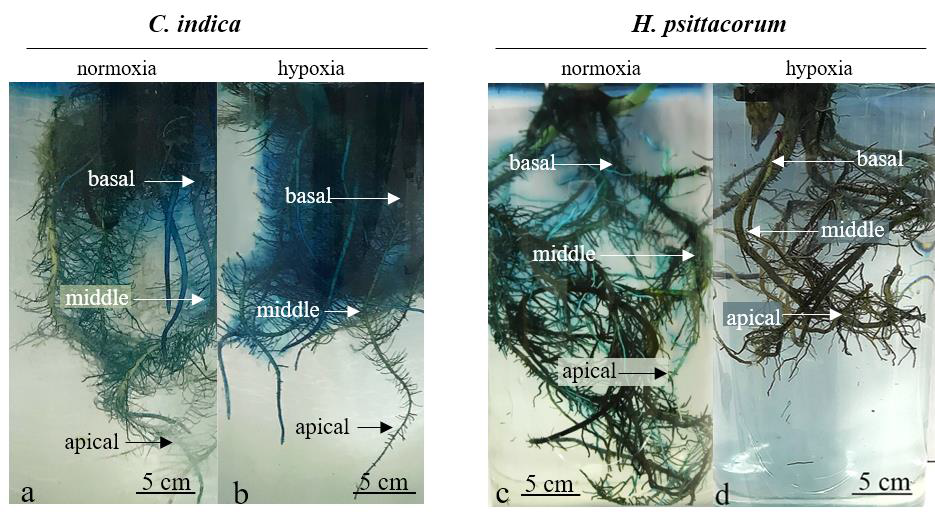
Figure 3. Oxygen released from roots of C. indica (a, b) and H. psittacorum (c, d) grown under normoxic and hypoxic conditions under Fe supplied. Blue color indicates zone of O2 released from root to rhizosphere.
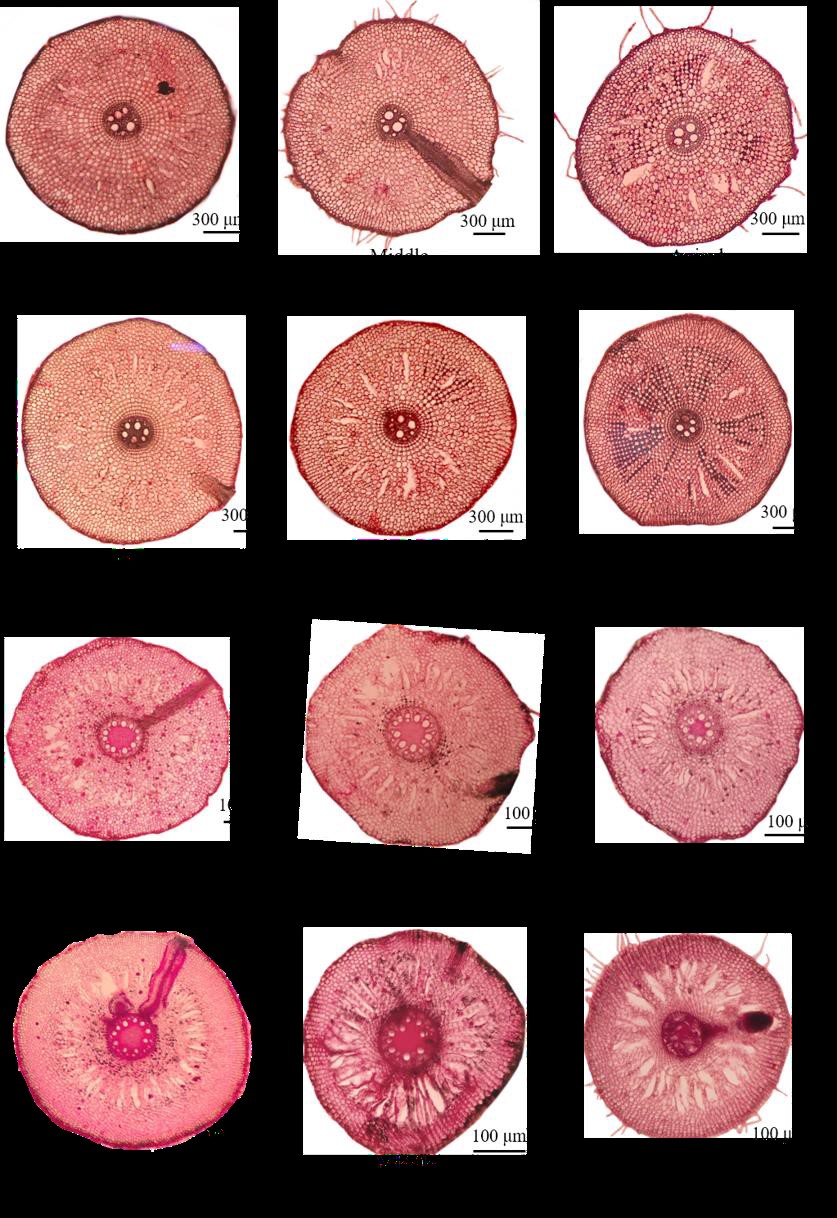
Figure 4. Root cross-sections at basal, middle and apical zone of C. indica and H. psittacorum grown under normoxic and hypoxic conditions under Fe supplied.
