
Effects of Supplemental Cations on Growth and Nitrogen Accumulation in Canna indica L.
Manutsawan Manokieng and Arunothai Jampeetong*Published Date : 2021-09-13
DOI : https://doi.org/10.12982/CMUJNS.2021.075
Journal Issues : Number 4, October-December 2021
Abstract The effects of supplemental cations on growth, nitrogen, and mineral accumulation were assessed in Canna indica L. Similar sized 45 days-old plants were grown on a nutrient solution modified from Hoagland and Arnon (1950). The different cations were added to generate 6 treatments (n=4): (i) control (no cation added), (ii) 2.5 mM K+, (iii) 2.5 mM Ca2+, (iv) 75 mM Na+, (v) 1.25 mM K+ + 1.25 mM Ca2+ and (vi) 2.5 mM Ca2+ + 75 mM Na+, respectively. An experiment was carried out in the greenhouse for 49 days. The study found that supplemental K+ and K++ Ca2+ increased plant growth and total biomass. The highest SER was found in plants receiving supplemental K+. In contrast, SERs, leaf areas, and total biomass decreased in Na+ or Na++Ca2+ supplemented plants. The accumulated NO3- concentration (at the whole plant level) was also highest in the plants with supplemental K+ and K++Ca2+. The total nitrogen accumulation was higher in the K+, Ca2+, and K++Ca2+ supplemented plants than in the control plants. The results suggest that supplemental cations particularly K+ can enhance plant growth and nitrogen accumulation in C. indica. Therefore, cation supplementation could be an alternative technique to stimulate plant growth and improve nitrate removal in constructed wetlands.
Keywords: Constructed wetland, Nitrate removal, Potassium, Tropical wetland plants
Funding: This research was supported by a grant from the Faculty of Science, Chiang Mai University.
Citation: Manokieng, M. and Jampeetong, A. 2021. Effects of supplemental cations on growth and nitrogen accumulation in Canna indica L. CMU J. Nat. Sci. 20(4): e2021075.
INTRODUCTION
Nitrogen (N) is an important nutrient affecting plant growth and productivity. A major source of nitrogen taken up by plants is inorganic nitrogen in forms of nitrate (NO3-) and ammonium (NH4+) (Vymazal, 2007; Hawkesford et al., 2012). Many plants with nutrient uptake ability have been used to remove nutrients from wastewater. Generally, nitrogen removal in constructed wetlands (CWs) mainly occurs by microorganism processes and plant uptake. For biodegradation by microorganism, nitrogen removal consists of 3 processes including ammonification (organic N→NH4+), nitrification (NH4+→NO2-→NO3-), and denitrification (NO3-→N2) (Vymazal, 2007; Lee et al., 2009). To completely remove nitrogen, both nitrification and denitrification are needed. However, nitrification occurs under aerobic conditions while denitrification occurs under anaerobic condition. The difference in oxygen requirements is a problem for nitrogen removal when using only one CW. For NH4+ reduction, increasing oxygen can enhance nitrification. But in NO3- reduction, not only the presence of oxygen but also insufficient carbon source inhibit denitrification. (Vymazel; 2007; Lee et al., 2009; Leverenz et al., 2010; Matheson and Sukias, 2010). Plant uptake is another mechanism for removing nitrogen from the wastewater. Moreover, plants provide surface area for microorganism attachment and release oxygen from their roots, which supports microorganism processes (Greenway, 2007). Several documents pointed out that plant-bed CWs can achieve more nitrogen removal than systems without plants (Cui et al., 2010; Borin and Salvato, 2012; Toscano et al., 2015; Wang et al., 2016). Through N removal by methods such as artificial aeration or planting or both, nitrogen removal can be enhanced but nitrate was produced and remained in the systems. A study by Cui et al. (2010) found the removal efficiency of total P (70%), NH4+-N (70%), and total N (60%) in CWs treated with Canna indica L. was higher than the treatment without plants (50%, 50%, and 20%, respectively). Nonetheless, high concentrations of NO3--N were observed in the effluent of both planted treatments (37.7 mg L-1) and unplanted treatments (40.78 mg L-1). Wu et al. (2016) evaluated effects of intermittent aeration on organic substances and nitrogen removal in CWs planted with Phragmites australis (Cav.) Trin. ex Steud. The results showed that the intermittent aeration systems had higher reduction in chemical oxygen demand (COD), NH4+-N, and total N (96-97%, 97-99% and 82-86 % removal efficiency, respectively) compared with the non-aerated systems (76-79%, 22-27%, and 28-32% removal efficiency, respectively). However, the concentration of NO3--N in the intermittent aeration systems increased to 6-7 mg L-1. Therefore, techniques to improve nitrate removal in CWs are still needed. The improvement of plant nitrate uptake is another option to gain higher nitrate removal in CWs. A previous study in crop plants showed that the presence of cations like potassium (K+), calcium (Ca2+), and sodium (Na+) can enhance NO3- uptake and translocation. These cations act as counter ions to balance ion charge with NO3- (Minotti et al., 1968; Blevins et al., 1978; Ivashikina et al., 1998; Ruiz and Romero, 2002; Kaburagi et al., 2014). Because it has not been investigated in wetland plants, application of cation supplements could be a new technique that can enhance plant growth, nitrate uptake and increase pollutants removal efficiency in CWs.
