
The Use of Hematological and Histopathological Biomarkers to Assess the Health of Aquatic Ecosystems in Koh Sichang, Thailand
Anek Sopon, Jes kettratad, Ajcharaporn Piumsomboon, Gen Kaneko, and Sinlapachai Senarat*Published Date : 2021-09-13
DOI : https://doi.org/10.12982/CMUJNS.2021.085
Journal Issues : Number 4, October-December 2021
Abstract Koh Sichang area in Thailand is a sink for a wide variety of contaminants such as heavily polluted water from industry and oil spills. This situation may affect the health status of fish living in the area, but such information remains scarce. In this study, we evaluated the health status of java rabbitfish Siganus javus, an important marine fish in Koh Sichang, using hematological and histopathological biomarkers. All fish samples were collected from the Koh Sichang area during December 2017 and January 2018. Although the salinity, pH, and dissolved oxygen levels at sampling points were all within the normal range, abnormal nuclei were observed in erythrocytes (up to ~6% of all erythrocytes) and in some leucocytes (neutrophil, lymphocyte and monocyte). Visceral organs (gill, kidney and liver) were apparently normal in terms of gross morphology, but a wide variety of the histopathological alterations were found at the microscopic level: epithelial hyperplasia and aneurysm in gills; blood congestion and melanomacrophage centers (MMCs) in the liver; renal degeneration, granuloma and MMCs together with unidentified parasites in kidney. Calculation of semi-quantitative parameters [histological alteration index (HAI) and the average value of alteration (AVA)] demonstrated the highest frequency of histopathological alterations in kidney, suggesting that kidney is a sensitive organ. Overall, our observations suggest that S. javus in Koh Sichang is under the pathological state and warrants conservation efforts.
Keywords: Erythrocytes, Fish Health, Hematology, Histopathology, Kidney
Funding: This project was financially supported by the Grant for Research and Development of The Thailand Research Fund (TRF) and Electricity Generating Authority of Thailand.
Citation: Sopon, A., Kettratad, J., Piumsomboon,A., Kaneko,G., and Senarat, S. 2021 The use of hematological and histopathological biomarkers to assess the health of aquatic ecosystems in Koh Sichang, Thailand. CMU J. Nat. Sci. 20(4): e2021085.
INTRODUCTION
Koh Sichang is a small island well known as a popular tourist destination, which is located in an economically important area of Thailand. Because of the location, this area receives wastewater from public transport, industry, agriculture and urban runoff (Wattayakorn and Rungsupa, 2012). The Koh Sichang area has also received catastrophic oil spills, resulting in chemical contaminations that might exert harmful effects on aquatic animals (Wattayakorn and Rungsupa, 2012; Senarat et al., 2018). It is therefore important to monitor the health status of aquatic animals in this area. Fish would be a good sentinel organism for this purpose because they are dominant in many aquatic environments including the Koh Sichang area, and they are very sensitive to environmental changes/problems (National Research Council, 1991; Beeby, 2001; Frame and Dickerson, 2006).
Hematology is one of the important tools to monitor the health status and has been extensively used for aquatic organisms (Hayashi et al., 1998; Hrubec et al., 2000; Rodriguez-Cea et al. 2003; Thrall et al., 2007). Especially, abnormal blood composition has been well associated with the impaired fish health caused by environmental problems (Castro et al., 2019). Increased numbers of erythrocytes and leucocytes as well as the increase in the packed erythrocyte volume have been recorded in rohu, Labeo rohita (Hamilton 1822), from the polluted Lakes of Bangalore, India (Zutshi et al., 2010). Frequent erythrocyte alterations were reported from Colossoma macropomum living in the anthropic areas (Castro et al., 2019). The formation of chromatin-containing bodies in erythrocyte cytoplasm has often been used to monitor the water quality and the health of the aquatic organisms (Carins et al., 1975; Brugs et al., 1977; Rodriguez-Cea et al., 2003).
Histopathology is also a valuable yardstick to evaluate fish health and ecosystem status (Dalzochio et al., 2016) that identifies histological abnormalities at cellular, tissue, and organ levels (Teh et al., 1997; Senarat et al., 2015; Mansouri et al., 2016; Barbieri et al., 2016; Senarat et al., 2018a; Senarat et al., 2018b; Senarat et al., 2019; Senarat et al., 2020). For example, Louiz (2018) has evaluated the effect of pollution on the liver in black goby Gobius niger Linnaeus 1758 and grass goby Zosterisessor ophiocephalus (Pallas, 1814) in Tunisian lagoons. Liver lesions were found in specimens from polluted areas, suggesting the reduced liver function caused by environmental problems. Hepatic histopathological alterations were also found in Micropterus salmoides (Lacépède, 1802) from the Pigeon River, USA, which include hyperplastic basophilic hepatocytes, severe lipidosis, vacuolated and basophilic foci (Teh et al., 1997). In addition to the liver, gill is an important target of histopathological analysis. For example, abnormal gill structures including aneurysm, edema, hyperplasia and fusion were found in Iberian barbel Luciobarbus bocagei (Steindachner, 1864), Iberian nase Pseudochon drostoma (Coelho, 1985) and rainbow trout Oncorhynchus mykiss (Walbaum, 1792), living in Northern Portuguese river, Portugal, contaminated with heavy metals (Fonseca et al., 2017). Along with the liver and gill, kidney has also been used in histopathological analysis in many fish species (Puttipong et al., 2021; Mangang et al., 2021).
The java rabbitfish Siganus javus (Linnaeus, 1766) is one of the most important economical marine fish in Southeast Asia, particularly in Thailand. This fish is widely found around Koh Sichang, but to our knowledge the current study is the first detailed record of hematological and histopathological features of S. javus from the area. Our study indicates potential health risks of S. javus in the Koh Sichang area, which warrants the environmental protection practices around the island.
MATERIALS AND METHODS
Materials
Fish samples and environmental factors
Thirty-eight samples of adult Siganus javus with 7.16 ± 2.41 (mean ± standard deviation) inches of total length were collected by using swing and fishing hooks from two main stations (station 1: 13° 8'9.41"N, 100°47'58.75"E and station 2: 13° 8'47.98"N, 100°47'42.26"E) around Koh Sichang, Thailand (Figure 1), during December 2017 and January 2018. Physical and chemical parameters, such as water depth, water temperature, salinity, pH and dissolved oxygen (DO) during the time of sampling were measured using an EC900 AMTAST Waterproof DO Kit 9-in-1 Meter (AMTAST, Lakeland, FL, USA). The experimental protocol was approved by the Institutional Animal Care and Use Committee, Aquatic Resources Research Institute (IACUC ARRI) [1931001].
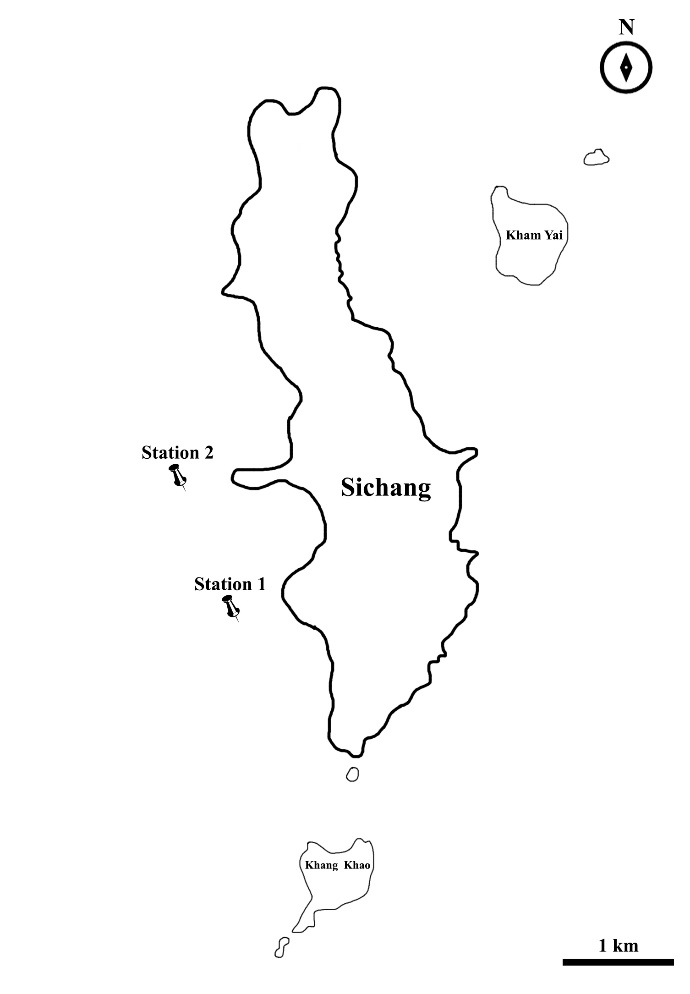
Figure 1. Sampling localities of Siganus javus from Koh Sichang, Thailand, including station 1 and station 2.
