
Fructans, Polyphenols and Antioxidant Activity in Edible Roots and Thistles from Seven Medicinal Plants
Nadezhda Petkova*, Ivanka Hambarlyiska, Elena Angelova and Ivan IvanovPublished Date : 2021-09-13
DOI : https://doi.org/10.12982/CMUJNS.2021.082
Journal Issues : Number 4, October-December 2021
Abstract The research purposed to evaluate the bioactive compounds and antioxidant content in water and 50 % ethanol extracts from different vegetal parts of seven herbs (black bryony, dandelion, leuzea, asparagus, St. Benedict's thistle, cotton thistle, and sarsaparilla). Sugars and total fructans (inulin ad fructooligosacchrides (FOS) were analysed by spectrophotometric and chromatographic methods. The total phenols, total flavonoids and derivatives of caffeic acid were also determined. The antioxidant activity was evaluated by DPPH and FRAP methods. Inulin and FOS were detected only in three plants (leuzea, dandelion and the cotton thistle). Dandelion roots were evaluated as the richest source of total fructans (18 g/100 g dw). The highest phenolic content was found in sarsaparilla roots 50 % ethanol extracts (21 mg GAE/g dw). Leuzea roots were evaluated as a rich source of dihydroxycinnamic acid derivatives and flavonoids. The high antioxidant activity demonstrated sarsaparilla water extracts, followed by water and 95 % ethanol of leuzea roots and cotton thistle flowеring heads (20-98 mM TE/g dw). The study demonstrated the use of some medicinal plants (especially leuzea, sarsaparilla and cotton thistle) as sources of antioxidants and inulin-type fructans in food and beverages.
Keywords: Antioxidant Activity, Fructan, Medicinal Plants, Polyphenols
Funding: The authors are grateful for the research funding provided by University of Food Technologies.
Citation: Petkova, N., Hambarlyiska, I., Angelova, E., and Ivanov, I. 2021. Fructans, polyphenols and antioxidant activity in edible roots and thistles from seven medicinal plants. CMU J. Nat. Sci. 20(4): e2021082.
INTRODUCTION
The open market and online trading, increased the use of medicinal plants in human nutrition as spices, beverages and additives. However, consumers should be aware of the quality, origin and labeling of medicinal plants as functional ingredients. The main interest in medicinal plants is due to the fact that they are a rich source of secondary metabolites, especially phenolic compounds with radical scavenging properties. The detailed characteristics of some herbs and their application were listed (Table 1).
Most of them are applied as tinctures, herbal infusions, decoctions for internal intake or as food supplements. However, some medicinal plants were applied in food technology, especially the roots of dandelion and sarsaparilla which is used as a colorant in alcoholic beverages as beer or whiskey (Schütz et al., 2006; Ranilla et al., 2010). Dandelion root is consumed also as a coffee substitute and is applied as а flavor enhancer in drinks (Wirngo et al., 2016). Other plant from Asteracea family that present interest for consumption as herbal infusions are leuzea, cotton thistle and St. Benedict's thistle.
Rhaponticum carthamoides (Willd) Iljin, commonly known as Maral root or Russian leuzea is traditionally used in Siberian medicine due to bioactive component as ecdysteroids, flavonoids, and phenolic acids (Kokoska and Janovska, 2009). Cotton thistle flowering heads are applied, because they are a rich source of phenolic acids, flavonoids and tocopherols, as well as they contain inulin (Angelov et al., 2012), soluble sugars, triterpenes, oils and coumarins (Al-Snafi et al., 2020). Other applications were listed in Table 1.
Table 1. Scientific name, common name, family and analyzed part of the medicinal plant.
|
Common name |
Family |
Plant organ |
Medicinal purposes |
References |
|
Black bryony Dioscorea communis L. |
Diascoreaceae |
Tubers |
used for heart, urinary and reproductive system |
Yuniastuti and Iswari, 2019 |
|
Leuzea Rhaponticum carthamoide s(Willd) Iljin |
Asteraceae |
Roots |
for fatigue, decreased mental performance, male sex stimulatnt, antitumor properties |
Kokoska and Janovska, 2009 |
|
Dandelion Taraxacum officinale Wigg. |
Asteraceae |
Roots |
Helps digestion and liver function; |
Schütz et al., 2006 |
|
Cotton thistle Onopordum acanthium L. |
Asteraceae |
Flower Heads |
Diuretic, help in skin diseases, cardiotonic and hemostatic |
Al-Snafi et al., 2020 |
|
St. Benedict’s thistle Cnicus benedictus L. |
Asteraceae |
Aerial Parts |
for liver disease, skin cancer, cholagogue, |
Szabó et al., 2009 |
|
Asparagus Asparagus officinalis L. |
Asparagaceae |
Rhizomes |
for liver, bile, urinary system, |
Zhang et al., 2018 |
|
Sarsaparilla Smilax officinalis Kunth. |
Smilacaceae |
Rhizomes |
immunostimulant, sexual stimulants, kidneys purifier |
Ranilla et al., 2010 |
The areal part of St. Benedict's thistle (Cnicus benedictus L.), known also as blessed thistle, holy thistle or spotted thistle is consumed as "bitter" tonic drinks that stimulate digestion and enhance appetite. Moreover, its flowering heads were used for food purposes similar like Globe artichoke (Szabó et al., 2009). Cnicus benedictus is a rich source of phenolic acids (vanilic, ferulic, hydroxycinnamic acids, chlorogenic and sinapic acids) and flavonoids (cynarin and rutin) (Can et al., 2017).
