
Investigation of Excellent ACE Inhibitor Agents from Scurrula atropurpure and Dendrophthoe pentandra for Anti-Hypertension
Nour Athiroh Abdoes Sjakoer*, Nurul Jadid Mubarakati, and Ahmad TaufiqPublished Date : 2021-04-28
DOI : https://doi.org/10.12982/CMUJNS.2021.068
Journal Issues : Number 3, July-September 2021
Abstract The objective of this study was to investigate the bioactivity, toxicity, and interaction of the prepared bioactive compounds from Scurrula atropurpurea and Dendrophthoe pentandra with angiotensin-converting enzyme (ACE) macromolecule via an in-silico route. The bioactivity was investigated through Way2Drug PASS Online program. The drug-likeness property and human intestinal absorption of the bioactive compounds were evaluated through absorption, distribution, metabolism, and excretion (ADME) analysis. The interaction of bioactive compounds with ACE was examined via a molecular-specific docking analysis. The crystal structure of ACE was evaluated using the protein database 1086 chain A. The results elucidated that the prepared compounds exhibited potential bioactive agents for vasodilation, vasoprotective, and cardioprotective applications based on PASS analysis. The ADME analysis also revealed that the flavonol 3-O-D-glucoside did not violate any of the Lipinski's rule and had high gastrointestinal absorption. Furthermore, the results also suggested that the flavonol 3-O-D-glucoside of mistletoes had a harmless LD50 but was not recommended to be used for a long period. The molecular docking revealed that the flavonol 3-O-D-glucoside had the lowest binding energy with a value of −8.3 kcal.mol−1, suggesting the potential inhibitor for ACE. Generally, the toxicity and molecular docking analysis showed the compounds inhibited the ACE macromolecule with a series of flavonol > kaempferol > casticin > quercetin > quercitrin > isoquercitrin. Therefore, the S. atropurpurea and D. pentandra from Indonesian natural resources open new excellent potency for traditional herbal medicines, especially to treat hypertension.
Keywords: ACE inhibitor, D. pentandra, hypertension, S. atropurpurea, traditional herbal
Funding: This study is funded by The Ministry of Research and Technology/National Research and Innovation Agency (KEMENRISTEK/BRIN) from 2019-2020 through Penelitian Terapan Unggulan Perguruan Tinggi (PTUPT) scheme with contract number 018/SP2H/AMD/LT/MULTI/L7/2020.
Citation: Sjakoer, N.A.A., Mubarakati, N.J., and Taufiq, A. 2021. Investigation of excellent ACE inhibitor agents from Scurrula atropurpurea and Dendrophthoe pentandra for Anti-Hypertension. CMUJ. Nat. Sci. 20(3): e2021068
INTRODUCTION
In the last decades, hypertension has become one of the most medical problems in many countries, including in Indonesia. Hypertension is a non-transferrable disease that becomes one of the early death causes globally. World Health Organization (WHO) estimated that the prevalence of hypertension is 22% of the global population. Less than 20% of them have done measures to control the blood pressure. Southeast Asia is in 3rd place with a 25% prevalence of its total population. WHO estimated that 1 in 5 women around the world has hypertension. The number is bigger in males, which is 1 in 4 (WHO, 2019). Hypertension is the most generally chronic disease that affects approximately one billion individuals; this disease is associated with chronic conditions that often increase the risks of stroke, diabetes mellitus, chronic kidney failure, and heart problems as the main causes of death (Winata, 2020).
To treat hypertension, several methods or treatments have been developed by many medical experts. One of the essential therapeutic methods that have been used is angiotensin-converting enzyme (ACE) inhibitor related to its advantages.
Theoretically, ACE plays an essential role in the regulation of blood pressure. This enzyme can hydrolyze angiotensin I into angiotensin II vasoconstrictor and deactivate the bradykinin vasodilator. The inhibition of ACE activity is deemed to be an essential therapeutic approach to treat hypertension. The currently available ACE inhibitors include captopril, enalapril, lisinopril, and ramipril, which are administered as treatment of hypertension in humans. However, these synthetic drugs have several side effects, including dry cough, skin rashes, and angioneurotic edema (Attique et al., 2019). Therefore, safer and natural ACE inhibitors are essential and urgent to be developed for future treatment and hypertension prevention. One source of potential inhibitor comes from the biodiversity of medicinal plants, namely Scurrula known as benalu in Indonesia (Ohashi et al., 2003).
Empirically, benalu is a parasitic plant used as traditional herbal medicine. In the present study, we utilized two types of benalu that attack tea (Thea sinensis L.) and mango (Mangifera indica L.), namely, Scurrula atropurpurea (BL.) DANS. or “benalu teh” and Dendrophthoe pentandra L. miq. or “benalu mangga,” respectively. The whole plant (stems and leaves) of benalu has been traditionally used to treat various diseases, including hypertension in Java, Indonesia. Based on our previous work, the methanolic extract of S. atropurpurea (MESA) can modulate superoxide dismutase (SOD), diminish oxidative stress, and decrease systolic blood pressure (Athiroh et al., 2014a). Furthermore, it was also reported that the MESA sub-chronic introduced did not interfere with rat liver function as indicated through analysis of cellular enzyme activities, including aspartate transaminase, alanine transaminase, concentrations of albumin, globulin, and total protein (Athiroh et al., 2015a). The MESA also raised arterial nitric oxide and decreased pulmonary malondialdehyde (MDA) (Athiroh et al., 2015b), modulated total plasma nitrate/nitrite levels, and diminished endothelial damage via increasing endothelial progenitor cells (Athiroh et al., 2014a), and raised SOD activity and decreased MDA concentration in hypertensive rats (Athiroh et al., 2017)
The development of herbs as therapeutic agents that can prevent, reduce, or cure disease is a central notion in the field of human health. The discovery of new drugs through screening using experimental animals and humans requires an enormous cost and a long time. Anchored by information technology advancement, virtual screening or in silico can accelerate the search, identification, and analysis of active compounds as candidate drug compounds. In silico studies using molecular docking techniques include a method that can be used to predict the bioactivity of a compound before laboratory analysis. This method entails an advantage, among others, that is, reducing the use of excessive tools and materials and saving provisional costs (Sliwosky et al., 2014).