DISCUSSION
Generally, C. indica and H. psittacorum grew well in the aerated nutrient solution with an Fe supply. However, plant growth and total biomass drastically decreased under hypoxic conditions. Similar results were found in Pennisetum purpureum x P. americanum cv. Pakchong 1; the plants not only decreased leaf biomass but also reduced transpiration rates, photosynthetic rates, and nutrient uptake rates when grown under hypoxia (Dat et al., 2018; Muenrew and Jampeetong, 2018). In this study, no signs of iron toxicity were observed in C. indica. The greater ROL of this species can oxidize Fe2+ to an insoluble form (Fe3+), so that less Fe2+ is taken up by the plants. In contrast, severe symptoms such as leaf chlorosis, leaf curling (epinasty), wilted leaves and defoliation were found in H. psittacorum, which has no ROL. Leaf chlorosis in H. psittacorum was probably caused by high Fe2+ taken up and accumulated in the tissue, which interferes with chlorophyll formation by generating reactive oxygen species (ROS), promoting chlorophyll degradation and diminishing photosynthesis (Saaltink et al., 2017). Leaf curling, corresponding to hypoxic stress, makes the plants decline in water uptake, and leaves wilted as has been found in Physalis peruviana L. grown under hypoxia (Aldana et al., 2014.). In general, root biomass is highly reduced under hypoxia (Jampeetong and Muenrew, 2016; Pederson et al., 2019) but in this study H. psittacorum and C. inidica had no significant change of root biomass under hypoxia. However, it was found that each species had different root responses to hypoxic conditions in terms of root number and lateral root production. The primary root of C. indica was shorter, but it produced more lateral roots. In contrast, the primary root of H. psittacorum was longer but produced lower numbers of new roots and shorter lateral roots. As available oxygen declines, decrease of root number may be one strategy to reduce root O2 requirements in order to maintain root growth and activity (Folzer et al., 2006; Jampeetong and Muenrew, 2016).
Ability to form aerenchyma is an important root trait that allows many wetland plant species to grow under hypoxic conditions. In this study, aerenchyma formation tended to increase in the basal and middle parts of the root in both species but was not significant. At the basal parts, most cells were loose parenchyma cells, providing intercellular space for air preservation (Jung et al., 2008). Normally, C. indica had greater root diameter than H. psittacorum regardless of O2 availability. Under hypoxia, C. indica greatly increased root diameters in the middle parts of roots. Due to the long roots of both plant species, air transported from shoots to roots by passive O2 diffusion is inadequate for root respiration. Increase in aerenchyma combined with increasing root diameter, leads to more space for air transportation (Brix, 1993; Khan et al., 2016; Li et al., 2013). Under hypoxia, C. indica well developed internal air space area (87,254 μm2) compared with H. psittacorum (22,055 μm2) likewise greater root porosity was found in C. indica than H. psittacorum. Moreover, both plants slightly increased root angle and developed internal air spaces; the roots containing air tends to be lighter than the roots containing less air, resulting in root growth upward to the water surface where O2 levels are sufficient for aerobic processes of roots. Similar results were found in Arabidopsis thaliana (L.) Heynh. and Oryza sativa L. exposed to hypoxia, both plants species increased root air space and adjusted their primary and adventitious root angles upward to the water surface to avoid the hypoxic soil layer (Pederson et al., 2019).