Among some plants, especially those from Asteracea and Asparagaceae family consists of fructans with potential prebiotic activity. Inulin and its short chains - fructooligosaccharides are fructans that consist mainly of β-(2↔1) fructosyl fructose units (Fm), and usually, but not always, the chain terminates with α-glucopyranosyl unit (1→2) (GFn) (Van Loo et al., 1995). The roots of dandelion contain carbohydrates (pectin, up to 20-45% inulin and sugars (as sucrose, glucose and fructose), carotenoids, fatty acids, minerals, vitamins, mucilage. inulin and (fructo-oligosaccharides) possessed many beneficial effects such as prebiotic activity, and repression of obesity and osteoporosis (Wirngo et al., 2016). Dioscorea sp. was considered as a very important alternative source of carbohydrate in Asia, especially of inulin, which content was in the range of 2.88% -14.77% (Winarti et al., 2011; Judprasong et al., 2011; Zubaidah and Akhadiana, 2013; Mudannayake et al. 2015; Yuniastuti and Iswari, 2019). Many studies were devoted to the evaluation of inulin and fructooligosacchrides in Dioscorea hispida, Diascorea alata L., Dioscorea bulbifera, however, inulin from tubers of Dioscorea esculenta (Lesser Yam) was isolated in the highest yield 21.33% with the degree of polymerization (DP) 6 (Winarti et al., 2011). Until now, the detailed analysis of fructan in some medicinal plants, as potential prebiotics still remained unrevealed.
The aim of the study was to evaluate the fructan, total polyphenols and antioxidant activity in water and 50% ethanol extracts were prepared from the roots and thistle of commercially available medicinal plants (wild yams, dandelion, leuzea, asparagus, St. Benedict's thistle, cotton thistle, and sarsaparilla).
MATERIALS AND METHODS
Medicinal plants materials
All medicinal plants were purchased in the dry state from herbal pharmacies as follows: black bryony (Dioscorea communis) flour and asparagus (Asparagus officinalis L.) were produced by Bilki Ltd., Sofia; bio flour from dandelion roots was purchased from an Internet Cafe-BG Ltd., Sofia. Maral root (leuzea) (Rhaponticum carthamoides) was produced by ООО "Tselebnie Travie Altaja", Russia. St. Benedict’s thistle (Cnicus benedictus L.) and sarsaparilla roots (Smilax officinalis) were purchased from Dikrassin Bulgaria Ltd., Sofia. Cotton thistle (Onopordum acanthium L.) flowering heads was purchased by Herbal Pharmacy № 1, Plovdiv. Some of them were additionally milled in a laboratory homogenizer to a particle size of 0.5 mm.
Moisture and ash content
The moisture content in the dry plant materials was determined at 105 ± 1ºC to the constant weight by oven drying method (AOAC, 2007). Ash content was performed in a crucible, ignited in a muffle furnace at 550ºC (AOAC, 2007).
Preparation of herbal extracts
The dried and finely ground roots and thistles of medicinal plants were weighted in 50 ml centrifuge tubes. The samples were extracted with distilled water and 50 % ethanol in the solvent to solid ratio was 1:10 (w/v). The extraction procedure was performed in duplicate in the ultrasonic bath Siel UST 5.7-150 (Gabrovo, Bulgaria) with frequency 35 kHz and 300 W power at 65°С for 20 min. The samples were filtered, the final volume was checked. The extracts were used for further analyses.
Thin layer chromatography (TLC)
TLC analysis was performed on silica gel G60 F254 plates (Merck, Germany) with a solvent system n-BuOH:i-Pro:H2O:CH3COOH (7:5:4:2) (v/v/v/v). The herbal extracts (5 µL) and the same volume of standards in concentration 3 mg/ml (glucose, fructose, sucrose, FOS (Frutafit CLR with DP 7-9) and inulin (Frutafit TEX DP 22) were spotted. The plates were dried under gentle warm air and place in the developing chamber. The TLC plates were dried, dipped for 10 seconds in diphenylamine-aniline-H3PO4–acetone (1:1:5:50 w/v/v/v) (Lingyun et al., 2007), heated at 110°C for 5 min and scanned.
Total fructans
The total fructans were determined spectrophotometrically. The hundred microliters water extract was added into a glass graduated tube of 10 mL. Then, 100 μL resorcinol (1% solution in 95% ethanol), 100 μL thiourea (0.1% solution in 95% ethanol), 800 μL 95% ethanol and 900 μL HCl was added. After heating for 8 min at 80°C, the samples were cooled and filled with water until 10 mL. The absorbance was measured at 480 nm against a blank and calculated (Petkova et al., 2017).
HPLC-RID analysis of inulin and sugars
The analysis of inulin and sugars in the water extracts were performed on an HPLC instrument Elite Chrome Hitachi with refractive index detector Chromaster 5450 at 35 °C. The separation was performed on a column Shodex® Sugar SP0810 (300 mm × 8.0 mm i.d.) with Pb2+ and a guard column Shodex SP - G (5 μm, 6 × 50 mm) operating at 85°C with mobile phase d. H2O with flow rate 1.0 mL/min and the injection volume of sample 20 μL.
Total polyphenols
Total phenolic contents (TPC) were analyzed using a five time diluted Folin-Ciocalteu reagent (Sansomchai et al., 2021) The reaction was performed as 200 μL herbal extracts mixed with 1 ml Folin-Ciocalteu reagent and then 800 μL of 7.5% Na2CO3 was added. After 20 min the absorbance was measured at 765 nm against the blank. The results were presented as mg equivalent gallic acid (GAE)/100 g dry sample (Stintzing et al., 2005).
