
Morphometry and Phylogeny of the Different Populations of Selaginella tamariscina (P. Beauv.) Spring and S. pulvinata (Hook. & Grev.) Maxim. in Northern Thailand
Udon Pongkawong, Jatupol Kampuansai, Rossarin Pollawatn, and Arunothai Jampeetong*Published Date : 2021-09-13
DOI : https://doi.org/10.12982/CMUJNS.2021.077
Journal Issues : Number 4, October-December 2021
Abstract “Dok Hin” is the Thai local name for Selaginella species that form rosettes. They commonly distributes in Siberia, Manchuria, southern China, Japan, the Philippines and Thailand. Morphology of Dok Hin is very resemble leading to misidentification. So, exactly number of species of Dok Hin in Thailand and their differences in morphological characteristics is not well understood. Thus, revision of morphological characters and phylogenetic confirmation of the taxonomic identification are needed. This study aims to examine morphological charateristics and phylogenetic patterns in eight populations of the Dok Hin in Northern Thailand. Morphology of Dok Hin from each populations was quantitatively examined using 15 vegetative and 6 reproductive characters meanwhile phylogenetic analyses was explored by DNA barcode ITS2. The results of the phylogenetic analysis revealed the existence of two species of Dok Hin, S. tamariscina and S. pulvinata. Selaginella tamariscina can be distinguished from S. pulvinata by its presence of a pseudotrunk above ground and ridges of dorsal leaves. On the other hand, the results of phylogenetic analysis indicated the differences among populations of S. pulvinata as well. Chiang Mai populations of S. pulvinata was characterized by peculiar set of characters long leaves and leaf apices look like caudate, while the rest of their populations have shorter leaves and leaf apices look like aristate. It indicates that S. pulvinata has genetic and phenotypic divergence among populations. However, additional studies of Dok Hin populations in other parts of Thailand and studies on different genetic markers are necessary to confirm the taxonomic status of S. pulvinata.
Keywords: Dok Hin, Morphometric, Phylogeny, Pseudotrunk, Resurrection plant
Funding: The authors are appreciated for the research funding partly provided by Faculty of Science, Chiang Mai University, Thailand.
Citation: Pongkawong,U., Kampuansai, J., Pollawatn, R., and Arunothai Jampeetong, A. 2021. Morphometry and phylogeny of the different populations of Selaginella tamariscina (P. Beauv.) Spring and S. pulvinata (Hook. & Grev.) Maxim. in Northern Thailand. CMU J. Nat. Sci. 20(4): e2021077.
INTRODUCTION
Selaginella is the only genus in the family Selaginellaceae, the largest group of fern allies. Approximately 750 known species of this genus distribute in all continents except Antarctica. The species diversity is high in tropical and subtropical regions (Zhou et al., 2015a). Members of this genus also have highly diverse growth forms, including erect, climbing, creeping, prostrate and special rosette forms (Zhou et al., 2015a).
“Dok Hin” is the Thai local name for Selaginella species having rosette forms. The first record of S. tamariscina (P. Beauv.) Spring in Thailand, was reported for Doi Chiang Dao, Chiang Mai in 1979, in Flora of Thailand (Tagawa & Iwatsuki, 1979). It is a perennial herb, with a pseudotrunk, and rosettes with evergreen or seasonally green branches at the top which resemble green flowers (Tagawa & Iwatsuki, 1979; Boonkerd & Pollawatn, 2000). Previous researches showed the distribution of this specie in several areas in Thailand, growing especially on sandy soil or limestone rock at 300 - 2,200 m AMSL altitude (Biodiversity-based Economy Development Public Organization [BEDO], 2011).
The recent study of the Dok Hin diversity in Thailand confirmed Selaginella pulvinata (Hook. & Grev.) Maxim. as a new record of this genus for Thailand (Jaruwattanaphan et al., 2015). Morphologically, it is similar to S. tamariscina. Consequently, mistakens in the species identification process have been often occurring, leaving the correct taxonomic status of plenty of voucher specimens of “Dok Hin” in several herbaria in Thailand under question.
Morphometry is the study of characterizing the biologically relevant forms and patterns of organisms using a combination of mathematics and statistics to provide a quantitative description and analysis of morphological variation (Speer and Hilu, 1999; Jearranaiprepame, 2014). Several studies using morphometric methods to determine the character differences within same species or genus of the plants with phenotypes that might vary with environments or geography (Baum, and Bailey, 1994: Boonkerd, 2003). A study by Boonkerd (2003) showed that cluster analysis and canonical discriminant analysis can classify Doryopteris ludens (Wall. Ex Hook.) J. Sm. between a normal form and a dwarf form, growing under different ecological conditions. Valdespino (2004) discovered three new species of Selaginella (S. myriostachya, S. nanophylla, and S. phiara) from Cuba using morphological data from scanning electron microscopic study. Similarly, Sirichamorn (2006) revised taxonomic status and unsuitability of species treatment in the genus Afgekia Craib (Fabaceae) using morphometrics and molecular systematics.
Morpho-anatomical characteristics are basically essential for identification. However, it should be confirmed by the molecular methods as well. DNA barcoding is presently method that can provides more precise identification. In plants, many regions represent standardized DNA barcoding useful for integrated taxonomic systems, such as matK + trnH-psbA (Newmaster et al., 2008), rbcL + matK (CBOL Plant working Group, 2009), and ITS2 (Chen et al., 2010) regions. The internal transcribed spacer 2 (ITS2) of the nuclear ribosomal repeat unit is one of the most common markers applied to phylogenetic analysis. Its fast mutation rate and high variation makes it appropriate for low taxonomic-level studies, whereas its well-conserved secondary structure can be used for tree reconstructions at high taxonomic levels (Keller et al., 2009). An earlier study by Chen et al. (2010) demonstrated that DNA barcoding obtained from the ITS2 region can discriminate more than 6,600 plant samples belonging to 4,800 species from 753 distinct genera. It indicated that the rate of successful identification using the ITS2 barcode was 92.7% at the species level. Furthermore, Gu et al. (2013) documented that the ITS2 barcode can be used to identify species in the family Selaginellaceae, providing a scientific basis for phylogenetic investigations of this family.
Thus, this study aims to clarify the taxonomic status of Dok Hin species in Thailand and to determine morphological and genetic variation between their populations. Consequently, it will provide more sufficient and accurate morphological and molecular information useful for Dok Hin identification in Thailand.
MATERIALS AND METHODS
Plant materials
Selaginella pulvinata and S. tamariscina were surveyed and collected from eight localities in Northern Thailand (Figure 1). Because these species are rare and in risk of extinction (Suksatan, 1998; BEDO, 2011), minimum but statistical sufficient number of plant samples (ten plants per population) were collected. Plant samples were identified using Flora of Thailand (Tagawa & Iwatsuki, 1979) and Flora of China (Zhang et al., 2013). The voucher specimens were deposited in the herbaria of the Biology Department of Chiang Mai University (CMUB) and Queen Sirikit Botanical Garden (QBG), Thailand.