Hematological observation
All fish specimens were euthanized by a rapid cooling shock (Wilson et al., 2009). The bloods were collected by venipuncture of the caudal vertebral vein using a 21G × 1” needle and a 1- or 2-mL syringe (NIPRO, Japan), following the standard method of Watson et al. (1989). Blood smears were prepared on glass slides using the manual wedge technique, fixed in methanol for 1 min, air-dried, and stained with the Wright-Giemsa solution for 25 min. The slides were washed three times with phosphate-buffered saline (PBS, pH 7.4) and mounted by the mounting medium. The blood cells were characterized by visual examination of the peripheral blood smear according to the guideline of standard hematology (Theml et al., 2004) and photographed using a Leica DM 1000 light microscope equipped with a 100× oil-immersion lens (Leica, Wetzlar, Germany). Additionally, the number of abnormal erythrocytes (30 cells/fish per slide) and leucocytes (20 cells/fish per slide) were quantified.
Histopathological observation
All fish samples were abdominally opened and assessed for the external morphology of visceral organs. Gill, kidney and liver were fixed with the 10% neural buffer formalin (NBF). The anterior, middle, and posterior parts of each organ were randomly selected, cut into small pieces (1 × 1 cm), and then processed by the permanent histological preparation method (Presnell and Schreibman, 1997; Suvarna et al., 2013). The paraffin-embedded tissues were sectioned at 4 µm thickness and stained by Harris's hematoxylin and eosin (H&E) (Presnell and Schreibman, 1997; Suvarna et al., 2013). Histopathological alterations of the organs were photographed using a Leica digital 750 light microscope using a digital camera (Leica, Wetzlar, Germany).
All tissues (gill, liver and kidney) were semi-quantitatively analyzed for their histopathological alterations and classified into several degrees of damage based on the histological alteration index (HAI) (Poleksic and Mitrovic-Tutundzic, 1994) and average value of alteration (AVA) (Poleksic and Mitrovic-Tutundzic, 1994; Schwaiger et al., 1997).
To calculate the HAI, degrees of alterations in each tissue were evaluated by the standard criteria of progressive tissue damage stage (Table 1) following previous publications (Poleksic and Mitrovic-Tutundzic, 1994; Paulo et al., 2012; Dos Santos et al., 2018; Barbierl et al., 2019). The HAI was calculated using the equation: HAI = 1 × ∑ I + 10 × ∑ II + 100 × ∑ III, where I, II, and III corresponded to alterations of stage I, II and III (Table 1), respectively. Based on the HAI, tissue damage was classified into five categories including 0 to 10 (normal organ/tissue functioning), 11 to 20 (slight alteration in the organ/tissue), 21 to 50 (moderate alteration in the organ/tissue), 51 to 100 (severe alteration in the organ/tissue) and values above 100 (irreparable alteration in the organ/tissue) [Poleksic and Mitrovic-Tutundzic, 1994].
The AVA was calculated according to the frequency of occurrence and severity of lesions, which can be classified into three categories; score 1 (no pathological alteration of organs, score 2 (slight or mild pathological alterations of organs), and score 3 (severe and extensive pathological alterations of organs), following Poleksic and Mitrovic-Tutundzic (1994).
Table 1. Classification of the severity of histopathological alterations in adult Siganus javus. Adapted from Poleksic and Mitrovic-Tutundzic (1994); Paulo et al. (2012); Dos Santos et al., (2018); Barbierl et al., (2019)
|
Histopathological alterations |
stage |
|
Gill |
|
|
Disorganization of secondary lamellae |
I |
|
Blood congestion |
I |
|
Epithelial hyperplasia |
I |
|
Lamellar aneurysm |
II |
|
Liver |
|
|
Vacuolar degeneration of hepatocyte |
I |
|
Sinusoidal dilatation |
I |
|
Blood congestion |
I |
|
Melanomacrophage centers (MMCs) |
I |
|
Degeneration of hepatic cytoplasm |
II |
|
Kidney |
|
|
Unidentified parasite |
I |
|
MMCs |
I |
|
Renal degeneration |
II |
|
Necrotic tissue |
III |
|
Granuloma |
III |
RESULTS
Environmental factors
Environmental factors were recorded to compare the station 1 and station 2 (Table 2). In both stations, temperature, pH, and DO were within the standard range for marine environmental resources (Pollution Control Department, 2017). We found no major differences in environmental factors and biological characteristics of S. javus (see below). We therefore described the results of fish from both stations together.
External and internal characteristics
No abnormalities were observed in the external and internal characteristics of all fish examined (Figures 2A-2B), but the pronounced visceral adipose accumulation was found in 19 out of the 38 samples (Figure 3, 50% prevalence).
Table 2. Environmental parameters collected from Koh Sichang, Thailand, during December 2017 and January 2018-2019.
|
Environmental parameters |
Stations |
Permissible limits and reference |
|
|
1 |
2 |
||
|
Water depth (m) |
14.3 |
15.6 |
- |
|
Water temperature (°C) |
28.3 |
28.3 |
28 – 32 (Pollution Control Department, 2017) |
|
Salinity (psu) |
28.56 |
28.8 |
- |
|
pH |
8.16 |
8.13 |
7.0 – 8.5 (Pollution Control Department, 2017) |
|
Dissolved oxygen (DO) (mg/L) |
4.31 |
4.50 |
≥ 4 (Pollution Control Department, 2017) |
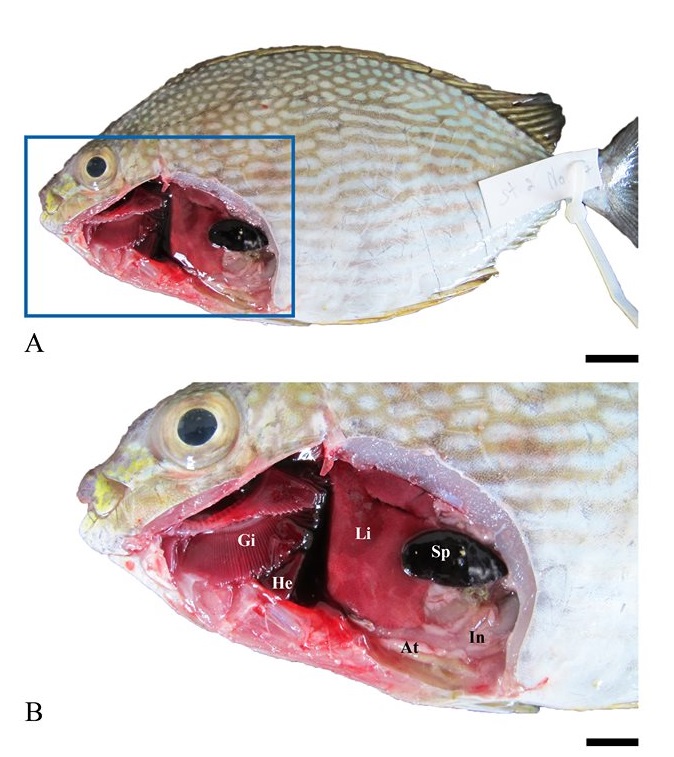
Figure 2. External morphology (A) and visceral organs (B) of adult Siganus javus from Koh Sichang. Abbreviations: At = adipose tissue, Gi = gill, In = intestine, Li = liver, He = heart, Sp = spleen. Scale bar A = 0.5 mm, B = 0.2 mm.
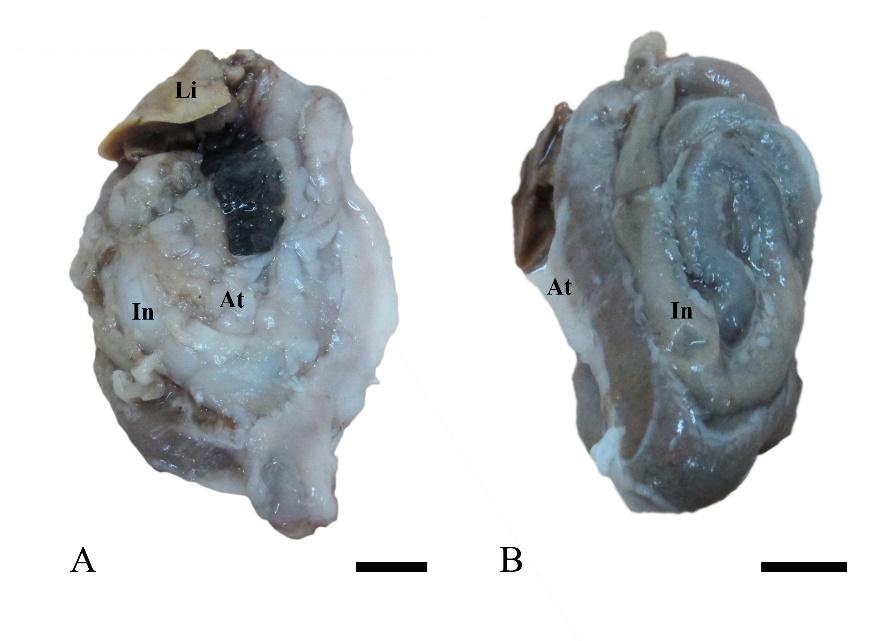
Figure 3. The prominent accumulation of adipose tissue (At) in the visceral organ of adult Siganus javus observed in 50% of individuals from Koh Sichang (A). The remaining individuals had normal adipose morphology (B). Abbreviation: In = intestine Scale bar A-B = 0.3 mm.
Hematological parameters
By the peripheral blood smear analysis, different types of blood cells including erythrocytes, leucocytes and platelets were identified (Figure 4).