Determination of total dihydroxycinnamic derivative (DCA)
The total dihydroxycinnamic acid (including caffeoyl derivatives) content was expressed as mg chlorogenic acid derivates per g dw (Fraisse et al., 2011). In brief, the extract (1 ml) was mixed with 2 ml 0.5 M HCl, 2 ml Arnow’s reagent (10 g NaNO3 and 10 g sodium molybdate dissolved in 100 ml distilled water), 2 ml 2.125 M NaOH and 3 ml water. Absorbance was measured at 525 nm against a blank sample without Arnow’s reagent.
Total flavonoids content
The total flavonoids content was determined as 500 μL herbal extracts were mixed with 50 μL 10% Al(NO3)3, 50μL 1M CH3COOK, and 1.95 mL distilled water (or ethanol). The absorbance was measured at 415 nm against blank sample without the addition of Al(NO3)3. The results were expressed as mg equivalents quercetin (QE) per g dry sample (Kivrak et al., 2009).
DPPH (2, 2-diphenyl-1-picryl-hydrazyl-hydrate) free radical method
The extract (150 μL) was added to 2.85 ml freshly prepared 0.1 mM DPPH in methanol. After 15 min at 37°C the reduction of absorbance was measured at 517 nm in comparison to the blank containing methanol. The % inhibition was calculated (Prasajak et al., 2021). Antioxidant activity was expressed as mM Trolox® equivalents (TE) per g dry weight (dw) (Ivanov et al., 2014).
Ferric reducing antioxidant power (FRAP) method
The method was performed according to Benzie and Strain (1996) with some modifications. The FRAP reagent was prepared before analysis by mixing 0.3 M acetate buffer (pH 3.6), 10 mM 2,4,6- tripyridyl-s-triazine (TPTZ) in 40 mM HCl and 20 mM aqueous solutions of FeCl3.6H2O in a ratio 10:1:1 (v/v/v). Sample (100 μL) was added to 3.0 ml FRAP reagent. After 10 min at 37°С in darkness, the absorbance was measured at 593 nm against a blank (Adekoya et al., 2021). Antioxidant activity was expressed as mM Trolox® equivalents (TE) per g dry weight (dw).
Statistical analysis
The data were expressed as mean values ± standard deviation (SD) from three replications. Statistical analysis was performed using using ANOVA, with the Tukey's range. A difference was considered statistically significant, when P < 0.05.
RESULTS
Moisture and ash content of initial medicinal plants
The results for the moisture and ash content in the medicinal plants were presented in Table 2. The moisture content did not exceed 10%. The lowest values were found in the cotton thistle flowering heads (7.9%), and the highest content was detected in the wild yam flour (10.1%). The mean values of ash content were about 5-6%. However, bio flour from dandelion roots and aerial parts of St. Benedict’s thistle demonstrated the high ash content - 19.4 and 13.9%, respectively. These observations could be explained by the nature of the samples.
Fructan and sugar composition
The detailed TLC quantitative test for presence of sugars and inulin in water extracts of medicinal plants was shown (Figure 1). The results confirmed that inulin and FOS were presented only in three plants – dandelion (spots 6 and 7), leuzea (spots 8 and 9) and cotton thistle (spots 10 and 11). Sucrose, fructose and glucose were the most abundant sugars detected in all samples except asparagus roots.
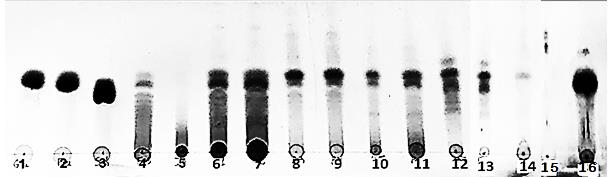
Figure 1. Thin layer chromatograms of herbal extracts, where 1 = glucose, 2 = fructose, 3 = sucrose, 4 = chicory fructooligosacchrides Frutafit CLR (DP 7-9), 5 = inulin (Frutafit TEX DP 22), water herbal extracts as follows: 6 and 7 = dandelion (Taraxacum officinale Wigg) flour; 8 and 9 = leuzea roots (Rhaponticum carthamoides), 10 and 11 = cotton thistle (Onopordum acanthium L.) flowering heads, 12 and 13 = sarsaparilla (Smilax officinalis Kunth.) roots, 14 St. Benedict’s thistle (Cnicus benedictus L.) leaves with flowering heads, 15= asparagus (Asparagus officinalis L.) roots, 16 = black bryony (Dioscorea communis) tuber flour.
The carbohydrate content in the water extracts from seven medicinal plants was summarized (Table 2).
Table 2. Moisture, ash, total fructans, sugars and inulin content in the water extracts of medicinal plants, g/100 g dw (mean ± SD).