Under normoxic conditions, C. indica had higher ROL than H. psittacorum even though both plant species had the same ROL pattern. Under hypoxic conditions, C. indica greatly increased O2 loss from its roots. The O2 release was observed from the main roots and lateral roots especially at the basal and middle parts of roots where there were great increases of aerenchyma formation and root porosity. In C. indica, higher ROL was clearly influenced by increasing root porosity. Likewise, the ROL was positively correlated to root porosity under hypoxia in some wetland plants such as Juncus effusus L., Rumex crispus L., Rumex palustris Sm. and Salix nigra Marshall (Visser et al., 2000; Li et al., 2006; Manzur et al., 2014). Unlike C. indica, O2 released from the roots of H. psittacorum under hypoxia was lower than normoxia even though aerenchyma formation increased. It might be because H. psittacorum produced fewer new roots and lateral roots when compared with normoxia. In many wetland plants, the greatest ROL has been found in subapical parts of active young roots and lateral roots compared to old roots. Moreover, leaf wilting and stomata closure leading to reduced O2 entering the aerial parts of the plants along with reducing lateral root density, result in less area for gas movement and limit permeability of ROL (Li et al., 2013; Pincam et al., 2020).
The results of growth, biomass production, aerenchyma formation, root porosity and ROL suggest that with sufficient oxygen, both C. indica and H. psittacorum could be used in CWs. Under low O2 condition, C. indica may be preferred over H. psittacorum as it overcomes hypoxia and Fe rich wastewater by developing extensive aerenchyma and increasing ROL greatly. According to Cheng et al. (2014), plant species with higher ROL correlate with higher growth and photosynthesis rate, leading to higher nutrient removal efficiency in CWs. Moreover, the oxygenation of the rhizosphere promotes the growth of microorganisms and facilitates nutrient uptake (Khan et al., 2016; Sandoval et al., 2019). In addition, C. indica has abundant lateral roots which provide surface area for microorganism attachment and might lead to improving nutrient removal by microbial nutrient cycle processes, e.g., nitrification. The higher ROL also leads to tolerance to pollutants as root released O2 neutralizes toxic substances such as Fe2+, Mn2+ and H2S through oxidation or aerobic microorganism in the rhizosphere. As plants permit O2 released from the roots, Fe2+ tends to be oxidized into Fe3+causing iron precipitation onto root surfaces, called iron plaque. Tai et al. (2018) pointed out that amounts of iron plaque on C. indica increase with the concentration of Fe2+ in the medium, varying from 0-200 mg L-1. It has been documented that iron plaque can be either a barrier or a reservoir. Functioning as a barrier, iron plaque can prevent excess uptake, leading to metal tolerance in plants (Jia et al. 2018; Khan et al., 2016). In this study, C. indica showed little rust-color on new roots when growing in hypoxic conditions and did not show leaf chlorosis from excessive iron accumulation in leaves. On the other hand, the iron plaque on the root surface provided more surface for nutrient uptake. As mentioned by Tai et al. (2018) root iron plaque formation can enhance phosphorous uptake. However, this role might result from Fe plaque thickness and composition (Zhang et al., 2020). In contrast, H. psittacorum should not be used for long-term hypoxia and Fe contaminated wastewater treatment since it showed toxic symptoms, leading to a releasing of organic matter from root to the solution. From this situation, DO was dramatically declined which could lead to a death of plant and worse water quality.
CONCLUSION
In summary, under low O2 availability, both species decreased growth rates, biomass, and leaf areas. C. indica responded effectively to hypoxia and Fe stress by increasing of internal air space and radial oxygen loss. Whereas H. psittacorum seems to be not well adapted to low O2 with high Fe availability. Under such conditions, this species showed stress symptoms e.g. leaf chlorosis, leaf curling, and root rotting combined with reduction of ROL. Thus, this study suggests that C. indica is more satisfactory for Fe contaminated wastewater treatment under low O2 conditions.
ACKNOWLEDGEMENTS
This research was supported by a Development and Promotion of Science and Technology Talents Project scholarship (DPST), Thailand and was also supported by Chiang Mai University, Thailand. Finally, the authors thank Mr. Alvin Yoshinaga for his kind help to check and correct the English text.
AUTHOR CONTRIBUTIONS
Tanapong Suriyakaew conducted the experiments, performed the statistical analysis, data visualization and wrote original draft of the manuscript. Arunothai Jampeetong designed and supervised T. Suriyakaew, conducted the experiments, commented and edited the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no conflict of interests.
REFERENCES
Aldana, F., Garcia, P.N., and Fischer, G. 2014. Effect of water logging stress on the growth, development and symptomatology of cape gooseberry (Physalis peruviana L.) plants. Ciencias Naturales. 38: 393-400.
Armstrong, J. and Armstrong, W. 1988. Phragmites australis – A preliminary study of soil-oxidizing sites and internal gas transport pathways. New Phytologist. 108: 373-382.
Brix, H. 1993. Macrophyte-mediated oxygen transfer in wetlands: transport mechanisms and rates. Constructed Wetlands for Water Quality Improvement. Lewis Publishers, Boca Raton, Florida.