Several previous studies have shown that the bioactive compounds from S. atropurpurea have the potential as antihypertension natural medicines. Conversely, D. pentandra leaf extract contains numerous potential agents, such as flavonoids, polyphenols, and saponins, for cancer prevention. Flavonoid from D. pentandra is also a potential antioxidant and antihypertension agent and a therapeutic agent for several other diseases (Mustarichie et al., 2016). It has been reported that quercetin, quercetin-3-O-glucoside, quercitrin, and kaempferol from tea mistletoe can play roles as antihypertensive drugs (Athiroh et al., 2014a), whereas the active compounds from mango mistletoe include quercetin, casticin, a flavonol glycoside, and quercitrin (Mustarichie et al., 2016).
Nevertheless, studies on ACE inhibitory activity and predicting interaction with ACE of those compounds have not been extensively reported. Therefore, we examined such potentials and determined the cytotoxic activity, toxicity, and physicochemical properties of S. atropurpurea and D. pentandra bioactive compounds via an in-silico approach. The results of this study could elucidate the active compounds from the analyzed plants that could be an antihypertension agent.
MATERIALS AND MENTHODS
Proteins/macromolecules and ligands structural data retrieval
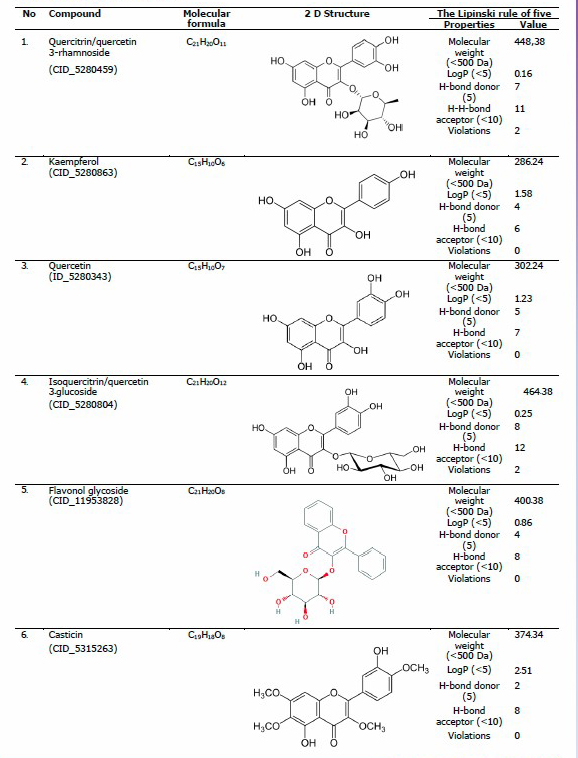
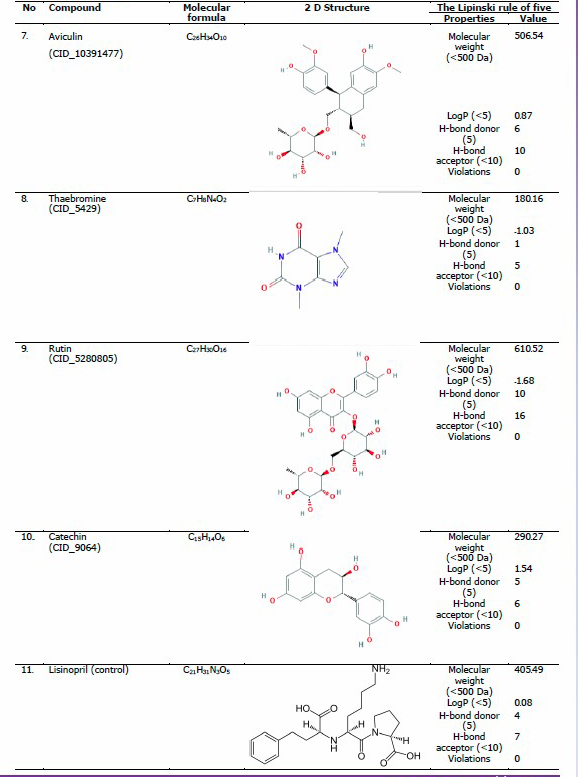
The crystal structure of ACE in complex with lisinopril was retrieved from Protein Data Bank (RSC Protein Data Bank, 2003) with the PDB ID of 1O86. PDB is a global repository of experimentally determined three-dimensional (3-D) structures of biological macromolecules. Additionally, the 3-D structures of ligands were obtained from PubChem (PubChem National Library of Medicine, 2017), in .sdf format. PubChem is an open chemistry database that contains information on chemical structures, identifiers, chemical and physical properties, biological activities, and bioassay, providing data to several million users worldwide. The compounds used in the present study were quercitrin/quercetin 3-rhamnoside (CID_5280459), kaempferol (CID_5280863), quercetin (CID_5280343), isoquercitrin/quercetin 3-glucoside (CID_5280804), flavonol 3-O-D-glucoside/flavonol glycoside (CID_11953828), casticin (CID_5315263), aviculin (CID_10391477), theobromine (CID_5429), rutin (CID_5280805), and cathecin (CID_9064).