Canna spp. are the tropical and subtropical perennial wetland plants belonging to family Cannaceae. They have been used as ornamental plants in urban areas and also to remove contaminants in CWs (Sandoval et al., 2019). A previous study found that C. siamensis Kraenzl. had a high potential to reduce suspended solids (SS) (84-94%), biochemical oxygen demand (BOD) (77-92%), NH4+-N (56-84%) and total P (69-86%) from municipal wastewater while NO3--N increased (Sirianuntapiboon and Jitvimolnimit, 2007). Moreover, the coupled system of microbial fuel cell (MFC) and integrated vertical flow CW (IVCW) planted with C. indica removed 88.1% COD, 75.0% NH4+-N, and 64.1% NO3--N (Liu et al., 2020). Recently, using ornamental wetland plants in CWs is become more popular because of their fast growth, high nutrient removal efficiency, and also they provide beautiful landscapes (Sandoval et al., 2019). Improving nitrate uptake by supplying cation supplements has never been studied in C. indica. Thus, this study aims to assess the growth response, nitrogen, and minerals accumulation in C. indica when receiving different supplemental cations.
MATERIALS AND METHODS
Experimental design and growth study
Similar size 45 days-old C. indica plants generated from rhizomes were selected. Individual plants (n=24) were planted in a plastic bag (127×280 mm) filled with sand. Then, the plants were placed in buckets containing 5 L nutrient solution modified from Hoagland and Arnon (1950) with 1.5 mM NO3--N, 100 µM NH4+-N and micronutrient fertilizer (Tropica, Denmark) added. All plants were acclimated under greenhouse conditions. After 14 days, initial plant height was recorded. The experimental plants were grown on nutrient solutions with different cation supplements including: (i) control (no cation added); (ii) 2.5 mM K+; (iii) 2.5 mM Ca2+; (iv) 75 mM Na+; (v) 1.25 mM K+ combined with 1.25 mM Ca2+ (K++Ca2+); and (vi) 2.5 mM Ca2+ combined with 75 mM Na+ (Ca2++Na+), respectively. Each treatment consisted of 4 replications. The nutrient solutions were renewed every 7 days. The pH was adjusted to 6.8 ± 0.2 using HCl or NaOH. The experiment was conducted under greenhouse conditions with 13:11h light: dark periods and 35-40ºC: 23-28ºC day: night temperatures.
All plants were harvested after 49 days. Average leaf area, number of new shoots, and total plant height were measured. Increase of total plant height was used for shoot elongation rate (SER) calculated using the formula, SER (mm•d-1) = (final height – initial height)/experimental days. Finally, the plants were separated into leaves, rhizomes, and roots. After that, all plant samples were dried using a hot air oven at 60 ºC, except for the samples used for plant tissue analyses, which were dried using a freeze dryer. Then, the total dry mass was recorded.
Plant tissue analyses
Chlorophylls (Chl a, Chl b, and total Chl a+b) and total carotenoids were analysed from 8 mg of fine pieces of leaf samples. The samples were soaked with 8 mL 98% ethanol and kept in the darkroom for 24 h. After that, the extractions were measured using a UV-VIS spectrophotometer (Lambda 25 version 2.85.04, USA). The concentrations of the chlorophylls and total carotenoids were calculated according to Lichtenthaler (1987).
Fine pieces of freeze-dried samples from leaves, rhizomes, and roots were assayed for NO3- and NH4+ concentrations in the tissues. The samples were extracted by the hot water method. The NO3- and NH4+ concentrations in the extracts were analyzed using the UV method (Oscarson et al., 1989) and the modified salicylate method (Quikchem Method No. 10-107-06-3-B; Lachat Instruments, Milwaukee, WI, USA), respectively.
Nutrients and minerals in the plant tissue were assayed in each plant part. Approximately 200 mg of freeze-dried samples were digested with acid solution at high temperature (100-380°C). Total N was analyzed using the Kjeldahl method (Nelson and Sommers, 1980). K+, Na+, and Ca2+ were analyzed by ICP-AES (Munter et al., 1981).
Statistics
Statistical analyses were performed using SPSS Statistics version 17.0 (SPSS Inc., Chicago, IL, USA). All data were tested by one-way analysis of variance (ANOVA) and the multiple range test was performed using Duncan’s test at the 5% significance level.