Erythrocytes of the elliptical shape were the prominent blood cell in this fish (Figure 4A). Their oval, dark-purple nucleus was centrally located in the cell, being surrounded by the light pink cytoplasm (Figure 4A). We found several erythrocytic nuclear abnormalities. Some nuclei showed notched (20.17% proportion) and blebbed (9.21% proportion) shapes (Figures 4B-4D, Table 3). Interestingly, the present study also identified potentially apoptotic cells in the blood smear (Figures 4E, 4G). On the other hand, thrombocytes occupied 8.02% of total blood cells in unhealthy fish (Table 3). These cells had a spindle-shape and contained a purple-stained nucleus (Figure 4F).
In this study, three important types of leucocytes including monocytes (Figures 4G, 4I), neutrophils (Figure 4H) and lymphocytes (Figure 4J) were observed. Monocytes were larger than lymphocytes. They had a large nucleus with a horseshoe or bean shape (Figure 4I) and the basophilic nucleoplasm. Monocytes occupied a low proportion in blood cells (9.60% proportion) and were rarely found in the blood smear. Neutrophils were found at a higher proportion (21.57%) (Table 3). Neutrophils had an oval shape and contained a multi-lobulated nucleus (Figure 4H). Lymphocytes were small agranulocytes of approximately 12-15 µm diameter (Figure 4J). Lymphocytes occupied the highest proportion in leucocytes in this study (35.65%) (Table 3). This cell had a large nucleus with dark purple color surrounded by a narrow layer of lightly basophilic cytoplasm without granules.
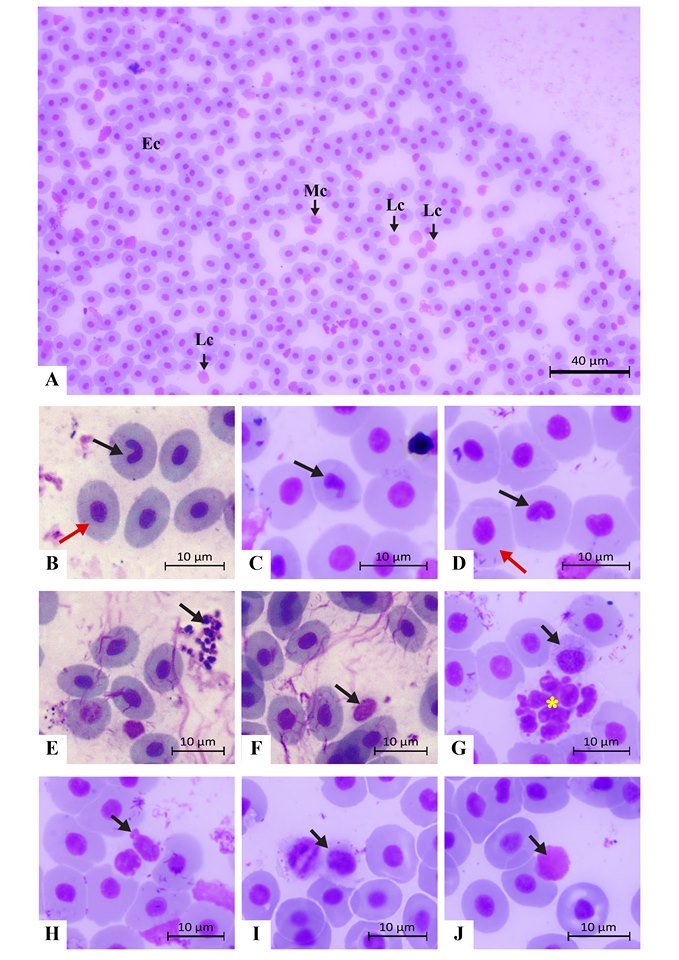
Figure 4. Blood cells of adult Siganus javus from Koh Sichang. A: An overview of a blood smear slide containing erythrocytes (Ec), monocytes (Mc) and lymphocytes (Lc). B: An erythrocyte containing a notched nucleus (arrow) with normal erythrocytes (red arrow indicates one of them). C: An erythrocyte containing a blebbed nucleus (arrow). D: An erythrocyte containing a large, notched nucleus (arrow) with normal erythrocytes (red arrow indicates one of them). E: Potentially apoptotic cells (arrow). F: A thrombocyte. G: A monocyte (arrow) located close to a large apoptotic cell (yellow asterisk). H: A neutrophil with two lobes of nucleus (arrow). I: A small monocyte (arrow). J: A lymphocyte (arrow).
Table 3. Proportion of blood cell types of adult Siganus javus from Koh Sichang during December 2017 and January 2018.
|
Blood cell types |
Unhealthy fish (Percentage proportion, %) N=38 (Mean ± SD) |
|
Notched nuclei of erythrocyte |
20.17 (mean = 6.05 ± 2.74 from total erythrocyte [n=30]) |
|
Blebbed nuclei of erythrocyte |
9.2 (mean = 2.76 ± 0.84 from total erythrocyte [n=30]) |
|
Thrombocytes |
8.02 (mean = 1.60 ± 0.71 from total blood cell [n=20]) |
|
Monocytes |
9.60 (mean = 1.92 ± 0.66 from total blood cell [n=20]) |
|
Neutrophils |
21.57 (mean = 4.31 ± 1.20 from total blood cell [n=20]) |
|
Lymphocytes |
35.65 (mean = 7.13 ± 2.24 from total blood cell [n=20]) |
Histological and histopathological examination of gill
The gill showed a comb-like structure consisted of the gill raker, gill arch and gill filaments. Many histopathological alterations were observed. The disorganization of secondary lamella was the most frequent gill lesion found (Figures 5A-5B). The lamellar aneurysm, which is the hypertrophic walls and blood congestion, were also identified from all individuals tested (Figures 5C-5D). The epithelial hyperplasia was also observed (Figure 5B).
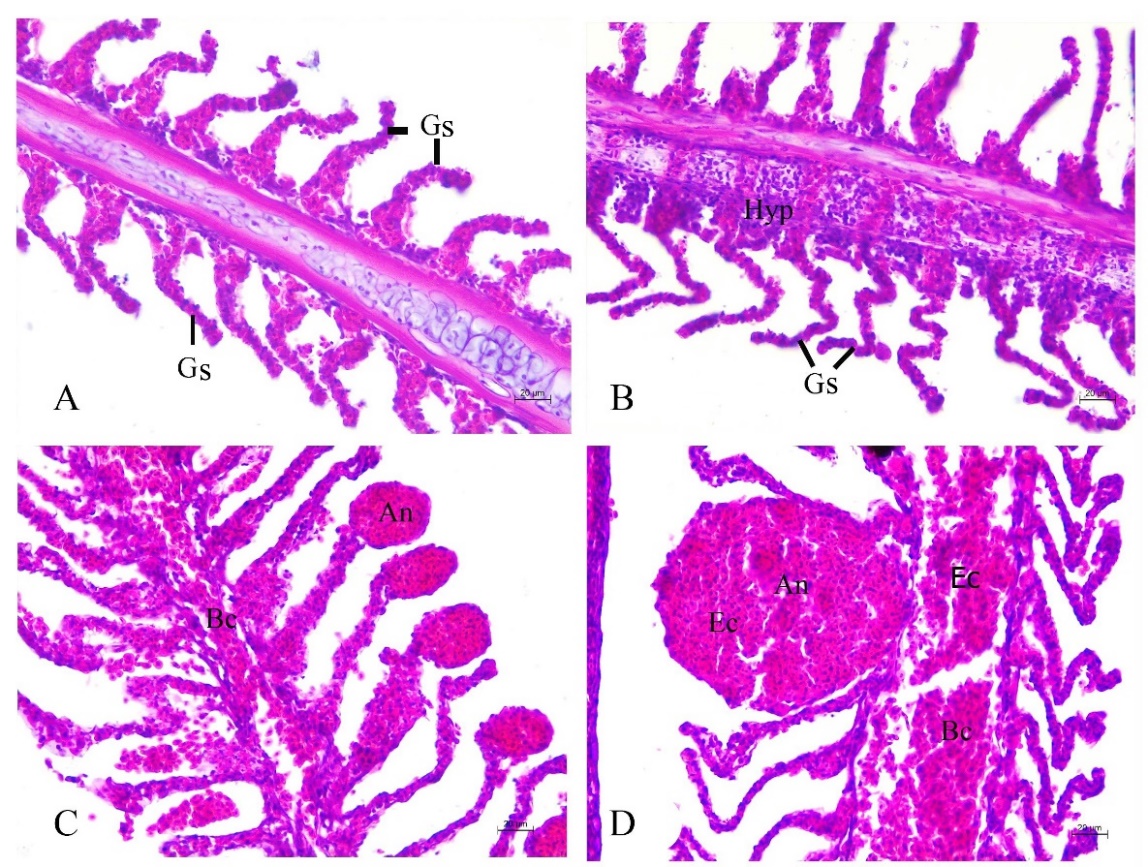
Figure 5. Histopathology of gill in adult Siganus javus from Koh Sichang. A: Disorganization of secondary lamellae (Gs). B: Disorganization of secondary lamellae (Gs) together with hyperplasia of gill epithelium (Hyp). C: Lamellar aneurysm (An) and blood congestion (Bc). D: High magnification showing lamellar aneurysm (An) with an erythrocyte (Ec).