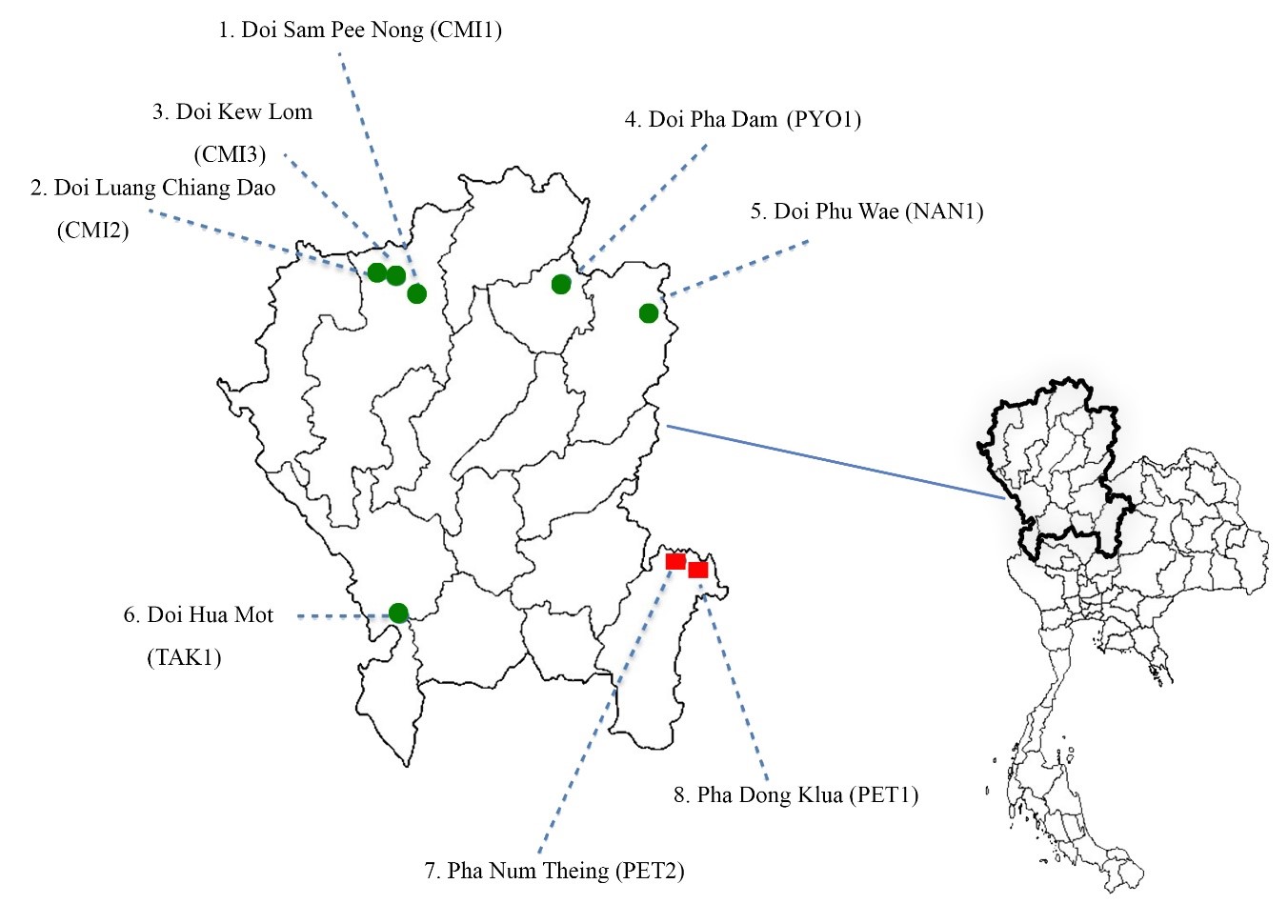
Figure 1. Distribution map of two Dok Hin species from Northern Thailand (Selaginella pulvinata-green dots, S. tamariscina-red squares).
Morphometric study
Morphological data were obtained from the collected specimens. Both vegetative and reproductive morphology were examined for ten specimens per population using a stereo microscope (Olympus SZ-30, Japan).
Pseudotrunks above ground length (PTL) was measured with a digital caliper. Vegetative and reproductive morphology including, primary branch width (PBW), dorsal ridge length (DRL), dorsal leaf length (DLL), dorsal leaf width (DLW), dorsal leaf apex length (DLAL), dorsal leaf marginal sinus depth (DLMD), dorsal leaf marginal sinus width (DLMW), ventral leaf length (VLL), ventral leaf width (VLW), ventral leaf apex length (VLAL), ventral leaf marginal membrane width (VLMW), axillary leaf length (ALL), axillary leaf width (ALW), axillary apex length (AAL), strobili length (SL), strobili width (SW), sporophyll length (SL) and sporophyll width (SW) were observed and determined from photos taken under microscope using the software ImageJ I (Schneider, et al., 2012).
Reproductive morphology including, megaspore diameter (MGD) and microspore diameter (MCD) was investigated using scanning electron microscopy (SEM). Mature microspores and megaspores were separated from sporangia and placed on aluminum stubs with double-sided tape. Each stub was spurt coated with a gold-palladium mixture for 2 minutes using an SPI-Module sputter coater. The spore morphology including shape and exine wall was observed under a JSM -IT300 scanning electron microscope (SEM; JEOL, Tokyo, Japan) at an accelerating voltage of 15 kV. Digital images were captured using the supplied ORION software (Version 1.72.1.0; JEOL USA, Peabody, Mass.).
Molecular study
DNA of the plants from each population (three specimens per area) was extracted from approximately 10 mg silica gel-dried leaves according to the protocol provided by the DNeasy Plant Mini Kit DNeasy (QIAGEN Inc, U.S.A.). Then, the ITS2 region was amplified using a pair of universal primers: ITSS2F (forward) and ITSS3R (reverse) (Gu et al., 2013). Polymerase chain reaction (PCR) amplification was performed using approximately 30 ng genomic DNA as a template in a 25 mL reaction mixture (2.5 mL 10x PCR buffer without MgCl2, 2 mL 25 mM MgCl2, 2 mL of each dNTP (2.5 mM), 1.0 mL of each primer (2.5 mM)), and 1.0 U of Taq DNA Polymerase (Vivantis).
The reactions were performed with the following cycling conditions: 94°C for 5 min and 40 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 45 s followed by 72°C for 10 min. (Gu et al., 2013). The 450 base pairs (bp) products were then purified and sequenced at Macrogen Co. (South Korea) using same pair of PCR primers. ITS2 sequences of each specimen were assembled using the BioEdit version 7.2 software (Hall, 1999).
Statistical analysis
Morphological statistic
Morphological data of S. pulvinata and S. tamariscina was tested for normal distribution and homogeneity of variance using Cochran’s C-test. If necessary, data was log-transformed to ensure homogeneity of variance. The data was tested by one-way analysis of variance (ANOVA). Differences between treatments were identified by the Tukey HSD post hoc procedure at the 1% significance level.
Morphometric study
Morphological variation among Dok Hin populations was estimated by principal components analysis (PCA). The PCA was performed using the PAST statistics software package (Ryan et al., 1995). Dok Hin populations were then grouped by cluster analysis using the average taxonomic distance among the 80 specimens from eight localities to generate a dendrogram using neighbor-joining (NJ) in PAST package version 4.03.
