
Effect of Vitamin A-Fortified Rice on the Gut Microbiota of Thai Lactating Women and Their Exclusively Breastfed Infants
Lukman Azis, Siwaporn Pinkaew*, and Santad WichienchotPublished Date : 2021-09-13
DOI : https://doi.org/10.12982/CMUJNS.2021.079
Journal Issues : Number 4, October-December 2021
Abstract The optimal vitamin A (VA) status of lactating women is important for mothers and their breastfed infants, especially in protecting against infectious diseases. Vitamin A fortified rice is one of the food-base intervention strategy which has the potential to improve VA status. Vitamin A and gut microbiota are interrelated in their effect on human health and immunity however no specific relationship has been proved in these groups of population. This study aimed to determine the effect of VA fortified rice on the gut microbiota changes of lactating woman-exclusively breastfed infant pairs. A double-blind, randomized controlled trial (RCT) of VA fortified rice was conducted in 70 lactating women-infants pairs for 14 weeks. Gut microbiota was measured using the fluorescent in situ hybridization (FISH) and next generation sequencing (NGS) technique. Based on the FISH technique, the numbers of Clostridium spp. /Enterobacter spp. were significantly lower (P < 0.05) in mothers fed VA-fortified rice at the end of the study. In contrast, the abundance of Bifidobacterium spp. and Lactobacillus spp. of infants whose mothers fed with VA-fortified rice was significantly higher (P < 0.05) than the control group. The NGS technique confirmed that results with the increasing of Lactobacillus, B. longum and B. Choerinum in the infant of intervention group. In conclusion, VA-fortified rice was efficacious in decreasing Clostridium spp. /Enterobacter spp. in lactating women and raising the number of Bifidobacterium spp. and Lactobacillus spp. in their breastfed infants.
Keywords: Breastfeeding, Gut microbiota, Lactating woman-infant pairs, Randomized controlled trial, Vitamin A
Funding: The research was funded by Halal Food Science Center, Department of Food Science and Nutrition, Faculty of Science and Technology, Prince of Songkla University, Thailand’s Education Hub for Southern Region of ASEAN Countries (TEH-AC) scholarship and Graduate School research funding 2018, Prince of Songkla University, Thailand.
Citation: Azis, L., Pinkaew, S., and Wichienchot, S. 2021. Effect of vitamin A-fortified rice on the gut microbiota of Thai lactating women and their exclusively breastfed infants. CMU J. Nat. Sci. 20(4): e2021079.
INTRODUCTION
Vitamin A (VA) is a micronutrient and a fat-soluble vitamin which has vital functions in human health, especially in the visual metabolism, prenatal and postnatal development, cellular differentiation and tissue repair (Tanumihardjo, 2011; Ross, 2014). However, the most crucial role of VA is as a substance which promotes immune function, that protects the body’s natural defense system against infections and diseases (Huang et al., 2018; Maggini et al., 2018). According to the Thai dietary recommended intake (DRIs), lactating women has the highest requirement of VA intake (975 µg/day) due to support and maintain their health as well as breast milk production which is important for the infant during the first six months of life (FAO & WHO, 2001; Garcia-Mantrana et al., 2018).
Newborn infants are born with a low level of VA stores and are therefore dependent on external sources, most importantly breast milk. Thus, maternal dietary intake during pregnancy and postpartum is an essential determinant of both maternal and infant VA status (World Health Organization, 2011a). Vitamin A is transferred from the mother to the infant via breast milk and is essential for the growth and development of the infant’s immune system during the first stage of life (Mccauley et al., 2015).
Vitamin A deficiency (VAD) is still remaining as a public health problem in many developing countries due to inadequate VA intake. Several health consequences, including infections, ocular manifestations diseases, inflammation and growth retardation can be provoked from the inadequate VA intake (West et al., 2011). Moreover, VAD is a leading cause of morbidity and mortality in pregnant/lactating women and infants. Many strategies have been implemented to improve VA status and combat VAD. One of the most direct, effective and low-cost approaches is food fortification. Vitamin A-fortified food products were successfully increased VA status have been reported in Vietnam, Indonesia, Bangladesh and Thailand (de Pee et al., 1995; Ezzati et al., 2004; Khan et al., 2007). Our preceding study found that the feeding of VA-fortified rice (890 µg/ day) for 2 months increased total body store of VA in Thai school-age children (Pinkaew et al., 2014b).
The human gastrointestinal (GI) tract is the habitat for more than thousand microbiotic species which form a complex ecological community that affects the host’s normal physiology (Karakochuk et al., 2017). The human health is susceptible to diseases through the collective metabolic activities of the community members consisting of bacteria, viruses and fungi and their interactions with the host. Kau et al. (2011) established a model of the interrelations between the gut microbiota and the development of malnutrition and VAD is one type of malnutrition known to affect the immune system, infection and impaired absorption of nutrients (Huang et al., 2018; World Health Organization, 2019b). A recent study reported VA inhibited Murine Norovirus (MNV) replication after VA administration by increasing Lactobacillus spp. stimulation in mice (Lee & Ko, 2016). However, the correlation between gut microbiota and VAD is by no means clear, and the connection between the role of the VA and the gut microbiota needs to be explored, especially in human.
Therefore, this study intended to determine the effect of VA-fortified rice including its effect in infants through the breast milk of lactating women, ascertained by the changes in representative species of gut bacteria, such as Bifidobacterium spp., Lactobacillus spp., Clostridium spp. and Enterobacter spp. in lactating woman-infant pairs.
MATERIALS AND METHODS
The study design was a double-blind, randomized controlled trial. The current study was conducted as one part of the main study protocol, namely “the efficacy of VA fortified rice in lactating Thai women” which the primary outcome was to measure vitamin A status (total body store, liver vitamin A concentration and serum retinol) of lactating women (Pinkaew et al., 2021a).
Study site and participants
The participants for this study were lactating women and their infants who lived in the peri-urban area of Muang district, Pattani province, located on the east coast of southern Thailand. The intervention was conducted from January 2017 to February 2018.
The inclusion criteria of participants were apparently healthy lactating women aged 20-40 years with a gestational age of 37-42 weeks, and infants from a singleton pregnancy with a birth weight greater than 2,500 g. The sample of 31 pairs/group was calculated based on a sample size calculation of the main study protocol for a primary objective (which could detect the difference of 12 µg retinol/g liver with an SD that is 1.25 times the difference) (Waddel Snedecor et al., 1989). To allow for dropouts, 70 lactating women were recruited (n=35/group). All lactating women planned to breastfeed for at least 4-6 months at the time of recruitment.
The exclusion criteria were not being exclusively breastfeeding (EBF) at the end of the intervention, unable to collect the stool samples of both mother and infant at the same day (within the specified period of time), suspected to have an infection, taken the antibiotic (a month before stool collection) and taken VA supplement. The details of the study protocol were explained to lactating women. Informed consent was obtained from mothers and on behalf of their infants.
The intervention
The details for preparation of VA fortified rice as component of the lunch meal was explained previously (Pinkaew et al., 2021a). Briefly, VA-premixed kernel (DSM Nutritional Products, Singapore) were mixed with normal rice at the ratio 4:96 to obtain the target value 500 µg retinol/50 g fortified rice. The participants were randomly assigned into 2 groups (n= 35/group). VA-fortified rice (500 µg retinol/day) was given to lactating women of the intervention group while unfortified/normal rice was given to the control group. The rice meals were freshly prepared daily and given to all the lactating women in the morning of the weekday for 14 weeks by the field staffs. Research assistants and a nurse visited the mothers once a week to monitor leftovers and compliance.