Histological and histopathological examination of liver
The liver tissue consisted of numerous hepatocytes and hepatic sinusoids (Figure 6A). Degeneration of hepatocytic cytoplasm and the progressive loss of sinusoidal structure (or dilation of venous sinus) were observed (Figure 6B) throughout the liver with blood congestion (Figure 6C). The vacuolar degeneration (or vacuolated cytoplasm) associated with the foamy appearance was observed (hepatocellular lipidosis, Figure 6B). Clusters of melanomacrophage centers (MMCs) containing yellowish-brown pigments were found in the liver (Figures 6C-6D).
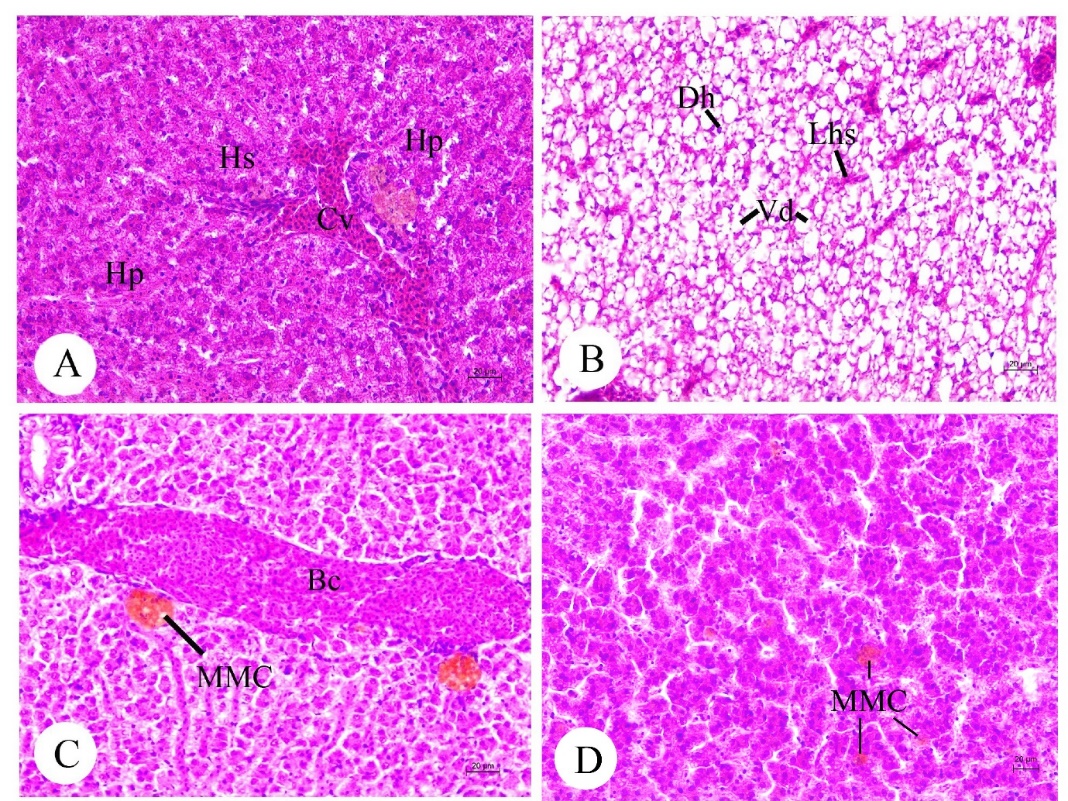
Figure 6. Histology and histopathology of liver (A-D) in adult Siganus javus from Koh Sichang. A: A lobular liver was composed of hepatocytes (Hp), hepatic sinusoid (Hs) and the central vein (Cv). B: High magnification showing the hepatic degeneration (Hd), loss of hepatic sinusoids (Lhs) and vacuolar degeneration (Vd). C-D: Blood congestion (Bc) and the clusters of melanomacrophage centers (MMCs) among the hepatocytes.
Histological and histopathological examination of kidney
The kidney parenchyma of S. javus was composed of the renal corpuscle (Bowman´s capsule and glomerulus) and renal tubule in the large area of hematopoietic tissue (Figure 7A). Histopathological alterations including the renal tubular degeneration and necrotic tissue were seen (Figures 7B-7C). The cluster of MMCs was observed in kidneys (Figure 7D). In addition to the histopathological alterations, unidentified parasites and granuloma were found in kidneys (Figures 7E-7F).
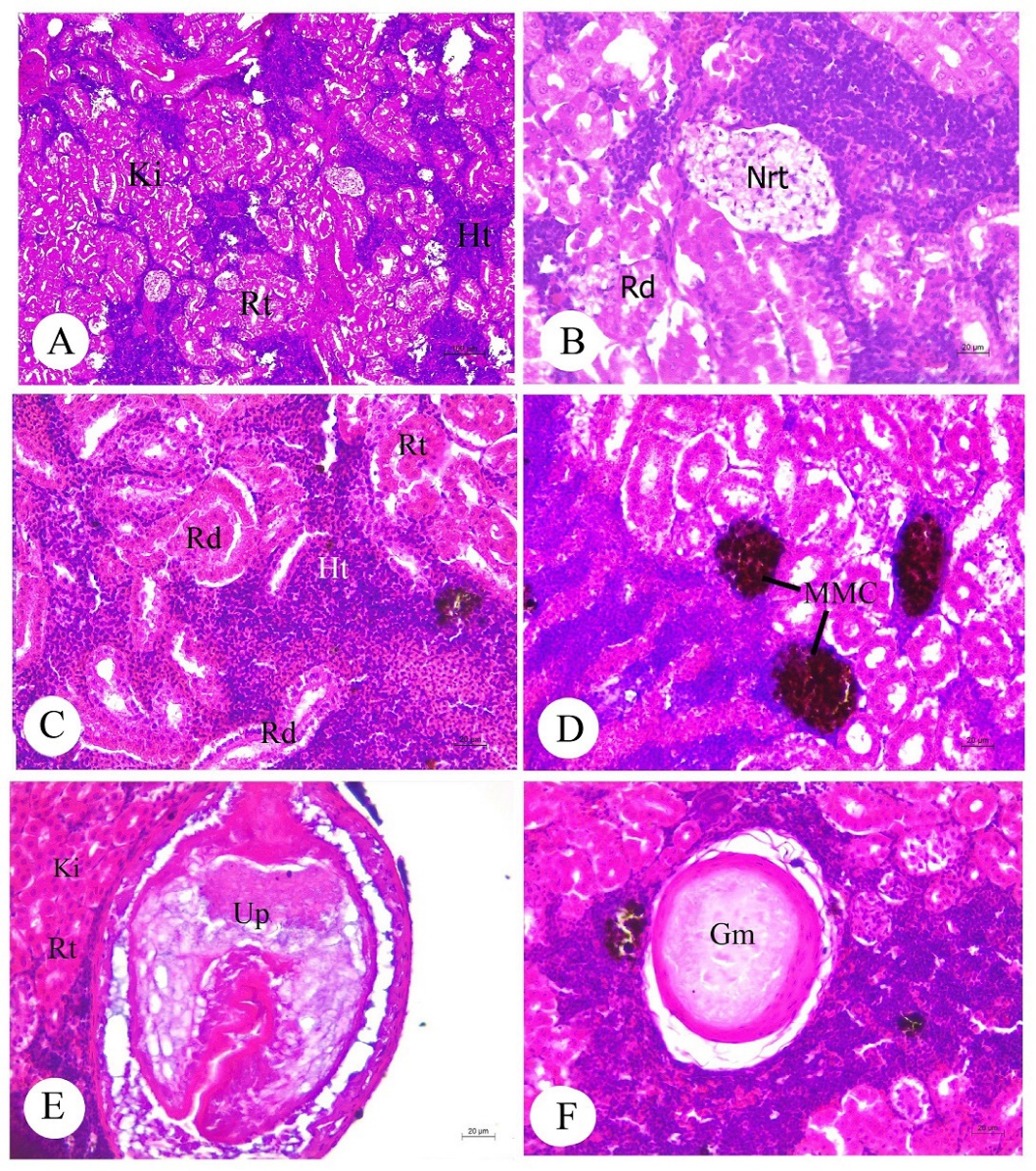
Figure 7. Histology and histopathology of kidney (A-F) in adult Siganus javus from Koh Sichang. A: The kidney parenchyma (Ki) contained the renal tubule (Rt)
and hematopoietic tissue (Ht). B-C: A large parenchyma of renal degeneration (Rd) and necrotic tissue (Nrt). D: Large clusters of melanomacrophage center (MMCs). E: Unidentified parasite (Up) found close to the renal tubule (Rt) of kidney (Ki).F: Granuloma (Gm). Abbreviations: Ht = hematopoietic tissue, Rt = renal tubule
Semi-quantitative analysis on histopathological alterations
Finally, we calculated the HAI and AVA for the accurate ecotoxicological assessments. Although many histopathological changes were observed, based on the HAI, the gill was classified as a normal organ/tissue functioning (9.42, Figure 8A). The AVA showed that the most individuals had no pathological alteration in the gill (76.31 %, n = 29), and 23.68 % of individuals (n = 9) had slight or mild pathological alterations (Figure 8B). The liver HAI was also classified this organ as a normal organ/tissue functioning (10.15, Figure 8A), and the liver AVA was found to be no pathological alteration (56.63%, n = 20) in many individuals. Individuals of slight or mild pathological alterations (42.10%) and severe and extensive pathological alterations (5.26%) were also found (Figure 8B). However, the kidney HAI was classified as a severe alteration (54.94, Figure 8A). The major kidney AVA was the slight or mild pathological alteration (55.26%, n = 21), 21.05% was classified as severe and extensive pathological alterations (Figure 8B).