Phylogenetic study
Twenty-four Dok Hin ITS2 sequences newly generated in this study were analysed together with 16 S. pulvinata and S. tamariscina accessions retrieved from GenBank. One ITS2 accession of S. stauntoniana Spring was used an outgroup. Voucher information and GenBank accession numbers for each sampled taxon are provided in Table 1-2. A total of 41 ITS2 sequences were aligned using the BioEdit software and 188 bp length were used for phylogenetic analysis. The genetic distances were then computed using MEGA 10 (Kumar et al., 2018) according to the Kimura 2-Parameter (K2P) model. The phylogenetic tree was reconstructed based on Maximum Likelihood (ML) using MEGA 10 with 1,000 bootstrap replicates.
Table 1. List of sampled taxa containing information related to the taxonomy (specimen voucher numbers, country, GenBank accession numbers and references).
|
Taxa |
Specimen voucher numbers |
Country |
GenBank accession number |
References |
|
S.pulvinata |
HS2401 |
China |
KC559845 |
Gu et al., 2013 |
|
S. pulvinata |
HS2402 |
China |
KC559846 |
Gu et al., 2013 |
|
S. pulvinata |
HS2403 |
China |
KC559847 |
Gu et al., 2013 |
|
S.pulvinata |
HS2404 |
China |
KC559848 |
Gu et al., 2013 |
|
S.pulvinata |
HS2405 |
China |
KC559849 |
Gu et al., 2013 |
|
S.pulvinata |
HS2406 |
China |
KC559850 |
Gu et al., 2013 |
|
S.tamariscina |
HS3001 |
China |
KC559863 |
Gu et al., 2013 |
|
S. tamariscina |
yyzw2015AQ27N1 |
China |
KX068993 |
Cai et al., 2016 |
|
S.tamariscina |
yyzw2015AQ27N2 |
China |
KX068990 |
Cai et al., 2016 |
|
S. tamariscina |
yyzw2015AQ27N3 |
China |
KX068991 |
Cai et al., 2016 |
|
S. tamariscina |
yyzw2015AQ27N4 |
China |
KX068992 |
Cai et al., 2016 |
|
S.tamariscina |
yyzw2015AQ27N5 |
China |
KX068993 |
Cai et al., 2016 |
|
S. tamariscina |
yyzw2015BH27N1 |
China |
KX068994 |
Cai et al., 2016 |
|
S.tamariscina |
yyzw2015BH27N2 |
China |
KX068995 |
Cai et al., 2016 |
|
S. tamariscina |
yyzw2015DK27N1 |
China |
KX068987 |
Cai et al., 2016 |
|
S.tamariscina |
yyzw2015DK27N2 |
China |
KX068988 |
Cai et al., 2016 |
|
S.stauntoniana |
Zhao 169 |
China |
KT161841 |
Zhou et al., 2015a |
RESULTS
Dok Hin distribution in Northern Thailand
Based on morphology, two species of Dok Hin, S. pulvinata and S. tamariscina were identified (Figure 2). S. pulvinata was found at Doi Sam Pee Nong, Doi Luang Chiang Dao, and Doi Kew Lom in Chiang Mai province; Doi Pha Dam in Phayao province; Doi Phu Wae in Nan province; Doi Hua Mot in Tak province, while S. tamariscina was found at Pha Dong Klua and Pha Num Theing in Phetchabun province (Figure 1).

Figure 2. Selaginella pulvinata from Doi Luang Chiang Dao, Chiang Mai province (A); Doi Hua Mot, Tak province (B); Doi Pha Dam, Phayao province (C); Doi Phu Wae, Nan province (D), respectively and S. tamariscina from Pha Dong Klua and Pha Num Theing, Phetchabun province (E).
Selaginella pulvinata was found in limestone habitats such as limestone crevices, limestone nooks in vertical, limestone cliffs, or open limestone areas near the summit at high elevations from 980 to 2,225 m AMSL. On the other hand, S. tamariscina was usually found on sandstone cliffs at lower elevations from 630 to 650 m AMSL (Table2).
Table 2. Dok Hin species investigated with their respective locality, province, elevation, habitat, specimen voucher numbers and GenBank accession numbers.
|
Dok Hin Species |
Locality
|
Province |
Elevation (AMSL) |
Habitat |
Specimen voucher numbers |
GenBank accession Numbers |
|
S.pulvinata |
Doi Sam Pee Nong (CMI1) |
Chiang Mai |
1,830 |
Limestone cliff, limestone crevices |
Udon-S042-044 |
MW594443-MW59445 |
|
S.pulvinata |
Doi Luang |
Chiang Mai |
2,103 |
Open limestone area near the summit |
Udon-S039-041 |
MW594446-MW59448 |
|
S.pulvinata |
Doi Kew Lom (CMI3) |
Chiang Mai |
1,856 |
Limestone cliff, limestone crevices |
Udon-S055, 060-061 |
MW594449-MW594451 |
|
S.pulvinata |
Doi Pha Dam (PYO1) |
Phayao |
1,030 |
Limestone cliff, limestone crevices |
Udon-S049-051 |
MW594461-MW594463 |
|
S.pulvinata |
Doi Phu Wae (NAN1) |
Nan |
1,535 |
Open limestone area near summit |
Udon-S046-048 |
MW594452-MW594454 |
|
S.pulvinata
|
Doi Hua Mot (TAK1) |
Tak |
984, 1,016 |
Limestone crevices |
Udon-S054, 062-064 |
MW594464-MW594466 |
|
S.tamariscina |
Pha Num Theing (PET1) |
Phetchabun |
634 |
Sandstone cliff |
Udon-S053, 058-059 |
MW594455-MW594457 |
|
S.tamariscina |
Pha Dong Klua (PET2) |
Phetchabun |
650 |
Sandstone cliff |
Udon-S052, 056-057 |
MW594458-MW594460 |
Morphometric study
The results of morphometric study are presented in Table 3. The first two principal components are explaining the total variability of the sample (Figure 3). The first principal component (PC 1) was responsible for 99.04% of the total variability whereas 0.86% of the total variability was represented by the second principal component (PC2). The morphometric variables contributing the to PC1 were: pseudotrunk above ground length (PTL) and dorsal ridge length (DRL). While the morphometric variables contributing the to PC2 were: ventral leaf length (VLL), dorsal leaf length (DLL), and dorsal leaf apex length (DLAL). Scatter plots of PC1 vs. PC2 separated the population of S. tamariscina from S. pulvinata. Additionally, the populations of S. pulvinata from Chiang Mai province (CMI1, CMI2, CMI3) also separated from TAK1, NAN1, and PYO1, respectively (Figure 4). Similarly, results from one way ANOVA showed that PTL and DRL were significantly different between S. tamariscina and S. pulvinata populations. Moreover, VLL, DLL, and DLAL of S. pulvinata from Chiang Mai populations (CMI1, CMI2, CMI3) significantly differed from Tak, Nan, and Phayao populations (TAK1, NAN1, PYO1).