|
Sample/ Characteristcs |
Black bryony |
Leuzea |
Dandelion |
Cotton thistle |
St. Benedict’s thistle |
Asparagus |
Sarsaparilla |
|
Moisture |
10.1 ± 0.1a |
8.6 ± 0.1b |
8.2 ± 0.2b |
7.9 ± 0.4d |
8.4 ± 0.8 b |
8.3 ± 0.5 b |
9.6 ± 0.2 c |
|
Ash |
5.1 ± 0.1b |
5.7 ± 1.7b |
19.4 ± 0.1a |
5.8 ± 0.7b |
13.9 ± 0.1d |
3.8 ± 0.2 c |
5.7 ± 0.8 b |
|
Total fructans |
0.3 ± 0.1a |
4.7 ± 1.0b |
18.1 ± 2.1c |
5.5 ± 0.1ns |
n.d |
n.d |
0.9 ± 0.1f |
|
Inulin |
n.d |
3.6 ± 0.8a |
16.1 ± 0.1c |
2.6 ± 0.4b |
n.d |
n.d |
n.d |
|
Nystose |
n.d |
0.2 ± 0.1a,b |
3.1 ± 0.1c |
0.4 ± 0.1a, b |
n.d |
n.d |
n.d |
|
1-Kestose |
n.d |
0.3 ± 0.1a |
1.6 ± 0.8b |
0.9 ± 0.2a, b |
n.d |
n.d |
n.d |
|
Sucrose |
0.1 ± 0.1a |
0.6 ± 0.1b |
3.4 ± 0.5c |
1.2 ± 0.2b,d |
tr |
n.d |
0.8 ± 0.1 b |
|
Glucose |
0.4 ± 0.1a |
0.4 ± 0.1c |
0.5 ± 0.2a,b |
0.6 ± 0.1d |
0.5 ± 0.1ns |
n.d |
1.2 ± 0.2f |
|
Fructose |
0.2 ± 0.1a,b |
1.0 ± 0.1c |
1.4 ± 0.2c,d |
1.8 ± 0.1e |
0.3 ± 0.1ns |
n.d |
0.8 ± 0.2f |
Notes: Values are mean ± standard deviation of three separate experiments. Different letters within each column indicate significant differences between treatments according to Tukey’s test at P < 0.05; n.d. – not detected, tr – traces, ns - not significant
From the investigated herbal extracts, inulin was detected only in roots of leuzea and dandelion, as well as in the flower heads of the cotton thistle. The detailed profiles of inulin, fructooligosacchrides and sugars in these three herbal extracts were shown on Figure 2.
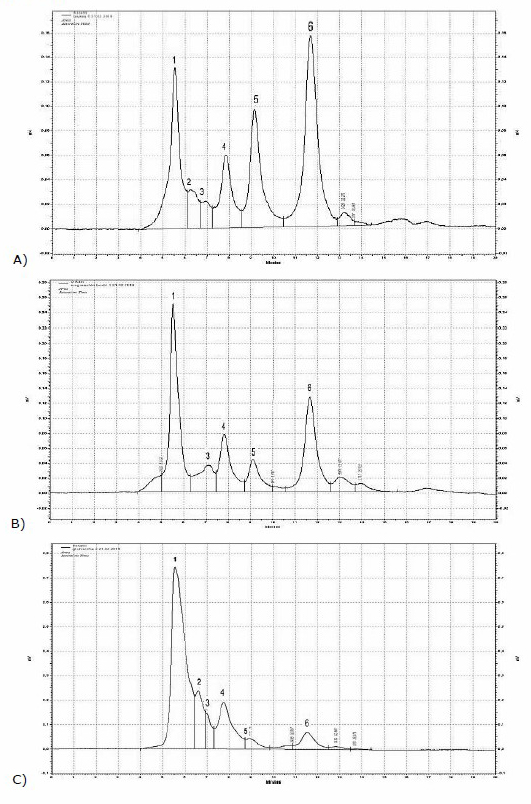
Figure 2. HPLC-RID chromatograms of water extracts from A) leuzea roots, B) cotton thistle flowering heads, C) dandelion roots where 1 = inulin, 2 = nystose, 3 = 1-kestose, 4 = sucrose 5 = glucose, 6 = fructose.
Fructan and inulin content decreased in the following order dandelion˃ leuzea ˃cotton thistle. The highest values of inulin 16 g/100 g dw were detected in dandelion bio flour, while the lowest values were found in the cotton thistle (2.6 g/100 g dw). The detected in our study total fructans in leuzea roots reached 4.6 g/100 g dw, that was approximately twice times lower than reported by Vasfilova et al. (2015), levels of polyfructants in Rhaponticum carthamoides (7-14%). Cotton thistle contained 1-kestose and nystose in higher amounts in comparison of leuzea roots. Nystose was detected in the highest vales in dandelion flour 3.1 g/100 g and the lowest values in leuzea roots - 0.2 g/100 g. Other substance with potential prebiotic activity 1-kestose dominated in the dandelion roots (1.6 g/100 g dw) and cotton thistle flowering heads (0.9 g/100 g dw).
The presence of inulin in the receptacles or flower head of the cotton thistle (Onopordum acanthium L.) was reported, but without any content or characteristics (Van Loo et al., 1995; Petit, 2012). Petkova and Mihaylova (2015) reported the fructan content in Onopordum tauricum 7.90 ± 0.34 g/100 g and inulin 4.5 g/100 g. Parzhanova et al. (2018) found 0.84 ± 0.17 g/100 g total fructans in water infusions of Onopordum acanthium. However, in the current research total fructan content reached 5.53 g/100 g as half part of it is due to the presence of inulin (2.63 g/100 g dw). The detailed profile of fructoooigosacchrides and total fructans was observed in water extracts of the cotton thistle (Onopordum acanthium L.). The quantity of nystose (0.4 g/100 g dw) and 1-kestose (0.9 g/100 g dw) present 25% from total fructan content in this plant. The water extracts from roots of Asparagus officinalis L. did not show any presence of sugars and inulin (Table 2). Black bryony and sarsaparilla contained only sugars (sucrose, glucose and fructose). Dioscorea communis roots did not contain inulin, nor fructoligosacchrides. Contrary to many Dioscorea species that was shown as a good source of inulin (Winarti et al., 2011), in Dioscorea communis, as well as in Dioscorea hispida Dennst inulin is absent in their underground parts.
Total phenolic content, and total flavonoids
The values of total phenol, total flavonoids, caffeic acid derivatives, as well as antioxidant activity of medicinal plants were presented (Table 3). The highest values of total phenolic content were detected in 50% ethanol extract in sarsaparilla and leuzea roots (21.33 and 7.45 mg GAE/g dw, respectively). The lowest content was found in water extracts from black Bryony (0.87 mg GAE/g dew). In general, water extracts of medicinal plants demonstrated lower levels of total phenolic content in comparison to 50% ethanol extracts (Table 4). Only leuzea and dandelion roots showed near phenolic content in water and 50% ethanol extracts. The phenolic content in 50% ethanol extracts decreased in the following order: sarsaparilla>leuzea> black bryony >asparagus> cotton thistle>St. Benedict’s thistle>dandelion. In water extracts the tendency was completely different following the order leuzea>sarsaparilla> St. Benedict’s thistle > cotton thistle>asparagus >dandelion> black bryony. In general, the proportional relation (%) of total phenols in selected medicinal plants comprised between 40 and 80% (Figure 3).