Bioactivity prediction of bioactive compound from S. atropurpurea and D. pentandra
Each bioactive compound's biological activity in mistletoes including quercitrin/quercetin 3-rhamnoside, kaempferol, quercetin, isoquercitrin/quercetin 3-glucoside, flavonol 3-O-D-glucoside/flavonol glycoside, and casticin was predicted through the Way2Drug PASS Online (PubChem National Library of Medicine, 2017). The potential active mistletoe compound was determined by comparing the similarity index (Pa value) between their bioactive structure and potential recorded compound in the database via the Way2Drug PASS Online (Way2Drug, 2020). A potentially active compound was reviewed based on its Pa value describing the potential compound that was being tested. Additionally, the 3-D structure was then constructed using the Discovery Studio software (PubChem National Library of Medicine, 2020).
The Lipinski's rule of five for drug-likeness of ADME (absorption, distribution, metabolism, and excretion) analysis
Drug-likeness properties, including absorption, distribution, metabolism, and excretion of the selected potential inhibitors within the body, were calculated using SwissADME based on the Lipinski rule of five. Molecules with poor permeation and oral absorption proposed should have molecular weights of 500, logP of 5, more than five hydrogen-bond donors, and 10 acceptor groups based on Lipinski rule of five (Attique et al., 2019).
In-silico toxic risk prediction
A computational simulation study was conducted to estimate possible toxicity risks of the six major compounds of mistletoes (quercitrin/quercetin 3-rhamnoside, kaempferol, quercetin, isoquercitrin/quercetin 3-glucoside, a flavonol glycoside, and casticin). Toxicity tests were conducted using the Pro-Tox server (http://tox.charite.de/protox_II/). The evaluated toxic risks, including LD50, mutagenic, hepatoxic, immune-toxic, and carcinogenic effects, were interpreted and expressed in a flexible manner of positive (+) and negative (−) values.
Docking and visualization between protein and ligand
Molecular docking modeling between S. atropurpurea and D. pentandra active compounds and target proteins was conducted using Auto-Dock Vina with PyRx version 9.5. The docking procedure comprised of three stages of visualization: rigid body energy minimization, semi-flexible repair, and finishing refinement in explicit solvent. The docking results were then visualized with PyMol version 2.3.1, whereas the molecular docking results were visualized using Discovery Studio 4.1 and LigPlot 2.1. Interactions between protein and ligand were analyzed to determine the formed number and the type of chemical bonds.
RESULTS
We conducted an in-silico investigation (Table 1) to determine the major mistletoe compounds' biological activities based on prediction. In-silico investigation is a rapid and low-cost preliminary analysis to identify the potential candidate for drugs with the advantage of reducing animal toxicity testing. The prediction of activity spectra for substances (PASS) analysis was performed to predict those selected compounds' drug-likeness nature.
The PASS analysis showed that the major active compounds (quercitrin/quercetin 3-rhamnoside, kaempferol, quercetin, isoquercitrin/quercetin 3-glucoside, flavonol glycoside, and casticin) from S. atropurpurea and D. pentandra have six potential biological activities, namely, cardioprotective, free radical scavenging, vasoprotective, antioxidant, anti-inflammatory, and vasodilating effects; the antioxidant and free radical scavenging properties of all compounds were assumed to have a Pa score of more
than 0.7. Accordingly, we suggested the selected six major compounds from these mistletoes as the potential candidates for hypertension treatment based on an ethnopharmacological viewpoint.
The evaluation of the drug-likeness and determining certain pharmacological or biological activity through oral administration in humans for the selected compounds were conducted according to the Lipinski rule of five predicted using Swiss-ADME. This rule depends on simple physiochemical parameters of five, including the MW of <500 g/mol, lipophilicity (LogP) of <5, and the number of hydrogen bond donors and acceptors of <5 and <10, respectively. These parameters are associated with intestinal permeability and aqueous solubility, determining the first step of oral bioavailability. The selected ligands that did not violate more than two standards of the Lipinski rule are potential candidates for further analyses such as molecular docking experiments with the target protein. The results showed that some tested compounds in this study were informed with the Lipinski rule of five, excluding quercitrin and isoquercitrin, which have lower logP, HBD, and HBA values than others (Table 2).
Table 1. The bioactivity prediction of active compound from S. atropurpurea and D. pentandra through PASS server.
