
The Attenuation of TNF-α-mediated Inflammatory Responses in Human Lung Adenocarcinoma Cell Line by Perilla Seed and Seed Meal Extract
Chakkrit Khanaree, Wanisa Punfa, Payungsak Tantipaiboonwong, Maitree Suttajit, Teera Chewonarin, Kanjana Pangjit, and Komsak Pintha*Published Date : 2021-09-13
DOI : https://doi.org/10.12982/CMUJNS.2021.074
Journal Issues : Number 4, October-December 2021
Abstract Thai perilla (Perilla frutescens) extracts, which contain a substantial quantity of bioactive substances including phenolics and flavonoids, have shown marked anti-inflammatory activities in several investigated models. In the present study, the effect of perilla seed extract (PSE) and seed meal extract (PSME) on TNF-α-induced inflammatory response in human lung adenocarcinoma A549 cells was investigated. The total phenolic and flavonoid contents in PSME was lower than PSE. Markedly, rosmarinic acid was identified as the main constituent in both extracts. However, the DPPH and ABTS assays indicated that the antioxidant capacity of PSME was equal to PSE. Moreover, the iron-binding activity of PSE and PSME were exhibited by complex formation with Fe3+-NTA, indicating that the extracts may inhibit hydroxyl radical production via Fenton reaction. In vitro cytotoxicity analysis showed that both PSE or PSME co-treated with TNF-α, at 24 h exposure, were not toxic to the A549 cells. Interestingly, PSE and PSME dramatically exhibited an anti-inflammatory activity by inhibiting the mRNA expression of pro-inflammatory cytokines, IL-1β, IL-6, IL-8, and TNF-α, but did not influence iNOS and COX-2 mRNA expressions. Moreover, both extracts significantly reduced reactive oxygen species (ROS) production in TNF-α-induced A549 cells. The findings presented in this paper suggest that PSE and PSME could mitigate TNF-α-mediated inflammatory responses via limiting pro-inflammatory cytokine expressions and decreasing ROS production. Thus, perilla seed and seed meal, the by-product of a perilla seed oil cold-pressed extraction process, could be developed as food supplements or functional foods for the prevention of inflammation-induced lung carcinogenesis development.
Keywords: Human lung adenocarcinoma cell line, Inflammation, Perilla seed, Perilla seed meal, Tumor necrosis factor-alpha (TNF-α)
Funding: This research was financially supported by the Thailand Research Fund (TRF) and Office of the Higher Education Commission (OHEC) [grant number MRG6180080], Unit of Excellence (FF64-UoE019) University of Phayao, University of Phayao research grant 2018 [RD61065].
Citation: Khanaree, C., Punfa, W., Tantipaiboonwong, P., Suttajit, M., Chewonarin, T., Pangjit, K., and Pintha, K. 2021. The attenuation of TNF-α-mediated inflammatory responses in human lung adenocarcinoma cell line by perilla seed and seed meal extract. CMU J. Nat. Sci. 20(4): e2021074.
INTRODUCTION
Globally, the most frequent cause of cancer-related mortality among both sexes is lung cancer. Lately, one of the most acute issues linked with lung tumorigenesis and disease progression is chronic inflammation. Intensified inflammatory responses through NF-κB and MAPK pathways, with increased the probabilities of morbidity and mortality, have been linked to the increasing prevalence of chronic respiratory diseases (Wedzicha, 2002; Comer, 2013).
The primary inflammatory cytokine, tumor necrosis factor-alpha (TNF-α), which stimulates lung inflammation by inducing the activation of NF-κB and MAPK pathways in alveolar epithelial cells, was metabolized by tumor-associated macrophages (Matera et al., 2010; Calcinotto et al., 2012; Knobloch et al., 2013). Alveolar epithelial cells can exacerbate inflammatory signals through the release of several inflammatory mediators, such as IL-1β, IL-6, IL-8, TNF-α, iNOS and COX-2 (Hardie et al., 2004). In addition, the prolonged inflammation and development of TNF-α will trigger the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) that contribute to DNA damage and facilitate tumor proliferation as well as invasion and metastasis (Woo et al., 2000).
In northern Thailand, Thai perilla (Perilla frutescens) is a long-established plant that is eaten often. The perilla seed is used in a traditional food called "Kao-Nuk-Nga" by grounding then mixing with sticky rice. In addition to ꞷ-3 fatty acid, perilla seed is also an essential source of phytochemical bioactive compounds including rosmarinic acid, apigenin, luteolin and glycosides (Asif, 2011; Scapin et al., 2017). Through the cold-pressed extraction process, oil extracted from the perilla seed produces a critical agricultural by-product, perilla seed meal. This by-product is normally disposed of or used in animal feed since it is a low-value material (Longvah and Deosthale, 1991). Intriguingly, various experiments have shown that perilla seed meal comprises bioactive phytochemical compounds equivalent to perilla seeds (Tang et al., 2014; Zhou et al., 2014). A recent study by Zhang et al. revealed that a fraction of cold-pressed perilla seed meal extract rich in rosmarinic acid demonstrated a possible impact on hepatic anti-oxidative disruption (Zhang et al., 2018). In addition, previous research by the authors showed that perilla seed extracts (PSE) and perilla seed meal extracts (PSME) exhibit anti-mutagenic, anti-oxidant and anti-inflammatory capacities (Chumphukam et al., 2018).
Therefore, the perilla seed and seed meal, having demonstrated several biological activities and active compounds, may reduce lung inflammation, pro-inflammatory mediator production and inflammation-induced ROS production. This recent study aimed to evaluate the effects of perilla seed and seed meal extracts on the synthesis of inflammatory mediators and ROS generation in TNF-α -induced human lung adenocarcinoma cell line (A549 cells).
MATERIALS AND METHODS
Plant material
Seed samples of Thai perilla (Perilla frutescens) were collected from Wiang-Sa district, Nan province, Thailand. The voucher number of plant material was prepared by Dr. Komsak Pintha and Dr. Payungsak Tantipaiboonwong and deposited at the Queen Sirikit Botanic Garden Herbarium, Chiang Mai, Thailand (Code: QSBG-K2).
Preparation of Thai perilla seed and seed meal extracts
The seeds of Thai perilla were collected and washed with water before drying by air. One hundred grams of miniaturized dried perilla seed were extracted in 1 L of 70% ethanol. The ethanolic solution was then stirred for 4 h and further left overnight. After that, the solution was filtered through filter paper (Whatman no.1) and the ethanol was removed by rotary evaporator and lyophilized to obtain crude perilla seed extract (PSE) powder. The perilla seed meal was collected after oil extraction. The 100 g of seed meal was extracted by following the same procedure as described in the seed extraction process. This fraction will be referred to as perilla seed meal extract (PSME). The two fractions were kept at -20°C until use.
Determination of total phenolic contents
Total phenolic contents of PSE and PSME were determined by using Folin-Ciocalteu assay as described by the previous study (Chumphukam et al., 2018) with some modifications. Briefly, PSE or PSME was dissolved in DMSO at assigned concentrations. In 96-well plate, 20 µl of PSE or PSME was added to 100 µl of 10% v/v Folin-Ciocalteu reagent and incubated for 3 min in the dark at room temperature. Next, 80 µl of 7.5% w/v sodium carbonate (Na2CO3) was added to each well. The incubation was then carried out for 30 min at room temperature in the dark, followed by measuring the absorbance at 765 nm using a Synergy™ HT Multi-Detection Microplate Reader (BioTek Instruments, Inc., VT, USA). A standard curve was obtained using various concentrations of standard gallic acid. The total phenolic contents were calculated by authentic gallic acid standard curve and expressed as milligrams of gallic acid equivalents (GAE) per gram dry weight of the extract (mg GAE/g extract).