RESULTS
Plant growth
Overall, the plants increased growth rates when grown with supplemental K+, Ca2+, or K++Ca2+. The plants that received supplemental K+ and K++Ca2+ had greater increase in total dry mass. Similarly, root dry mass increased in the plants that received supplemental K++Ca2+ or K+. In contrast, supplemental Na+ had negative effects on plant growth and morphology. The plants supplied with Na+ and Na++Ca2+ had significantly decreases in total dry mass and total height, resulting in lower SERs. Also, these plants had smaller leaf areas (Figure 1).

Figure 1. Shoot elongation rates (SERs) (a), average leaf area (b), leaf dry mass (c), rhizome dry mass (d), root dry mass (e), and total dry mass (f) (mean ± S.D.) of Canna indica supplied with different cations. Different letters above columns represent significant differences among treatments (P <0.05).
Chlorophylls and total carotenoids
Supplemental cations did not affect pigments in leaves. There were no significant differences in Chl a, Chl b, total Chl a+b, and total carotenoids concentrations across the treatments (Figure 2).
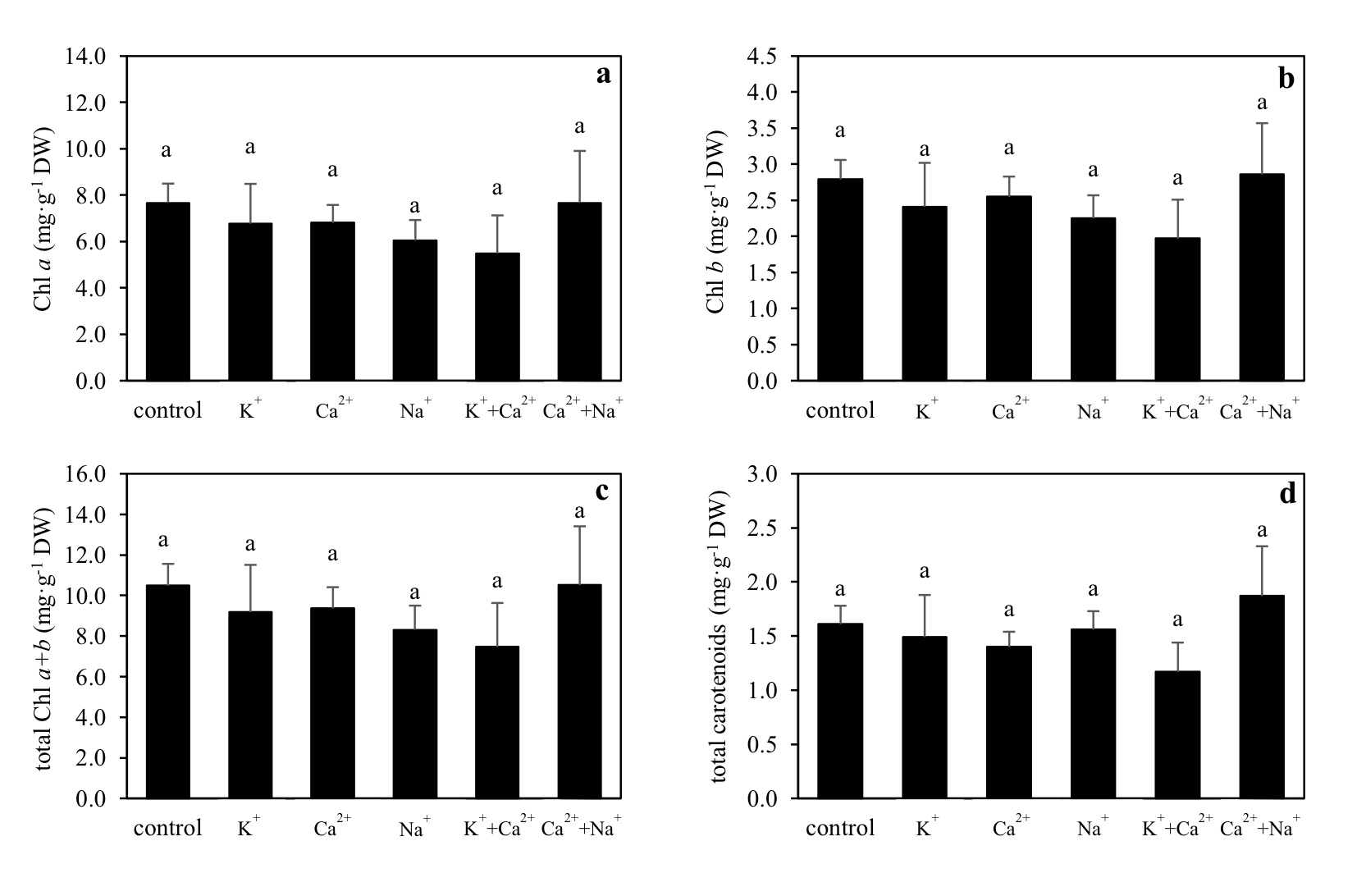
Figure 2. Chlorophyll a (Chl a) (a), chlorophyll b (Chl b) (b), total chlorophylls (total Chl a+b) (c), and total carotenoids (d) (mean ± S.D.) of Canna indica supplied with different cations. Different letters above columns represent significant differences among treatment (P <0.05).