|
Major compound |
Main bioactivity-predicted properties |
Pa* |
Pi* |
|
Quercitrin/ |
Cardioprotective |
0.979 |
0.001 |
|
Quercetin 3-rhamnoside |
Free radical scavenging |
0.974 |
0.001 |
|
(CID_5280459) |
Vasoprotective |
0.966 |
0.001 |
|
|
Antioxidant |
0.915 |
0.003 |
|
|
Anti-inflammatory |
0.754 |
0.010 |
|
|
Vasodilating |
0.658 |
0.010 |
|
|
|
|
|
|
Kaempferol |
Antimutagenic |
0.948 |
0.001 |
|
(CID_5280863) |
Cardioprotective |
0.814 |
0.003 |
|
|
Free radical scavenging |
0.771 |
0.003 |
|
|
Vasoprotective |
0.807 |
0.005 |
|
|
Antioxidant |
0.856 |
0.003 |
|
|
Vasodilating |
0.658 |
0.021 |
|
|
|
|
|
|
Quercetin |
Cardioprotective |
0.833 |
0.003 |
|
(CID_5280343) |
Free radical scavenging |
0.811 |
0.003 |
|
|
Vasoprotective |
0.824 |
0.004 |
|
|
Antioxidant |
0.872 |
0.003 |
|
|
Hepatoprotective |
0.706 |
0.007 |
|
|
Vasodilating |
0.472 |
0.025 |
|
|
|
|
|
|
Isoquercitrin / |
Cardioprotective |
0.984 |
0.001 |
|
Quercetin 3-glucoside |
Free radical scavenging |
0.978 |
0.001 |
|
(CID_5280804) |
Vasoprotective |
0.947 |
0.002 |
|
|
Antioxidant |
0.913 |
0.003 |
|
|
Anti-inflammatory |
0.739 |
0.011 |
|
|
Vasodilating |
0.613 |
0.012 |
|
|
|
|
|
|
Flavonol 3-O-D-glucoside / |
Cardioprotective |
0.963 |
0.002 |
|
Flavonol Glycoside |
Free radical scavenging |
0.895 |
0.002 |
|
(CID_11953828) |
Vasoprotective |
0.919 |
0.002 |
|
|
Antioxidant |
0.839 |
0.003 |
|
|
Anti-inflammatory |
0.713 |
0.014 |
|
|
Vasodilating |
0.646 |
0.01 |
|
|
|
|
|
|
Casticin |
Cardioprotective |
0.698 |
0.004 |
|
(CID_5315263) |
Free radical scavenging |
0.845 |
0.002 |
|
|
Vasoprotective |
0.735 |
0.008 |
|
|
Antioxidant |
0.742 |
0.004 |
|
|
Anti-inflammatory |
0.714 |
0.014 |
|
|
Vasodilating |
0.581 |
0.015 |
|
Aviculin |
Cardioprotective |
0.482 |
0.012 |
|
(CID_10391477) |
Free radical scavenging |
0.557 |
0.007 |
|
|
Vasoprotector |
0.878 |
0.003 |
|
|
Antioxidant |
0.438 |
0.009 |
|
|
Anti-inflammatory |
0.653 |
0.022 |
|
|
Vasodilating |
0.541 |
0.035 |
|
Theobromine |
Cardioprotective |
0.197 |
0.194 |
|
(CID_5429) |
Vasoprotector |
0.309 |
0.147 |
|
|
Vasodilating |
0.602 |
0.013 |
|
Rutin |
Cardioprotective |
0.988 |
0.001 |
|
(CID_5280805) |
Free radical scavenging |
0.988 |
0.001 |
|
|
Vasoprotector |
0.980 |
0.001 |
|
|
Antioxidant |
0.923 |
0.003 |
|
Quercitrin/ |
Anti-inflammatory |
0.728 |
0.013 |
|
|
Vasodilating |
0.740 |
0.006 |
|
Catechin |
Cardioprotective |
0.647 |
0.005 |
|
(CID_9064) |
Vasoprotector |
0.652 |
0.014 |
|
|
Antioxidant |
0.810 |
0.003 |
|
|
Anti-inflammatory |
0.548 |
0.044 |
|
|
Cardioprotective |
0.647 |
0.005 |
|
|
Vasodilating |
0.350 |
0.054 |
Note : *Pa = probable activity; Pi = probable inactivity. The PASS prediction results were interpreted and used as follows: (i) only activities with Pa > Pi are deemed to be possible for a particular compound; (ii) if Pa > 0.7, there is a high chance of experimentally finding the activity.
Table 2. Properties of the ACE potential inhibitor from S. atropurpurea and D. pentandra compounds as anti-hypertension candidate.
The Pro-Tox server evaluated the toxic risks (LD50, mutagenic, hepatoxic, immune-toxic, and carcinogenic effects) for the selected compounds. The results showed that all compounds, except quercetin and quercitrin, have low toxicity probability (Table 3). The evaluation results of quercetin showed a high mutagenic and carcinogenic probability, low LD50 value, and medium risk for others. Thus, Flavonol glycoside presented the lowest risk based on all evaluated parameters.
Table 3. Toxicity prediction for the six major mistletoe compounds obtained via the Pro-Tox server
|
Compound |
Toxicity test |
|||||
|
LD50 (mg/kg) |
Toxicity class |
Mutagenicity |
Hepato toxicity |
Immuno-toxicity |
Carcinogenicity |
|
|
Quercitrin/quercetin 3-rhamnoside (CID_5280459) |
2300 |
5 |
− |
− |
+ |
+ |
|
Kaempferol (CID_5280863) |
3919 |
5 |
− |
− |
− |
− |
|
Quercetin (CID_5280343) |
159 |
3 |
+ |
− |
− |
+ |
|
Isoquercitrin/quercetin 3-glucoside (CID_5280804) |
5000 |
5 |
− |
− |
+ |
− |
|
Flavonol glycoside (CID_11953828) |
5000 |
5 |
− |
− |
− |
− |
|
Casticin (CID_5315263) |
5000 |
5 |
− |
− |
+ |
− |
|
Aviculin(CID_10391477) |
823 |
4 |
_ |
_ |
+ |
_ |
|
Theobromine(CID_5429) |
837 |
4 |
_ |
_ |
_ |
_ |
|
Rutin(CID_5280805) |
5000 |
5 |
_ |
_ |
+ |
_ |
|
Catechin(CID_9064) |
10000 |
6 |
_ |
_ |
_ |
_ |
|
Lisinopril (control) |
8500 |
6 |
− |
− |
− |
− |
In the present study, a silico molecular docking analysis was performed to predict the potency of activity and interaction between ACE macromolecule with the isolated active compounds of mistletoe as ligand molecules lisinopril as a control. All selected compound candidates were predicted to have a relatively low binding affinity with ACE; the list of compounds showing from the lowest binding affinity score in the sequence includes quercitrin (−9.3 kcal/mol), isoquercitrin (−8.6 kcal/mol), quercetin (−8.3 kcal/mol), flavonol glycoside (−8.3 kcal/mol), kaempferol (−8.1 kcal/mol), casticin (−7.5 kcal/mol), aviculin (−8.1 kcal/mol), Theobromine (−5.8 kcal/mol), Rutin (−9.7 kcal/mol), and Catechin (−8.3 kcal/mol) (Table 3). Most of the compounds, excluding kaempferol and casticin, have a lower binding affinity than lisinopril as a control (8.2 kcal/mol), indicating to be potential candidates as ACE inhibitors, since the lower the binding affinity value of the active compound (ligand), the stronger the interaction that occurred with ACE.