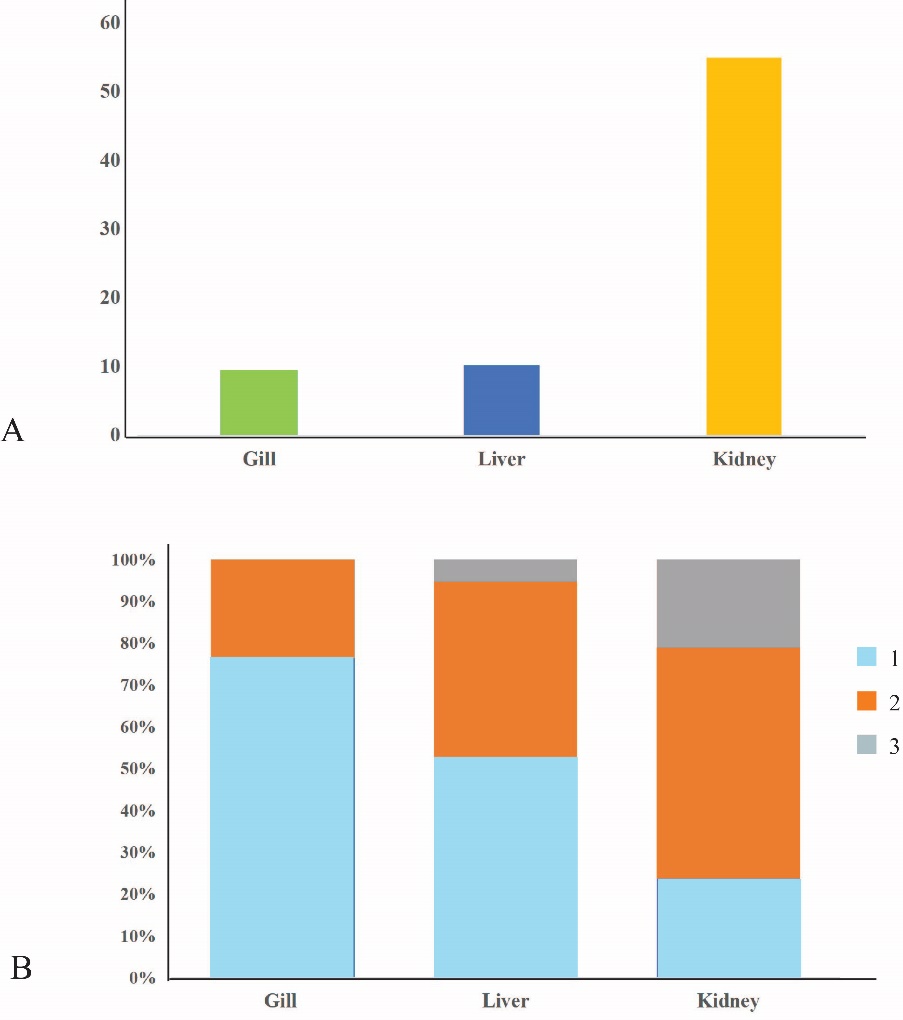
Figure 8. The mean of histological alteration index (HAI) [A] and relative frequency of average value of alteration (AVA) [B] of the selected organs (gill, liver and kidney) of Siganus javus from Koh Sichang, Thailand.
Note: 1 = no pathological alteration of organs, 2 = slight or mild pathological alterations of organs), and 3 = severe and extensive pathological alterations of organs)
DISCUSSION
Our observation showed pronounced visceral adipose tissue accumulation in some S. javus individuals. This phenotype is observed under a relatively favorable nutritional condition (Hillestad et al., 1998; Xiong et al., 2018). Because the Koh Sichang area receive water from urban area, this area may be rich in nutrients. Since nutritional status also causes hematological and histopathological alterations, it is important to investigate the nutritional status of S. javus in the Koh Sichang area. Proximate analysis will be a crucial next step in further studies.
Hematological parameters have become a promising biomarker for environmental stressors (Borges et al., 2007; Sudova et al., 2009; Li et al., 2011), although these parameters are also influenced by nutrition and disease (Adams et al., 1996). The features of S. javus erythrocytes were found to resemble those previously reported in other teleosts (Kousar and Javed, 2015; Okomoda et al., 2018; Singkhanan et al., 2019). However, the erythrocytic nuclear abnormalities in both notched and blebbed shapes were recorded in this study. Such nuclear abnormalities are known to be increased by copper and cadmium toxicity (Jiraungkoorskul et al., 2007; Summak et al., 2010; Guner et al. 2011; Kousar and Javed, 2015) and genotoxic damage (Kousar and Javed, 2015; Ali et al., 2008; Summak et al., 2010), which might reduce the respirational functions. The detailed monitoring of water quality will be required for the Koh Sichang area.
In this study, we found thrombocytes and leucocytes from the blood smear slide. Thrombocytes play an important role in clotting (Stosik et al., 2019; Singkhanan et al., 2019), inflammatory exudates and phagocytic activity (Tavares-Dias et al., 1999), whereas leucocytes are involved in the innate and acquired immune defense (Ballarin et al., 2004). The leucocytes found in S. javus contain monocytes, neutrophils and lymphocytes. The characteristic of monocytes is similar to those in a series of previous studies on C. chanos, C. subviridis, D. pusilla and in C. subviridis (Singkhanan et al., 2019), but the proportion of monocytes was relatively low in this study (9.60%). Because the monocyte is a marker of phagocytosis (Secombes and Fletcher, 1992), antigen presentation (Vallejo et al, 1992) and the production of cytokines (Secombes, 1991; Secombes and Fletcher, 1992), the low occurrence of monocyte of S. javus may indicate the normal inflammation condition. Neutrophils of S. javus were visually similar to those in other fish (Rowley et al., 1988; Ikeda, 1986; Ellis, 1977). It is well known that neutrophils are the first line of innate immune against infectious diseases and play a major role in the resolution of antimicrobial molecules (Ellis, 1977; Ranzani-Paiva et al., 2004). Neutrophils are also involved the T-cell immune response to protect the host against the attack of various pathogens (Ranzani-Paiva et al., 2004; Garcia et al., 2007). The small agranular lymphocytes showed the highest proportion in of S. javus as reported in other species (Modra et al., 1998). This blood cell plays an important role in the immune response and the biological defense system (Galagarza et al., 2017; Singh and Tandon, 2009) by producing antibodies and chemical substances that serve as a defense against the pathogen and parasite infections (Jalali et al. 2009; Musa et al., 2013). However, little data has been available for lymphocytes used for a monitoring purpose. An increase in lymphocyte numbers was found in fish collected from polluted river (Singh et al., 2009) and in fish after injected with Corynebacterium sp., a gram-positive bacterial species (Silveira-Coffigny et al., 2004).
Histopathologically, the disorganization of gill was the most frequently observed lesion in S. javus. It is suggested that such lesions are the consequence of a variety of water pollution problems (Cantanhêde et al., 2014; Paruruckumani et al., 2015). The appearance of lamellar aneurysm was also identified from all individuals. These lesions have been related to the exposure to a high concentration of heavy metals (Fonseca et al., 2017), for example copper in Solea senegalensis Kaup, 1858 (Arellano et al., 1999) and Poronotus triacanthus (Peck, 1804) (Jiraungkoorskul et al., 2007), and particularly impair the cellular defense mechanisms and physiological response against biotic and abiotic stresses (Arellano et al., 1999). In addition, the epithelial hyperplasia was also noted. Previous observations suggest that the increased lamellar epithelium is a defense against water-borne pollutants that allows more distance from the environment across the membrane.
Histopathological changes observed in the liver of S. javus are also a common abnormality in fish under nutritional and environmental stresses (Greenfield et al., 2008; Senarat et al., 2018; Senarat et al., 2019). It is suggested that the underlying mechanism for the hepatic lipidosis is the disruption of carbohydrate, lipid and protein metabolism (Hinton and Laure´n, 1990). Previous observations showed that hepatocellular lipidosis can be induced by several pollutants, typically chlorinated hydrocarbon and anthropogenic contaminations (Hendricks et al., 1984; Hinton et al., 1992; Robertson and Bradley 1992; Schrank et al., 1997), including PCBs (Teh et al., 1997; Anderson et al., 2003) and TiO2 nanoparticle (Diniz et al., 2013). Additionally, age, overnutrition and nutritional imbalance likely cause the hepatocellular lipidosis in fish (Hinton et al., 1992; Robertson and Bradley, 1992; Yilmaz and Akyurt, 2005; Yilmaz and Genc, 2006; Senarat et al., 2015; Ruiz-Ramírez et al., 2019). Thus, the above results indicate the presence of hepatic abnormalities associated with environmental or nutritional stress in S. javus from Koh Sichang.