Data collection and laboratory analysis
General characteristics and anthropometric measurements
At the baseline, the lactating women were asked for general information about themselves and their socioeconomic status, such as their age, education, occupation, marital status, number of children, their expected duration of breastfeeding and their health status, as well as the information about their infants. Moreover, anthropometric measurements were assessed at baseline for both lactating women and infants. The body dimensions (weight, height or length and head circumference (HC) of all participants were measured. Their height/length and HC were recorded in centimetres (cm ± 0.1 cm), and their weights were recorded in kilograms (kg ± 0.1 kg). The weight of the participants was measured using a digital scale (Camry, USA) which was calibrated daily. The height/length of lactating women and infants were measured with a stadiometer (Seca, Japan) and a length board (Ro-chain, Germany) respectively. The HC was measured with a measuring tape (Kanko, Thailand). All the measurements were done for 2 replicated and the average values were calculated from the two measurements.
Stool samples collection
The stool samples were obtained from lactating woman-infant pairs on the same day at baseline and endpoint in order to determine the gut microbiota composition. Instructions relating to the collection of the stool samples were explained to participants at the time of recruitment. The stool samples of both lactating woman and their infant must be taken at the same day (before-after intervention) with a plastic teaspoon then kept in a clean small plastic box. The fresh stool sample was immediately brought to the laboratory by research assistant where it was kept in a freezer at -80°C for gut microbiota analysis. All of the supply materials that contact with the stool sample were sterilized.
Gut microbiota determination
Enumeration of gut microbiota by fluorescent in situ hybridization (FISH)
All the stool samples from the lactating woman-infant pairs were diluted in Phosphate-buffered saline (PBS) and analyzed at baseline and endpoint. The analysis was carried out using DNA probes for the specific hybridization of Bifidobacterium spp. (Bif164) (Langendijk et al., 1995) and Lactobacillus spp. (Lab158) (Harmsen et al., 1999) as representatives of mostly benign or beneficial bacteria and Clostridium spp. /Enterobacter spp. (Chis150) (Franks et al., 1998) as representative of mostly non-beneficial bacteria (Plongbunjong et al., 2017). The DNA probes were purchased from Sigma-Aldrich (St. Louis, USA). Data were expressed as log10 cell/g faecal sample.
Identification of gut microbiota by next-generation sequencing (NGS)
The NGS technique (MiSeq, Illumina, USA) was performed in the subset of the infants’ stool samples (three from the intervention and three from the control group). Stool samples were randomly selected for the sequencing of bacterial 16S rRNA gene of gut microbiota at baseline and endpoint. This identification technique used V3--V4 regions of 16S rRNA gene. The primer sequences standard used IUPAC nucleotide nomenclature with the 16S amplicon PCR forward primer = 5'TCGTCGG CAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG and 16S reverse primer = 5'GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC. The data analysis from MiSeq Reporter software was aligned with 16s reference of bacteria and archaea in the Greengenes 16S rRNA gene database (http://greengenes.lbl.gov/). The pipeline of NGS data analysis is on‐board primary analysis, and secondary analysis using MiSeq Reporter software. The amplicon primers were used for the V3 and V4 region that create a single amplicon of approximately ~460 bp (Klindworth et al., 2013).
The output of this workflow is a classification of reads at several taxonomic levels: kingdom, phylum, class, order, family, genus, and species. This protocol was referenced by Illumina (Illumina, 2020). For quality control of identification, the minimum read number of each sample was set to at least 100,000. The chimeric sequences were cleaned up by using AMPure XP beads to purify the 16S V3 and V4 amplicon away from free primers and primer dimer species. The sequence data was deposited to National Center for Biotechnology Information (NCBI) with GenBank submission: SUB8713875.
Ethical clearance
The study’s protocol was approved by the Research Ethics Committee for Science, Technology and Health Science, Prince of Songkla University, Pattani campus (REC No. psu.pn.1-005/59) and the protocol was registered for ClinicalTrials.gov (ID: NCT03056625).
Statistical analysis
The R program i386 version 3.5.2 was used for data analysis. The normality was checked by the Shapiro-Wilk test (P > 0.05) and normal distributed data were presented as mean ± standard deviation (SD). The gut microbiota enumeration by the FISH technique was analyzed using repeated measures to determine the effects of time x treatment. The data were compared between the intervention and control groups using independent sample t-tests, data of the same group at different time point using paired t-test and the chi-square test for categorical data. Differences were considered to be significant at P < 0.05.
RESULTS
Baseline characteristics of lactating women and infants
At the end of the study, there were a total of 27 pairs of lactating women-infants whose stool samples were completely collected at pre vs post intervention (14 pairs belonging to the intervention group and 13 pairs belonging to control group, respectively). Beside unable to collect stool samples of mother and infant at the same day (n=14), the reasons for dropout as follows: (i) moved out of the study area (n=4), (ii) cannot breastfeed (n=2), (iii) late neonatal mortality (n=1), (iv) refused to give blood samples (n=1), (v) not EBF (n=21),
The baseline characteristics of the 27 lactating woman-infant pairs were found to be comparable between the two groups (Table 1). The average age of the mothers was 29.2 years, with mean BMI of 22.5 kg/m2, all of them were Muslim. More than 40% of the lactating women did not work and approximately half (51.8%) had completed a bachelor’s degree, with the average of schooling year by 13.5 (data not shown in Table 1). The average family income per month was ≈ 500 USD which supported around 5 members of their family. Most women (70%) had a normal labour. The parity was 2.5 and approximately half of the infants were male and their average age at baseline was 1 month. The weight, length and HC of infants were 4.4 kg, 53.7 cm and 37.1 cm, respectively, at baseline.
Table 1. Baseline characteristics of lactating women (n=27) and infants (n=27) participated in the study.
|
Characteristics |
Total (n=27) |
Intervention (n=14) |
Control (n=13) |
|
Mother |
|
|
|
|
Age, y |
29.20 ± 4.60 |
27.60 ± 4.40 |
30.80 ± 4.30 |
|
Religion, % Muslim |
100.00 |
100.00 |
100.00 |
|
Household size, n |
5.40 ± 1.70 |
5.30 ± 1.50 |
5.60 ± 1.90 |
|
Gestation age, wk |
38.80 ± 1.20 |
39.00 ± 1.30 |
38.70 ± 1.00 |
|
Mode of delivery, % Normal labor |
70.40 |
64.30 |
76.90 |
|
Parity, n |
2.50 ± 0.90 |
2.30 ± 0.90 |
2.80 ± 0.80 |
|
Weight, kg |
54.30 ± 9.70 |
52.20 ± 11.40 |
56.80 ± 6.90 |
|
Height, m |
1.57 ± 0.04 |
1.58 ± 0.02 |
1.56 ± 0.04 |
|
Body mass index, kg/m2 |
22.50 ± 3.90 |
21.60 ± 4.50 |
23.40 ± 3.20 |
|
Infant |
|
|
|
|
Gender, % boy |
51.80 |
50.00 |
53.80 |
|
Age, mo |
1.00± 0.20 |
1.10 ± 0.10 |
1.10 ± 0.20 |
|
Weight, kg |
4.40 ± 0.50 |
4.30 ± 0.50 |
4.40 ± 0.50 |
|
Length, cm |
53.70 ± 1.80 |
54.00 ± 1.80 |
53.40 ± 1.90 |
|
Head circumference, cm |
37.10 ± 1.00 |
37.30 ± 0.90 |
37.00 ± 1.10 |
Note: Data expressed as mean ± SD
Not significantly different at P > 0.05, between intervention and control group
Lactating women and infants’ gut microbiota enumeration by FISH technique
At the baseline, the number of Bifidobacterium spp., Lactobacillus spp., Clostridium spp. /Enterobacter spp. in faecal samples between the 2 groups of lactating women and infants were comparable (P > 0.05) (Figure 1 and Figure 2).