The schematic representation of the interaction between each candidate and control with ACE macromolecule using LigPlot showed that quercitrin has the most similar interaction characteristic with lisinopril as a control (Figures 1 and 2). The binding mode of lisinopril in the ACE macromolecule complex was examined from its crystal structure (PDB ID: 1O86). The analysis of interaction characteristic between lisinopril with ACE macromolecule showed that this ligand can bind to the active site of the ACE via hydrogen bonds with Glu162, Asp377, His353, His513, Try523, Gln281, and Asn277 and hydrophobic bonds with Glu376, Val 380, His383, Tyr520, Phe527, Asp415, Phe457, and Trp279. Based on the interaction with the ACE binding site, quercitrin showed the most similar interaction with the lisinopril ligand compared with other compounds.
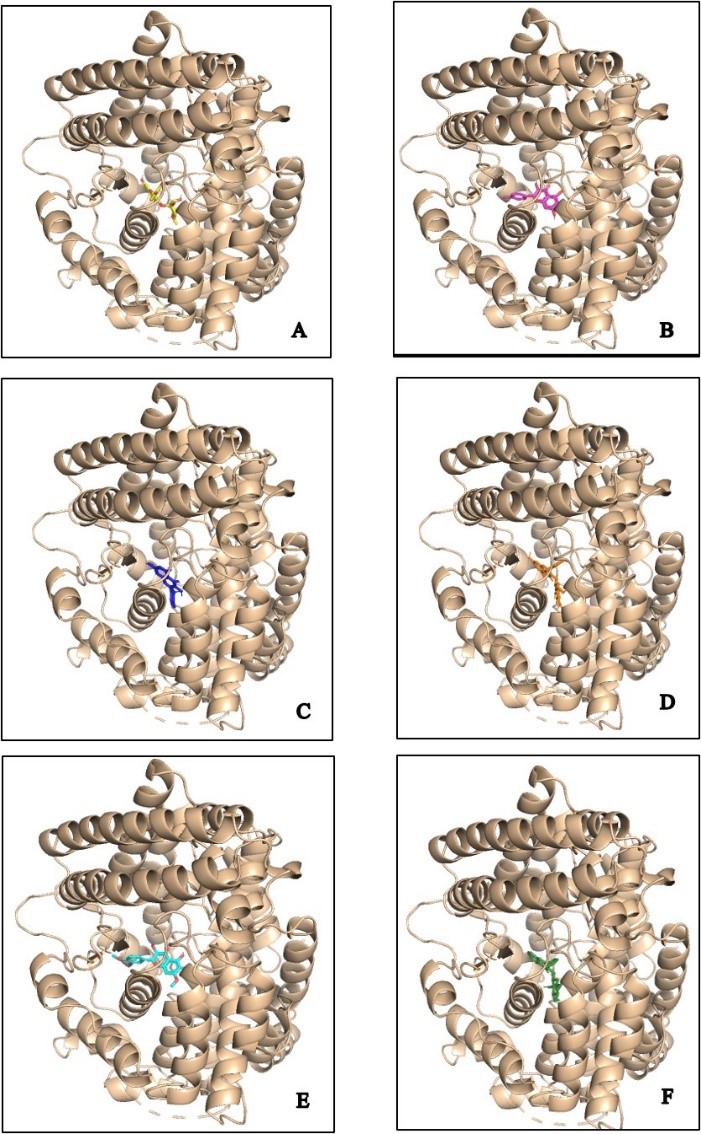
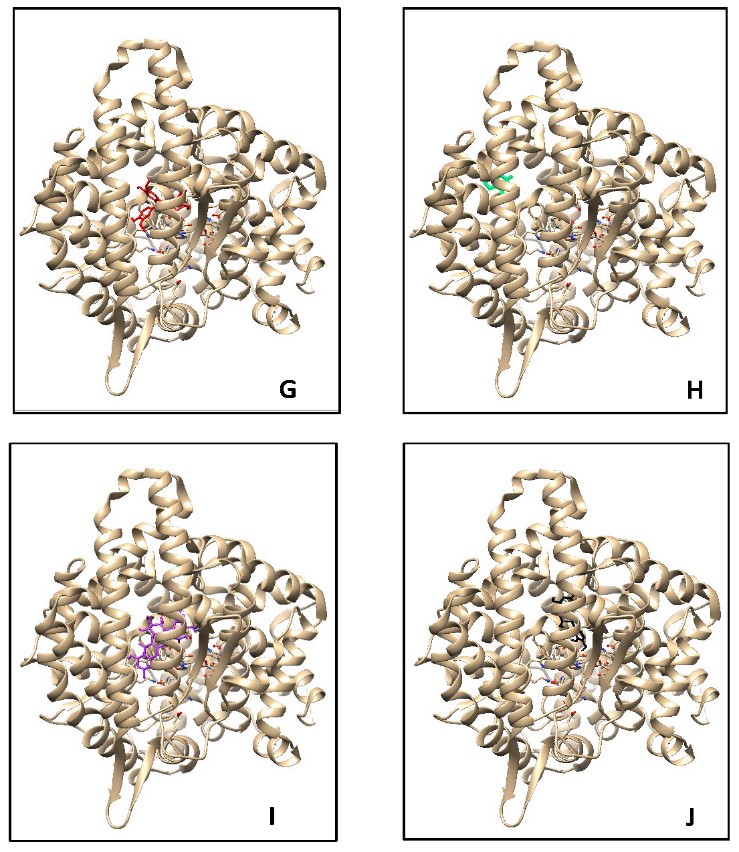
Figure 1. Docking analysis visualization of ACE binding with several compounds: casticin (cyan), kaempferol (violet), quercitrin (yellow), isoquercetine (orange), flavonol 3-O-D-glucoside (green), and quercetin (blue) (A–F), Aviculin (Red) (G), Theobromine (Cyan) (H), Rutin (Purple) (I), dan Catechin (Black) (J) using PyMOL
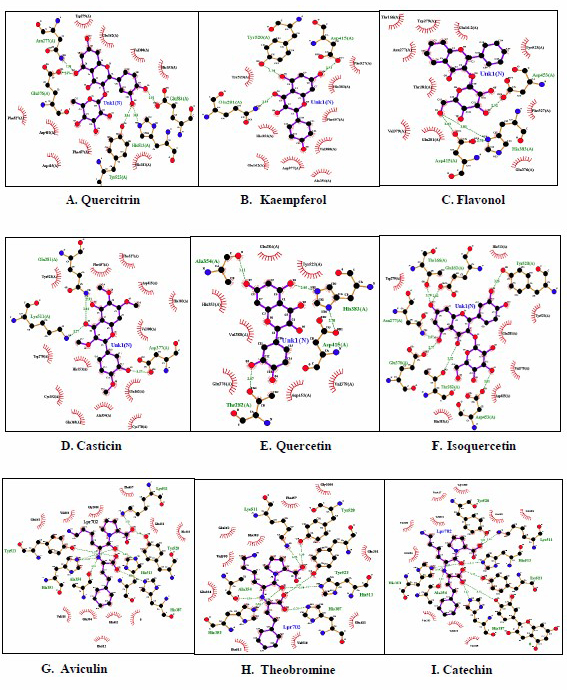
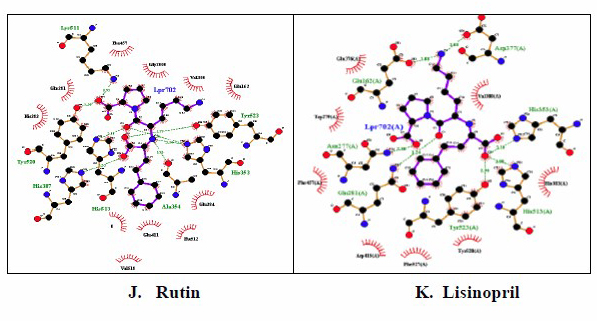
Figure 2. Visualization of docking results of active compounds from mistletoes (S. atropurpurea and D. pentandra) and ACE macromolecule receptor (PDB ID: 1O86) by Ligplot 2.1 software. Quercitrin (A), Kaempferol (B), Flavonol (C), Casticin (D), Quercetin (E), Isoquercetin (F), Aviculin (G), Theobromine (H), Catechin (I), Rutin (J), Lisinopril (K).