Determination of total flavonoid contents
Total flavonoid contents in PSE and PSME were determined by using aluminum chloride colorimetric assay (Min et al., 2011) with slight adjustments. In brief, PSE or PSME was dissolved in DMSO at assigned concentrations. The mixture of 25 µl extract and 125 µl deionized water was mixed with 7.5 µl of 5% NaNO2 in 96-well plate. After 6 min of incubation at room temperature in the dark, 15 µl of 10% w/v aluminum chloride (AlCl3) was added and incubated for another 6 min. Color development was performed by adding 50 µl of 1 M NaOH. The final volume of the reaction mixture was adjusted to 250 µl using deionized water. The mixture was incubated for 15 min at room temperature in the dark before measuring the absorbance at 532 nm using the microplate reader. A standard curve was obtained using various concentrations of standard catechin. The total flavonoid contents were calculated by authentic catechin standard curve and expressed as milligrams of catechin equivalents (CE) per gram dry weight of the extract (mg CE /g extract).
Determination of phenolic derivatives content in PSE and PSME
Phenolic constituents of PSE and PSME were determined by using reverse-phase-high performance liquid chromatography (RP-HPLC) according to the previously published method (Pintha et al., 2014). The PSE and PSME were dissolved in HPLC grade methanol to obtain 5 mg/ml concentration. The HPLC was performed using reverse-phase ODS-3-C18 column (4.6 mm x 250 mm, 5 µm particle diameters). The assay was performed using two solvents including 0.1% trifluoroacetic acid (TFA) in water as a mobile phase A and 100% methanol as a mobile phase B. The gradient was performed as follows: 0-35 min, 90-10% mobile phase A and 10-90% mobile phase B; 35-40 min, 10-90% mobile phase A and 90-10% mobile phase B. Twenty microliters of the 5 mg/ml PSE or PSME were injected into the column with a flow rate 1.0 ml/min. Total phenolic content peaks were detected at 280 nm. Peak area and retention time of each fraction were compared with a calibration curve of various concentrations of phenolic standards. The phenolic constituents were calculated and expressed as mg/g extract.
Determination of antioxidant activity by DPPH radical scavenging assay
The antioxidant activity of PSE and PSME was assessed by using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay based on the method previously described (Wu et al., 2020), with a few modifications. Briefly, the 20 µl of PSE or PSME at assigned concentrations were mixed with 180 µl of 0.2 mM DPPH reagent in 96-well plate. The reaction mixture was incubated for 30 min at room temperature and protected from light. The absorbance was measured at 517 nm using the microplate reader. Methanol was used as a reagent blank of control and mixing methanol with various concentrations of the extract was used as a reagent blank of test. The percentage of scavenging inhibition was calculated and presented as the concentration of the extracts which scavenged free radicals by 50% (SC50).
Determination of antioxidant activity by ABTS radical scavenging assay
The antioxidant activity of PSE and PSME was also evaluated using the 2,20-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) radical scavenging assay, according to the previous method (Wu et al., 2020) with slight modification. Ten microliters of PSE or PSME at assigned concentrations were mixed with 990 µl of working ABTS solution. The reaction mixture was incubated at room temperature for 6 min in the dark and then the absorbance of the samples was measured at 734 nm using a UV-vis spectrophotometer. Water was used as a reagent blank of control and mixing water with various concentrations of the extract was used as a reagent blank of test. The percentage of scavenging inhibition was calculated and presented as the concentration of the extracts which scavenged free radicals by 50% (SC50).
Determination of antioxidant activity by iron binding assay
Iron binding activity was determined by the spectrophotometric technique according to the previously published method (Tipsuwan and Chaiwangyenwith, 2018), with a few modifications. In brief, PSE or PSME was dissolved in 10 mM MOPS buffer (pH 7.0) at 50 mg/ml. The extract was incubated with 0-400 µM of ferric nitrilotriacetate (Fe3+-NTA), pH 7.0 at room temperature for 60 min. The absorbance of the iron-PSE/PSME complexes was measured in the range of 250-900 nm by a scanning double-beam UV-vis spectrophotometer. PSE or PSME at 50 mg/ml was used as a blank.
Cell lines and culture condition
The human lung adenocarcinoma A549 cell line was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). The cells were grown and maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
Determination of cytotoxicity by MTT Assay
A549 cells (1.5 x 103 cells/well) were seeded into a 96-well plate for 24 h and were then treated with various concentrations of PSE or PSME (25, 50, 100 and 200 µg/ml) with 100 ng/ml TNF-α for 24 h. Then, the cell viability was determined by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, as previously described (Phannasorn et al., 2017). Briefly, 15 µl of 5 mg/ml MTT dye were added and the incubation was carried out at 37ºC for 4 h. All solutions were discarded and a 100 µl DMSO was added to dissolve formazan crystal. The absorbance (OD) of the formazan dye was measured at 540/630 nm using the microplate reader. The percentage of cell viability was calculated and non-cytotoxic concentrations (≤ IC20) were selected for further experiments.
Measurement of pro-inflammatory mediator gene expression by RT-qPCR
The effect of PSE or PSME on the mRNA expression of pro-inflammatory mediators including IL-1β, IL-6, IL-8, TNF-α, iNOS and COX-2 in TNF-α-treated A549 cells was measured by Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR). The A549 cells were plated for 24 h in 6-well plate and incubated with PSE or PSME for 2 h, and then stimulated with TNF-α (100 ng/ml) for 24 h. Total RNA was extracted using Nucleospin RNA (Macherey-nagel, Germany), according to the manufacturer’s instructions. Total mRNA was reversed to cDNA using ReverTra Ace qPCR RT master mix (TOYOBO, Japan). The target cDNA was subsequently used as the template for RT-qPCR amplification using SensiFAST™ SYBR® Lo-ROX (Bioline Ltd., London, UK). The oligonucleotide primers were obtained from Bio Basic Inc (Toronto, ON, Canada) and the primer sequences for the pro-inflammatory mediators were as shown in Table 1.
Table 1. Primer used in RT-qPCR.
|
Gene |
Sequence |
|
IL-1β |
Forward: 5’-AAA CAG ATG AAG TGC TCC TTC CAG G-3’ |
|
Reverse: 5’-TGG AGA ACA CCA CTT GTT GCT CCA-3’ |
|
|
IL-6 |
Forward: 5’-ATG AAC TCC TTC TCC ACA AGC-3’ |
|
Reverse: 5’-GTT TTC TGC CAG TGC CTC TTT G-3’ |
|
|
IL-8 |
Forward: 5’-AGA TAT TGC ACG GGA GAA-3’ |
|
Reverse: 5’-GAA ATA AAG GAG AAA CCA-3’ |
|
|
TNF-α |
Forward: 5’-CCC AGG CAG TCA GAT CAT CTT C-3’ |
|
Reverse: 5’-AGC TGC CCC TCA GCT TGA-3’ |
|
|
iNOS |
Forward: 5’-TCC GAG GCA AAC AGC ACA TTC A-3’ |
|
Reverse: 5’-GGG TTG GGG GTG TGG TGA TGT-3’ |
|
|
COX-2 |
Forward: 5’-CCC TTG GGT GTC AAA GGT AA-3’ |
|
Reverse: 5’-GCC CTC GCT TAT GAT CTG TC-3’ |
|
|
GAPDH |
Forward: 5’-GAA GGT GAA GGT CGA GTC A-3’ |
|
Reverse: 5’-GCT CCT GGA AGA TGG TGA T-3’ |
The reaction conditions were followed by 40 cycles of 70°C for 5 min, 4°C for 1 min, and 42°C for 60 min. The level of target cDNA was normalized by the expression of GAPDH and analyzed using the comparative threshold (ΔΔCt) method (7,500 software v2.0.5; Applied Biosystem, Thermo Fisher Scientific Inc., Foster City, CA, USA). Quantitative assays were calculated based on the following equation: relative quantitation (RQ) =2−ΔΔCt. Then the values were represented as relative mRNA expression to the non-treated control (Phannasorn et al., 2017).
Measurement of intracellular reactive oxygen species (ROS) level
The generation of intracellular ROS in A549 cells was investigated using the 2',7'-dichlorofluorescein-diacetate (DCFH-DA) dye. For measuring total ROS level in the cells, A549 cells were incubated with DCFH-DA for 2 h, then 100 ng/ml of TNF-α and the PSE or PSME at non-toxic concentrations were added. The co-incubation was carried out at 37°C for 30 min. In the presence of ROS, the DCFH-DA was de-esterified to form a measurable fluorescent product. The fluorescent intensity presenting in proportion to the intracellular ROS levels was read a using fluorescent microplate reader with an excitation wavelength of 480 nm and an emission wavelength of 525 nm (Banjerdpongchai and Khaw-On, 2013).