Total dihydroxycinnamic acids derivatives
Total dihydroxycinnamic acids derivatives were detected in the highest amount in leuzea and sarsaparilla roots in both water and 50% ethanol extracts (Table 3). In leuzea water extracts their content was 8.78 mg CAE/g dw, while in sarsaparilla 50% root extracts their level reach to 9.54 mg CAE/g dw. Miliauskas et al. (2005) reported that the caffeoylquinic acid derivatives are the main group of biologically active constituents in leuzea roots, especially mono-, di-(1,3-dicaffeoylquinic acid, 3,4-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid, 1,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid) and tri-caffeoylquinic acid (1,4,5-tricaffeoylquinic acid). Proportional relation (%) of total dihydroxycinnamic derivative in selected medicinal plants varied between 20 and 50%, as in the leuzea roots their content was 50 % of all phenolic content (Figure 3).
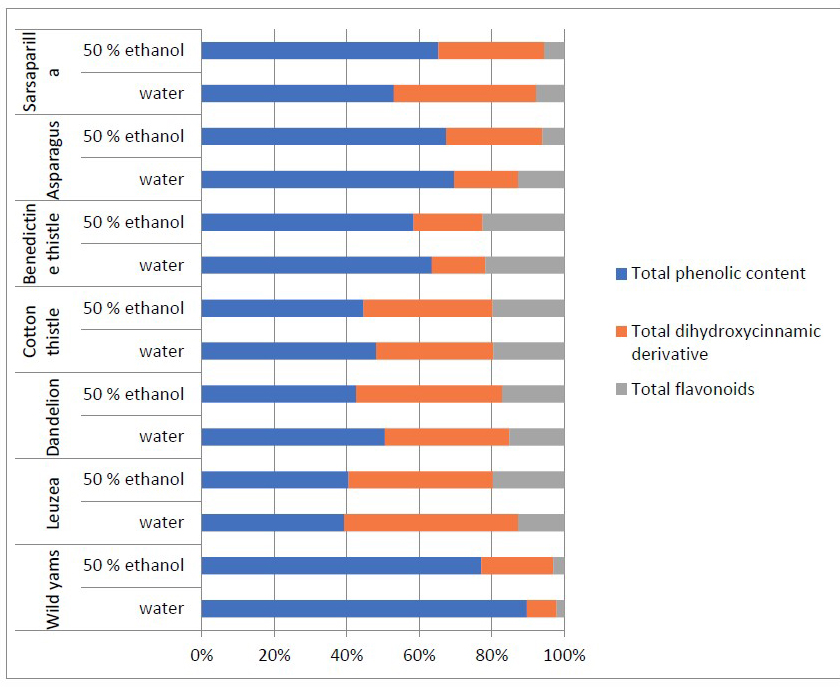
Figure 3. Proportional relation (%) of total phenols to total dihydroxycinnamic derivative and total flavonoids content in the selected medicinal plants.
Table 3. Total phenolic content and antioxidant activity of medicinal plants (mean ± SD). Physical symptoms.
|
Sample
|
TPC, mg GAE/g |
DCA, mg CAE/g |
TF, mg QE/g |
Antioxidant activity, mM TE/g |
|
|
DPPH |
FRAP |
||||
|
Black bryony A |
0.87 ± 0.04a |
0.08 ± 0.06a |
0.02 ± 0.04a |
12.86 ± 1.27a |
26.99 ± 1.27a |
|
Black bryony B |
6.30 ± 0.36ns |
1.62 ± 0.04a |
0.25 ± 0.05ns |
0.08 ± 0.04c |
2.59 ± 0.14a |
|
Leuzea A |
7.16 ± 0.04b |
8.78 ± 0.24b |
2.32 ± 0.04b |
60.74 ± 4.93b |
31.04 ± 4.81b |
|
Leuzea B |
7.45 ± 1.01ns |
7.34 ± 0.69b |
3.62 ± 0.04b |
50.74 ± 1.22a |
46.51 ± 4.07b |
|
Dandelion A |
2.10 ± 0.03c |
1.43 ± 0.13a |
0.63 ± 0.05b |
4.25 ± 0.13c |
9.38 ± 2.34c |
|
Dandelion B |
2.21 ± 0.06c |
2.09 ± 0.16a |
0.89 ± 0.03c |
5.71 ± 1.42b |
6.58 ± 0.38c |
|
Cotton thistle A |
4.06 ± 0.26d |
2.73 ± 0.19a |
1.65 ± 0.14d |
22.73 ± 5.07d |
30.81 ± 1.89b |
|
Cotton thistle B |
5.44 ± 0.03ns |
4.33 ± 0.17c |
2.41 ± 0.26e |
20.51 ± 4.12d |
21.81 ± 2.45d |
|
St. Benedict’s thistle A |
4.63 ± 0.03d,e |
1.08 ± 0.74a |
1.59 ± 0.29d,e |
10.76 ± 4.05a |
20.11 ± 0.11d |
|
St. Benedict’s thistle B |
5.87 ± 0.23ns |
1.91 ± 0.11a |
2.26 ± 0.03b |
9.22 ± 1.13e |
21.09 ± 0.73d |
|
Asparagus A |
2.68 ± 0.15c,f |
0.68 ± 0.35d |
0.49 ± 0.03a |
24.81 ± 0.10d |
39.08 ± 0.13b |
|
Asparagus B |
5.88 ± 0.49ns |
2.31 ± 0.03a |
0.53 ± 0.15c |
5.18 ± 2.24b |
14.47 ± 0.88e |
|
Sarsaparilla A |
7.01 ± 0.77b,g |
5.20 ± 1.99b |
1.03 ± 0.20d,f |
98.13 ± 5.13e |
82.31 ± 5.04e |
|
Sarsaparilla B |
21.33 ± 2.46a,g |
9.54 ± 3.95b |
1.82 ± 0.77d |
49.31 ± 5.80e |
35.66 ± 2.73f |
Note: A- water extract, B - 50% ethanol extract, Values are mean ± standard deviation of three separate experiments.. Different letters within each column indicate significant differences between treatments according to Tukey’s test at P < 0.05; n.d. – not detected Values are mean ± SD of three independent experiments.