Cheng, X.-Y., Wang, M., Zhang, C.-F., Wang, S.-Q., and Chen, Z.-H. 2014. Relationships between plant photosynthesis, radial oxygen loss and nutrient removal in constructed wetland microcosms. Biochemical Systematics and Ecology. 54: 299-306.
Dat, J.F., Folzer, H., Parent, C., Badot, P.-M., and Capelli, N. 2018. Hypoxia stress: understanding and perspective. Global Science Books. Besançon, France.
Deng, H., Ye, Z.H., and Wong, M.H. 2009. Lead, zinc and iron (Fe2+) tolerances in wetland plants and relation to root anatomy and spatial pattern of ROL. Environmental and Experimental Botany. 65: 353-362.
Evans, C.G. 1972. The quantitative analysis of plant growth. Oxford: Blackwell Scientific.
Folzer, H., Dat, J.F., Rieffel, D., and Badot, P.M. 2006. Response of sessile oak seedlings (Quercus petraea) to flooding: an integrated study. Tree Physiology. 26: 759-766.
Huang, P., Zhang, D., Bai, S. and Qin, S. 2017. Application of combined emergent plants in floating bed for phytoremediation of landscape pond in South China. International Journal of Environmental Technology and Management. 20: 22-36.
Inamori, R., Wang, Y., Yamamoto, T., Zhang, J., Kong, H., Xu, K. and Inamori, Y. 2008. Seasonal effect on N2O formation in nitrification in constructed wetlands. Chemosphere. 73: 1071–1077.
Jampeetong, A., and Muenrew, J. 2016. Interactive effects of NH4+ concentration and O2 availability on growth, morphology, and mineral allocation of hybrid Napier grass (Pennisetum purpureum × P. Americanum cv. Pakchong 1). Ecological Engineering. 91: 409-418.
Jia, X., Otte, M.L., Liu, Y., Qin, X., Lu, X., Jiang, M., and Zou, Y. 2018. Performance of iron plaque of wetland plants for regulating iron, manganese, and phosphorus from agricultural drainage water. Water. 10: 42.
Jiang, X., Tian, Y., Ji, X., Lu, C., and Zhang, J. 2020. Influences of plant species and radial oxygen loss on nitrous oxide fluxes in constructed wetlands. Ecological Engineering. 142: 105644.
Jung, J., Lee, S.C., and Choi, H.-K. 2008. Anatomical pattern of aerenchyma in aquatic and wetland plants. Journal of Plant Biology. 51(6): 428-439.
Khan, N., Seshadri, B., Bolan, N., Saint, C.P., Kirkham, M.B., Chowdhury, S., Yamaguchi, N., Lee, D.Y., Li, G., Kunhikrishnan, A., Qi, F., Karunanithi, R., Qiu, R., Zhu, Y.-G., and Syu, C.H. 2016. Root iron plaque on wetland plants as a dynamic pool of nutrients and contaminants. Advances in Agronomy. 138: 1-96.
Konnerup, D., Koottatep, T., and Brix, H. 2009. Treatment of domestic wastewater in tropical, subsurface flow constructed wetlands planted with Canna and Heliconia. Ecological Engineering. 35: 248-257.
Latune, R.L., Laporte-Daube, O., Fina, N., Peyrat, S, Perus, L. and Molle, P. 2017. Which plants are needed for a French vertical-flow constructed wetland under a tropical climate?. Water Science and Technology. 75:1873-1881.
Lemonie, D.G., Mermillod-Blondin, F., Barrat-Segretain, M.-H., Masse, C., and Malet, E. 2012. The ability of aquatic macrophytes to increase root porosity and radial oxygen loss determines their resistance to sediment anoxia. Aquatic Ecology. 46: 191-200.
Lesley, B., Daniel, H., and Paul, Y. 2008. Iron and manganese removal in wetland treatment systems: rate, processes and implications for management. Science for the Total Environment. 394: 1-8.
Li, S., Reza Pezeshiki, S., and Douglas Shields, F. 2006. Partial flooding enhances aeration in adventitious roots of black willow (Salix nigra) cuttings. Journal of Plant Physiology. 163: 619-628.
Li, L., Yang, Y., Tam, N.F.Y., Yang, L., Mei, X.-Q., and Yang, F.-J. 2013. Growth characteristics of six wetland plants and their influences on domestic wastewater treatment efficiency. Ecological Engineering. 60: 382-392.
Liu, W.J., Zhu, Y.G., Smith, F.A., and Smith, S.E. 2004. Do phosphorous nutrition and iron plaque alter arsenate (As) uptake by rice seedlings in hydroponic culture?. New Phytologist. 162:481-488.