Compared to the gill and liver, less information is available for histopathological alterations in kidney, although lesions of renal tubular degeneration and necrosis of S. javus have been associated with heavy metals, particular mercury and cadmium (Robert, 2000). An important function of the renal epithelium is to excrete metabolic wastes and the divalent ions (Genten et al., 2008). It is possible that the kidney function of S. javus in this study is impaired.
The occurrence of MMCs in S. javus were documented, which is well known to play crucial roles in responses against pathogen invasion (Agius and Roberts, 2003; Louiz et al., 2018). Previous observations suggested that the MMCs are more frequently observed in unhealthy fish, possibly in relation to parasitic infestation (Roganovic-Zafirova and Jordanova, 1998). The prevalence and intensity of MMCs might be a biomarker for stress, environmental degradation and pollution (Couillard and Hodson, 1996).
Although we found several histopathological changes in gill, liver and kidney, the semi-quantitative analysis indicated that S. javus from Koh Sichang is under normal conditions in terms of gill and liver HAI and AVA. However, the kidney data indicate that the specimens have severe alterations in consistent with our observation of severe and frequent pathological alterations compared to other organs. These results suggest that the kidney is the most sensitive organ to the environmental stressors, at least in S. javus. To further investigate this possibility, laboratory exposure experiments with cell area measurements should be useful (Campos-Garcia, 2016; Mansouri et al., 2017; Pirsaheb et al., 2019; Sayadi et al., 2020). It will also be useful to compare hematological and histopathological changes between sites of different pollution levels, which we could not do in this study because of the no major environmental difference in the two sampling sites.
CONCLUSION
The health status of java rabbitfish Siganus javus from Koh Sichang, Thailand has been diagnosed using a multi-biomarker approach. Abnormal erythrocytes and the presence of some leucocytes, along with the histopathological data, indicate that the fish live challenging natural environment. Investigations on the pollutants in fish and environment are being considered for future research.
AUTHOR CONTRIBUTIONS
Anek Sopon, Jes kettratad, Ajcharaporn Piumsomboon, Gen Kaneko and Sinlapachai Senarat assisted in conducting the experiments, performed the statistical analysis and data visualization and wrote the manuscript. Anek Sopon, Jes kettratad and Sinlapachai Senarat designed and conducted all of the experiments and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Adams, S.M., Ham, K. D., Greeley, M. S., LeHew, R. F., Hinton, D. E., and Saylor, C. F. 1996. Downstream gradients in bioindicator responses: point source contaminant effects on fish health. Canadian Journal of Fisheries and Aquatic Sciences. 53: 2177-2187
Ali, D., Nagpure, N.S., Kumar, S., Kumar, R., and Kushwaha, B. 2008. Genotoxicity assessment of acute exposure of chlorpyrifos to freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Chemosphere. 71: 1823-1831.
Agius, C., and Roberts, R.J. 2003. Melano-macrophage centers and their role in fish pathology. Journal of Fish Diseases. 26(9):499-509.
Anderson, M.J., Cacela, D., Beltman, D., Teh, S.J., Okihiro, M.S., Hinton, D.E., Denslow, N., and Zelikoff, J.T. 2003 Biochemical and toxicopathic biomarkers assessed in smallmouth bass recovered from a polychlorinated biphenyl-contaminated river. Biomarkers. 8: 371- 393.
Arellano, J.M., Storch, V., and Sarasquete, C. 1999. Histological changes and copper accumulation in liver and gills of the Senegales sole, Solea senegalensis. Ecotoxicology and Environmental Safety. 44: 62-72.
Ballarin, L., Dalloro, M., Bertotto, D., Libertini, A., Francescon, A., and Barbaro, A. 2004. Haematological parameters in Umbriana cirrosa (Teleostei, Scianidae): a comparison between diploid and triploid specimen. Comparative Biochemistry & Physiology A 183: 45-51.
Barbieri, E., Campos-Garcia, J., Martinez, D.S.T., da Silva, J.M.C., Alves, L.O., and Rezende, K.F.O. 2016. Histopathological effects on gills of nile tilapia (Oreochromis niloticus, Linnaeus, 1758) exposed to Pb and carbon nanotubes. Microscopy and Microanalysis. 22: 1162-1169.
Barbierl, E., Rezende, K.F.O., Carneiro, J.S., and Henriques, M.B. 2019. Metabolic and histological alterations after exposing Deuterodon iguape to different salinity. Boletim do Instituto de Pesca. 45: e.410.
Beeby A. 2001. What do sentinels stand for?. Environmental Pollution. 112: 285–298.
Borges, A., Scotti, L.V., Siqueira, D.R., Zanini, R., and Do Amaral, F. 2007. Changes in hematological and serum biochemical values in jundia Rhamdia quelen due to sublethal toxicity of cypermethrin. Chemosphere. 69: 920-926.
Brungs, W.A., McCormick, J.H., Neiheisel, T.W., Neiheisel, R.L., Spehar, C.E., and Stephan, G.N. 1977. Effect of pollution on freshwater fish. Journal of the Water Pollution Control Federation. 49: 1425-1493.
Campos-Garcia, J., Martinez, D.S.T., Rezende, K.F.O., da Silva, J.R.M.C., Alves, O.L., and Barbieri, E. 2016. Histopathological alterations in the gills of Nile tilapia exposed to carbofuran and multiwalled carbon nanotubes. Ecotoxicology and environmental safety, 133: 481-488.
Cantanhêde, S.M., Medeiros, A.M., Ferreira, F.S., Alves, L.M.C., Cutrim, M.V.J., and Santos, D.M.S. 2014. Uso de biomarcador histopatológico em brânquias de Centropomus undecimalis (Bloch, 1972) na avaliação da qualidade da água doParque Ecológico Laguna da Jansen, São Luís - MA. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 62: 593-601.
Carins, J., Dickson, K.L., and Westlake, G.F. 1975. Biological monitoring of water and effluent quality. ASTM Publ., Philadelphia.
Castro, J.S., Sodre, C.F.L., Souza, C.B., Sousa, D.B.P., Neta, R.N.F.C. 2019. Histopathological and hematological biomarkers in tambaqui Colossoma macropomum (Cuvier, 1816) from an environmental protection area of Maranhão, Brazil. An Interdisciplinary Journal of Applied Science. 14:1-10.
Couillard, C. M., and Hodson, P. V. 1996. Pigmented macrophage aggregates: a toxic response in fish exposed to bleached-kraft mill effluent? Environmental Toxicology and Chemistry. 15: 1844-1854.
Dalzochio, T., Zimmermann, G., Petry, IE., Gehlen, G., and da Silva, L.B. 2016. The use of biomarkers to assess the health of aquatic ecosystems in Brazil: a review. Journal of Aquatic Research. 8:289–298.
Diniz, M.S., de Matos, A.P., Lourenço, J., Castro, L., Peres, I., Mendonça., E., and Picado. A. 2013. Liver Alterations in Two Freshwater Fish Species (Carassius auratus and Danio rerio) Following Exposure to Different TiO2 Nanoparticle Concentration. Microsc. Microanal. 19: 1131-1140.
Dos Santos, I.V.F., de Souza, G.C., Santana, G.R., Duarte, J.L., Fernandes, C.P., Keita, H., Velázquez-Moyado, J.A., Navarrete, A., Ferreira, I.M., Carvalho, H.O., and Carvalho, C.T. 2018. Histopathology in zebrafish (Danio rerio) to evaluate the toxicity of medicine: An anti-inflammatory phytomedicine with Janaguba Milk (Himatanthus drasticus Plumel). Biology. Chapter 3 Histopathology - An Update. Pp. 39-64.
Ellis, A.E. 1977. The leucocytes of fish: a review. Journal of Fish Biology. 11: 453-491.
Fonseca, A.R., Sanches Fernandes, L.F., Fontainhas-Fernandes, A., Monteiro, S.M., and Pacheco, FAL. 2017. The impact of freshwater metal concentrations on the severity of histopathological changes in fish gills: A statistical perspective. Science of The Total Environment. 599-600: 217-226.
Frame, L., and Dickerson, R.L. 2006. Fish and wildlife as sentinels of environment contamination. In D.O. Norris and J.A. Carr (Eds.). Endocrine disruption: biological bases for health effects in wildlife and humans (pp. 202-222), New York: Oxford University Press.
Galagarza, O.A., Kuhn, D.D., Smith, S.A., and Hrubec, T.C. 2017. Hematologic and plasma chemistry RIs for cultured Striped catfish (Pangasius hypophthalmus) in recirculating aquaculture systems. Veterinary Clinical Pathology. 46: 457-465.
Garcia, F., Pilarski, F., Onaka, E.M., Moraes, F. R., and Martins, M. L. 2007. Hematology of Piaractus mesopotamicus fed diets supplemented with vitamins C and E, challenged by Aeromonas hydrophila. Aquaculture. 271: 39-46.
Genten, F., Terwinghe, E., and Danguy, A. 2008. Atlas of Fish Histology. Science Publishers, Enfield. New Hampshire, U.S.A.
Greenfield, B., Teh, S., Ross, J., Hunt, J., Zhang, J., Davis, J., Ichikawa, G., Crane, D., Hung, S., D. Deng, F., and Green, P. 2008. Contaminant concentrations and histopathological effects in Sacramento Splittail (Pogonichthys macrolepidotus). Archives of Environmental Contamination and Toxicology. 55: 270-281.