Similarly, a dendrogram of neighbor-joining (NJ) approach using the same 5 characters, (PTL, DRL, DLL, DLAL, and VLL) classified Dok Hin populations into 3 groups (Figure 4). Group 1 consisted of S. tamariscina populations from Phetchabun province (PET1 and PET2), with a pseudo trunk above ground and a ridge on the adaxial surface of dorsal leaves as unique characters (Figure 5). Group 2 consisted of S. pulvinata populations from Chiang Mai province (CMI1, CMI2, and CMI3), with longer dorsal leaves, longer dorsal leaf apices, and longer ventral leaves compared to Group 3. Group 3 consisted of S. pulvinata populations from Tak province (TAK1), Nan province (NAN1), and Phayao province (PYO1) (Figure 6).
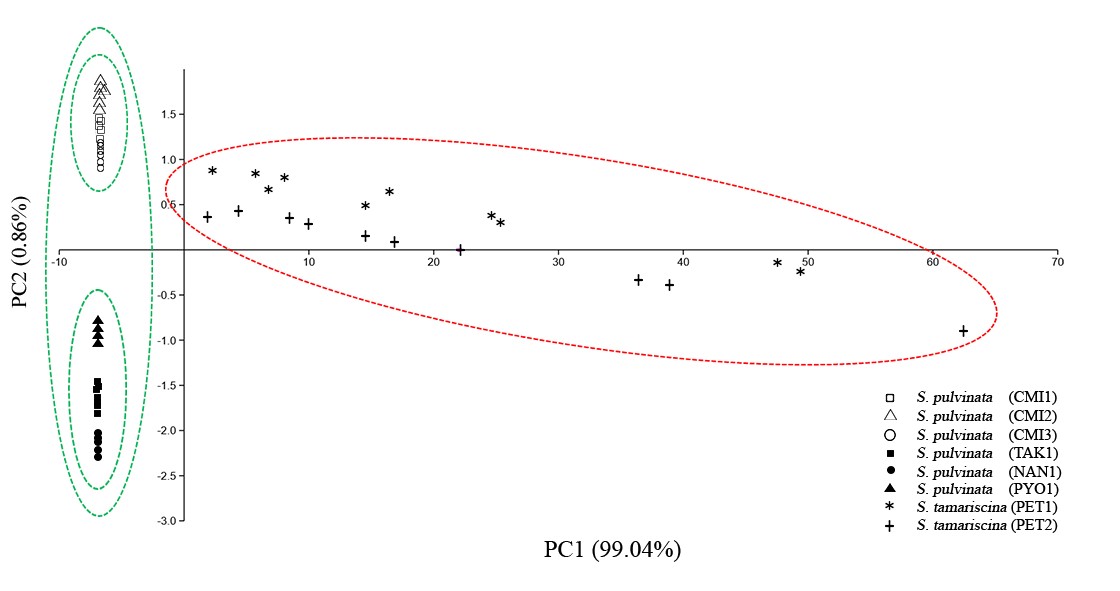
Figure 3. Scatter plot of a principal component analysis (PCA) showing the source of inter and intraspecific variability of Selaginella pulvinata (green line) and S. tamariscina (red line).
Table 3. Results of morphometric study of Selaginella pulvinata and S. tamariscina (mean ± SD values of the characters) from investigated localities. Different letter superscripts between rows indicate significant differences among populations (P < 0.01), as analyzed by one-way ANOVA.
|
Character |
S. pulvinata |
|
S. tamariscina |
|||||||||||
|
|
CMI1 |
CMI2 |
CMI3 |
NAN1 |
PYO1 |
TAK1 |
|
PET1 |
PET2 |
|||||
|
1. Pseudotrunk above ground length (mm) |
0.00 ± 0.00a |
0.00 ± 0.00a |
0.00 ± 0.00a |
0.00 ± 0.00a |
0.00 ± 0.00a |
0.00 ± 0.00 a |
|
26.84 ± 16.80b |
28.36 ± 18.99b |
|||||
|
2. Primary branch width (mm) |
3.03 ± 0.09c |
3.28 ± 0.08d |
3.04 ± 0.04c |
2.52 ± 0.05b |
2.15 ± 0.04a |
2.54 ± 0.08b |
|
2.63 ± 0.03b |
2.60 ± 0.03b |
|||||
|
3.Dorsal ridge length (mm) |
0.00 ± 0.00a |
0.00 ± 0.00a |
0.00 ± 0.00a |
0.00 ± 0.00a |
0.00 ± 0.00a |
0.00 ± 0.00a |
|
0.63 ± 0.08b |
0.62 ± 0.04b |
|||||
|
4.Dorsal leaf length (mm) |
3.17 ± 0.08c |
3.28 ± 0.16cd |
3.02 ± 0.06c |
2.33 ± 0.12b |
1.54 ± 0.11a |
1.84 ± 0.44a |
|
3.64 ± 0.14de |
3.73 ± 0.06e |
|||||
|
5. Dorsal leaf width (mm) |
1.28 ± 0.04e |
1.41 ± 0.00f |
1.15 ± 0.02d |
0.94 ± 0.02b |
0.91 ± 0.01b |
0.75 ± 0.06a |
|
1.07 ± 0.03c |
1.09 ± 0.12cd |
|||||
|
6.Dorsal leaf apex length (mm) |
1.12 ± 0.04 d |
1.22 ± 0.01e |
1.12 ± 0.00d |
0.83 ± 0.00c |
0.29 ± 0.00a |
0.55 ± 0.07b |
|
1.54 ± 0.00f |
1.47 ± 0.07f |
|||||
|
7. Marginal sinus of dorsal leaf depth (mm) |
0.19 ± 0.00d |
0.29 ± 0.01e |
0.17 ± 0.00c |
0.18 ± 0.00cd |
0.08 ± 0.00b |
0.08 ± 0.00b |
|
0.08 ± 0.00b |
0.06 ± 0.00a |
|||||
|
8. Marginal sinus of dorsal leaf width (mm) |
0.12 ± 0.00de |
0.13 ± 0.00e |
0.11 ± 0.00d |
0.07 ± 0.00b |
0.10 ± 0.01c |
0.08 ± 0.00b |
|
0.06 ± 0.00a |
0.06 ± 0.00a |
|||||
|
9.Ventral leaf length (mm) |
3.77 ± 0.00g |
3.81 ± 0.00g |
3.69 ± 0.04f |
2.27 ± 0.00c |
1.85 ± 0.04 a |
1.95 ± 0.03b |
|
3.47 ± 0.00e |
3.33 ± 0.04d |
|||||
|
10.Ventral leaf width (mm) |
1.32 ± 0.00d |
1.43 ± 0.04f |
1.31 ± 0.00d |
1.13 ± 0.00 c |
0.94 ± 0.00 a |
1.07 ± 0.00 b |
|
1.37 ± 0.00e |
1.31 ± 0.00d |
|||||
Table 3. Continued.