Lux, A., Morita, S., Abe, J., and Ito, K. 2005. An improved method for clearing and staining free-hand sections and whole-mount samples. Annals of Botany. 96: 989-996.
Manzur, M.E., Grimoldi, A.A., Insausti, P., and Striker, G.G. 2014. Radial oxygen loss and physical barriers in relation to root tissue age in species with different types of aerenchyma. Functional Plant Biology. 42: 9-17.
Marin-Rivera, J.V., Martinez-Giron, J., and Quintero-Angel, M. 2019. Effectiveness of vertical subsurface wetlands for iron and manganese removal from wastewater in drinking water treatment plants. Universitas Scientiarum. 24: 135-163.
Muenrew, J. and Jampeetong, A. 2018. Interactive effects of O2 levels and Fe supply on growth, morphology, and mineral allocation of hybrid Napier grass (Pennisetum purpureum × P. Americanum cv. Pakchong 1). Songklanakarin Journal of Science and Technology. 40(6): 1271-1280.
Pederson, O., Sauter, M., Colmer, T.D., and Nakazono, M. 2019. Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytologist. 229: 42-49.
Pincam, T., Brix, H., and Jampeetong, A. 2020. Growth performance of tropical wetland species (Cyperus involucratus Rottb. and Thalia geniculate L.) in anaerobic digester effluent and their water treatment efficiency. Ecological Engineering. 143(105667): 1-10.
Saaltink, R.M., Dekker, S.C., Eppinga, M.B., Griffioen, J., and Wassen, M.J. 2017. Plant-specific effects of iron-toxicity in wetlands. Plant Soil. 416: 83-96.
Sandoval, L., Zamora-Castro, S.A., Vidal-Álvarez, M., and Marín-Muñiz, J.L. 2019. Role of wetland plants and use of ornamental flowering plants in constructed wetlands for wastewater treatment: a review. Applied Sciences. 9: 685.
Sidek, N.M., Ahmad, N.U., Abdullah, S.R.S., and Draman, S.F.S. 2020. Phytoremediation of ex mining lake water in constructed wetland by perennial plants. Chiang Mai University Journal of Natural Sciences. 19: 580-594.
Smart, R.M. and Barko, J.W. 1985. Laboratory culture of submerged freshwater macrophytes on natural sediments. Aquatic Botany. 21: 251-263.
Sojka, R.E. 1988. Measurement of root porosity (volume of root air space). Environmental and Experimental Botany. 28: 275-280.
Tai, Y., Tam, N.F., Wang, R., Yang, Y., Lin, J., Wang, J., Yang, Y., Li, L., and Sun, Y. 2018. Iron plaque formation on wetland roots accelerates removal of water-borne antibiotic. Plant and Soil. 433: 323-338.
Tjandraatmadja, G., Diaper, C., Gozukara, Y., Burch, L., Sheedy, C., and Price, G. 2008. Sources of critical contaminants in domestic wastewater: contaminant contribution from household products. CSIRO: Water for a Healthy Country National Research Flagship, Australia.
Tran, H.D., Vi, H.M.T., Dang, H.T.T., and Narbaitz, R.M. 2019. Pollutant removal by Canna generalis in tropical constructed wetlands for domestic wastewater treatment. Global Journal of Environment Science and Management. 5: 331-344.
Visser, E.J.W., Colmer, T.D., Blom, C.W.P.M., and Voesenek, L.A.C.J. 2000. Change in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant, Cell and Environment. 23: 1237-1245.
Wu, S., Vymazal, J., and Brix, H. 2019. Critical review: biogeochemical networking of iron in constructed wetlands for wastewater treatment. Environmental Science & Technology. 53(14): 7930-7944.
Zhai, X., Piwpuan, N., Arias, C.A., Healey, T., and Brix, H. 2013. Can root exudates from emergent wetland plants fuel denitrification in subsurface flow constructed wetland systems?. Ecological Engineering. 61: 555-563.
Zhang, Q., Yan, Z., and Li., X. 2020. Ferrous iron facilitates the formation of iron plaque and enhances the tolerance of Spartina alterniflora to artificial sewage stress. Marine Pollution Bulletin. 157: 111379.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Tanapong Suriyakaew1 and Arunothai Jampeetong2,*
1 Graduate Master’s Degree Program in Biology, Faculty of Science, Chiang Mai University, Chiang Mai, 50200, Thailand
2 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, 50200, Thailand
Corresponding author: Arunothai Jampeetong, E-mail: Arunothai.2519@gmail.com
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: February 16, 2021;
Revised: July 4, 2021;
Accepted: July 19, 2021;