At the end of the intervention, for the mothers, it was found that the VA-fortified rice only had an effect on the number Clostridium spp./Enterobacter spp. (Figure 1) which had decreased and was significantly lower (P < 0.01) in the intervention group (9.57 ± 0.57 log10 cell/g faecal sample) compared to the control group (9.73 ± 0.35 log10 cell/g faecal sample). On the other hand, the increases in benign or beneficial bacteria were found in the group of exclusively breastfed infants whose mothers had been fed with VA-fortified rice (Figure 2). Bifidobacterium spp. (10.12 ± 0.25 log10 cell/g faecal sample vs 9.93 ± 0.65 log10 cell/g faecal sample) and Lactobacillus spp. (9.88 ± 0.37 log10 cell/g faecal sample vs 9.58 ± 0.37 log10 cell/g faecal sample) were significantly higher (P < 0.05) in the intervention group when compared to the control group of infants respectively at the endpoint.
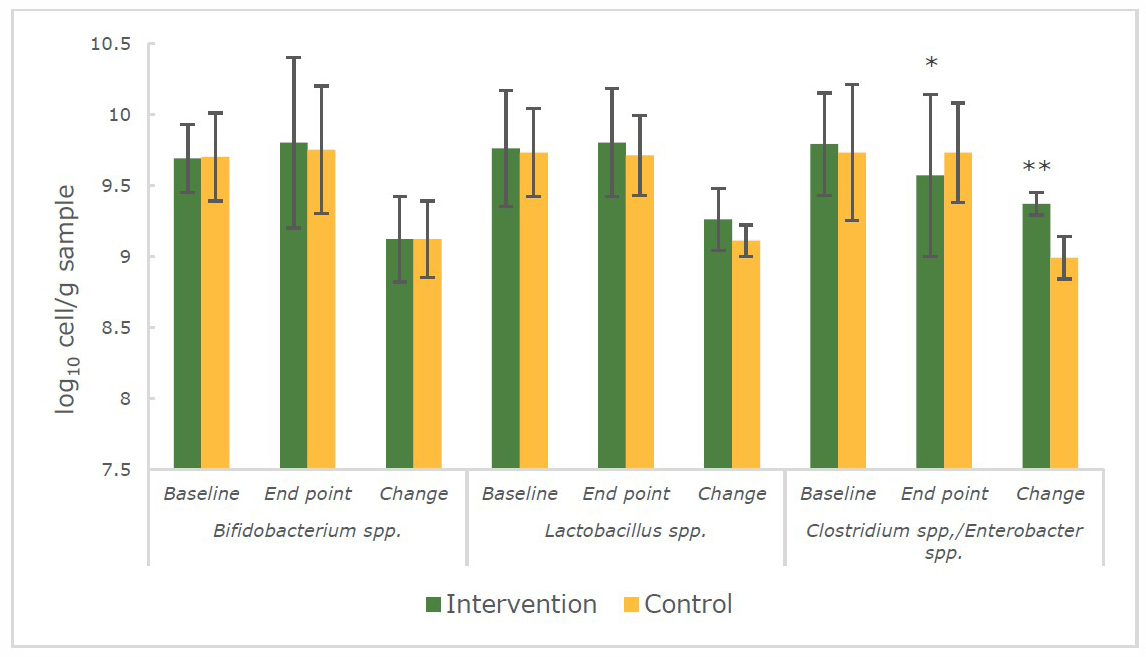
Figure 1. The number (log10 cell/g faecal sample) of Bifidobacterium spp., Lactobacillus spp., Clostridium spp./Enterobacter spp. in faecal samples of lactating women during 14 weeks of intervention.
Note: *significant difference (P < 0.05) between intervention and control group (repeated measures and independent sample t-test)
**significant difference (P < 0.01) between intervention and control group (independent sample t-test)
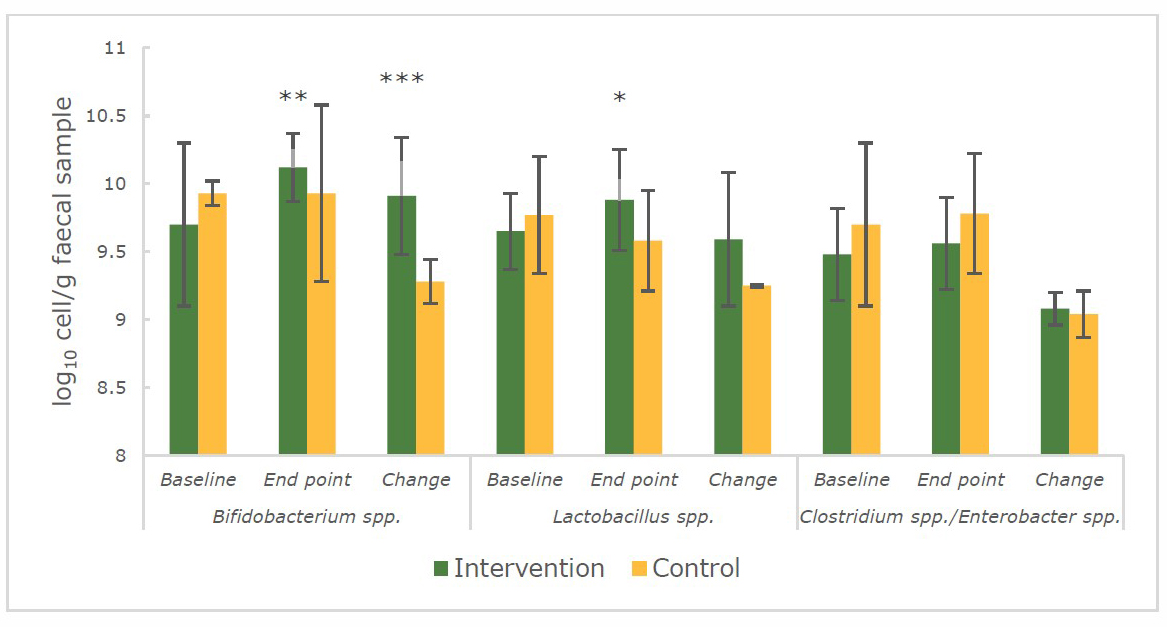
Figure 2. The number (log10 cell/g faecal sample) of Bifidobacterium spp., Lactobacillus spp., Clostridium spp./Enterobacter spp. in infants’ faecal samples during 14 weeks of intervention.
Note: *significant difference (P < 0.05) between intervention and control group (repeated measures and independent sample t-test)
**significant difference (P < 0.01) between intervention and control group (repeated measures and independent sample t-test)
***significant difference (P < 0.001) between intervention and control group (independent sample t-test)
Infants’ gut microbiota identification by NGS technique
Figure 3 to 8 depict the relative percentage abundance of the gut microbiota composition measured by the NGS technique at phylum (Figure 3-4), genus (Figure
5-6)and species (Figure 7-8) level in the infants’ faecal samples (n= 3/group) at baseline and endpoint.