|
Character |
S. pulvinata
|
|
|
|
S. tamariscina
|
|
||||||
|
|
CMI1 |
CMI2 |
CMI3 |
NAN1 |
PYO1 |
TAK1 |
|
PET1 |
PET2 |
|||
|
11. Ventral leaf apex length (mm) |
1.54 ± 0.03f |
1.75 ± 0.04g |
1..51 ± 0.04f |
0.69 ± 0.00c |
0.43 ± 0.00a |
0.52 ± 0.00b |
|
1.27 ± 0.00e |
1.13 ± 0.01d |
|||
|
12. Marginal membrane of ventral |
0.59 ± 0.00f |
0.64 ± 0.00g |
0.50 ± 0.01d |
0.55 ± 0.00e |
0.44 ± 0.00b |
0.47 ± 0.00c |
|
0.43 ± 0.00a |
0.48 ± 0.01c |
|||
|
13. Axillary leaf length (mm) |
3.46 ± 0.03f |
3.51 ± 0.00f |
3.38 ± 0.00e |
2.57 ± 0.01c |
2.08 ± 0.00a |
2.13 ± 0.07a |
|
3.06 ± 0.02d |
2.50 ± 0.04b |
|||
|
14. Axillary leaf width (mm) |
1.44 ± 0.00e |
1.56 ± 0.00f |
1.41 ± 0.00d |
1.60 ± 0.00g |
1.41 ± 0.00d |
1.36 ± 0.00b |
|
1.40 ± 0.00c |
1.17 ± 0.00a |
|||
|
15. Axillary leaf apex length (mm) |
1.51 ± 0.01f |
1.62 ± 0.03g |
1.47 ± 0.00e |
0.81 ± 0.00c |
0.54 ± 0.01a |
0.52 ± 0.00a |
|
1.08 ± 0.00d |
0.76 ± 0.00b |
|||
|
16.Strobilus length (mm) |
8.49 ± 2.57a |
7.93 ± 2.62a |
7.88 ± 2.36a |
6.68 ± 1.96a |
16.88 ± 0.59 b |
No data |
|
6.47 ± 1.78a |
7.00 ± 1.32a |
|||
|
17.Strobilus width (mm) |
2.65 ± 0.55a |
2.75 ± 0.60a |
2.72 ± 0.58a |
2.61 ± 0.46 a |
2.21 ± 0.01 a |
No data |
|
2.55 ± 0.48a |
2.65 ± 0.41a |
|||
|
18.Sporophyll length (mm) |
3.28 ± 0.17ab |
3.89 ± 0.68a |
3.53 ± 0.54a |
3.49 ± 0.56a |
2.14 ± 0.01 a |
No data |
|
3.46 ± 0.57a |
3.76 ± 0.64a |
|||
|
19.Sporophyll width (mm) |
2.99 ± 0.02b |
2.99 ± 0.02b |
2.98 ± 0.02b |
2.97 ± 0.02b |
1.40 ± 0.05 a |
No data |
|
2.98 ± 0.02b |
2.77 ± 0.39b |
|||
|
20.Megaspore diameter (µm) |
204.84 ± 23.22a |
211.95 ± 17.32a |
204.84 ± 23.22a |
194.15 ± 11.55a |
No data |
No data |
|
184.48 ± 9.03a |
184.2 ± 5.00a |
|||
|
21.Microspore diameter (µm) |
35.35 ± 0.57a |
37.35 ± 1.80a |
36.55 ± 2.36a |
No data |
No data |
No data |
|
36.85 ± 2.17a |
37.35 ± 1.80a |
|||
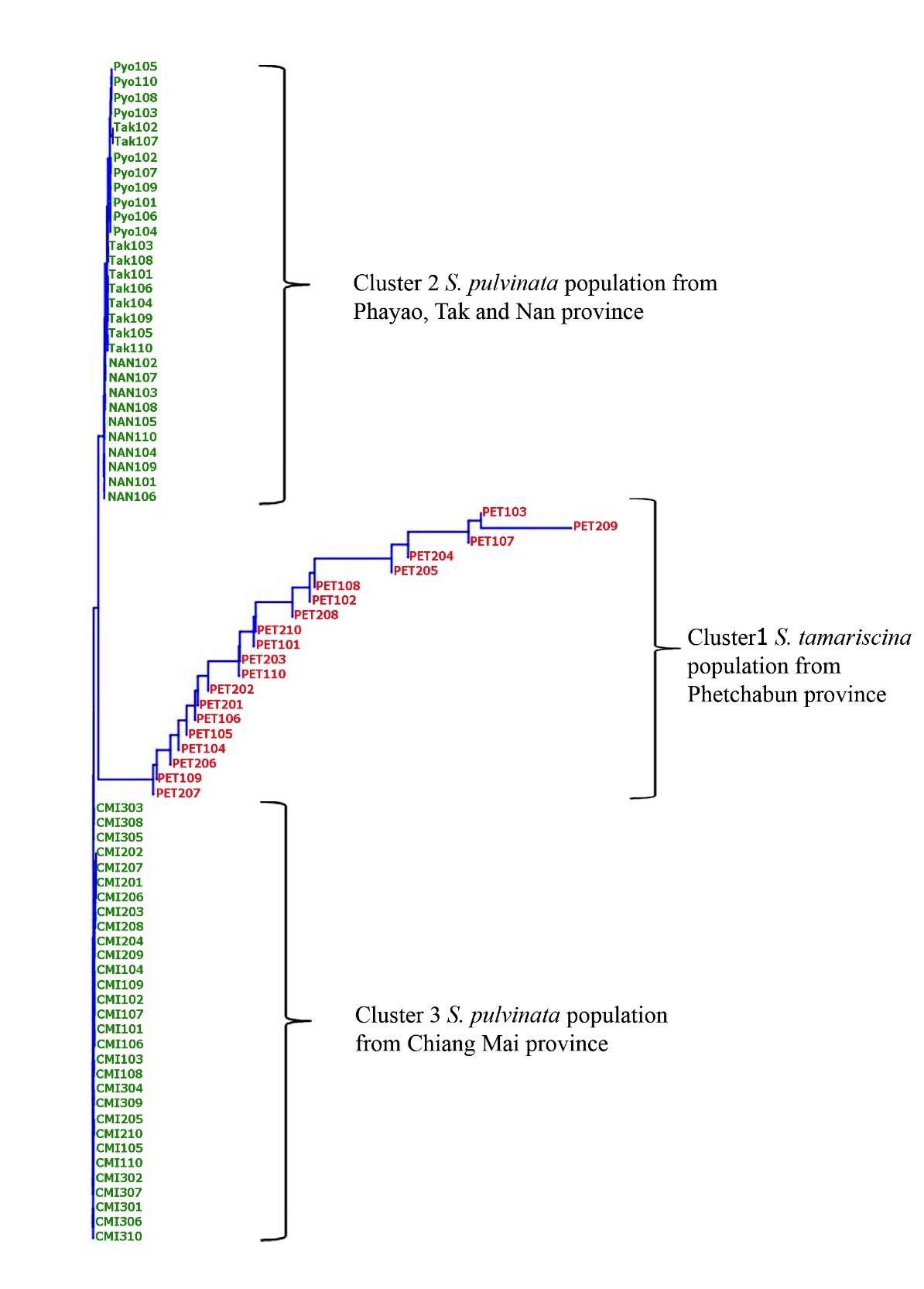
Figure 4. Dendrogram of neighbor-joining clustering based on 5 morphological characters including pseudotrunk above ground length (PTL), dorsal ridge length (DRL), dorsal leaf length (DLL), dorsal leaf apex length (DLAL), and ventral leaf length (VLL).

Figure 5. Pseudotrunk above ground (A) and ridge on dorsal leaf (B) of Selaginella tamariscina (red arrows) compared with S. pulvinata, with no pseudotrunk above ground (C) and no ridge on dorsal leaf (D).