Statistical analysis
Data were presented as mean ± standard deviation (SD) of three independent experiments. Statistical analysis was analyzed by Prism version 6.0 software using one-way ANOVA with Dunnett’s test and applied for multiple comparisons at the P < 0.05, P < 0.01, P < 0.001 levels.
RESULTS
Phytochemical constituents of PSE and PSME
Extraction of perilla seed and seed meal by 70% ethanol yielded 5.26% for PSE and 8.08% for PSME. Total phenolic contents of PSE and PSME was 211.36 ± 1.5 and 27.96 ± 6.0 mg GAE/g extract, respectively. Total flavonoid content was found to be 346.86 ± 9.6 and 2.97 ± 0.22 mg CE/g extract for PSE and PSME, respectively. The hydrophilic phytochemical contents of PSE and PSME were analyzed in Table 2. The major constituent of PSE and PSME shown in the HPLC chromatogram was rosmarinic acid (Figure 1). Small contents of apigenin and luteolin were also detected in PSE and PSME.
Table 2. The phytochemical contents in PSE and PSME.
|
Extracts |
Hydrophilic phytochemical contentsa |
||
|
Rosmarinic acid |
Apigenin |
Luteolin |
|
|
PSE |
37.4 ± 1.3 |
2.4 ± 0.1 |
8.3 ± 1.3 |
|
PSME |
37.4 ± 1.2 |
2.5 ± 0.1 |
6.0 ± 0.1 |
|
Concentration ratiob |
1.0 |
1.0 |
1.4 |
Note: amg/g extract, bConcentration ratio: Value from PSE/value from PSME
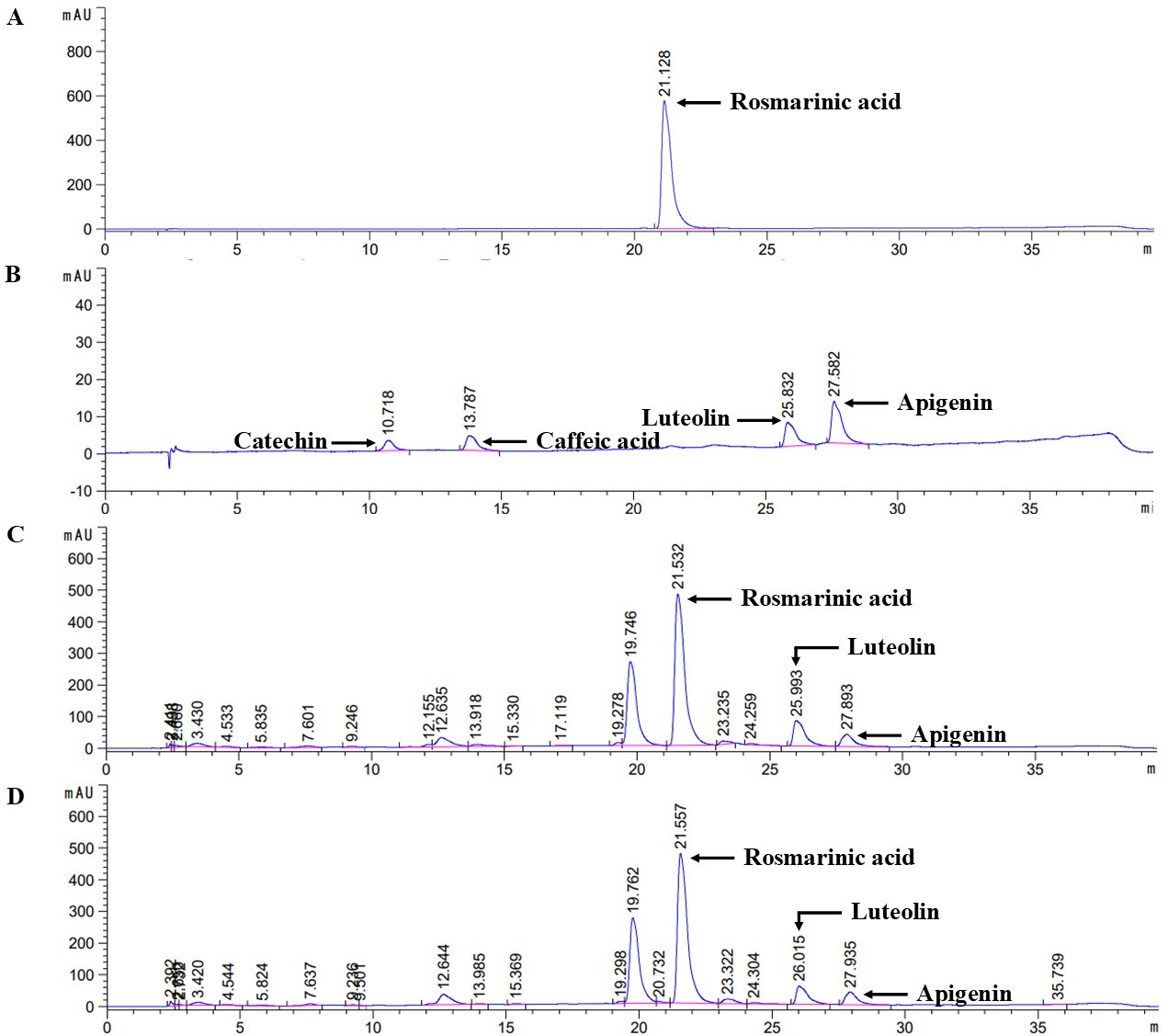
Figure 1. The HPLC chromatogram of (A) standard rosmarinic acid, (B) standard mix (catechin, caffeic acid, luteolin and apigenin), (C) PSE and (D) PSME
Antioxidant capacities of PSE and PSME
The antioxidant activity determination demonstrated that PSE and PSME could scavenge DPPH radicals in dose-dependent manner and the SC50 of PSE and PSME was 114.46 ± 15.37 and 130.43 ± 17.92 µg/ml, respectively. Similarly, PSE and PSME could inhibit the stable ABTS•+ radicals with an SC50 of 20.07 ± 4.14 and 21.40 ± 2.26 µg/ml, respectively. In addition, the spectral analysis of the iron-PSE extract complexes exhibited the maximum absorption at 255, 368 and 600 nm whereas the maximum absorption of iron-PSME extract complexes revealed at 260, 360 and 675 nm (the respective major and minor peaks as shown in Figure 2). The absorbance of complexes was increased in a dose-dependent manner (0–400 µM Fe3+-NTA).