Gut microbiota at the phylum level
At the phylum level, the infants’ faecal samples were dominated by Actinobacteria, Proteobacteria, Bacteroides and Firmicutes (Figure 3 and 4). The percentage of bacteria were not significantly (P > 0.05) different when compared with the intervention and control group at 2 time points. However, only Actinobacteria in the intervention group significantly (P < 0.01) increased at endpoint compared to the baseline and no changed had been detected for the control group. At the baseline, for the intervention group (Figure 3), the abundance of Proteobacteria (41.6%) was higher than that of Actinobacteria (26.4 %), Bacteroides (23.2%), Firmicutes (5.7%) and unclassified bacteria (3.0%). Meanwhile, for the control group, the abundance of Actinobacteria (57.3%) was higher than that of Proteobacteria (25.7%), Firmicutes (12.9%), Bacteroides (3.6%) and unclassified bacteria (0.5%). At the endpoint of the intervention (Figure 4), the most abundant phylum for intervention and control group was changed to Actinobacteria (beneficial bacteria) and Proteobacteria (pathogenic bacteria) respectively.
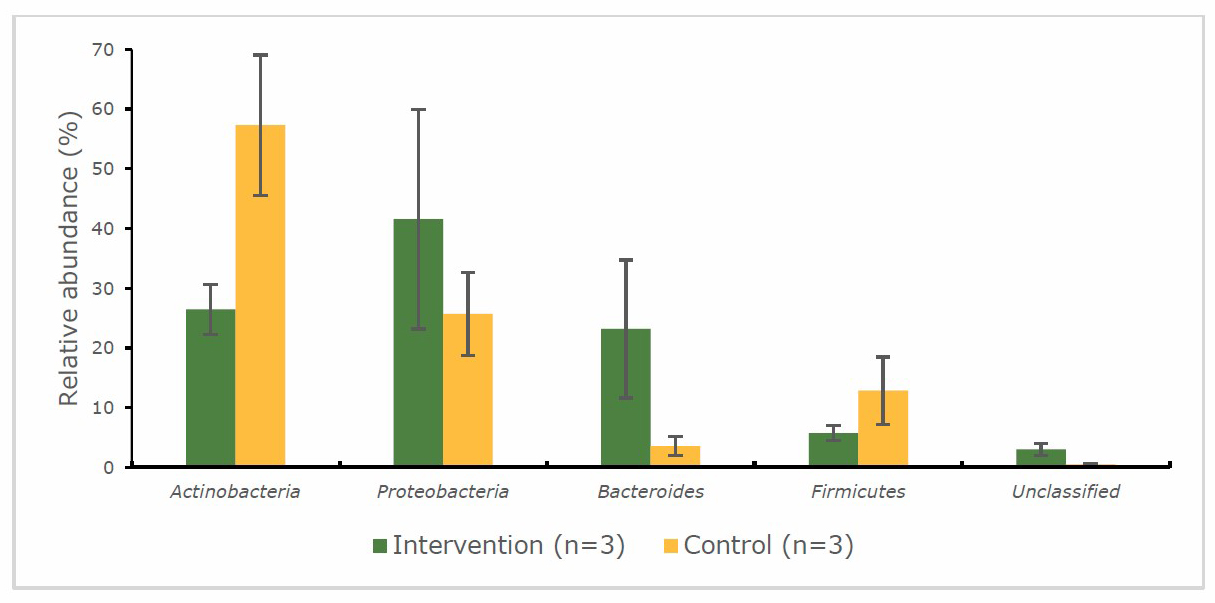
Figure 3. Percentage abundance of gut microbiota at the phylum level in infants’ faecal samples during 14 weeks of intervention measured by 16S rRNA sequencing (NGS) at baseline
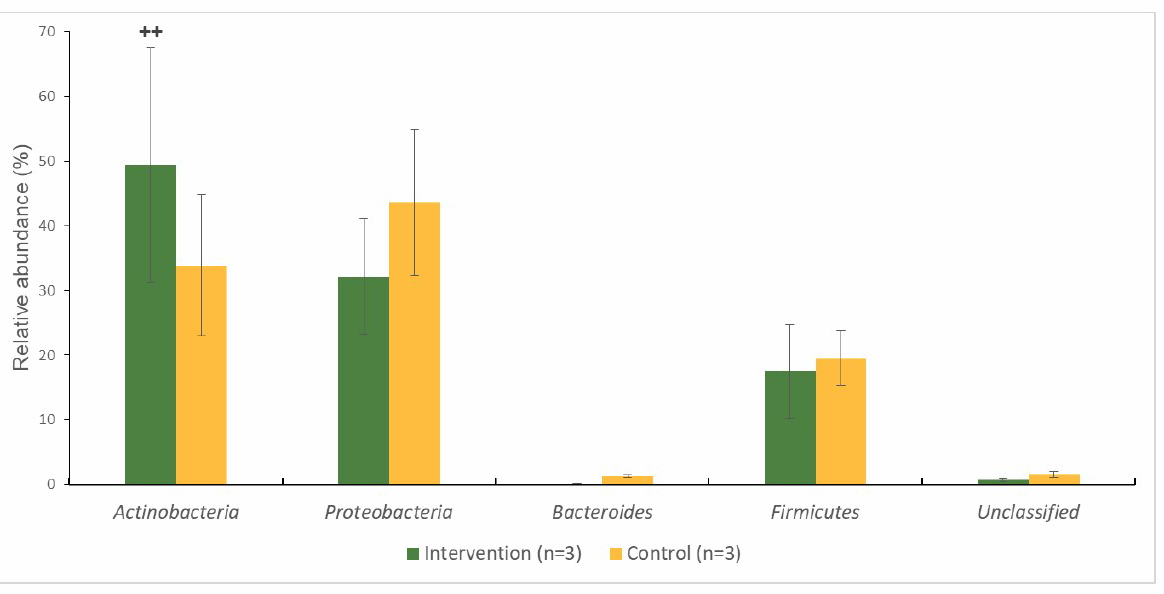
Figure 4. Percentage abundance of gut microbiota at the phylum level in infants’ faecal samples during 14 weeks of intervention measured by 16S rRNA sequencing (NGS) at endpoint.
Note: ++ significant different (P < 0.01) at 2 timepoints (baseline and endpoint) (pair-t-test)
Gut microbiota at the genus level
The results of the genus level enumeration are shown in Figure 5 and 6. Benign or beneficial bacteria such as Bifidobacterium, Lactobacilli and pathogenic bacteria such as Escherichia, Klebsiella, Bacteroides, Streptococcus, Enterobacter, Serratia and Veillonella were found in the 2 groups at the baseline and endpoint.
At baseline (Figure 5), both intervention and control group were not significantly (P > 0.05) different in the proportion of gut bacteria. Escherichia (25.0%) and Bifidobacterium (24.7%) dominated at the baseline of the intervention group. At the endpoint (Figure 6) the abundance at genus level of the intervention group had changed to Bifidobacterium (48.3%) which was significantly (P < 0.01) increase after intervention. The Lactobacilli appeared in both groups; however, the intervention group had a significantly (P < 0.01) higher than the control group (26.3% vs 1.4%, respectively). Moreover, Bacteroides was no longer detected in the intervention group at the end of the study. At the baseline (Figure 5), the gut microbiota of control group was dominated by Bifidobacterium (56.6%) (the beneficial bacteria) which decreased to 33.3% at the end point (Figure 6) while the pathogenic bacteria Klebsiella (27.6%) was significantly increase and higher than the intervention group (P < 0.01).