Nutrients and minerals in the plant tissues
The concentrations of NO3-, NH4+, and total N in the plant tissue were significantly affected by supplemental cations. Generally, NO3- concentrations in the plant tissues were higher than NH4+ concentrations. Compared with the control, the plants with K+ supplement increased NO3- concentrations in their leaves, whereas the plants with Na+ or Ca2++Na+ supplements increased NO3- accumulation in their leaves and roots (Figure 3a-c). In contrast, the plants that received supplemental K+ had lower NH4+ concentrations in their leaves. Whereas the plants with Na+ supplement had higher NH4+ concentrations in their rhizomes than the control plants (Figure 3d-f). The Ca2+ supplemented plants increased total N concentrations in their leaves, while the Na+ and Ca2++Na+ supplemented plants increased total N concentrations in their leaves and roots compared with the control treatment (Figure 3g-i).
The K+ concentration in all plant parts increased when the plants received supplemental K+ and K++Ca2+ (Figure 4a-c). Similarly, Na+ concentration in the plant tissues increased as a result of receiving supplemental Na+ and Ca2++Na+ (Figure 4g-i). However, Ca2+ concentrations increased only in the roots of the plants supplied with supplemental Ca2+ (Figure 4d-f).
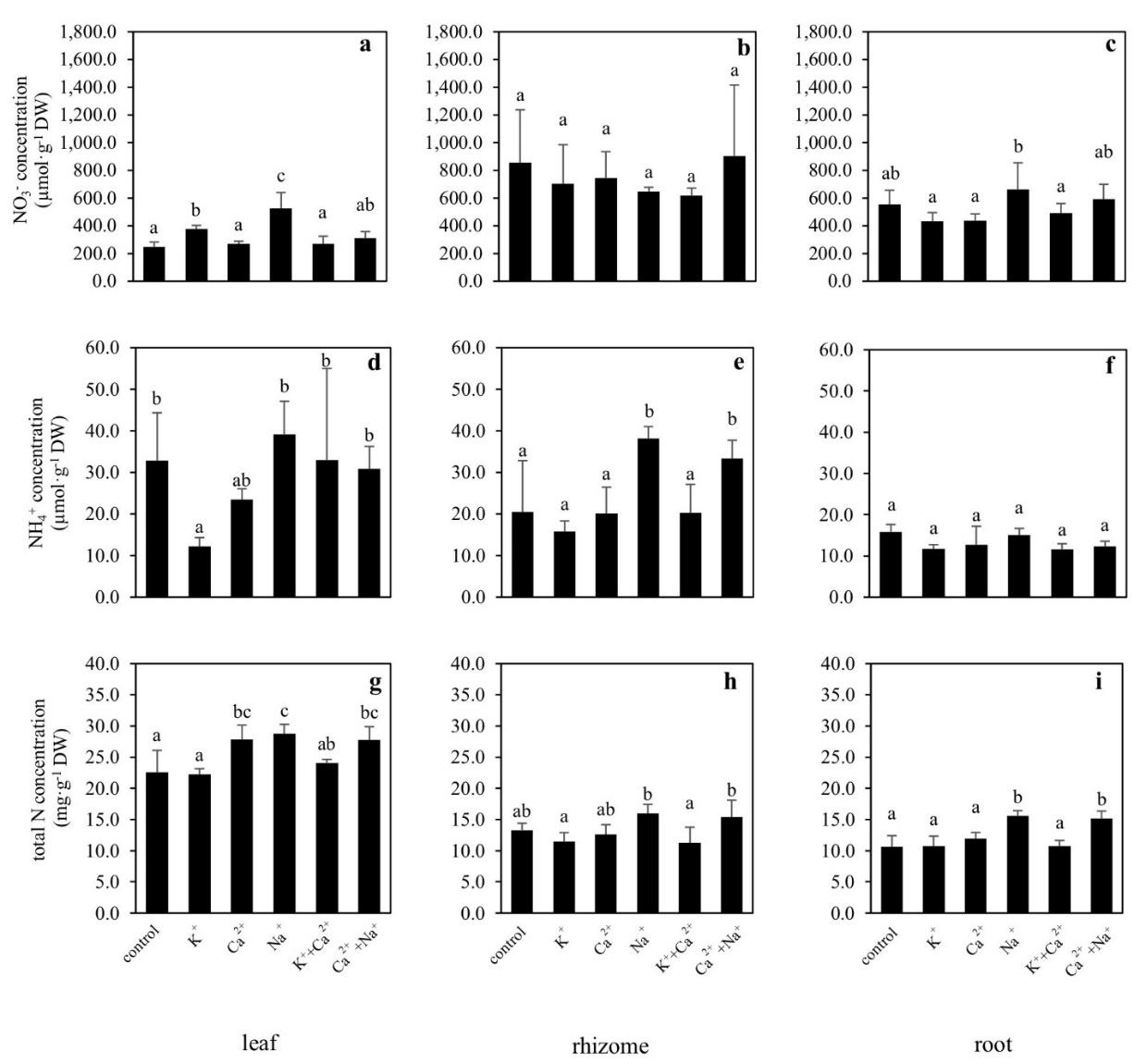
Figure 3. NO3- concentration (a-c), NH4+ concentration (d-f), and total N concentration (g-i) (means ± S.D.) in leaf, rhizome, and root tissues of Canna indica supplied with different cations and N concentration in the media is 1.6 mM (NO-3-N: 1.5 mM and NH4+-N: 0.1 mM). Different letters above columns represent significant differences among treatment (P <0.05).