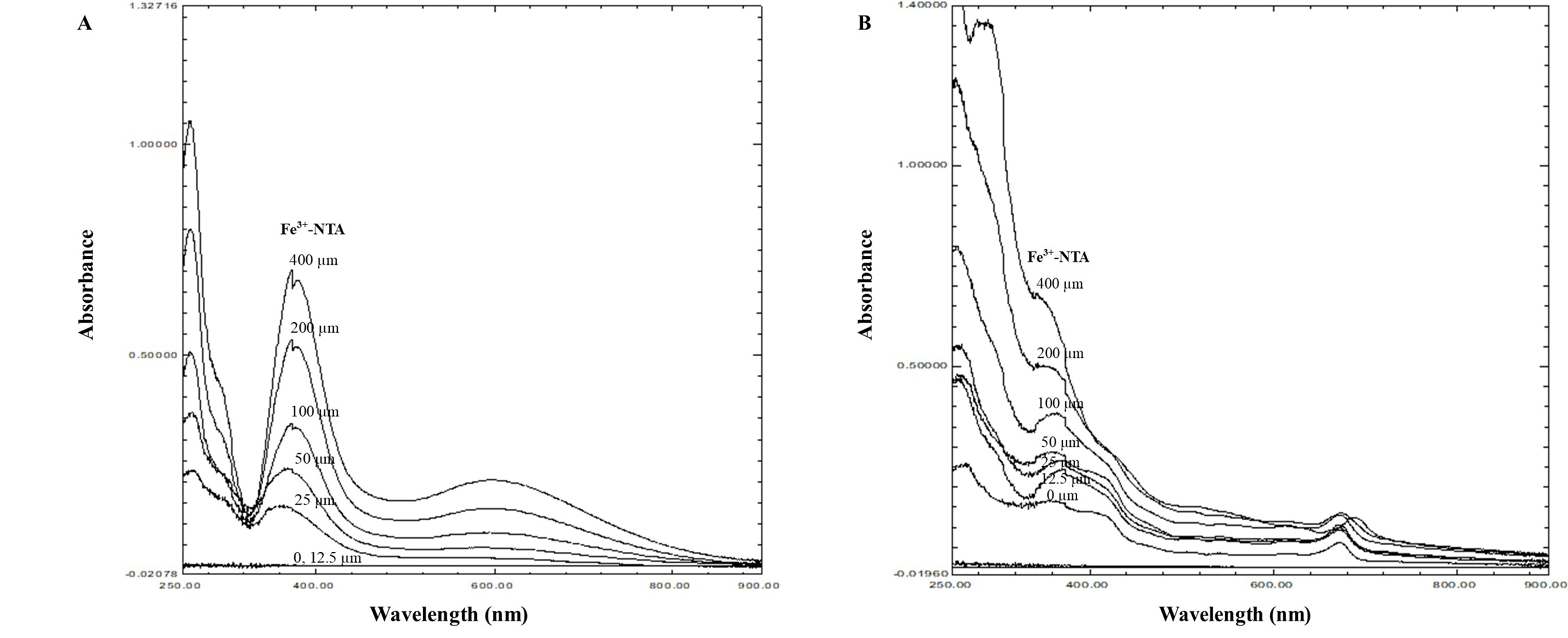
Figure 2. The spectral analysis of the iron-PSE complex (A) and iron-PSME complex (B) resulting from complexation of Fe3+-NTA (0-400 µM)
Cytotoxicity of PSE and PSME on TNF-α-treated A549 cells
To examine the cytotoxic effect of PSE and PSME, A549 cells were co-treated with different concentrations of the extracts (0-200 µg/ml) and 100 ng/ml TNF-α for 24 h compared with non-treated cells, and the cell viability was determined by MTT assay. PSE and PSME at concentrations of up to 200 µg/ml with 100 ng/ml TNF-α did not affect the cell viability of A549 cells (Figure 3). The results indicated that the concentrations of PSE and PSME up to 200 µg/ml and 100 ng/ml TNF-α were non-toxic to cells and could be used in the following experiments.
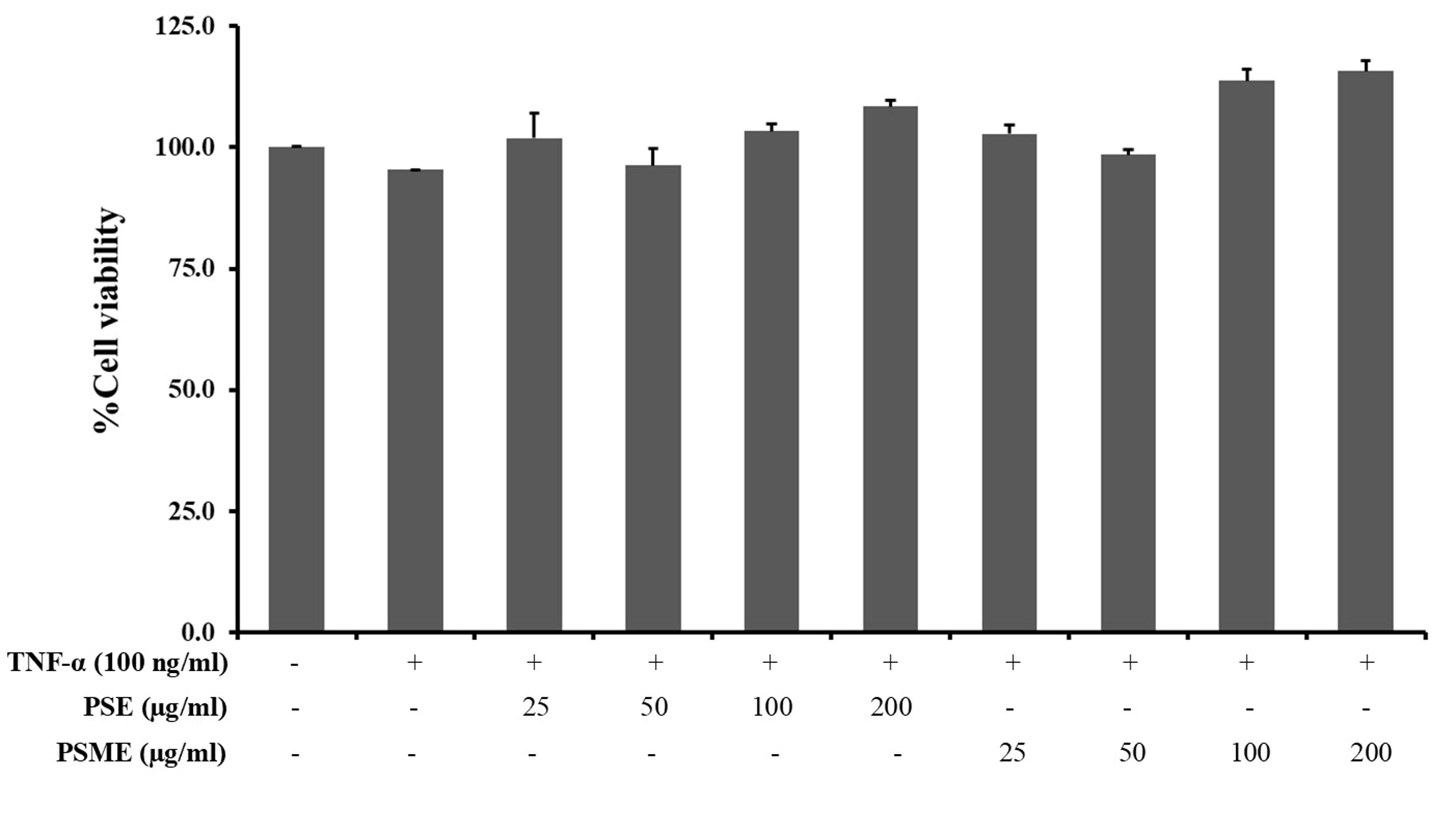
Figure 3. The cytotoxic effect of PSE and PSME on TNF-α-treated A549 cells. Data were represented as a percentage of viable cells relative to the control. All data are expressed as the mean ± SD of three independent experiments.
Effect of PSE and PSME on inflammatory cytokine gene expression in TNF-α-treated A549 cells
The inhibitory effect of PSE and PSME on inflammatory cytokine mRNA expressions, including IL-1β, IL-6, IL-8 and TNF-α in TNF-α-treated A549 cells was determined using RT-qPCR technique (Figure 4). PSE significantly inhibited the mRNA expression of IL-1β, IL-6, IL-8 and TNF-α in TNF-α-treated A549 cells. In addition, PSME could significantly decrease IL-1β, IL-6 and IL-8 expression. While the mRNA level of TNF-α in TNF-α-treated A549 cells was not significantly changed by PSME treatment.