Guner, U., Dilek, F., and Muranl, G. 2011. Micronucleus Test, Nuclear Abnormalities and Accumulation of Cu and Cd on Gambusia affinis (Baird & Girard, 1853). Turkish Journal of Fisheries and Aquatic Science. 11: 615-622.
Hayashi, M., Ueda, T., Uyeno, K., Wada, K., Kinae, N., Saotome, K., Tanaka, N., Takai, A., Sasaki, Y.F., and Asno, N. 1998. Development of genotoxicity assay systems that use aquatic organisms. Mutation Research. 399: 125-33.
Hendricks, J.D., Meyers, T.R., and Shelton, D.W. 1984. Histological progression of hepatic neoplasia in rainbow trout (Salmo gairdneri). Journal of the National Cancer Institute. 65: 321-336.
Hillestad, M., Johnsen, F., and Austreng, E. 1998. Long-term effects of dietary fat level and feeding rate on growth, feed utilization and carcass quality of Atlantic salmon. Aquaculture Nutrition. 4: 89-97.
Hinton, D.E., Baumann, P.C., Gardner, G.R., Hawkins, W.E., Hendricks, J.D., Murchelano, R.A., and Okihiro, M.S. 1992. Histopathologic biomarkers. biochemical, physiological, and histological markers of anthropogenic stress. Biomarkers (pp. 155-209). Lewis Publishers, Boca Raton, FL.
Hinton, D.E., and Laure´n, D.J. 1990. Liver structural alterations accompanying chronic toxicity in fishes: potential biomarkers of exposure. In J. F. McCarthy and L.R. Shugart (Eds.). Biomarkers of Environmental Contamination (pp. 17-57). Lewis, Boca Raton, FL.
Hrubec, T.C., Cardinale, J.L., and Smith, S.A. 2000. Haematology and plasma chemistry reference intervals for cultured tilapia (Oreochromis hybrid). Veterinary Clinical Pathology. 29: 7-12.
Ikeda, Y., Ozaki, H., and Hamazaki, K. 1986. Blood Atlas of Fishes. Midarishobou, Tokyo. p. 361 (in Japanese).
Jalali, M.A., Ahmadifar, E., Sudagar, M., and Azari Takami, G.H. 2009. Growth efficiency, body composition, survival and haematological changes in great sturgeon (Huso huso Linnaeus, 1758) juveniles fed diets supplemented with different levels of Ergosan. Aquaculture Research. 40: 804-809.
Jiraungkoorskul, W., Kosai, P., Sahaphong, S., Kirtputra, P., Chawlab, J., and Charucharoen, S. 2007. Evaluation of micronucleus test’s sensitivity in freshwater fish species. Research Journal of Environmental Sciences. 1(2): 56-63.
Kousar, S., and Javed, M. 2015. Studies on induction of nuclear abnormalities in peripheral blood erythrocytes of fish exposed to copper. Turkish Journal of Fisheries and Aquatic Sciences. 15: 879-886.
Li, Z.H., Velisek, J., Grabic, R., Li, P., and Kolarova, J. 2011. Use of hematological and plasma biochemical parameters to assess the chronic effects of a fungicide propiconazole on a freshwater teleost. Chemosphere. 83: 572-578.
Louiz, I., Palluel, O., Ben-Attia, M., Aït-Aïssa, A., and Hassine, O.K.B. 2018. Liver histopathology and biochemical biomarkers in Gobius niger and Zosterisessor ophiocephalus from polluted and non-polluted Tunisian lagoons (Southern Mediterranean Sea). Marine Pollution Bulletin. 128: 248-258.
Mangang, Y.A., Pandey, P.K. 2021. Hemato-biochemical responses and histopathological alterations in the gill and kidney tissues of Osteobrama belangeri (Valenciennes, 1844) exposed to different sub-lethal unionized ammonia. Aquaculture 542, 736887.
Mansouri, B., Maleki, A., Davari, B., Johari, S.A., Shahmoradi, B., Mohammadi, E., and Shahsavari, S. 2016. Histopathological effects following short-term coexposure of Cyprinus carpio to nanoparticles of TiO2 and CuO. Environmental Monitoring and Assessment. 188: 575.
Mansouri, B., Maleki, A., Johari, S.A., Shahmoradi, B., Mohammadi, E., and Baravi, B. 2017. Histopathological effects of copper oxide nanoparticles on the gill and intestine of common carp (Cyprinus carpio) in the presence of titanium dioxide nanoparticles. Chemistry and Ecology, 33: 295-308.
Modra, H., Svobodova, Z. and Kolarova, J. 1998. Comparation of differential leckocyte counts in fish of economic and indicator importance. Acta Veterinaria Brno. 67: 215-226.
Musa, S., Aura, C., Ogello, E., Omondi, R., and Charo-Karisa, H. 2013. Haematological response of African Catfish (Clarias gariepinus, Burchell 1822) fingerlings exposed to different concentrations of Tobacco (Nicotiana tobaccum) Leaf Dust. Zoology 7: 1-7.
National Research Council. 1991. Animals as sentinel of environmental health hazards. National Academy Press, Washington D.C.
Okomoda, V.T., Koh, I.C.C., Hassan, A., Amornsakun, T., Khairul, A.B.K., Rajamad, R.Y., Shuhaimi, A.D., Shafiq, M.R., and Shahreza, M.S. 2018. Erythrocyte characteristics of the progenies of pure and reciprocal crosses of Pangasianodon hypophthalmus (Sauvage, 1878) and Clarias gariepinus (Burchell, 1822). Comparative Clinical Pathology 27: 301-312.
Paulo, D.V., Fontes, F.M., and Flores-Lopes, F. 2012. Histopathological alterations observed in the liver of Poecilia vivipara (Cyprinodontiformes: Poeciliidae) as a tool for the environmental quality assessment of the Cachoeira River, BA. Brazilian Journal of Biology. 72: 131-140.
Paruruckumani, P.S., Maharajan., A., and Ganapiriya, V. 2015. Surface ultrastructural changes in the gill and liver tissue of Asian sea bass Latescal carifer (Bloch) exposed to copper. Biological Trace Element Research. 168: 500-507.
Pirsaheb, M., Azadi, N.A., Miglietta, M.L., Sayadi, M.H., Blahova, J., Fathi, M., & Mansouri, B. 2019. Toxicological effects of transition metal-doped titanium dioxide nanoparticles on goldfish (Carassius auratus) and common carp (Cyprinus carpio). Chemosphere 215: 904-915.
Pollution Control Department. 2017. Quality of marie environment. Available from http://www.pcd.go.th/info_serv/reg_std_water02.html
Poleksic, V., and Mitrovic-Tutundzic, V. 1994. Fish gills as a monitor of sublethal and chronic effects of pollution. p. 339-352. In R. Müller and R. Lloyd (eds) Sublethal and Chronic effects of Pollutants on Freshwater Fish. Cambridge Univ, Press Cambridge.
Presnell, J.K., and Schreibman, M.P. 1997. Humason’s Animal Tissue Techniques. 5th ed. US, Johns Hopkins University Press, 600 pp.
Puttipong, T., Senarat, S., Kettratad, J., Chantangsi, C., Kaneko, G., Siriwong, W. 2021. Evaluation of health status in the striped catfish Pangasianodon hypophthalmus (Sauvage, 1878) from Khlong Saen Saep, Thailand: The use of integrated biomarkers. Human and Ecological Risk Assessment. 27: 938-953.
Ranzani-Paiva, M. J. T., Ishikawa, C.M., Eiras, A. C., Silveira, V. R. 2004. Effects of an experimental challenge with Mycobacterium marinum on the blood parameters of Nile Tilapia, Oreochromis niloticus (Linnaeus, 1757). Brazilian Archives of Biology and Technology. 47: 945-953.
Roberts, J.R. 2000. Fish Pathology. 4th ed. London: Bailliere Tindall.
Robertson, J.C., and Bradley, T.M. 1992. Liver ultrastructure of juvenile Atlantic salmon (Salmo salar). Journal of Morphology. 211: 41-54.
Rodriguez-Cea, A., Ayllon, F., and Garcia-Vazquez, E. 2003. Micronucleus test in freshwater fish species: an evaluation of its sensitivity for application in field surveys. Ecotoxicology and Environmental Safety. 56: 442-448.
Roganovic-Zafirova, D., and Tavcioska-Vasileva, I. 1998. Liver lesions in Ohrid minnow moranec (Pachychilion pictus Heck. Et Kner) collected from some contaminated sites of lake Ohrid. A histopathological evidence. Ekologija i Zaštita na Životnata Sredina. 6: 19-27.
Rowley, A. F., Hunt, T. C., Page, M., and Mainwaring, C. 1988. Fish. In A.F. Rowley and N.A. Ratcliffe (Eds.). Vertebrate Blood Cells (pp. 19-127). Cambridge University Press, New York.
Ruiz-Ramírez, J.A., Ramírez-Ayala, E., Tintos-Gómez, A., Salas-Villaseñor, C., García-Márquez, L.J., Borja-Gómez, I., Sepúlveda-Quiroz, C.A., Lezama-Cervantes, C., Morales-Blake, A.R. 2019. Hepatocellular steatosis as a response to nutritional stressors in Lutjanus guttatus (Steindachner, 1869) grown in floating cages: a case study. Latin American Journal of Aquatic Research. 47: 709-715.