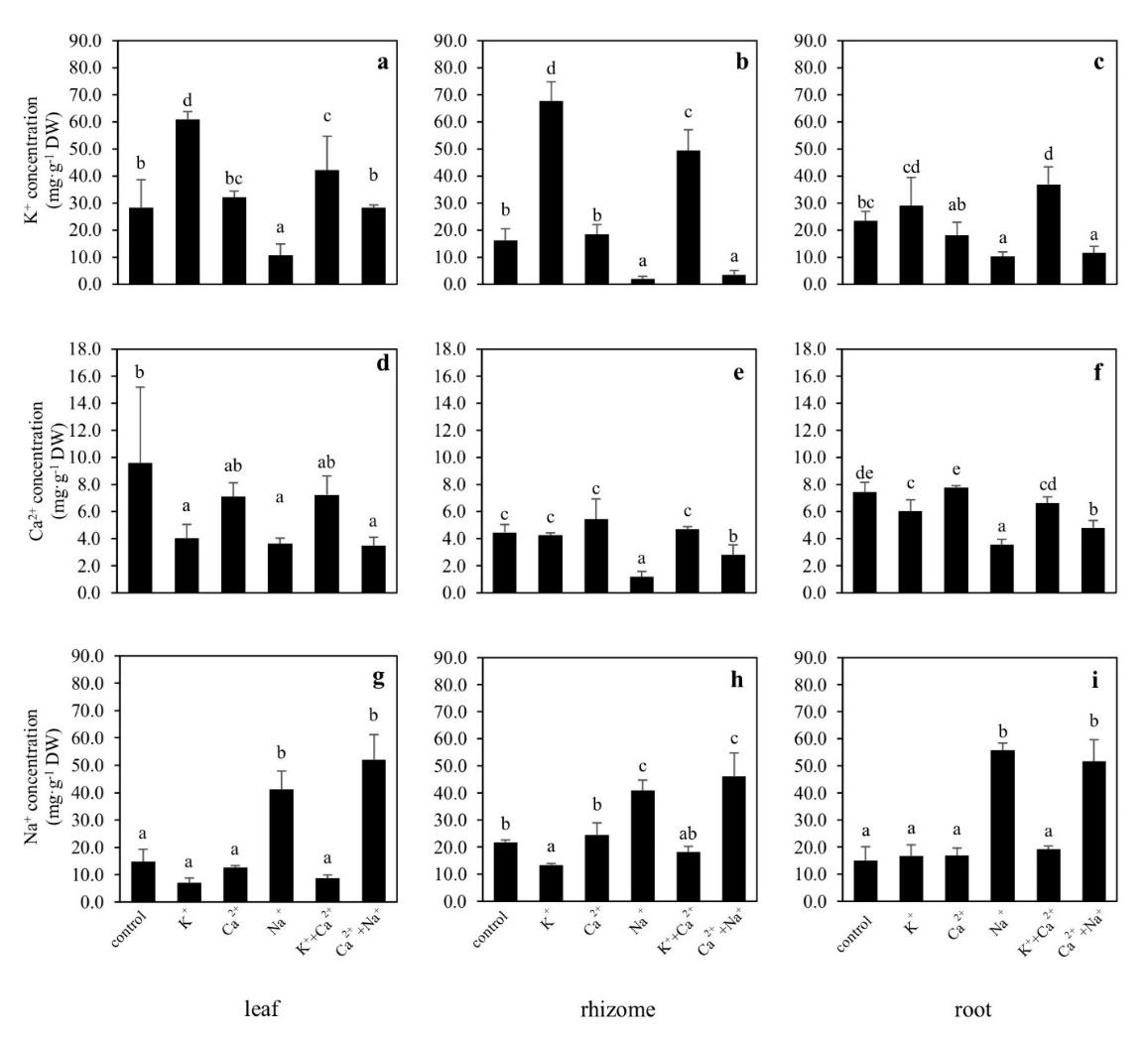
Figure 4. K+ concentration (a-c), Ca2+ concentration (d-f), and Na+ concentration (g-i) (means ± S.D.) in leaf, rhizome, and root tissues of Canna indica supplied with different cations. Different letters above columns represent significant differences among treatment (P <0.05).