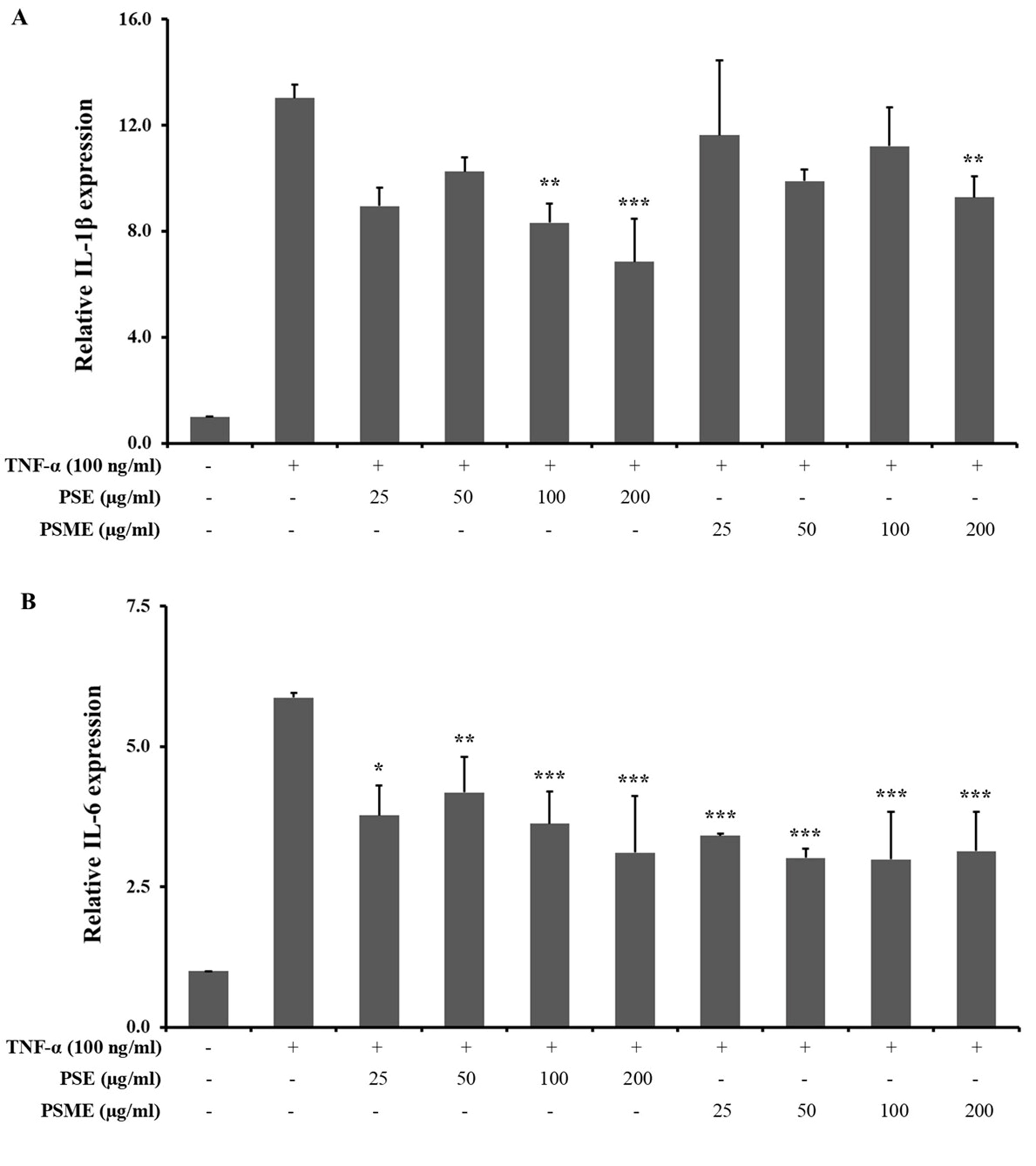
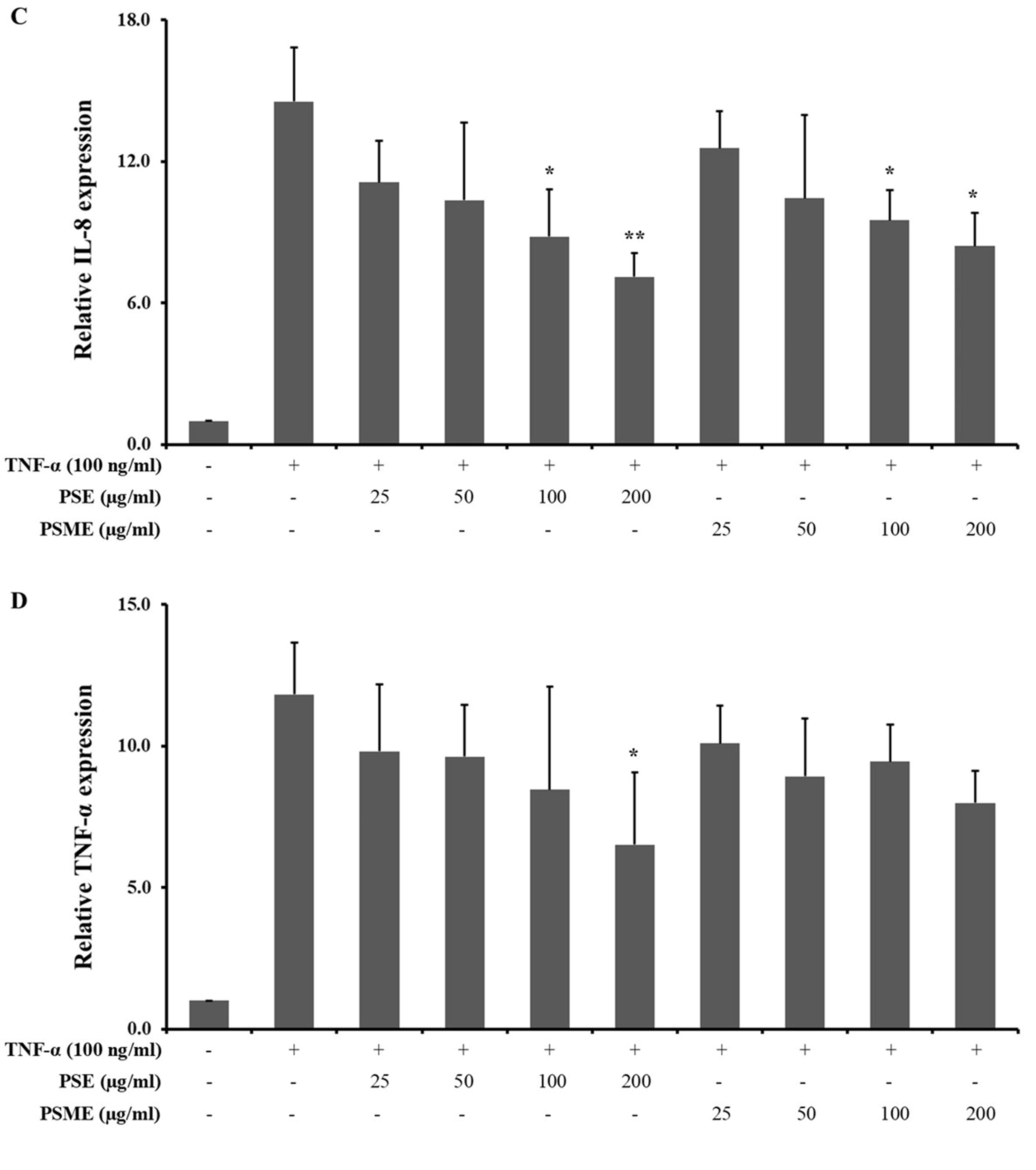
Figure 4. The effect of PSE and PSME on the inflammatory cytokine mRNA expression. Bar graph revealed the relative mRNA expression of (A) IL-1β, (B) IL-6, (C) IL-8, and (D) TNF-α in TNF-α-treated A549 cells after treated with PSE and PSME at indicated concentrations. All data are expressed as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. TNF-α-treated alone.
Effect of PSE and PSME on pro-inflammatory enzyme gene expression in TNF-α-treated A549 cells
The inhibitory effect of PSE and PSME on pro-inflammatory enzyme mRNA expressions, including iNOS and COX-2 in TNF-α-treated A549 cells was determined using the RT-qPCR technique. All concentrations of PSE and PSME were not significantly changed the mRNA expression iNOS and COX-2 in TNF-α-treated A549 cells (as shown in Figure 5).