Total flavonoids
The highest content of total flavonoids was detected in leuzea roots, cotton thistle flowering heads and St. Benedict's thistle areal parts from 2 to 3.6 mg QE/g dw (Table 3). Proportional relation (%) of total flavonoids comprised 10-20% from all total polyphenols. Black bryony demonstrated the lowest values - below 0.1 mg QE/g dw, followed by Asparagus officinalis.
Antioxidant activity
The results from antioxidant activity were summarized in Table 3. The highest antioxidant activity evaluated by both methods (DPPH and FRAP) was found in 50% ethanol extracts of sarsaparilla roots (98.13 ± 5.13 and 82.31 ± 5.04 mM TE/g dw), followed by leuzea (60.74 ± 4.93 and 46.51 ± 4,07 mM TE/g dw). The extracts of the cotton thistle also demonstrated strong antioxidant potential. However, Black bryony tuber extracts demonstrated the lowest values of antioxidant activity that could be explained with the lowest level of total flavonoids and total polyphenols content. The correlation between total antioxidant activity evaluated by DPPH and FRAP methods and total phenolic content, total dihydroxycinnamic derivatives, total flavonoids in medicinal plant extracts were presented in Table 4.
Table 4. Correlation coefficient (r2) between total phenolic content, caffeic acid derivatives, total flavonoids, and antioxidant activities (DPPH and FRAP assays)
|
|
DPPH |
FRAP |
Total dihydroxycinnamic derivatives |
Total flavonoids |
|
Total phenols |
0.9002 |
0.5437 |
0.7796 |
0.4173 |
|
Total dihydroxycinnamic derivatives |
0.9429 |
0.5312 |
- |
0.7080 |
|
Total flavonoids |
0.5864 |
0.1713 |
0.7080 |
- |
A high correlation between the amount of total phenols and caffeic acid derivatives and the antioxidant activity by the DPPH method existed with r2>0.90. Total flavonoids are weakly correlated with the antioxidant methods DPPH and FRAP. Therefore, the radical scavenging activity determined by the DPPH method was most directly influenced by the amount of total phenols and caffeic acid derivatives.
DISCUSSION
In addition, Bagaoutdinova et al. (2001) detected in the rootstock of Rhaponticum carthamoides 6.8% low-molecular carbohydrates (fructose and oligofructanes) and 7.2 % high-molecular carbohydrates (polyfructanes). The possible explanation for this change could be explained by the harvest time of leuzea and the age of the collected plants. However, this is the first detailed study about leuzea fructan composition. The detected inulin content of leuzea roots was higher than results for reported for inulin content in Rhaponticum uniflorum, while some sugar composition was near to reported values in root of Rhaponticum uniflorum (Olennikov, 2018). However, this is the first detailed report for presence of inulin (3.61 g/100 g dw), nystose, and 1-kestose in the leuzea roots and cotton thistle flower heads. About other plants, our observation was in accordance with Judprasong et al., (2011) who did not find kestose and inulin in edible portions of asparagus (Asparagus officinalis). However, some Asian representatives as Asparagus falcatus L. and Asparagus racemosus Willd. showed inulin content 11-17 g/100 g fresh weight (Mudannayake et al., 2015). However, St. Benedict’s thistle belongs to Asteracea family where fructans are typical, in aerial parts only gucse and fructose were detected.
Ranilla et al. (2010) also reported for total phenolic content in water extracts of sarsaparilla roots from Peru (20 mg/g dw). However, in our case water extracts form Bulgarian sarsaparilla showed approximately three times lower values as their content did not exceed (7 mg GAE/g dw). Water and 50% ethanol extracts from the cotton thistle flowering heads and St. Benedict’s thistle aerial parts (from 4.06 to 5.87 mg GAE/g dw) were close to reported values for St. Benedict’s thistle leaves - 635.10 mg GAE/100 g (Can et al., 2017), water and 50% ethanol extracts from cotton thistle (Angelov et al., 2012) (3 and 4 mg GA/g extract) and other thistles (Petkova and Mihaylova, 2016). In our case the leuzea root extracts demonstrated lower values (7.45 mg GAE/g dw) in comparison to the reports of Miliauskas et al. (2005) for R. carthamoides root extracts culture – from 1,908 mg to 3,520 mg/100 g dw. The lowest values of total phenolic content were observed in dandelion roots that could be with the observation for Cnicus benedictus. The lower content in of phenolic compounds is due to the fact that their roots store more of the reserve carbohydrates of the plants (Can et al., 2017). The obtained results for total phenolic content were lower in comparison with other extraction approach as infusion and microwave extraction (Petkova et al., 2017).
However, the obtained results for the total phenols in roots of asparagus 2.68 mg GAE/g dw was twice higher than reported water extracts from Asparagus officinalis 1.12 mg GAE/g dw (Kapoor et al., 2019).