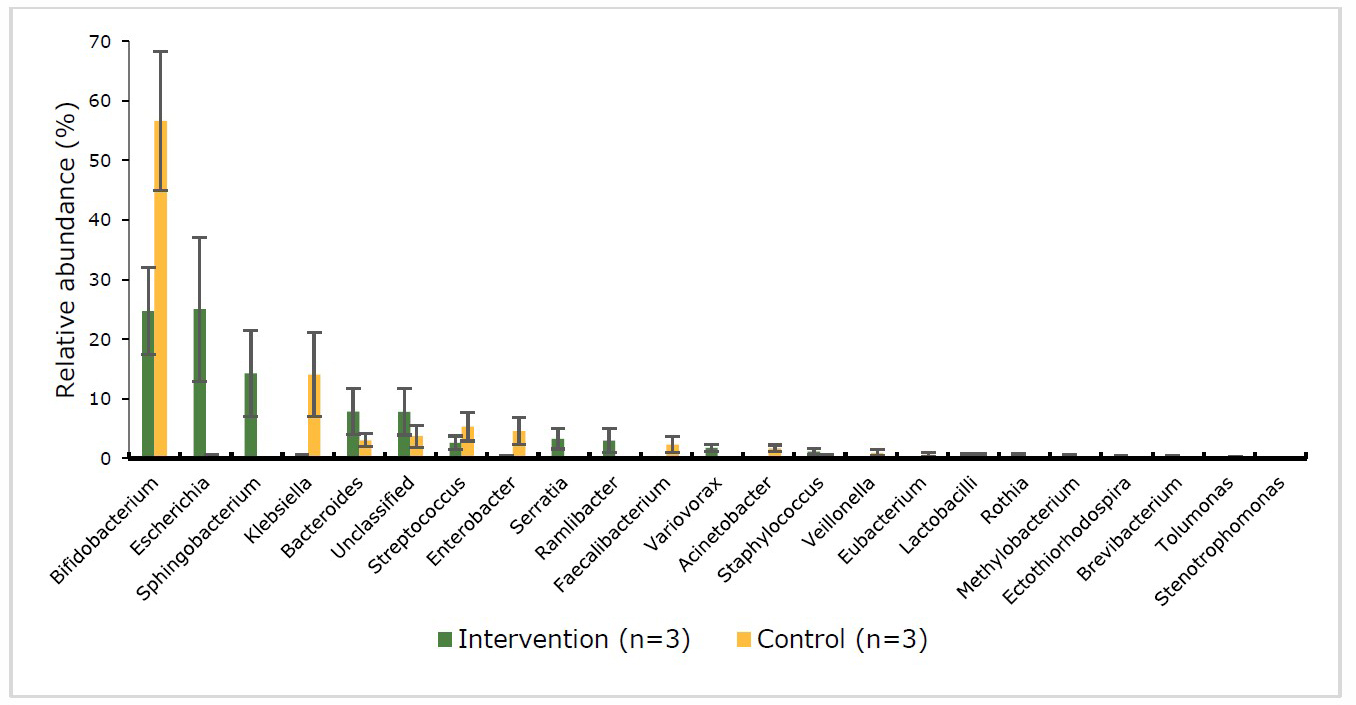
Figure 5. Percentage abundance of gut microbiota at the genus level in infants’ faecal samples during 14 weeks of intervention measured by 16S rRNA sequencing (NGS) at baseline
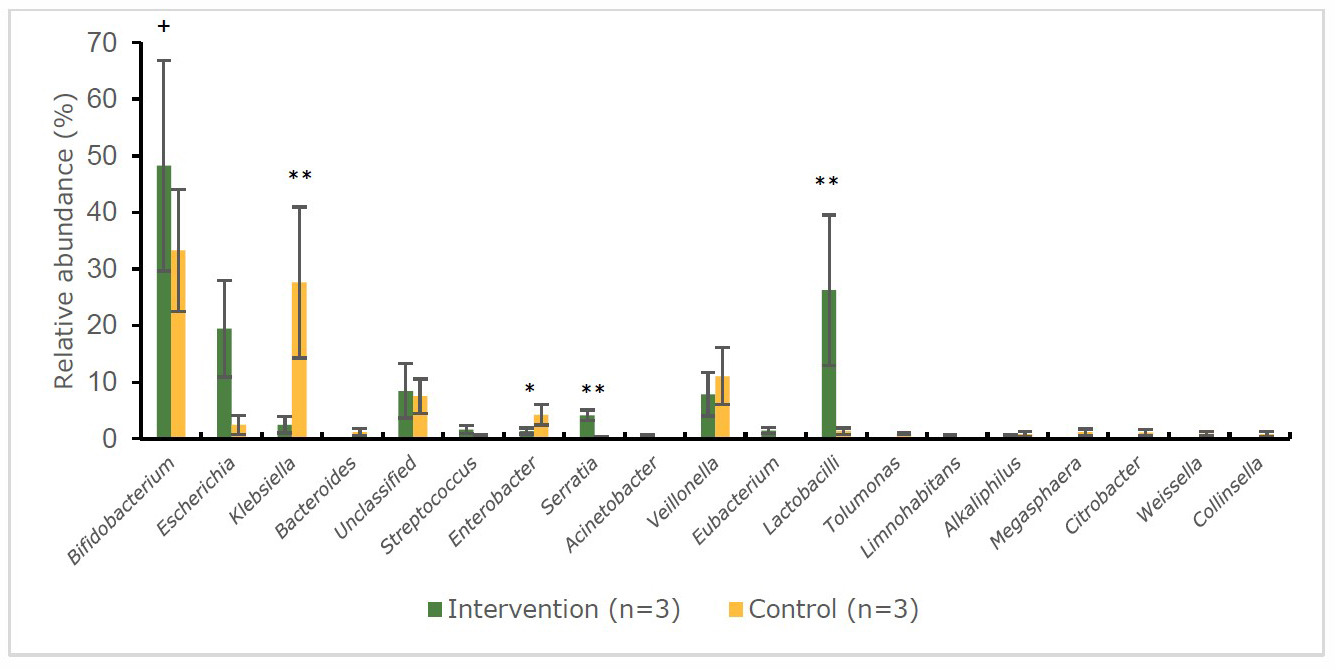
Figure 6. Percentage abundance of gut microbiota at the genus level in infants’ faecal samples during 14 weeks of intervention measured by 16S rRNA sequencing (NGS) at endpoint.
Note: *significant difference (P < 0.05) between intervention and control group at the same time point (independent t-test)
**significant difference (P < 0.01) between intervention and control group at the same time point (independent t-test)
+significant difference (P < 0.05) at 2 time points (baseline and endpoint) (pair t-test)
++significant difference (P < 0.01) at 2 time points (baseline and endpoint) (pair t-test)
Gut microbiota at the species level
Figure 7 and 8 show the relative abundance (%) of gut bacteria at the species level. At the baseline (Figure 7) of the intervention group, infant’s stool samples were more populate with the pathogenic bacteria, Escherichia albertii (23.4%) and Sphingobacterium multivorum (11.3%) than the beneficial bacteria: Bifidobacterium breve (7.0%) and Bifidobacterium longum (5.8%). On the other hand, beneficial bacteria such as Bifidobacterium catenulatum (16.6%), and Bifidobacterium longum (13.8%) were dominated at the baseline of the control group. The changes were found after intervention in the pattern of bacteria (Figure 8) for the intervention group significantly increased (P < 0.05) in beneficial bacteria e.g. Bifidobacterium longum (22.2%), Bifidobacterium choerinum (3.4%) and decreased in pathogenic bacteria, such as Escherichia albertii (17.9%). Moreover, Bifidobacterium longum and Bifidobacterium choerinum were significantly higher (P < 0.05) compared to control group. Meanwhile, in the control group at the endpoint, the pathogenic bacteria, Klebsiella variicola (18.4%) dramatically increased (P < 0.01) with a decrease in the abundance of the beneficial bacteria, Bifidobacterium longum (10.3%).