DISCUSSION
The results showed that SERs and total dry mass of C. indica increased after receiving supplemental K+ and K++Ca2+. They indicate that K+ and K++Ca2+ supplements have positive effects on NO3- uptake and nitrogen metabolism. When NO3- is taken up, it is converted to NH4+ by nitrate reductase and nitrite reductase, and then NH4+ is assimilated to amino acids and organic compounds which are necessary for plant growth (Hawkesford et al., 2012). Previous study showed that increasing K+ supply can enhance NO3- uptake, increase rates of NO3- translocation from roots to shoots, resulting in high NO3- accumulation and reduction in the shoots (Minotti et al., 1968; Blevins et al., 1978; Ivashikina et al., 1998; Coskun et al., 2017; Xu et al., 2020). Our study found that K+ supplemented plants increased NO3- accumulation in the tissue, particularly in the leaves. Whole-plant NO3- concentrations (calculated as tissue NO3- concentrations multiplied by biomass) showed NO3- concentrations in leaves increased approximately 123.3% while they only increased 2.5% in roots. Moreover, we found K++Ca2+ supplemented plants had 44.7% and 98.4% increase in NO3- concentrations in their leaves and in their roots, respectively. Plants slightly increased their accumulative NO3- concentrations (23.3%) in their leaves when Ca2+ added. The accumulative total N of the whole-plant was higher in K+, Ca2+, and K++Ca2+ supplemented plants than in the control plants (28.9%, 26.9%, and 33.9% increasing from control, respectively). It indicates that the Ca2+ supplement had less effect on NO3- uptake compared with the K+ supplement. It may be because Ca2+ functions as a second messenger that can mediate nitrate transporters (NRTs). Previous study found that Ca2+ is involved in N availability and NRT regulation. Under low N conditions, protein kinase, CIPK23 is activated by a Ca2+ signal, and NRT1.1 is efficiently regulated by phosphorylation. As a result, the NO3- uptake is mediated from low to high affinity (Ho et al., 2009, Thor, 2019). So, it seems that supplemental Ca2+ could enhance NO3- uptake particularly at low N conditions. It was found that the Ca2+ signal was well activated in response to high NO3- concentration, but this signal is not involved in the NO3- uptake mechanism (Hu et al., 2009). However, the role of Ca2+ in NO3- signaling pathway was not clearly understood until recently (O’Brien et al., 2016).
The NaCl added into the growth medium decreases osmotic potential leading to water deficiency and decrease in plant growth (Parida and Das, 2005). Thus, plants have some defense responses to maintain the osmotic potential like exclusion and compartmentation of Na+, increase of NO3- accumulation in roots or accumulation of the compatible solutes such as amino acids and amides. Nevertheless, long-term NaCl exposure still decreased plant growth in many plant species (Greenway and Munns, 1980; Munns, 2002; Parida and Das, 2005; Chun et al., 2012; Wang et al., 2012). In our study, C. indica dramatically decreased growth when Na+ and Na++Ca2+ were supplied although the plants had high accumulation of NO3-, NH4+ and total N in their tissue. Decreased K+ concentration in the plant tissues was also found in Na+ and Na++Ca2+ supplemented plants. The antagonistic effect with another cation like K+ can cause K+ deficiency leading to decrease of K+-coupled enzyme activity and protein synthesis and cause stunted growth. In addition, photosynthetic pigments usually decrease under NaCl stress in many plant species (Parida and Das, 2005; Kaburagi et al., 2014). In this study, however, the chlorophylls and total carotenoids concentrations were not affected by supplemental Na+.
In CWs, plant biomass harvesting is a nutrient removal process. The more biomass is harvested, the more nutrients will be removed from CWs. High plant biomass production indicates high nutrient uptake and storage capacity (Greenway, 2007; Vymazal, 2020). Luo et al. (2018) showed that the harvested total N mass of Myriophyllum aquaticum (Vell.) Verdc. was in the range of 120-222 g N m-2yr-1. The N taken up by this plant accounted for 15.2-57.3% of total N removal from swine wastewater treatment. Other studies of Canna × generalis L. Bailey and Heliconia spp. found that Canna had higher biomass production than Heliconia. The above ground biomass harvest of Canna and Heliconia could remove 85 and 12 g N m-2 yr-1, respectively. The plant uptake of N into Canna above ground biomass accounted for 41% of N removal from domestic wastewater treatment compared to 12% for Heliconia (Konnerup et al., 2009). Therefore, K+ application to stimulate plant growth and biomass production could be an appropriate method to enhance plant nitrate removal in CWs. However, the further study of effects of cations on nitrate removal efficiency must be performed in real wastewater to assess the actual ability of plants to remove NO3- after using this technique in CWs.