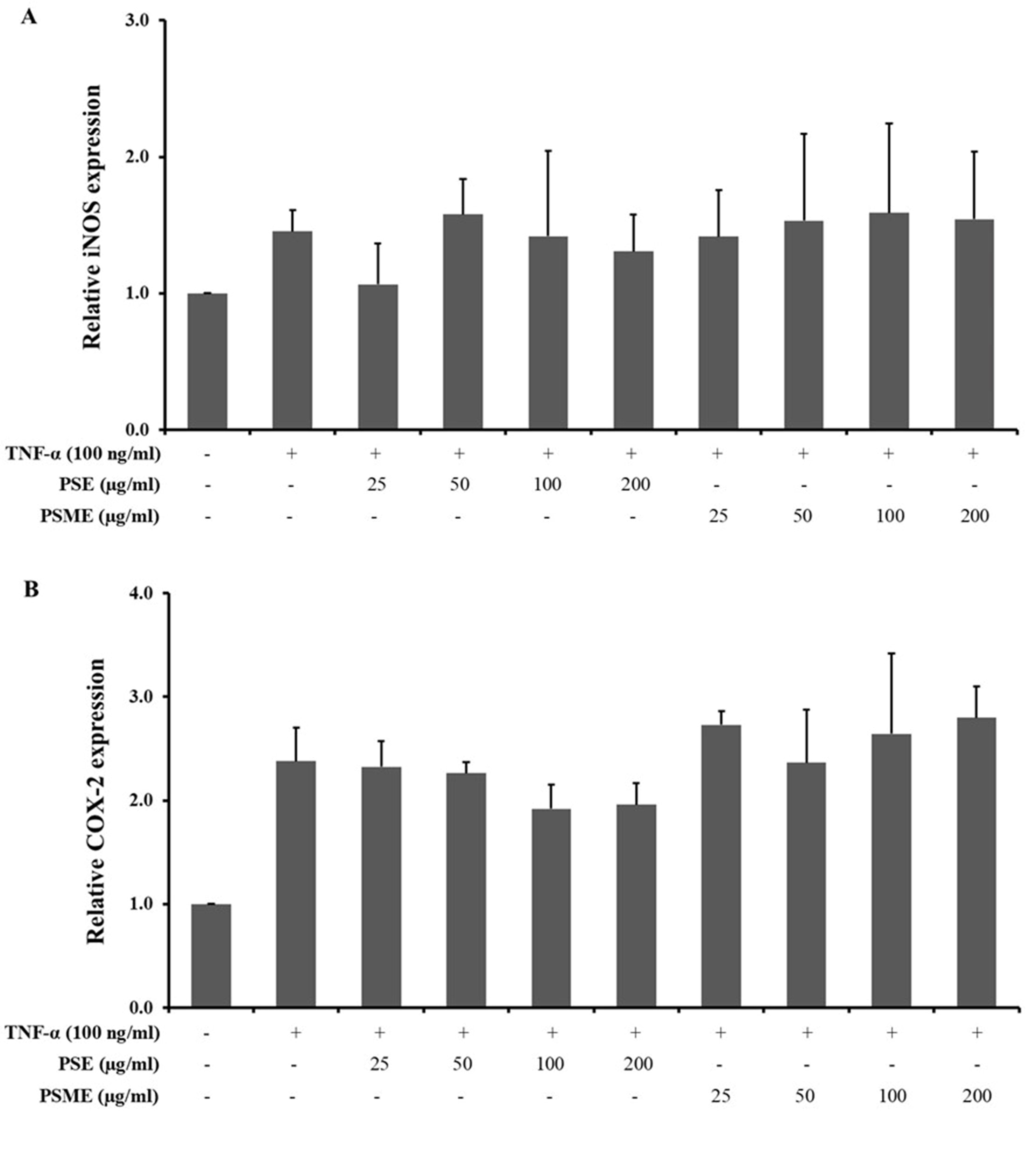
Figure 5. The effect of PSE and PSME on the pro-inflammatory enzyme mRNA expression. Bar graph revealed the relative mRNA expression of (A) iNOS, and (B) COX-2 in TNF-α-treated A549 cells after treatment with PSE and PSME at indicated concentrations. All data are expressed as the mean ± SD of three independent experiments.
Effect of PSE and PSME on TNF-α-induced intracellular reactive oxygen species production in A549 cells
An intracellular ROS was significantly induced in A549 cells after treatment with 100 ng/ml TNF-α. In the presence of PSE or PSME (50-200 µg/ml), the relative levels of intracellular ROS were reduced in a dose-dependent manner when compared to the TNF-α-treated alone. The significant ROS reduction results were shown when treated with the PSE and PSME at 100-200 µg/ml.
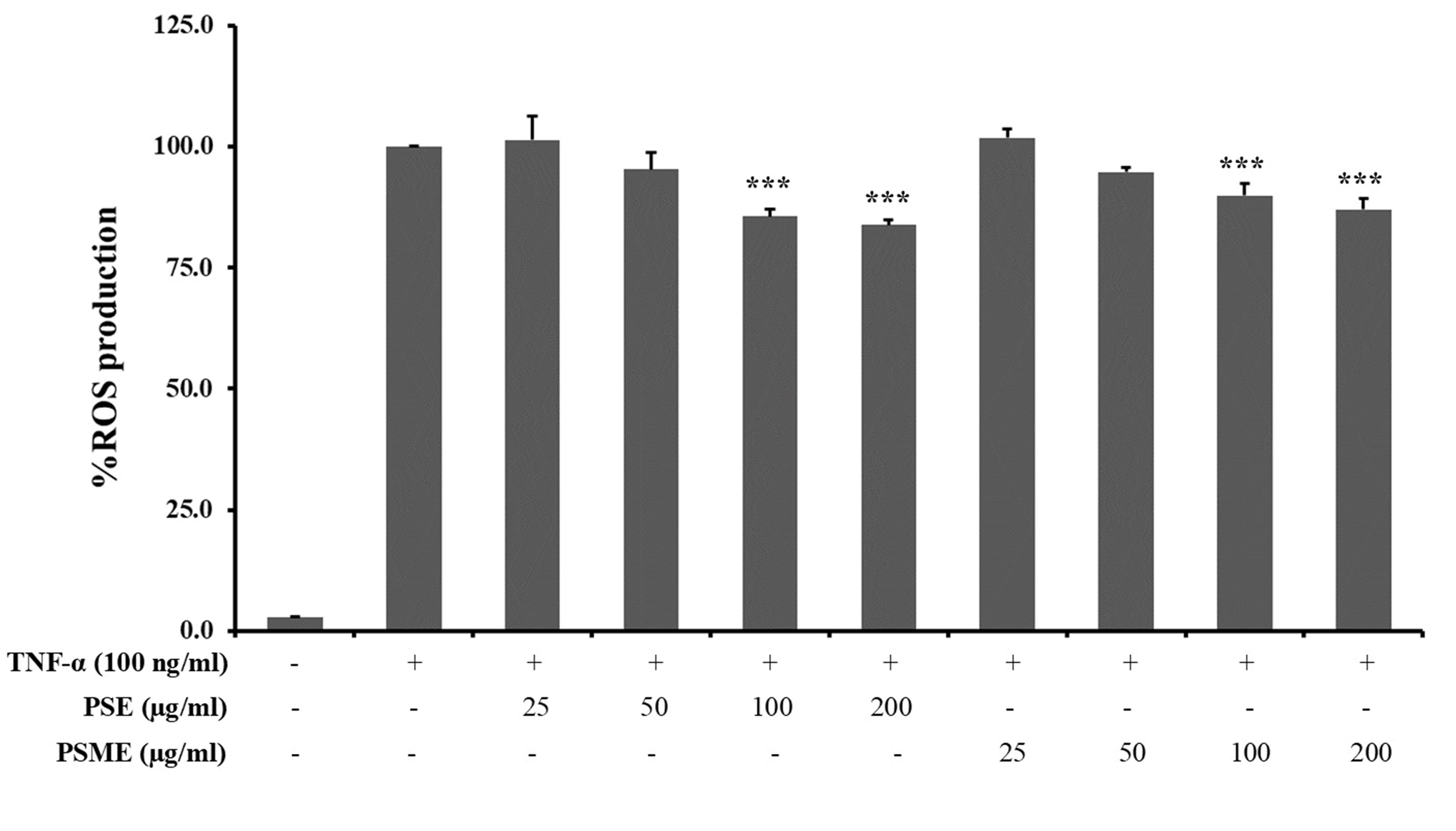
Figure 6. The effect of PSE and PSME on intracellular ROS production in TNF-α-treated A549 cells. The bar graph reveals the percentage of ROS production in TNF-α-treated A549 cells after treatment with PSE and PSME at indicated concentrations. All data are expressed as the mean ± SD of three independent experiments. ***P < 0.001 vs. TNF-α-treated alone.