DISCUSSIONS
The present study was conducted to examine the potential activity, toxicity, and interaction confirmation from phytocompounds of S. atropurpurea and D. pentandra via an in-silico approach. The findings of the present study supported the traditional uses of mistletoes for hypertension treatment and suggested the six major compounds (quercitrin/quercetin 3-rhamnoside, kaempferol, quercetin, isoquercitrin/quercetin 3-glucoside, a flavonol glycoside, and casticin) as ethnopharmacological agents.
The computational study for predicting pharmacokinetics, bioavailability, drug-likeness, and medicinal chemistry friendliness of natural or synthetic small molecules may ease the discovery of novel drugs; Swiss-ADME is one of the tools that aid such study (PubChem, 2020; Karmaka et al., 2019). It is well known that the potential of a small molecule as a successful drug candidate can be predicted through its physicochemical properties such as solubility and lipophilicity (Tripathi et al., 2019; Daina, et al., 2017). With the aid of the SwissADME online tool, the present study predicted the potential of S. atropurpurea and D. pentandra phytocompounds as the known synthetic drugs for hypertension treatment as compared with lisinopril. Previously, an experimental study has proposed that the crude leaf extract of S. atropurpurea and D. pentandra can be used as the prevention agent for hypertension but the exact phytocompounds that have such anti-hypertension activity are unknown (Athiroh et al., 2017; Sliwosky, et al., 2014; Daina et al., 2017).