CONCLUSION
In conclusion, cation supplements particularly K+, can enhance growth and nitrogen accumulation in C. indica. K+ supplemented plants can increase both NO3- and total nitrogen accumulation in their plant tissue. In contrast, plants decreased growth and showed K+ deficiency in their tissue when they received supplemental Na+. Thus, supplemental K+ application could be an appropriate technique to improve plant growth and NO3- uptake. However, further study on supplemental K+ application to plants used in CWs and their performance in nutrient removal from real wastewater is still needed.
ACKNOWLEDGEMENTS
This research was supported by a grant from the Faculty of Science, Chiang Mai University. This work was supported by the Development and Promotion of Science and Technology Talents Project (DPST) and the Institute for the Promotion of Teaching Science and Technology (IPST), Thailand. We also thank Mr. Alvin Yoshinaga for his kind help to correct the English text.
REFERENCES
Blevins, D.G., Barnett, N.M., and Frost, W. B. 1978. Role of potassium and malate in nitrate uptake and translocation by wheat seedling. Plant Physiology. 62: 784-788.
Borin, M., and Salvato, M. 2012. Effects of five macrophytes on nitrogen remediation and mass balance in wetland mesocosms. Ecological Engineering. 46: 34-42.
Chun, C.Z., Lv, X.F., Li, J.Y., Yi, H.Y., and Gong, J.M. 2012. Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiology. 159: 1582–1590.
Coskun, D., Britto, D.T., and Kronzucker, H.J. 2017. The nitrogen-potassium intersection: membranes, metabolism, and mechanism. Plant, Cell and Environment. 40: 2029-2041.
Cui, L., Quyang, Y., Lou, Q., Yang, F., Chen, Y., Zhu, W., and Luo, S. 2010. Removal of nutrients from wastewater with Canna indica L. under different vertical-flow constructed wetland conditions. Ecological Engineering. 36: 1083-1088.
Greenway, M. 2007. The role of macrophytes in nutrient removal using constructed wetlands. In: Singh S.N., Tripathi R.D. (Eds.). Environmental Bioremediation Technologies (pp. 331-351). Berlin, Heidelberg: Springer.
Greenway, H., and Munns, R. 1980. Mechanisms of salt tolerance in nonhalophytes. Annual Review of Plant Physiology. 31: 149-190.
Hawkesford, M., Horst, W., Kichey, T., Lambers, H., Schjoerring, J., Moller, I.S. and White, P. 2012. Functions of macronutrients. In P. Marschner (Ed.). Marschner's Mineral Nutrition of Higher Plants (3rd ed) (pp. 135-189). Pergamon.
Hoagland, D.R., and Arnon, D.I. 1950. The water culture method for growing plant without soil. California Agricultural Experiment Station, Circular No. 347. California: University of California Berkley Press.
Ho, C.H., Lin, S.H., Hu, H.C., and Tsay, Y.F. 2009. CHL1 functions as a nitrate sensor in plants. Cell. 138: 1184-1194.
Hu, H.C., Wang, Y.Y., and Tsay, Y.F. 2009. AtCIPK8, a CBL-interacting protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant Journal. 57: 264-278.
Ivashikina, N.V., and Feyziev, Ya.M. 1998. Regulation of nitrate uptake in maize seedling by accompanying cations. Plant Science. 131: 25-34.
Kaburagi, E., Morikawa, Y., Yamada, M., and Fujiyama, H. 2014. Sodium enhances nitrate uptake in Swiss chard (Beta vulgaris var. cicla L.). Soil Science and Plant Nutrition. 60: 651-658.
Konnerup, D., Kootatep, T., and Brix, H. 2009. Treatment of domestic wastewater in tropical, subsurface flow constructed wetlands planted with Canna and Heliconia. Ecological Engineering. 35: 248-257.
Lee, C. Fletcher, T.D., and Sun, G. 2009. Nitrogen removal in constructed wetland systems. Engineering in Life Sciences. 9: 11-22.
Leverenz, H.L., Haunschild, K., Hopes, G., Tchobanoglous, G., and Darby, J.L. 2010. Anoxic treatment wetlands for denitrification. Ecological Engineering. 36: 1544-1551.
Lichtenthaler, H.K. 1987. Chlorophylls and carotenoids: Pigments of photosynthetic membranes. Methods in Enzymology. 148: 350-382.
Liu, F., Sun, L., Wan, J., Shen, L., Yu, Y., Hu, L., and Zhou, Y. 2020. Performance of different macrophytes in the decontamination of and electricity generation from swine wastewater via an integrated constructed wetland-microbial fuel cell process. Journal of Environmental Sciences. 89: 252-263.