DISCUSSION
In terms of the stimulation of lung inflammation, TNF-α is a conventional pro-inflammatory cytokine with an important function. Monitoring and servicing of neutrophilic airway inflammation and systemic symptoms contributing to lung tissue damage has been linked with the inappropriate release or spillover of TNF-α. Mechanistically, TNF-α plays a crucial role in initiating and exacerbating the inflammatory response by stimulating the inflammatory signaling pathways in alveolar epithelial cells, such as NF-κB and MAPKs (Takabatake et al., 2000; Pitsiou et al., 2002; Antoniu et al., 2008). Through inflammatory cell chemotaxis and inflammatory mediator release, alveolar epithelial cells can enhance inflammatory signals, triggering the release of multiple pro-inflammatory mediators such as IL-1β, IL-6, IL-8, TNF-α, iNOS and COX-2 (Hardie et al., 2004). These mediators represent major carcinogenic activities, including their ability to develop reactive oxygen and nitrogen species, their possible mutagenic influence and their role in epithelial mesenchymal transformation, angiogenesis and metastasis processes. Consequently, the attenuation of inflammatory responses induced by TNF-α may yield important therapeutic results (Wang et al., 2016).
The inhibitory influence of perilla seed and seed meal extract on TNF-α-induced A549 cells has been exhibited in the current study. Perilla is a herbal medicine regularly used and cultivated in nations throughout south-east Asia. It has been used for the prevention of asthma, colds, allergies and other human disorders (Kamei et al., 2017). As an outstanding source of ꞷ-3 essential fatty acids, and phytochemical bioactive compounds such as rosmarinic acid, apigenin, luteolin and glycosides, perilla seed is also noteworthy (Asif, 2011). The cold-pressed perilla seed oil operation, however, has produced lots of perilla seed meal by-products that have usually been disposed of after oil extraction. Nevertheless, several analyses have revealed that high-quality digestible proteins and a large amount of phytochemicals are still found in the perilla seed meal (Longvah and Deosthale, 1991; Zhu and Fu, 2012; Tang et al., 2014; Zhou et al., 2017).
The phytochemical substance of HPLC techniques from this study indicated that the bioactive components of PSE and PSME are identical. Rosmarinic acid and modest quantities of apigenin and luteolin were the key components. In addition, the rosmarinic acid, apigenin and luteolin levels in PSE and PSME were quantitatively similar at a concentration ratio of about 1. The same content of rosmarinic acid in PSE and PSME in this study was consistent with previous study that the content of rosmarinic acid in 70% ethanolic extract of PSE and PSME were 158.8 mg/g extract and 141.2 mg/g extract, respectively with the concentration ratio of 1.1 (Chumphukam et al., 2018). In contrast, the percent yield, rosmarinic acid, apigenin and luteolin of PSE and PSME had different value comparing to previously study. The difference of nutrition values, phytochemical and biologically active constituents of each PSE and PSME depend on many factors such as moisture content (Kongkeaw et al., 2015), maturation period, harvesting time, ripening stage of the seed, environmental conditions, cultivation climate, season and location (Siriamornpun et al., 2006; Kang and Lee, 2011; Radácsi, 2017).
When matched with PSE based on the DPPH and ABTS assay, the antioxidant activities of PSME were identical. Moreover, this research likewise reinforced that, by disrupting the ferric nitrilotriacetate complex, PSE and PSME exhibit an iron-binding property. These data verified the influential iron-binding activity of PSE and PSME, which could inhibit the formation of hydroxyl radicals (•HO) from reactive iron through the Fenton reaction (Lin et al., 2014). Even so, the concentration in PSME of total phenolic and flavonoid compounds was lower than PSE as bioactive compounds were discarded during the cold-press process to perilla seed oil.
Other pro-inflammatory mediators, such as interleukin, iNOS, and COX-2, which regulate cell proliferation, angiogenesis, tumor promotion and metastasis, may be induced by the overproduction of TNF through use of NF- κB activation (Liu et al., 2017). As such, this study assessed whether PSE and PSME have the requisite ability to suppress pro-inflammatory mediator expression in A549 TNF-α-induced cells. It was found that PSE and PSME could substantially reduce the expression of IL-1β, IL-6, IL-8, and TNF-α, but did not affect the expression of iNOS and COX-2 mRNA. By limiting interleukin and TNF-α expression, the PSE and PSME were also able to mitigate TNF-α-mediated inflammatory responses. These results followed Chumphukam et al. in that PSE and PSME substantially decreased the expression of LPS-treated RAW 264.7 cells by IL-6 and TNF-α mRNA (Chumphukam et al., 2018).
The DNA of epithelial cells could be impaired by continuous NF-κB activity stimulating the creation of reactive oxygen species. Preserving the balance of cellular ROS production at manageable levels may be demanding. This research therefore investigated whether PSE and PSME have enough power to restrict ROS overproduction. PSE and PSME at non-toxic levels were able to decrease TNF-α-induced ROS in A549 cells in the sample used. Past research by Chumphukam et al. (2018), which revealed that PSE and PSME decreased ROS production in LPS-induced RAW 264.7 cells (Chumphukam et al., 2018), also confirmed these findings.
Remarkably, PSME obviously exhibited in vitro anti-inflammatory and antioxidant activities similar to PSE. The quantity of bioactive compounds in PSME, critical features for its bioactivity, may be the cause. Therefore, PSE and PSME may be regarded as biologically-safe and highly-nutritious sources of natural anti-inflammatory and antioxidant activities that might be developed as food supplements for health promotion. To afford additional comprehension of the causal unique beneficial health effects, more research is necessary concerning the insight underlying biochemical mechanism.
CONCLUSION
This study revealed the attenuation of TNF-α-mediated inflammatory responses with perilla seed by its anti-inflammatory and antioxidant activities. In addition, the perilla seed meal, by-product of a perilla seed oil cold-pressed extraction process, could reduce inflammatory responses, like perilla seed, due to its bioactive compounds and bioactivities. Thus, perilla seed and perilla seed meal might be developed as food supplements or functional foods for the prevention of inflammation-induced lung carcinogenesis development.
ACKNOWLEDGMENTS
The authors wish to acknowledge the School of Medical Sciences, University of Phayao, the Faculty of Medicine, Chiang Mai University, and the School of Traditional and Alternative Medicine, Chiang Rai Rajabhat University for the use of their facilitates and support.
AUTHOR CONTRIBUTIONS
Chakkrit Khanaree assisted in conducting the experiments, performed the statistical analysis and data visualization and wrote the manuscript. Komsak Pintha and Payungsak Tantipaiboonwong prepared plant material and extracted PSE and PSME. Maitree Suttajit and Teera Chewonarin designed and conducted all of the experiments. Kanjana Pangjit performed the iron binding assay. Wanisa Punfa conducted all of the experiments and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICTS OF INTEREST
There are no conflicts of interest.
REFERENCES
Antoniu, S.A., Mihaltan, F., and Ulmeanu, R. 2008. Anti-TNF-alpha therapies in chronic obstructive pulmonary diseases. Expert Opinion on Investigational Drugs. 17: 1203-1211.
Asif, M. 2011. Health effects of omega-3,6,9 fatty acids: Perilla frutescens is a good example of plant oils. Oriental Pharmacy and Experimental Medicine. 11: 51-59.
Banjerdpongchai, R., and Khaw-On, P. 2013. Terpinen-4-ol induces autophagic and apoptotic cell death in human leukemic HL-60 cells. Asian Pacific Journal of Cancer Prevention. 14: 7537-7542.