The results of the Lipinski rule assessment showed that most of the active compounds, including kaempferol, quercetin, a flavonol glycoside, and casticin, have drug-likeness properties. Of the clinically approved drugs, 95% have met the range of the following physicochemical properties: molecular weight (130–725 kDa), HBD (0–6), HBA (2–20), logP (–2 to 6.5), and the number of torsions (0–15), but this provision is not fulfilled by quercitrin and isoquercitrin. Conversely, human intestinal absorption (HIA) predicts the number of compounds that can be absorbed by the gastrointestinal (GI) tract. A compound is predicted to have a good or a bad absorption value through an HIA of >80% and <30%, respectively (Mustarichie et al., 2017). Our results indicated that all compounds except quercitrin and isoquercitrin can be absorbed well in the intestine based on GI absorption value. Mendis et al. (2011) explained that the ligand that fails to fulfill the parameters of the Lipinski rule of five is highly probable to cause trouble if ingested.
In this work, we also predicted the major mistletoe compounds' toxicity via an in-silico approach. Mutagenic, hepatoxic, immune-toxic, carcinogenic, and LD50 effects were evaluated based on the Pro-Tox server. Generally, our data indicated that mistletoe compounds commonly have low toxicity probability (Table 3), except for quercitrin and quercetin, which showed medium to high mutagenic, immune-toxic, and carcinogenic potentials.
Most of the compounds contained in S. atropurpurea and D. pentandra are bioactive flavonoids. Flavonoids are a group of phenolic compounds that occur widely in plants and have multiple biological, pharmacological, and medicinal properties, including anti-inflammatory, antiviral, antiallergic, antithrombotic, and anticancer effects (Mendis et al., 2011). Phytocompounds such as flavonoids have been reported to exert beneficial effects in cardiovascular disease, including hypertension.
Flavones, flavonols, flavanones, and flavonols can modulate blood pressure by restoring endothelial function, directly affecting nitric oxide levels, or indirectly through other pathways. Several flavonoids indicate antihypertensive and cardioprotective activities that protect against endothelial dysfunction. The relationship between hypertension and endothelial dysfunction contributes to atherosclerosis progression and other health complications of cardiovascular disease. Based on rodent and human studies, the antihypertensive effects of certain flavonoids appear to be due to enhanced endothelial function, which is achieved by inducing vasodilation via nitric oxide, by inhibiting the effects of vasoconstrictors, or by affecting pathways involved in vasorelaxation. Additionally, flavonol-3-O-glycosides and flavonol aglycones manifest their pancreatic lipase-inhibitory activities as anti-obesity agents (Khaki et al., 2011).
Casticin is one of the major flavonoids in mistletoe extracts. This compound has been shown to have several biological activities, but most studies have focused on its anti-tumor effects in different cancer types. The anti-tumor activity mechanism of the flavonoid casticin occurs through either cell growth arrest in G2/M or apoptotic cell death. As a tubulin-binding agent, casticin induces p21, which consequently inhibits Cdk1 and downregulates cyclin A. Following casticin exposure, Bcl-2 depletion occurs in cancer cells, and a sub-G1 accumulation occurs in the cell cycle. Furthermore, following transient transfection with Bcl-2, MN1 cells are resistant to casticin (Park et al., 2019).
Another study demonstrated that quercitrin and isoquercitrin have antioxidant effects using the mechanistic analysis role of the 6-OH group in antioxidant processes. The cytoprotective effect analysis of these two model compounds using mesenchymal stem cells (MSCs) provided some biological evidence of these compounds' antioxidant effects. Li et al. (2016) suggested that both quercitrin and isoquercitrin possessed higher antioxidant ability than the positive control Trolox. In the MTT assay, MSCs were initially oxidatively damaged using the Fenton reagent (FeCl2 plus H2O2), which was used to generate •OH radicals. The results revealed that quercitrin and isoquercitrin both protected the MSCs from •OH radical-induced damage at concentrations in the range of 0–100 µg/mL. However, isoquercitrin exhibited much stronger protective activity than did quercitrin at the same concentrations. For instance, quercitrin can protect osteoblastic MC3T3-E1 cells against H2O2-induced dysfunction (Li et al., 2016) and reduce ultraviolet B (UVB)-induced cell death and apoptosis in HaCaT cells (Eun et al., 2010). These results can be explained in the sense that H2O2 can result in oxidative damage. In contrast, UVB irradiation can result in large quantities of •OH radicals that can exert considerable toxicity. Furthermore, isoquercitrin can inhibit the H2O2-induced apoptosis of EA.hy926 cells (Yang et al., 2012).
Phenolic compounds exhibited a range of inhibitory effects on platelet activation, related signal transduction pathways, enhancement of NO production, and receptors' inhibition. An in vitro study by Yang et al. (2012) demonstrated that delphinidin-3-O-glucoside inhibited platelet aggregation in platelet-rich plasma and purified platelets from humans and mice, induced by collagen, thrombin, thrombin receptor-activating peptide, and adenosine diphosphate. Furthermore, quercetin inhibited collagen-induced fibrinogen binding to its receptor, perhaps by causing conformational changes to the GPIIb IIIa receptor, thus reducing the affinity of fibrinogen for the receptor binding site (Zhu et al., 2016). Simple phenolic acids, such as gallic acid, also inhibit platelet aggregation and activation via the inhibition of the phosphorylation of PKCα/p38 MAPK and Akt/GSK3β (Oh et al., 2012). Besides, Mubarakati et al., (2019) showed that based on molecular docking analysis, phenolic compounds such as dimethyl-oleuropein in olives have acetylcholinesterase (AChE) inhibitory activity for the treatment of Alzheimer's disease.