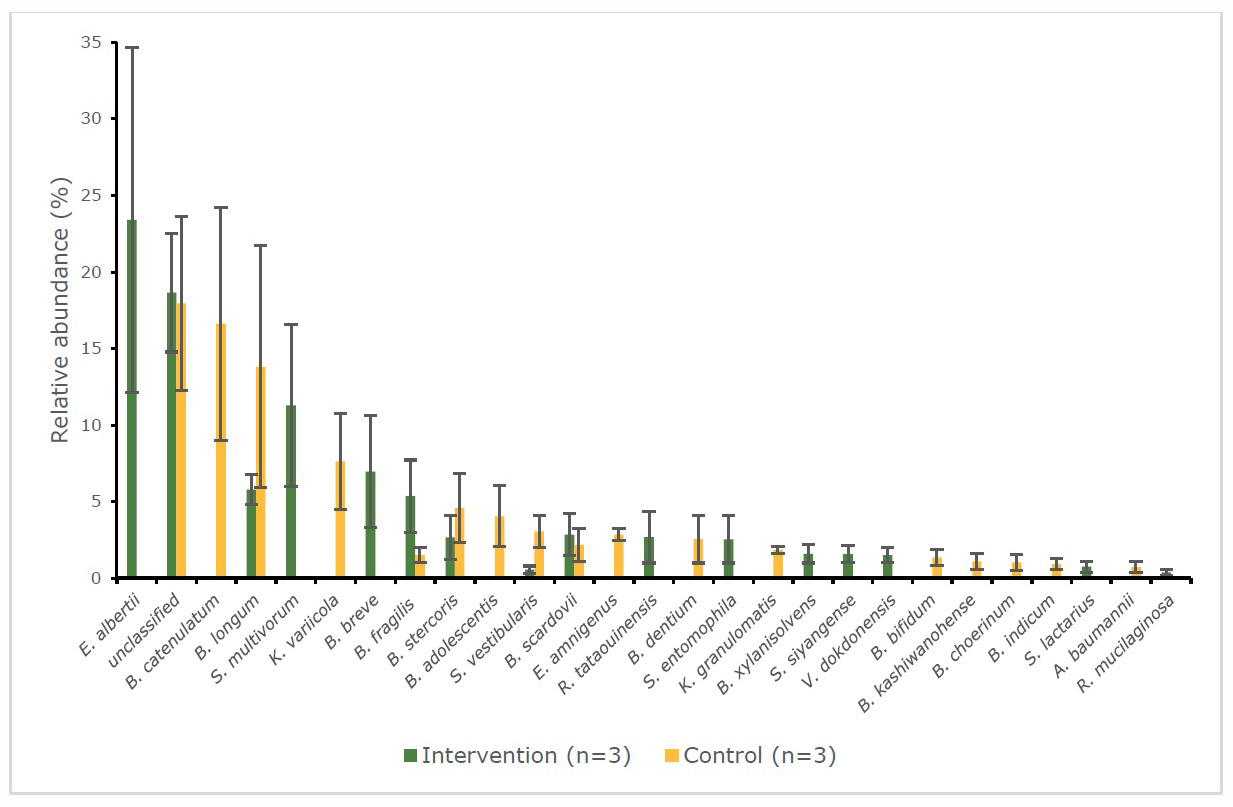
Figure 7. Percentage abundance of gut microbiota at the species level in infants’ faecal samples during 14 weeks of intervention measured by 16S rRNA sequencing (NGS) at baseline.
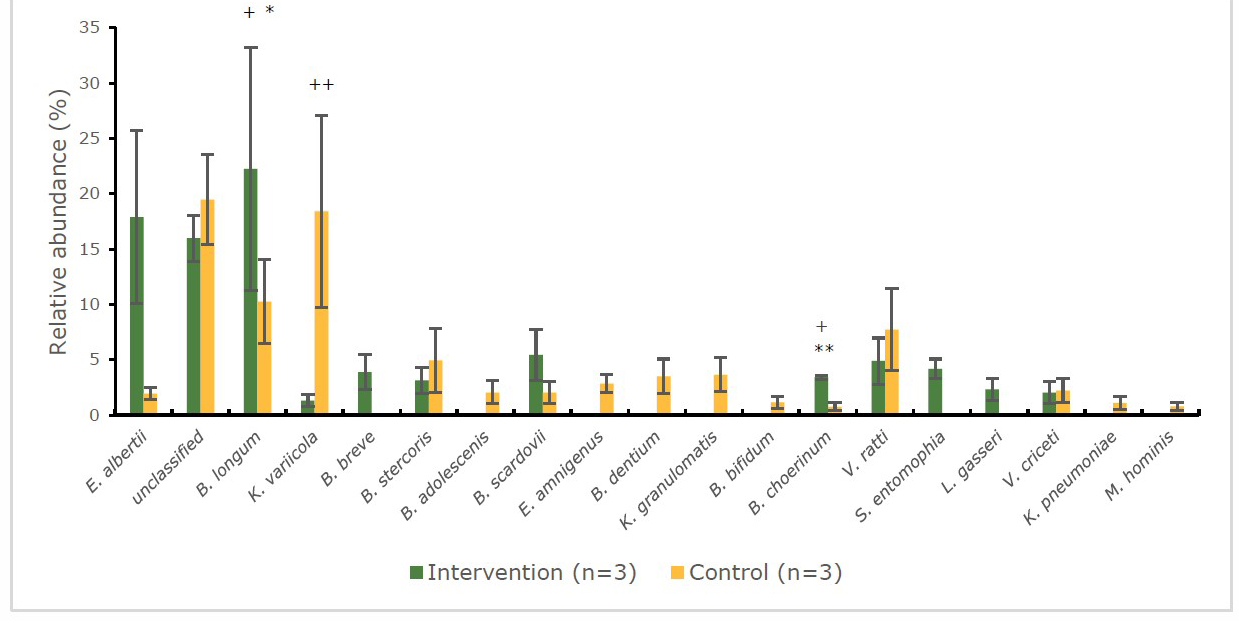
Figure 8. Percentage abundance of gut microbiota at the species level in infants’ faecal samples during 14 weeks of intervention measured by 16S rRNA sequencing (NGS) at end point.
Note: *significant difference (P < 0.05) between intervention and control group at the same time point (independent t-test)
**significant difference (P < 0.01) between intervention and control group at the same time point (independent t-test)
+significant difference (P < 0.05) at 2 time points (baseline and endpoint) (pair t-test)
++significant difference (P< 0.01) at 2 time points (baseline and endpoint) (pair t-test)
DISCUSSION
The effect of VA fortified rice had been evaluated in lactating women-infant pairs with 2 methods. For the FISH method, the results showed that lactating women who fed with VA-fortified (intervention group) had lower number Clostridium spp./Enterobacter spp. (representative of harmful bacteria) and their infants showed higher of the population of Bifidobacterium spp. and Lactobacillus spp. (benign or beneficial bacteria) compared to control group. This result was again confirmed with the NGS technique in the subset of infant’s stool samples which showed the increasing of Lactobacillus (genus level), B. longum and B. choerinum (species level). Infants in control group showed that Clostridium spp./ Enterobacter spp. increased (analysed by FISH method). And it was confirmed by NGS technique that its belong to genus of Escherichia in dominant species of E. albertii and genus of Klebsiella in dominant species of K. variicola.