Calcinotto, A., Grioni, M., Jachetti, E., Curnis, F., Mondino, A., Parmiani, G., et al. 2012. Targeting TNF-alpha to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. Journal of Immunology. 188: 2687-2694.
Chumphukam, O., Pintha, K., Khanaree, C., Chewonarin, T., Chaiwangyen, W., Tantipaiboonwong, P., et al. 2018. Potential anti-mutagenicity, antioxidant, and antiinflammatory capacities of the extract from perilla seed meal. Journal of Food Biochemistry. 42: e12556.
Comer, D.M., Kidney, J.C., Ennis, M., and Elborn, J.S. 2013. Airway epithelial cell apoptosis and inflammation in COPD, smokers and nonsmokers. European Respiratory Journal. 41: 1058-1067.
Hardie, W.D., Lecras, T.D., Jiang, K., Tichelaar, J.W., Azhar, M., and Korfhagen, T.R. 2004. Conditional expression of transforming growth factor-alpha in adult mouse lung causes pulmonary fibrosis. American Journal of Physiology Lung Cellular and Molecular Physiology. 286: 741-749.
Kamei, R., Fujimura, T., Matsuda, M., Kakihara, K., Hirakawa, N., Baba, K., et al. 2017. A flavanone derivative from the Asian medicinal herb (Perilla frutescens) potently suppresses IgE-mediated immediate hypersensitivity reactions. Biochemical and Biophysical Research Communications. 483: 674-679.
Kang, N.S., and Lee, J.H. 2011. Characterisation of phenolic phytochemicals and quality changes related to the harvest times from the leaves of Korean purple perilla (Perilla frutescens). Food Chemistry. 124: 556-562.
Knobloch, J., Lin, Y., Konradi, J., Jungck, D., Behr, J., Strauch, J., et al. 2013. Inflammatory responses of airway smooth muscle cells and effects of endothelin receptor antagonism. American Journal of Respiratory Cell and Molecular Biology. 49: 114-127.
Kongkeaw, S., Riebroy, S., and Chaijan, M. 2015. Comparative studies on chemical composition, phenolic compounds and antioxidant activities of brown and white perilla (Perilla frutescens) seeds. Chiang Mai Journal of Science. 42: 896-906.
Lin, E.S., Li, C.C., and Chou, H.J. 2014 Evaluation of the antioxidant and antiradical activities of perilla seed, leaf and stalk extracts. Journal of Medical Plant Research. 8: 109-115.
Liu, T., Zhang, L., Joo, D., and Sun, S. 2017. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy. 2: 17023.
Longvah, T., and Deosthale, Y.G. 1991. Chemical and nutritional studies on Hanshi (Perilla frutescens), a traditional oil seed from Northeast India. Journal of the American Oil Chemists’ Society. 68: 781–784.
Matera, M.G., Calzetta, L., and Cazzola, M. 2010. TNF-alpha inhibitors in asthma and COPD: we must not throw the baby out with the bath water. Pulmonary Pharmacology and Therapeutics. 23: 121-128.
Min, B., McClung, A.M., and Chen, M.H. 2011. Phytochemicals and antioxidant capacities in rice brans of different color. Journal of Food Science. 76: C117-126.
Phannasorn, W., Khanaree, C., Wongnoppavich, A., and Chewonarin, T. 2017. The effect of purple rice (Oryza sativa L. indica) extract on the inflammatory response in a colon cancer cell line and dextran sulfate-induced tumor promotion in the rat colon. Molecular and Cellular Toxicology. 13: 433-442.
Pintha, K., Yodkeeree, S., Pitchakarn, P., and Limtrakul, P. 2014. Anti-invasive activity against cancer cells of phytochemicals in red jasmine rice (Oryza sativa L.). Asian Pacific Journal of Cancer Prevention. 15: 4601-4607.
Pitsiou, G., Kyriazis, G., Hatzizisi, O., Argyropoulou, P., Mavrofridis, E., and Patakas, D. 2002. Tumor necrosis factor-alpha serum levels, weight loss and tissue oxygenation in chronic obstructive pulmonary disease. Respiratory Medicine. 96: 594-598.
Radácsi, P., Sárosi, S., Szomor, L.A., and Németh-Zámbori, E. 2017. Comparison of the production and chemical constituents of five Perilla frutescens (L.) Britt. accessions. Acta Biologica Hungarica. 68: 453-465.
Scapin, G., Vendruscolo, R.G., Wagner, R., da Rosa, C.S., Abaide, E.R., Martins, R.F., et al. 2017. Quality of perilla oil (Perilla frutescens) extracted with compressed CO2 and LPG. The Journal of Supercritical Fluids. 130: 176–182.
Siriamornpun, S., Li, D., Yang, L., Suttajit, S., and Suttajit, M. 2006. Variation of lipid and fatty acid compositions in Thai Perilla seeds grown at different locations. Songklanakarin Journal of Science and Technology. 28: 17-21.
Takabatake, N., Nakamura, H., Abe, S., Inoue, S., Hino, T., Saito, H., et al. 2000. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 161: 1179-1184.
Tang, W., Sun, B., and Zhao, Y. 2014. Preparative separation and purification of rosmarinic acid from perilla seed meal via combined column chromatography. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 1: 947-948:41–48.
Tipsuwan, W. and Chaiwangyen, W. 2018. Preventive effects of polyphenol-rich perilla leaves on oxidative stress and haemolysis. ScienceAsia. 44: 162-169.
Wang, W., Guan, W., Huang, R., Xie, Y., Zheng, J., Zhu, S., et al. 2016. Carbocisteine attenuates TNF-α-induced inflammation in human alveolar epithelial cells in vitro through suppressing NF-κB and ERK1/2 MAPK signaling pathways. Acta Pharmacologica Sinica. 37: 629–636.
Wedzicha, J.A. 2002. Exacerbations: etiology and pathophysiologic mechanisms. Chest Journal. 121: 136-141S.
Woo, C.H., Eom, Y.W., Yoo, M.H., You, H.J., Han, H.J., Song, W.K., et al. 2000. Tumor necrosis factor-alpha generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. The Journal of Biological Chemistry. 275: 32357-32362.
Wu, Q., Er-Bu, A., Liang, X., Luan, S., He, C., Yin, L., et al. 2020. Determination of the main naphthoquinones in Onosma hookeri Clarke. var. longiforum Duthie and its optimization of the ultrasound-assisted extraction using response surface methodology. Journal of Food Science. 86: 357-365.
Zhang, Y., Mei, X., Zhang, Q., Li, M., Wu, X., and Ma, C. 2018. Protective effect of a rosmarinic acid–rich extract from cold-pressed Perilla frutescens seed flour on oxidative hepatotoxicity in vitro and in vivo. Journal of Food Biochemistry. 42: e12492.
Zhou, X.J., Yan, L.L., Yin, P.P., Shi, L.L., Zhang, J.H., Liu, Y.J., et al. 2014. Structural characterization and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chemistry. 164: 150–157.
Zhu, J. and Fu, Q. 2012. Optimization of ultrasound-assisted extraction process of perilla seed meal proteins. Food Science and Biotechnology. 21: 1701–1706.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Chakkrit Khanaree1, Wanisa Punfa1, Payungsak Tantipaiboonwong2, Maitree Suttajit2, Teera Chewonarin3, Kanjana Pangjit4, and Komsak Pintha2,*
1 School of Traditional and Alternative Medicine, Chiang Rai Rajabhat University, Chiang Rai 57100, Thailand
2 Division of Biochemistry, School of Medical Sciences, University of Phayao, Phayao 56000, Thailand
3 Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand
4 College of Medicine and Public Health, Ubon Ratchathani University, Ubon Ratchathani 34190, Thailand
Corresponding author: Komsak Pintha, E-mail: komsakjo@gmail.com
Total Article Views
Editor: Nisit Kittipongpatana,
Chiang Mai University, Thailand
Article history:
Received: March 29, 2021;
Revised: April 21, 2021;
Accepted: May 11, 2021;