Our results for total flavonoids in water extracts (0.49 mg QE/g dw) from Asparagus officinalis coincided with reported data for aqueous extracts 0.49 mg RU/g dw (Kapoor et al., 2019). Moreover, Koc et al. (2015) demonstrated high values of flavonoids in different extracts from cotton thistle 30 and 42 mg/L. Parzhanova et al. (2018) and Petkova and Mihaylova (2016) reported high level of total flavonoids in the flowering heads of some edible thistles.
A similar tendency for a high correlation between the antiradical capacity and the reducing power with phenols was (R > or = 0.9) reported for the other different asparagus cultivars (Rodriaguez et al., 2015), as well as other medicinal plants (Parzhanova et al., 2018).
CONCLUSION
The fructan and sugar content was determined in water extracts obtained from roots and thistles of seven medicinal plants. For the first time detailed sugar and inulin profile of leuzea root was demonstrated. The presence of prebiotics inulin, nystose and 1-kestose was found only in three water herbal extracts from dandelion, cotton thistle and leuzea. However, in roots of Asparagus officinalis L. any sugars were not detected. The total phenols, total flavonoids and total dihydroxycinnamic derivatives were evaluated in water and 50% ethanol herbal extracts. Among them the extracts from roots of sarsaparilla and leuzea showed the highest values of total phenols and total dihydroxycinnamic derivatives. In 50% ethanol extracts of leuzea roots were detected the highest content of total flavonoids (3.62 ± 0.84 mg QE/g dw). From all studied extracts, 50% ethanol extracts of leuzea and sarsaparilla roots showed the highest antioxidant activity evaluated by DPPH and FRAP assays. Therefore, the herbal extracts from roots of leuzea and sarsaparilla roots are the richest source of bioactive compounds with antioxidant potential.
ACKNOWLEDGEMENTS
The authors thank the Technological Faculty, University of Food Technologies for providing an HPLC instrument.
AUTHOR CONTRIBUTIONS
Nadezhda Petkova planed the experiment and assisted in conducting the experiments, performed the statistical analysis and data visualization and wrote the manuscript. Ivanka Hambarlyiska and Elena Angelova conducted all of the experiments. Ivan Ivanov designed and participate in the writing of the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Adekoya, A.E., Chusri, S., Beng, E.O.B., and Idown, A.T. 2021. Antioxidant capacities of traditionally formulated Thai herbal decoction and its effect on cell growth using Saccharomyces cerevisiae model. Chiang Mai University Journal of Natural Sciences. 20: e2021012.
Al-Snafi, A. E. 2020. Constituents and pharmacology of Onopordum acanthium. IOSR Journal of Pharmacy. 10: 07–14.
Angelov, G., Georgieva, S., and Petkova-Parlapanska K. 2012. Antioxidant activity of extracts from cotton thistle (Onopordum acanthium L.). Science and Technologies. 3: 19–23.
AOAC. 2007. Official methods of the association of official analytical chemists. Association of Official Analytical Chemists, Washington D.C.
Bagaoutdinova, I., Fedoseyeva, P., and Okoneshnikova, F. 2001. Fructose-containing carbohydrates in plants of different families localization and content. Chemistry and Computational Simulation. Butlerov Communications. 2: 13-16.
Benzie, F., and Srain, J., 1996. Ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 239: 70-77.
Can, Z., Baltaş, N., Keskin, Ş., Yıldız, O., and Kolaylı, S. 2017. Properties of antioxidant and anti-inflammatory activity and phenolic profiles of şevketi bostan (Cnicus benedictus L.) cultivated in Aegean Region from Turkey. Turkish Journal of Agriculture - FoodScience and Technology. 5: 308–314.
Fraisse, D., Felgines, C., Texier, O., and Lamaison, J-L., 2011. Caffeoyl derivatives: Major antioxidant compounds of some wild herbs of the Asteraceae family. Food and Nutrition Sciences. 2: 181-19.
Ivanov, I.G., Vrancheva, R.Z., Marchev, A.S., Petkova, N.T., Aneva, I.Y., Denev, P.P., Georgiev, V.G., and Pavlov, A.I. 2014. Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. International Journal of Current Microbiology and Applied Sciences. 3: 296-306.
Judprasong, K., Charoenkiatkul, S., Sungpuag, P., Vasanachitt, K., and Nakjamanong, Y. 2006. Total and soluble oxalate contents in Thai vegetables, cereal grains and legume seeds and their changes after cooking. Journal of Food Composition and Analysis. 19: 340-347.
Kapoor, M., Mawal, P., and Gupta R.C. 2019. Antioxidant potential, total phenolic and flavonoid content of roots of seven asparagus species from North-west India. International Journal of Pharmaceutical Sciences and Research. 10: 3837-3842.
Kivrak, I., Duru, M.E., Öztürk, M., Mercan, N., Harmandar, M. and Topçu, G. 2009. Antioxidant, anticholinesterase and antimicrobial constituents from the essential oil and ethanol extract of Salvia potentillifolia. Food Chemistry. 116: 470-479.
Koc, S., Isgor, B. S., Isgor, Y. G., Shomali, M. N., and Yildirim, O. 2015. The potentialmedicinal value of plants from Asteraceae family with antioxidant defense enzymes asbiological targets. Pharmaceutical Biology. 53: 746–751
Kokoska, L., and Janovska, D. 2009. Chemistry and pharmacology of Rhaponticum carthamoides: A review. Phytochemistry. 70: 842-855.
Lingyun, W., Jianhua, W., Xiaodong, Zh., Da, I., Yalin, Y., Chenggang, C., Tianhua, F., and Fan, Zh. 2007. Studies of the extraction technical conditions of inulin from Jerusalem artichoke tubers. Journal of Food Engineering. 79:1087-1093.