Figure 6. Dorsal and ventral leaves of Selaginella pulvinata from Nan population (A, B); Phayao population (C, D); Tak population (E, F) and Chiang Mai population (G, H).
Phylogenetic study
The genetic relationship of Dok Hin populations from Northern Thailand was revealed by a maximum likelihood tree based on the ITS2 region sequences (Figure 7). The result showed that Dok Hin populations of Northern Thailand belong to 4 clades separated from their outgroup species (S. stauntoniana). All S. tamariscina samples from Phetchabun province formed a unique cluster (Clade IV) which obviously separated from S. pulvinata (Clades I, II). However, there is no clear distinction between S. tamariscina from Phetchabun province (Clade IV) and some S. pulvinata from Northern Thailand, since S. pulvinata (Clades III) represents of its sister clade. Among the S. pulvinata populations, all samples from Chiang Mai province formed a unique cluster in Clade I. Clade II consisted of samples from Tak, Nan, and Phayao provinces. The one sample from Nan province and two samples from Phayao province are grouped in Clade III.
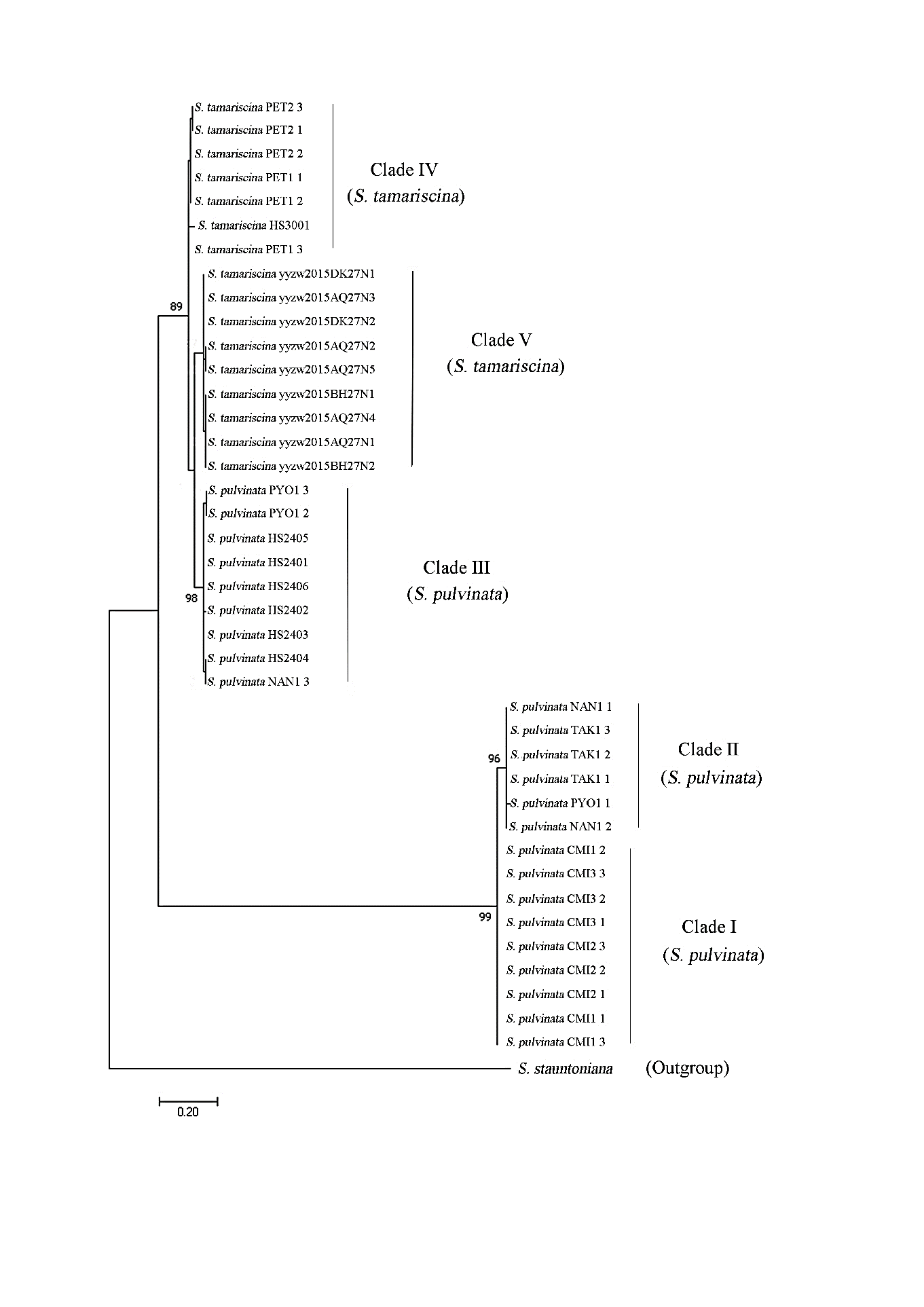
Figure 7. Maximum Likelihood (ML) tree based on ITS2 sequences of Selaginella pulvinata and S. tamariscina from Northern Thailand. Bootstrap values greater than 75% are labeled at the branches. Branches with bootstrap values less than 75% are collapsed. (CMI: Chiang Mai province, PYO: Phayao province, NAN: Nan province, PET: Phetchabun province).
DISCUSSION
Dok Hin distribution in Northern Thailand
Selaginella pulvinata and S. tamariscina were found in Russia (Siberia), Mongolia, China, Japan, Korea, India, Nepal, Vietnam, the Philippines, and Thailand (Alston, 1935; Takawa & Iwatsuki, 1979; Dahlen, 1982; Zhang et al., 2013; Bautista et al., 2018; Shalimov et al., 2019). In Northern Thailand, we found that these two species have patchy distributions in different geographical ranges. Selaginella pulvinata was usually found on limestone habitats such as limestone crevices, limestone nooks in vertical, limestone cliffs, or open limestone areas near the summit at high elevations about 980 – 2,225 m AMSL. In contrast, S. tamariscina was usually found on sandstone habitats at lower elevations than S. pulvinata, about 630 – 650 m AMSL. It seems that elevation can limit the distribution of these two species. Likewise, Zhang et al. (2013) wrote that S. tamariscina usually occurred at lower elevations (500 – 1,500 m AMSL) compared to S. pulvinata (1,000 – 3,000 m AMSL). Similar results have been documented by Shalimov et al. (2019) who found that S. pulvinata in Nepal usually grew on open rock, rooting on crevices at high elevations, 1,800 – 4,400 m AMSL.
Ecogeographic isolation between S. pulvinata and S. tamariscina may influence their adaptation to edaphic conditions in different environments and niche differentiation leading to the creation of reproductive isolation or reproductive barriers between populations (Nosil, 2012; Baack et.al. 2015). Li and Tan (2005) mentioned that S. tamariscina forms scattered small communities on dry, nutrient-poor, rocky, and shaded cliff representing one of the pioneering plant species in the so-called Danxia geologic formation. While S. pulvinata may represent a species adapted to the colder and drier inland conditions (Li and Tan, 2005; Zhou et al. 2015b)
Morphometric and phylogenetic study
The morphometric study showed that the length of pseudotrunk was an important key character to separate S. tamariscina and S. pulvinata. A previous study also reported that the main distinct character of these two species was presence of pseudotrunk. It was well documented that the pseudotrunk of S. tamariscina was formed by the matted roots (or rhizophores), while the roots of S. pulvinata remained spreading out (Alston, 1934; Dahlen, 1982). Furthermore, our study discovered that dorsal ridge was found only in S. tamariscina, thus the presence of dorsal ridge can be another key character to distinguish these two species. In short, both characters, i.e. pseudotrunk and dorsal ridge are useful for identification of S. tamariscina and S. pulvinata.