Sayadi, M.H., Mansouri, B., Shahri, E., Tyler, C.R., Shekari, H., and Kharkan, J. 2020. Exposure effects of iron oxide nanoparticles and iron salts in blackfish (Capoeta fusca): Acute toxicity, bioaccumulation, depuration, and tissue histopathology. Chemosphere, 247: 125900.
Schrank, C.S., Cormier, S.M., and Blazer, V.S. 1997. Contaminant exposure, biochemical, and histopathological biomarkers in white suckers from contaminated and reference sites in the Sheboygan River, Wisconsin. Journal of Great Lakes Research 23:119 -130.
Schwaiger, J., Adam, S., Pawert, M., Honnen, W., and Triebskorn, R. 1997. The use of histopathological indicators to evaluate contaminant related stress in fish. Journal of Aquatic Ecosystem Stress and Recovery. 6: 75-86.
Secombes, C. J. 1991. The phylogeny of cytokines. In A.W. Thomson (ed.). The cytokine handbook (pp. 387-412), London: Academic Press.
Secombes, C. J., and Fletcher, T. C. 1992. The role of phagocytes in the protective mechanisms offish. Annual Review of Fish Diseases. 2: 53-71.
Senarat, S., Kettratad, J., Siriwong, W., Bunsomboonsakul, S., Kenthao, A., Kaneko, G., Sopon, A., Sudtongkong, C., and Jiraungkoorskul, W., 2020. Oogenesis and ovarian health problems in economically important fishes from different habitats potentially affected by pollution in Thailand. Asian Fisheries Science 33: 274 -286.
Senarat, S., Kettratad, J. Plumley, F.G., Wangkulangkul, S., Jiraungkoorskul, W., Boonyoung, P., and Poolprasert, P. 2019. Pathological microscopy in liver parenchyma of gray-eel catfish, Plotosus canius, from the Ang-Sila area, Chonburi Province, Thailand. Veterinary Integrative Sciences. 17: 255-261.
Senarat, S., Kettretad, J., Poolprasert, P., Tipdomrongpong, S., Plumley, F.G., and Jiraungkoorskul, W. 2018a. Health status in wild and captive Rastrelliger brachysoma from Thailand: Histopathology. Songklanakarin Journal of Science and Technology 40: 1090-1097.
Senarat, S., Kettratad, J., Tipdomrongpong, S., Pengsakul, T., Jiraungkoorskul, W., Boonyoung, P., and Huang, S. 2018b. Histopathology of kidney and liver in the captive broodstock (Rastrelliger brachysoma) during its juvenile stage. Veterinary Integrative Sciences 16: 87-93.
Senarat, S., Kettratad, J., Poolprasert, P. Jiraungkoorskul, W., and Yenchum, W. 2015. Histopathological findings of liver and kidney tissues of the yellow mystus, Hemibagrus filamentus (Fang and Chaux, 1949), from the Tapee River, Thailand. Songklanakarin Journal of Science and Technology. 37: 1-5.
Senarat, S, Suksai, S., Kamnurdnin, T., Kettratad, J, Thongboon, L., Jiraungkoorskul, W., Para, C., Poolprasert, P., and Pengsakul, T. 2018. Gamete alteration of the Long-Spined Sea Urchin Diadema setosum (Leske, 1778) from Sichang Island, Chonburi Province, Thailand: A Preliminary Study. YRU Journal of Science and Technology. 3: 109-114.
Silveira-Coffigny, R., Prieto-Trujillo, A., and Ascencio-Valle, F. 2004. Effects of different stressors in haematological variables in cultured Oreochromis aureus. Comparative Biochemistry & Physiology C. 139: 245-250.
Singh, B.P., and Tandon, P.K. 2009. Effect of river water pollution on hematological parameters of fish, Wallago attu. Research in Environment and Life Sciences 2: 211-214.
Singkhanan, N., Kettratad, J., Senarat, S., Theerakamol, P., Para, C., Kaneko, G. 2019. Morphological characterization of blood cells in five important estuarine fish species in Thailand during juvenile stages. EnvironmentAsia 12: 79-86.
Sitja-Bobadilla, A. 2008. Fish immune response to myxozoan parasites. Parasitology. 15: 420-425.
Steinel, N.C., and Bolnick, D.I. 2017. Melanomacrophage centers as a histological indicator of immune function in fish and other poikilotherms. Frontiers in Immunology. 8: 827.
Stosik, M., Tokarz-Deptuła, B., and Deptuła, W. 2019. Characterisation of thrombocytes in Osteichthyes. Journal of Veterinary Research. 63: 123-131.
Sudova, E., Piackova, V., Kroupova, H., Pijacek, M., and Svobodova, Z. 2009. The effect of praziquantel applied per os on selected haematological and biochemical indices in common carp (Cyprinus carpio L.). Fish Physiology and Biochemistry. 35: 599-605.
Summak, S., Aydemir, N.C., Vatan, O., Yilmaz, D., Zorlu, T., and Bilaloglu, R. 2010. Evaluation of genotoxicity from Nilufer stream (Bursa/Turkey) water using piscine micronucleus test. Food Chemistry and Toxicology. 48: 2443-2447.
Suvarna, K.S., Layton, C., and Bancroft, J.D. 2013. Bancroft’s Theory and Practice of Histological Techniques. 7th ed. Canada, Elsevier, 654 pp
Tavares-Dias, M., Martins, M.L., Silva, E.D., Moraes, F.R., and Perecin, D. 1999. Hematologia de teleósteos brasileiros com infecção parasitária. I. Variáveis do Leporinus macrocephalus Garavello & Britski, 1988 (Anastomidae) e Piaractus mesopotamicus Holmberg, 1887 (Characidae). Acta Scientiarum, Biological Sciences. 21: 337-342.
Teh, S.J., Adams, S.M., and Hinton, D.E. 1997. Histopathologic biomarkers in feral freshwater fish populations exposed to different types of contaminant stress. Aquatic Toxicology. 37: 51-70.
Theml, H., Haferlach, T., and Diem H. 2004. Color atlas of hematology: practical microscopic and clinical diagnosis. Thieme.
Thrall, M. A., Baker, D. C., Campbell, T. W., DeNicola, D., Fettman, M. J., Lassen, E. D., Rebar, A., and Weiser, G. 2007. Hematologia e bioquímica clínica veterinária. São Paulo, Roca. 582 p.
Tsujii, T., and Seno, S. 1990. Melano-macrophage centers in the aglomerular kidney of 309 the sea horse (teleosts): morphologic studies on its formation and possible function. Anatomical Records. 226: 460-470.
Vallejo, A. N., Miller, N. W., and Clem, L.W. 1992. Antigen processing and presentation in teleost immune responses. Annual Review of Fish Diseases. 2: 73-89.
Watson, C.F., Baer, K.N., and Benson, W.H. 1989. Dorsal gill incision: a simple method for obtaining blood samples in small fish. Environmental Toxicology and Chemistry. 8: 457-461.
Wattanayakorn, G., and Rungsupa, S. 2012. Petroleum hydrocarbon residues in the marine environment of Koh Sichang-Sriracha, Thailand. Coastal Marine Science. 35: 122-128.
Wilson, J.M., Bunte, R.M., and Carty, A.J. 2009. Evaluation of rapid cooling and tricaine methanesulfonate (MS 222) as methods of euthanasia in zebra fish (Danio rerio). Journal of the American Association for Laboratory Animal Science. 48: 785-789.
Xiong, S., Krishnan, J., Peuß, R., and Rohner, N. 2018. Early adipogenesis contributes to excess fat accumulation in cave populations of Astyanax mexicanus. Developmental Biology. 441: 297-304.
Yilmaz, E., and Genc, E. 2006. Effects of alternative dietary lipid sources (soy-acid oil and yellow grease) on growth and hepatic lipidosis of common carp (Cyprinus carpio) fingerling: A preliminary study. Turkish Journal of Fisheries and Aquatic Sciences. 6: 37-42.
Yilmaz, E.C.E., and Akyurt, I. 2005. Effects of dietary fish oil, soy-acid oil, and yellow grease on growth and hepatic lipidosis of hybrid Tilapia rry. The Israeli journal of aquaculture. 57: 90-96.
Zutshi, B., Prasad, S.G., and Nagaraja, R. 2010. Alteration in hematology of Labeo rohita under stress of pollution from Lakes of Bangalore, Karnataka, India. Environmental Monitoring and Assessment. 168: 11-19.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Anek Sopon1, Jes kettratad1, 2, Ajcharaporn Piumsomboon1, Gen Kaneko3, and Sinlapachai Senarat4,*
1 Marine Ecology and Marine Resources Utilization Research Unit, Aquatic Resources Research Institute, Chulalongkorn University, Bangkok 10330, Thailand
2 Department of Marine sciences, Faculty of Science, Chulalongkorn University, Bangkok, 10330 Thailand
3 School of Arts and Sciences, University of Houston-Victoria, Victoria, Texas 77901, USA
4 Department of Marine Science and Environment, Faculty of Science and Fisheries Technology, Rajamangala University of Technology Srivijaya, Trang 92150, Thailand
Corresponding author: Sinlapachai Senarat, E-mail: sinlapachai.s@rmutsv.ac.th
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: May 7, 2021;
Revised: July 4, 2021;
Accepted: July 19, 2021;