Miliauskas, G., van Beek, T.A., de Waard, P., Venskutonis, R.P., and Sudhölter, E.J. 2005. Identification of radical scavenging compounds in Rhaponticum carthamoides by means of LC-DAD-SPE-NMR. Journal of Natural Products.68:168-172.
Mudannayake, D.C., Wimalasiri, K.M.S, Silva, K.F.S.T, and Ajlouni, S. 2015. Selected Sri lankan food plants and other herbs as potential sources of inulin-type fructans. Journal of the National Science Foundation of Sri Lanka. 43: 35–43.
Olennikov D. N. 2018. Free carbohydrates, glucofructans, and other polysaccharides from Rhaponticum uniflorum. Chemistry of Natural Compounds. 54: 751-754.
Parzhanova, A.B., Petkova, N.T., Ivanov, I., and Ivanova, S. D. 2018. Evaluation of biologically active substance and antioxidant potential of medicinal plants extracts for food and cosmetic purposes. Journal of Pharmaceutical Sciences and Research. 10: 1804-1809.
Petit, D. 2012. Significance of spines in Cardueae (Asteraceae), bioclimatic stages, and mammalian grazing. Revue Agrobiologia. 3: 27–32
Petkova, N., and Mihaylova, D. 2016. Flower heads of Onopordum tauricum Willd. and Carduus acanthoides L. – source of prebiotics and antioxidants. Emirates Journal of Food and Agriculture. 28: 732–736.
Petkova, N., Ivanova, L., Filova, G., Ivanov, I., and Denev P. 2017. Antioxidants and carbohydrate content in infusions and microwave extracts from eight medicinal plants. Journal of Applied Pharmaceutical Science. 7: 055–061.
Prasajak, P., Renumarn,P., Sriwichai, W., and Detchewa, P. 2021. Antioxidant and antimicrobial properties of Moringa oleifera leaves and pods extracts in pork meatballs during cold storage. Chiang Mai University Journal of Natural Sciences. 20: e2021033
Ranilla, L. G., Kwon, Y.-I., Apostolidis, Em., and Shetty, K. 2010. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America, Bioresourses Technology. 101: 4676–4689.
Rodriaguez, R., Jaramillo, S., Rodriaguez, G., Espejo, J.A., Guillea, R., Ndez-Bolan, J.F., Heredia, A., and Nez A.J. 2005. Antioxidant activity of ethanolic extracts from several Asparagus cultivars. Journal of Agriculture and Food Chemistry. 53: 5212-5217.
Sansomchai, P., Jumpatong, K., Lapinee, C., and Utchariyajit, K. 2021. Melientha suavis Pierre. Extract: antioxidant and sunscreen properties for future cosmetic development. Chiang Mai University Journal of Natural Sciences. 20: e2021008
Schütz, K., Carle, R., and Schieber A. 2006. Taraxacum - а review on its phytochemicaland pharmacological profile, Journal of Ethnopharmacology. 107: 313–323.
Stintzing, F.C., Nerbach, K.M., Mosshammer, M., Carle, R., Yi, W., Sellappan, S., Acoh, C.C., Bunch, R. and Felker, P. 2005. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. Journal of Agricultural and Food Chemistry. 53: 442-451.
Szabó, I., Pallag, A., and Blidar, F. 2009. The antimicrobial activity of the Cnicus benedictus L. extracts. Analele Universitatii din Oradea Fascicula Biologie.16: 126-128.
Van Loo, J., Coussement, P., De Leenheer, L., Hoebregs, H., and Smits, G. 1995. On the presence of inulin and oligofructose as natural ingredients in the Western diet. Critical Reviews in Food Science and Nutrition. 35: 525–552.
Vasfilova, Е., Bagautdinova, R., and Okoneshnikova, T. 2015. Phisiologia and Biohimia rasgenii, Tomsk State University Journal, Biology. 2: 96–112. In Russian
Winarti, S., Harmayani, E., and Nurismanto, R. 2011. Karakteristik dan profil inulin beberapa jenis uwi (Dioscorea app.). Agritech. 31: 378-383.
Wirngo, F. E., Lambert, M. N., and Jeppesen, P.B. 2016. The physiological effects of dandelion (Taraxacum officinale) in Type 2 Diabetes. The Review of Diabetic Studies. 13: 113–131.
Yuniastuti, A., and Iswari, R. S. 2019. Isolation and Identification of Inulin and Fos from Dioscorea esculenta in UNNESInternational Conference on Research Innovation and Commercialization 2018. KnE Social Sciences. 41–46.
Zhang, H., Birch, J., Yang, H., Xie, C., Kong, L., Dias, G., and Bekhit, A.E.‐D. 2018. Effect of solvents on polyphenol recovery and antioxidant activity of isolates of Asparagus Officinalis roots from Chinese and New Zealand cultivars. International Journal of Food Science Technology. 53: 2369-2377.
Zubaidah, E., and Akhadiana, W. 2013. Comparative study of inulin extracts from dahlia, yam, and gamble tubers as probiotics. Food and Nutrition Sciences.
4: 8–12.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Nadezhda Petkova*, Ivanka Hambarlyiska, Elena Angelova and Ivan Ivanov
Department of Organic Chemistry and Inorganic Chemistry, Technological Faculty, University of Food Technologies 26 Maritza Blvd., 4002, Plovdiv, Bulgaria
Corresponding author: Nadezhda Petkova, E-mail: petkovanadejda@abv.bg
Total Article Views
Editor: Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: April 23, 2021;
Revised: June 29, 2021;
Accepted: July 2, 2021;