In addition, the morphometric study also showed the differences within S. pulvinata. Chiang Mai population of S. pulvinata (CMI1, CMI2, and CMI3) was characterized by a peculiar set of characters longer dorsal leaves and ventral leaves and leaf apices look like caudate. Whereas, other populations (TAK1, NAN1, and PYO1) presented shorter leaves and aristate leaf apices.
The evolutionary history of Dok Hin from Northern Thailand was revealed by the ITS2 region sequences. Maximum likelihood tree showed that S. tamariscina from Phetchabun province (Clade IV) is a monophyletic group while S. pulvinata from Chiang Mai, Nan, Tak, and Phayao provinces are a polyphyletic group included in Clades I, II, and III. The polyphyletic origin of S. pulvinata was consistent with the within species variation of phenotypic traits demonstrated by our morphometric data as mentioned above.
A unique cluster of S. tamariscina samples from Phetchabun province (Clade IV) consisted of one S. tamariscina HS3001 from China, which is a sister of S. tamariscina from China (Clade V) and S. pulvinata from Thailand (Clade III). The phylogenetic tree pointed out the close relationship and probably share ancestor among these three clades (Clade III, IV, V). The distribution of both S. tamariscina and S. pulvinata is in the wide geographic range from eastern China to southern Malaysia (Tropicos.org, 2021) where limestone rocks at high elevations are common.
All S. pulvinata samples from Chiang Mai province formed a monophyletic group in Clade I which is distinctly separated from Clade II (S. pulvinata from Phayao, Nan province and China). We observed the specific characters of this clade which having longer dorsal leaves, longer dorsal leaf apices and longer ventral leaves compared to S. pulvinata in Clades II and Clade III. Both morphometric and molecular data pointed out that the S. pulvinata populations in Chiang Mai province are likely to be a new species or new subspecies. Monophyletic speciation of this clade may occur from the unique geographical and ecological patterns of Doi Chiang Dao Wildlife Sanctuary. At the top of Doi Chiang Dao where Dok Hin has spread, the area was covered by sub-alpine vegetation which cannot be found in other areas of Thailand (Suksatan, 1998). Various plant endemic taxa, including new species and new records for Thailand were discovered in this most remarkable locality (Thapyai et al., 2005, Santisuk et al., 2006). For example, Sirindhornia pulchella H.A. Pedersen & Indham.), Strobilanthes chiangdaoensis Terao, Impatien chiangdoaensis T. Shimizu, Swertia chiangdaoensis P. Suksathan, Gentiana leptoclada ssp. australis (Craib) Toyokuni, Scabiosa siamensis Craib, Primula siamensis Craib (Terao, 1981; Pedersen and Indhamusika, 2002;; Santisuk et al., 2006).
Clade II and Clade III of S. pulvinata consisted of combined samples from Nan and Phayao provinces. Although a paraphyletic branch was observed, there was no morphological difference among these clades. This may result from convergent evolution under similar environments or ecology. There is the possibility that plants have developed similar morphology even though they are genetically different; this process can be found among various plant species (Korall and Kenrick, 2002; Zhou et al., 2015a). For example, the rosette-forming branches that curl inwards into a ball when dry are normally observed in xerophytic Selaginella species but phylogenetic studies confirmed that this rosette habitus appears to have evolved independently at least three times in three groups of Selaginella, including S. lepidophylla (southwestern USA and Mexico), S. tamariscina (eastern Asia) and S. pilifera (southern USA and Mexico), S. pallescens (southern USA and Mexico) (Korall & Kenrick, 2002; Zhou et al., 2015).
CONCLUSION
This study showed stem and leaf morphology can be used to identify two species of Dok Hin in Thailand. Having a pseudotrunk above ground and ridge on dorsal leaves are species recognition characters that separated S. tamariscina from S. pulvinata. Moreover, other leaf characters such as length of leaf and leaf apex showed variation within the populations of S. pulvinata. There were distinct differences between leaves of the plants from the Chiang Mai population compared with the populations from Phayao, Tak, and Nan. Our findings were well supported with phylogenetic results which basically separated these from each other into two species and showed that S. pulvinata from the Chiang Mai population has unique characters that differed from other populations. Therefore, S. pulvinata in Chiang Mai province should be a new species or new subspecies. However, additional broad scale studies of S. pulvinata using different methods or other parts of Thailand are needed to obtain full insight into its phenotypic variation.
ACKNOWLEDGEMENTS
This research was supported by Faculty of Science, Chiang Mai, University, Thailand. The authors would like to thank the director of the Department of National Parks, Wildlife and Plant Conservation for permission to conduct field surveys and collect samples in protected areas of national parks. We are grateful to directors and staffs at Chiang Dao Wildlife Sanctuary, Umphang Wildlife Sanctuary, Phu Sang National Park, and Doi Phu Kha National Park for their kind help during field investigation. We also thank directors, curators, and staffs of AAU, BKF, CMUB, QBG herbaria for permission to consult, loan and take photos of specimens for references. Finally, we are grateful to Mr. Alvin Yoshinaga for his kind help to improve the English text.
AUTHOR CONTRIBUTIONS
Udon Pongkawong surveyed, collected samples and conducted the experiments, performed the statistical analysis, data visualization and wrote original draft of the manuscript. Arunothai Jampeetong designed and supervised U. Pongkawong, conducted the experiments, commented and edited the manuscript. Jatupol Kampuansai advised for phylogenetic study and analysis, and commented the manuscript. Rossarin Pollawatn advised for plant survey and collection. All authors have read and approved of the final manuscript
CONFLICT OF INTEREST
The authors declare that they hold no conflict of interests.
REFERENCES
Alston, A.H.G. 1935. The Philippine species of Selaginella. Philippine Journal of Science. 58: 359-382.
Baack, E., Melo, M.C., Rieseberg, L.H. and Ortiz‐Barrientos, D. 2015. The origins of reproductive isolation in plants. New Phytologist. 207: 968-984.
Baum, B.R. and Bailey, L.G. 1994. Taxonomy of Hordeum caespitosum, H. jubatum, and H. lechleri (Poaceae: Triticeae). Plant Systematics and Evolution. 190: 97-111.
Bautista, M.G., Coritico, F.P., Acma. F.M., and Amoroso, V. 2018. Spikemoss flora (Selaginella) in Mindanao Island, the Philippines: species composition and phenetic analysis of morphological variations. Philippine Journal of Systematic Biology. 12: 45-56.
Biodiversity-based Economy Development Public Organization: BEDO. (2011). Development of fern database. In Boonkorkaew, P. (ed.). Biodiversity-based Economics Development Office (Public Organization) (1st ed). pp. 384.