Luo, P., Liu, F., Zhang, S., Li, H., Yao, R., Jiang, Q., Xiao, R., and Wu, J. 2018. Nitrogen removal and recovery from lagoon-pretreated swine wastewater by constructed wetlands under sustainable plant harvesting management. Bioresource Technology. 258: 247-254.
Matheson, F.E., and Sukias, J.P. 2010. Nitrate removal processes in a constructed wetland treating drainage from dairy pasture. Ecological Engineering. 36: 1260-1265.
Minotti, P.L., Williams, D.C., and Jackson, W.A. 1968. Nitrate uptake and reduction as affected by calcium and potassium. Soil Science Society of America Journal. 32: 692-698.
Munns, R. 2002. Comparative physiology of salt and water stress. Plant, Cell and Environment. 25: 239-250.
Munter, R.C., and Grande, R.A. 1981. Plant analysis and soil extracts by ICP-atomic emission spectrometry. In: R.M. Barnes (Ed.). Developments in atomic plasma spectrochemical analysis (pp. 653-672). Heyden and Son. Ltd., London, England.
Nelson, D. W., and Sommers, L. E. 1980. Total nitrogen analysis of soil and plant tissues. Journal of Association of Official Analytical Chemists. 63: 770-778.
O’Brien, J.A., Vega, A., Bouguyon, E., Krouk, G., Gojon, A., Coruzzi, g., and Gutierrez, R.A. 2016. Nitrate transport, sensing and responses in plants. Molecular Plant. 9: 837-856.
Oscarson, P., Ingemarsson, B., Ugglas, M., and Larsson, C.M. 1989. Characteristics of NO3- uptake in Lemna and Pisum. Plant and Soil. 111: 203–205.
Parida, A.K., and Das, A.B. 2005. Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety. 60: 324-349.
Ruiz, J.M., and Romero, L. 2002. Relationship between potassium fertilisation and nitrate assimilation in leaves and fruits of cucumber (Cucumis sativus) plants. Annals of Applied Biology. 140: 241-245.
Sandoval, L., Zamora-Castro, S.A., Vidal-Alvarez, M., and Marin-Muniz, J.L. 2019.Role of wetland plants and use of ornamental flowering plants in constructed wetlands for wastewater treatment: a review. Applied Sciences. 9: 685.
Sirianuntapiboon, S., and Jitvimolnumit, S. 2007. Effect of plantation pattern on the efficiency of subsurface flow constructed wetland (sfcw) for sewage treatment. African Journal of Agricultural Research. 2: 447-454.
Thor, K. 2019. Calcium-Nutrient and Messenger. Frontiers in Plant Science. 10: 440.
Toscano, A., Marzo, A., Milani, M., Giuseppe L.C., and Barbagallo, S. 2015. Comparison of removal efficiencies in Mediterranean pilot constructed wetlands vegetated with different plant species. Ecological Engineering. 75: 155-160.
Vymazal, J. 2007. Removal of nutrients in various types of constructed wetlands. Science of the Total Environment. 380: 48-65.
Vymazal, J. 2020. Removal of nutrients in constructed wetlands for wastewater treatment through plant harvesting – Biomass and load matter the most. Ecological Engineering. 155: 105-962.
Wang, H., Wu, Z., Zhou, Y., Han, J. and Shi, D. 2012. Effects of salt stress on ion balance and nitrogen metabolism in rice. Plant, Soil and Environment. 58: 62-67.
Wang, W., Ding, Y., Ullman, J.L., Ambrose, R.F, Wang, Y., Song, X., and Zhao, Z. 2016. Nitrogen removal performance in planted and unplanted horizontal subsurface flow constructed wetlands treating different influent COD/N ratios. Environmental Science and Pollution Research. 23: 9012–9018.
Wu, H., Fan, J., Zhang, J., Ngo, H.H., Guo, W., Liang, S., Lv, J., Lu, S., Wu, W., and Wu, S. 2016. Intensified organics and nitrogen removal in the intermittent-aerated constructed wetland using a novel sludge-ceramsite as substrate. Bioresource Technology. 210: 101-107.
Xu, X., Du, X., Wang, F., Sha, J., Chen, Q., Tian, G., Zhu, Z., Ge, S., and Jiang, Y. 2020. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Frontiers in Plant Science. 11: 904.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Manutsawan Manokieng1 and Arunothai Jampeetong2,*
1 Graduate Master’s Degree Program in Biology, Faculty of Science, Chiang Mai University, Chiang Mai, 50200, Thailand
2 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, 50200, Thailand
Corresponding author: Arunothai Jampeetong, E-mail: arunothai.2519@gmail.com
Total Article Views
Editor: Nisit Kittipongpatana, Chiang Mai University, Thailand
Article history:
Received: December 4, 2020;
Revised: May 7, 2021;
Accepted: May 13, 2021;