The knowledge of interrelationship between nutrient especially vitamin A and gut microbiota is relatively new therefore, the direct mechanism to explain the role of VA on gut microbiota has not been confirmed yet. Previously, there were limited numbers of study focusing on the relationship of VA/VAD and gut microbiota on infection/health. The study of Lee and Ko (2016) showed the administration of VA inhibited the replication of murine norovirus (MNV) through stimulation of the development of Lactobacillus in mice. Another cohort study conducted in patients with persistent diarrhoea found that subjects with both VAD and dysbiosis accounted for 78.7% (Wang et al., 2016). Furthermore, a study in VAD rats found reduction of Lactobacillus spp. in the tissues of the jejunum (62%), ileum (82%) and colon (86%) with a more marked increase in Escherichia coli strains (Amit-Romach et al., 2009).
Lee and Ko (2016) proposed a model to explain the possible relationship of gut microbiota, immunity, infection and the absorption of nutrients in the GI tract. A proper balance of the gut microbiota is vital for human health, and the diet is one of the main factors that cause changes in the composition, diversity and activity of the gut microbiota (Graf et al., 2015). Different in microbiota composition profiles have been associated with dietary patterns (Wu et al., 2011). Specific nutritional components and dietary patterns influence the composition, diversity and activity of the gut microbiota (Garcia-Mantrana et al., 2018).
Vitamin A is one of the important micronutrient which has a multiple biological functions for growth and development. Thus, the proposed mechanism of the relationship between VA and gut microbiota is that VA could be help in the maintaining of villi cells which support the growth of gut microbiota, especially probiotic bacteria, and also help in the absorption of nutrients. This is in the relation to the essential role of the VA which is an important for the differentiation of epithelial and other cells in our body (Favennec & Cals, 1988; Klaus Kraemer, 2010).
Another possible mechanism is that VA may be necessary for the growth of specific bacteria like Bifidobacterium spp. and Lactobacillus spp. Previously, Snell (1993) and Gu and Li (2016) reported that water-soluble vitamins are used for nutritional proposes in many prokaryotes like Lactobacillus and Bifidobacterium. However, the mechanism mentioned above has not yet been observed in fat-soluble vitamins, such as VA. Therefore, this will be in the needed to be confirmed in the future research.
Vitamin A has the important role in the promotion of the immune system by development of T-cells, especially Th1 and Th2 in the human body (García, 2012) which directly act as natural defenses against pathogenic bacteria. About one third of ingested VA is transferred into breast milk from maternal diet, then it is becoming the primary source of VA for breastfed infants (Vahlquist & Nilsson, 1984). This could be one explanation by which VA-fortified rice resulted in a decrease of representative pathogenic bacteria, such as Clostridium spp./Enterobacter spp. in lactating women as well as in infants e.g., Enterobacter, Klebsiella (NGS method).
The body microbiota and gut microbiota of mother contribute to the initial exposure of an infant to viable microbiota, in the initial stage by the mother holding, cuddling and kissing (Karakochuk et al., 2017). Breastfed infants are also exposed to the mother’s microbiota which contains Lactobacillus, Staphylococcus and Bifidobacterium (Rautava, 2016; Yang et al., 2019) as showed in the present study.
To our knowledge this is the first RCT study related to VA intervention and gut microbiota in lactating women-infant pairs. The composition of their gut microbiota was measured using the FISH technique together with the NGS technique being used to determine the species level biota of a subsample (≈ 22% of total stool samples) of the infants. NGS provides significant advantages such as its high-performance capacity and high accuracy in the data generated (Berglund et al., 2011; Grotta et al., 2015).
Vitamin A fortified rice (500 µg/d) not only improved vitamin A status of lactating women as measured by total body vitamin A stores and total liver reserve (as primary outcome of the main protocol) (Pinkaew et al., 2021a) but also has the beneficial effect on the gut microbiota of both mothers and breastfed infants.
One limitation of this study was the sample size, which was rather low, partly because some of the participants initially recruited in the study were not exclusively breastfeeding at the end of the study. This could be one explanation for not finding the changes of beneficial bacteria; Bifidobacterium spp. and Lactobacillus spp. in lactating women who received VA fortified rice even though the number of Bifidobacterium spp. and Lactobacillus spp. were higher at the endpoint when compare to the control group. Therefore, for the study in the future, a larger group of the participant base on the number of gut microbiota, which were found in this study could be used for the sample size calculation. Moreover, confounding factors, i.e. the illness and health consequences during the period of intervention should be monitored and addressed by the health officers. The results from this study could be used as the supported evidence for the role of vitamin A on gut microbiota and to promote exclusively breastfeeding during the first 6 months of life.
CONCLUSION
The feeding of VA-fortified rice (500 µg/day) to Thai lactating women during 14 weeks resulted in a significant decrease in Clostridium spp./Enterobacter spp. representing harmful bacteria in mothers while breast milk of the lactating women who were fed with VA-fortified rice appeared to promote the growth of benign or beneficial bacteria (Bifidobacterium spp. and Lactobacillus spp.) in EBF infants especially Lactobacillus, B. longum and B. choerinum when measuring with NGS method.
ACKNOWLEDGEMENTS
Special thanks to Assoc. Prof. Dr. Emorn Udomkesmalee for the contribution of the main study protocol.
AUTHOR CONTRIBUTIONS
Siwaporn Pinkaew and Santad Wichienchot designed the research and critically reviewed the manuscript; Siwaporn Pinkaew and Lukman Azis conducted the intervention; Lukman Azis measured gut microbiota enumeration; Santad Wichienchot supervised gut microbiota determination; Siwaporn Pinkaew and Lukman Azis statistically analysed the data; Lukman Azis drafted the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
Lukman Azis, Siwaporn Pinkaew and Santad Wichienchot have no conflicts of interest.
REFERENCES
Amit-Romach, E., Uni, Z., Cheled, S., Berkovich, Z., and Reifen, R. (2009). Bacterial population and innate immunity-related genes in rat gastrointestinal tract are altered by vitamin A-deficient diet. Journal of Nutritional Biochemistry, 20: 70–77.
Berglund, E.C., Kiialainen, A., and Syvänen, A.C. (2011). Next-generation sequencing technologies and applications for human genetic history and forensics. Investigative Genetics, 2: 1–15.
de Pee, S., West, C.E., Hautvast, J.G.A.J., Muhilal, Karyadi, D., and West, C. E. (1995). Lack of improvement in vitamin A status with increased consumption of dark-green leafy vegetables. The Lancet, 6736: 92111–92117.
Ezzati, M., Lopez, A.D., Rodgers, A., and Murray, J.L.C. (2004). Comparative Quantification of Health Risks Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. In A. R. Majid Ezzati, Alan D. Lopez & and C. J. L. Murray (Eds.), Who (1st ed.). Geneva: WHO Library Cataloguing-in-Publication Data.
FAO, & WHO. (2001). Human Vitamin and Mineral Requirements. In Human vitamin and mineral requirements (1st ed.). Rome: Food and Nutrition Division.
Favennec, L. and Cals, M.J. (1988). The Biological Effects of Retinoids on Cell Differentiation and Proliferation. Clinical Chemistry and Laboratory Medicine, 26: 479–490.
Franks, A.H., Harmsen, H.J.M., Raangs, G.C., Jansen, G.J., Schut, F., and Welling, G. W. (1998). Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Applied and Environmental Microbiology, 64: 3336–3345.
Garcia-Mantrana, I., Selma-Royo, M., Alcantara, C., & Collado, M.C. (2018). Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Frontiers in Microbiology, 9: 1–11.
García, O.P. (2012). Effect of vitamin A deficiency on the immune response in obesity. Proceedings of the Nutrition Society, 71: 290–297.
Graf, D., Di Cagno, R., Fåk, F., Flint, H. J., Nyman, M., Saarela, M., and Watzl, B. (2015). Contribution of diet to the composition of the human gut microbiota. Microbial Ecology in Health & Disease, 26: 1–11.