Boonkerd, T. and Pollawatn, R. 2000. Pteridophytes in Thailand. Office of Environment Policy and Planning (1st ed). Bangkok, Thailand. pp. 57.
Boonkerd, T. 2003. Morphometric analysis of variation among three populations of Doryopteris ludens (Adiantaceae: Pteridophyta) in Thailand. The Fern Gazette. 17: 11-19.
Cai, Z.J., Xu, L., Wang, B., Wu,Y.N., and Kang,T.G. 2016. Direct submission sequence ITS2 for S. tamariscina. Botany Department, Liaoning University of Traditional Chinese Medicine, Double D Port, Dalian, Liaoning 116600, China.
CBOL Plant Working Group. 2009. A DNA barcode for land plants. Proceedings of the National Academy of Sciences of the United States of America. 106: 12794-12797.
Chen, S., Yao, H., Han, J., Liu, C., Song, J., Shi, L., Zhu, Y., Ma, X., Gao, T., Pang, X., Luo, K., Li, Y., Li, X., Jia, X., Lin, Y., and Leon, C. 2010. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLOS ONE. 5: e8613.
Dahlen, M.A. 1982. A taxonomic reassessment of the Hong Kong fern allies, with special reference to Selaginella Beauv. Doctoral thesis, University of Hong Kong, Pokfulam, Hong Kong SAR.
Gu, W., Song, J., Cao, Y., Sun, Q., Yao, H., Wu, Q., Chao, J., Zhou, J., Xue, W., and Duan J. 2013. Application of the ITS2 region for barcoding medicinal plants of Selaginellaceae in Pteridophyta. PLOS ONE. 8: e67818.
Hall, T.A. 1999. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 41: 95-98.
Jaruwattanaphan, T., Longchuphon, S., and Chulaka, P. 2015. Effect of growing media on growth of Selaginella pulvinata (Hook. et Grev.) Maxim. leafy shoot cutting. Thai Journal of Agricultural Science. 46: 209-212.
Jearranaiprepame, P. 2014. Morphometrics in zoology: Basic of morphometrics. KKU Science Journal. 42: 485-498.
Keller, A., Schleicher, T., Schultz, J., Müller, T., Dandekar, T., and Wolf, M. 2009. 5.8S-28S rRNA interaction and HMM-Based ITS2 annotation. Gene 430: 50-57.
Korall, P. and Kenrick, P. 2002. Phylogenetic relationships in Selaginellaceae based on rbcL sequences. American Journal of Botany. 89: 506-517.
Kumar, S., Stecher, G., Li, M., Knyaz, C. and Tamura, K. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35: 1547-1549.
Li, Z.J., and Tan, B.C. 2005. A review of the species diversity of Selaginella in Fujian province of China. Acta Phytotaxonomica Sinica 43: 50-59.
Newmaster, S.G., Fazekas, A.J., Steeves, R.A.D., and Janovec, J. 2008. Testing candidate plant barcode regions in the Myristicaceae. Molecular Ecology Resources. 8: 480-490.
Nosil, P. 2012. Ecological speciation. Oxford: Oxford University Press. p. 1-280. ISBN: 978-0199587117.
Pedersen, H.A., Suksathan, P., and Indhamusika, S. 2002. Sirindhornia, a new genus from Southeast Asia. Nordic Journal of Botany 22: 391-403.
Ryan, P.D., Harper, D.A.T. and Whalley, J.S. 1995. PALSTAT, Statistics for palaeontologists. Chapman & Hall (now Kluwer Academic Publishers).
Santisuk, T., Chayamarit, K., Pooma, P., and Suddee, S. 2006. Thailand Red Data: Plants. Office of Natural Resources and Environmental Policy and Planning (ONEP), Thailand. pp. 256. ISBN: 978-974-286-183-4.
Schneider, C.A., Rasband, W.S., and Eliceiri, K.W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature methods 9: 671-675.
Shalimov, A., Wu, Y.D., and Zhang, X.C. 2019. A taxonomic revision of the genus Selaginella (Selaginellaceae) from Nepal. PhytoKeys. 133: 1-76.
Sirichamorn, Y. 2006. Morphometrics and molecular systematics of the genus Afgekia Craib. (Fabaceae). Thesis, Chulalongkorn University.
Speer, W.D. and Hilu, K.W. 1999. Relationships between two infraspecific taxa of Pteridium aquilinum (Dennstaedtiaceae). I. Morphological evidence. Systematic Botany 23: 305-312.
Suksatan, P. 1998. Taxonomy and ecology of ephiphytic and lithophytic pteridophytes at Doi Chang Dao, Chiang Mai. Thesis, Kasetsart University.
Tagawa, M. and Iwatsuki, K. 1979. Flora of Thailand, Vol. 3(1). In Smitinand, T. and Larsen, K. (eds). Thailand Institute of Scientific and Technological Research Press. pp. 14-32.
Terao, H. 1981. Notes on some species of Strobilanthes (Acanthaceae) from Thailand (2)*. Acta Phytotaxonomica et Geobotanica. 32: 31-36.
Thapyai, C., Wilkin, P., and Chayamarit, K. 2005. The Dioscorea species of Doi Chiang Dao with particular reference to Dioscorea collettii Hook.f. (Dioscoreaceae), a new record for Northern Thailand. Thai Forest Bulletin (Botany). 33: 213-219.
Tropicos.org. 2021. Missouri Botanical Garden. Retrieved from http://www.tropicos.org. (12 May 2021)
Valdespino, I.A., López, C. and Góes-Neto, L.A., 2014. Additions to Cuban Selaginella (Selaginellaceae). Phytotaxa 184: 235-244.
Zhang, X.C., Nooteboom, H.P. and Kato, M. 2013. Flora of China, Vol. 2-3 (Pteridophytes) Selaginellaceae. In Wu, Z.Y., Raven P.H. & Hong, D.Y. (eds). Beijing Science Press and St. Louis: Missouri Botanical Garden Press. pp. 37-66.
Zhou, X.M., Rothfels, C.J., Zhang, L., He, Z., Le Péchon, T., He, H., Lu, N.T., Knapp, R., Lorence, D., He, X., Gao, X., and Zhang, L. 2015a. A large‐scale phylogeny of the lycophyte genus Selaginella (Selaginellaceae: Lycopodiopsida) based on plastid and nuclear loci. Cladistics 32 (4): 360-389.
Zhou, X.M., Jiang, L.J., Zhang, L., Gao, X.F., He, Z.R., and Zhang, L.B. 2015b. Spore morphology of Selaginella (Selaginellaceae) from China and its systematic significance. Phytotaxa 273: 1-67.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Udon Pongkawong1, Jatupol Kampuansai1, Rossarin Pollawatn2, and Arunothai Jampeetong1,*
1 Department of Biology, Faculty of Science, Chiang Mai University, Meuang, Chiang Mai, 50200, Thailand
2 Department of Botany, Faculty of Science, Chulalongkorn University, Pathumwan, Bangkok, 10330, Thailand
Corresponding author: Arunothai Jampeetong, E-mail: Arunothai.2519@gmail.com
Total Article Views
Editor: Korakot Nganvongpani, Chiang Mai University, Thailand
Article history:
Received: Mach 17, 2021;
Revised: Mach 12, 2021;
Accepted: May 13, 2021;