Grotta, S., D’Elia, G., Scavelli, R., Genovese, S., Surace, C., Sirleto, P., … Angioni, A. (2015). Advantages of a next generation sequencing targeted approach for the molecular diagnosis of retinoblastoma. BMC Cancer, 15: 1–9.
Gu, Q., and Li, P. (2016). Biosynthesis of Vitamins by Probiotic Bacteria. In Probiotics and Prebiotics in Human Nutrition and Health (pp. 135–148).
Harmsen, H.J.M., Elfferich, P., Schut, F., and Welling, G.W. (1999). A 16S rRNA-targeted Probe for Detection of Lactobacilli and Enterococci in Faecal Samples by Fluorescent In Situ Hybridization. Microbial Ecology in Health & Disease, 11: 3–12.
Huang, Z., Liu, Y., Qi, G., Brand, D., and Zheng, S.G. (2018). Clinical Medicine Role of Vitamin A in the Immune System. 7: 1–16.
Illumina. (2020). 16S Metagenomic Sequencing Library Preparation. Retrieved from https://support.illumina.com/documents/documentation/chemistry_documentation/
16s/16s-metagenomic-library-prep-guide-15044223-b.pdf.
Karakocuk, C. D., Whitfield, K. C., Green, T. J. and Kraemer, K. 2017. The Biology of the Fist 1,000 Days. New York: CRC Press
Kau, A.L., Ahern, P. P., Griffin, N. W., Goodman, A.L., and Gordon, J.I. (2011). Human nutrition, the gut microbiome and the immune system. Nature, 474: 327–336.
Khan, N., West, C., de Pee, S., Bosch, D., Phuong, H., Hulshof, P., … Hautvast, J. (2007). The contribution of plant foods to the vitamin A supply of lactating women in Vietnam: a randomized controlled trial. American Journal of Clinical Nutrition, 85: 1112–1120.
Klaus Kraemer, D. M. (2010). SIGHT AND LIFE: manual on vitamin A deficiency disorders (VADD) (2nd editio). Switzerland: Sight and Life Press.
Klindworth, A., Pruesse, E., Schweer, T., Peplies, J., Quast, C., Horn, M., and Glöckner, F.O. (2013). Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Research, 41: 1–11.
Langendijk, P.S., Schut, F., Jansen, G.J., Raangs, G.C., Kamphuis, G.R., Wilkinson, M. H. F., and Welling, G. W. (1995). Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Applied and Environmental Microbiology, 61: 3069–3075.
Lee, H. and Ko, G.P. (2016). Antiviral effect of Vitamin A on norovirus infection via modulation of the gut microbiome. Scientific Reports, 6:1–9.
Maggini, S., Pierre, A., and Calder, P.C. (2018). Immune function and micronutrient requirements change over the life course. Nutrients, 10: 1–27.
Mccauley, M.E., van den Broek, N., Dou, L., and Othman, M. (2015). Vitamin A supplementation during pregnancy for maternal and newborn outcomes. In Cochrane Database of Systematic Reviews. Liverpool, UK.
Pinkaew, S., Udomkesmalee, E., Davis, C.R., and Tanumihardjo, S.A. (2021a). Vitamin A–fortified rice increases total body vitamin A stores in lactating Thai women measured by retinol isotope dilution: a double-blind, randomized, controlled trial. The American Journal of Clinical Nutrition.
Pinkaew, S., Wegmuller, R., Wasantwisut, E., Winichagoon, P., Hurrell, R. F., & Tanumihardjo, S. A. (2014b). Triple-Fortified Rice Containing Vitamin A Reduced Marginal Vitamin A Deficiency and Increased Vitamin A Liver Stores in School-Aged Thai Children. The Journal of Nutrition, 144: 519–524.
Plongbunjong, V., Graidist, P., Knudsen, K.E.B., and Wichienchot, S. (2017). Isomaltooligosaccharide synthesised from rice starch and its prebiotic properties in vitro. International Journal of Food Science and Technology, 52: 2589–2595.
Rautava, S. (2016). Early microbial contact, the breast milk microbiome and child health. Journal of Developmental Origins of Health and Disease, 7: 5–14.
Ross, C. (2014). Modern Nutrition in Health and Diseases. 11th ed. (11th editi, Vol. 40; T.R.Z. Ross Catherine, Benjamin Caballero, Robbet J. Cousins, Katherine L. Tucker, Ed.). Philadelphia: Lippincott Williams & Wilkins (LWW).
Snell, E. (1993). From Bacterial Nutrition to Enzyme Structure: A Personal Odyssey. Annual Review of Biochemistry, 62: 1–26.
Tanumihardjo, S.A. (2011). Vitamin A: Biomarkers of nutrition for development. American Journal of Clinical Nutrition, 94(Suppl. 1): 658S–65S.
Vahlquist, A., & Nilsson, S. (1984). Vitamin A transfer to the fetus and to the amniotic fluid in rhesus monkey (Macaca mulatta). Annals of Nutrition and Metabolism, 28: 321–333.
Waddel Snedecor, George and Gemmel Cochran, W. (1989). Statistical methods / George W. Snedecor, William G. Cochran. In SERBIULA (sistema Librum 2.0). Ames, Lowa: Lowa State University Press.
Wang, Y., Gao, Y., Liu, Q., Zhan, X., Li, Z., Hu, H., … Chen, J. (2016). Effect of vitamin A and Zn supplementation on indices of vitamin A status, haemoglobin level and defecation of children with persistent diarrhea. Journal of Clinical Biochemistry and Nutrition, 59: 58–64.
West, K.P., Christian, P., Labrique, A.B., Rashid, M., Shamim, A.A., Klemm, R.D.W., … Sommer, A. (2011). Effects of vitamin A or beta carotene supplementation on pregnancy-related mortality and infant mortality in rural Bangladesh: A cluster randomized trial. JAMA - Journal of the American Medical Association, 305: 91986–91995.
World Health Organization. (2011a). Guideline: Vitamin A supplementation in postpartum women. Retrieved from https://www.who.int/elena/titles/vitamina_postpartum/en/
World Health Organization. (2019b). Vitamin A supplementation in children 6–59 months of age with severe acute malnutrition. Retrieved from https://www.who.int/elena/titles/vitamina_sam/en/
Wu, G.D., Chen, J., Hoffmann, C., Bittinger, K., Chen, Y.Y., Keilbaugh, S.A., … Lewis, J.D. (2011). Linking long-term dietary patterns with gut microbial enterotypes. Science, 334: 105–118.
Yang, B., Chen, Y., Stanton, C., Ross, R. P., Lee, Y.-K., Zhao, J., … Chen, W. (2019). Bifidobacterium and Lactobacillus Composition at Species Level and Gut Microbiota Diversity in Infants before 6 Weeks. International Journal of Molecular Sciences, 20: 3306–3322.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Lukman Azis1, Siwaporn Pinkaew1,*, and Santad Wichienchot2
1 Department of Food Science and Nutrition, Faculty of Science and Technology, Prince of Songkla University, Pattani Campus, Pattani, 94000 Thailand
2 Center of Excellence in Functional Foods and Gastronomy, Faculty of Agro-Industry, Prince of Songkla University, Hat Yai Campus, Songkhla, 90110 Thailand
Corresponding author: Siwaporn Pinkaew, E-mail: siwaporn.p@psu.ac.th
Total Article Views
Editor:
Korakot Nganvongpanit,
Chiang Mai University, Thailand
Article history:
Received: April 19, 2021;
Revised: June 8, 2021;
Accepted: June 22, 2021;