The hyperbolical production of ROS may cause tissue injury that may result in the inflammatory process. Polyphenol antioxidant activities are dependent on the structure of their functional groups. The number of hydroxyl groups significantly affects several antioxidant activity mechanisms such as scavenging radicals and metal ion chelation ability (Chang et al., 2012). Polyphenol antioxidant activities are related to their capacity to scavenge a wide range of ROS. Indeed, the mechanisms involved in polyphenols' antioxidant capacity include the suppression of ROS formation by either inhibition of enzymes involved in their production, scavenging of ROS, or upregulation or protection of antioxidant defenses. Polyphenols may reduce the catalytic activity of ROS generation enzymes and protect against oxidative damage (Hussain et al., 2016). ROS formation has been reported to enhance free metal ions by reducing hydrogen peroxidase with the generation of the highly reactive hydroxyl radical. Lower redox potentials of the polyphenols can thermodynamically reduce highly oxidizing free radicals because of their capacity to chelate metal ions and free radicals. For example, quercetin has iron-chelating and iron-stabilizing properties (Kumar et al., 2012).
The docking between six bioactive compounds of mistletoes (S. atropurpurea and D. pentandra), flavonol, casticin, kaempferol, quercetin, quercitrin, and isoquercitrin, against ACE in the present study, indicated that those secondary metabolites were suggested to be of natural compounds for ACE inhibitors. ACE inhibitors from natural ingredients are considered safer than synthetic ACE inhibitors. Indeed, synthetic ACE inhibitors such as captopril, ramipril, and lisinopril have side effects, including dry cough, hyperkalemia, rash, dizziness, and taste changes. Based on docking analysis, quercitrin has an affinity score (ΔG) of −9.3 kcal/mol, which indicated the lowest energy conformation of bond representing the most stable and optimum drug candidate among mistletoe compounds (Table 4). The free energy change (ΔG) indicates the stability of interaction (bonding) between the ACE receptor and ligands in the binding site. Hydroxy groups (–OH), ketone groups (=O), and ether groups (–O–) in all compounds were predicted to play roles as amino acid residue interactors at the active site of ACE. The high affinity of drug compounds depends on the type and amount of bonding that occurs with the protein's active site. In Figure 2, lisinopril forms several chemical bonds with ACE, including hydrogen bonds and hydrophobic bonds, and other active compounds. Although quercitrin appeared to be the most stable and optimum drug candidate based on docking analysis, the most potential drug candidate when sorted by the combination of the affinity, the Lipinski rule, GI absorption, and toxicity test is as follows: flavonol > casticin > kaempferol > quercetin > quercitrin > isoquercitrin. Thus, toxicity and molecular docking analysis have shown that flavonol 3-O-D-glucoside is the most potent active mistletoe compound as an ACE inhibitor (Table 4). According to Eff et al. (2020), the standardization of the Indonesian traditional antihypertensive medicine (jamu) is a combination of active compounds in medicinal plants that have been tested and met standard extract parameters, both specific and nonspecific, and have potential in vitro activity as an ACE inhibitor. This combination is expected to have a synergistic effect from several medicinal plants' chemical content in lowering blood pressure via different mechanisms of action.
Table 4. Summary of toxicity test and molecular docking analysis of bioactive ligand with ACE macromolecule
|
Compound |
Lipinski result |
GI absorption |
Toxicity class |
Binding affinity (kcal/mol) |
|
Quercitrin/quercetin 3-rhamnoside (CID_5280459) |
No |
Low |
5 |
−9.3 |
|
Kaempferol (CID_5280863) |
Yes |
High |
5 |
−8.1 |
|
Quercetin (CID_5280343) |
Yes |
High |
3 |
−8.3 |
|
Isoquercitrin/quercetin 3-glucoside (CID_5280804) |
No |
Low |
5 |
−8.6 |
|
Flavonol glycoside (CID_11953828) |
Yes |
High |
5 |
−8.3 |
|
Casticin (CID_5315263) |
Yes |
High |
5 |
−7.5 |
|
Aviculin(CID_10391477) |
Yes |
Medium |
4 |
-8,1 |
|
Thaebromine(CID_5429) |
Yes |
Medium |
4 |
-5,8 |
|
Rutin(CID_5280805) |
No |
Low |
5 |
-9,7 |
|
Catechin(CID_9064) |
Yes |
Medium |
6 |
-8,3 |
|
Lisinopril (control) |
Yes |
High |
6 |
−8.2 |
This study applied the basics of molecular interaction that could be used to screen and develop an antihypertension drug with low toxicity. Three main objectives, namely, to predict the bond model of the known ligand is active, to search for new ligands through virtual screening, and to predict the binding affinity of some active compounds. Virtual screening is the process of evaluating a compound structure using software to find candidate compounds that have potential as drugs. Theoretically, the application of the methods of virtual screening is limited to the structures of the compounds that can be counted and to compounds known to have ties with other compounds. There are a lot of studies doing computer screening and active compound molecular docking to a target protein (Kumar, et al., 2017; Mubarakati, et al., 2019; Sarkar, et al., 2021). Nevertheless, these predictive results require validation through in vitro and in vivo toxicological and pharmacological assays and experimental bioavailability tests for this main compound concerning hypertension prevention.
CONCLUSION
Quercitrin appeared to have the most similar interaction characteristic with the lisinopril ligand based on the sole docking analysis. However, the Lipinski rule, GI absorption, and toxicity analysis showed that flavonol 3-O-D-glucoside is the most recommended compound as a potential inhibitor of ACE because, practically, it is nontoxic. Based on the overall data, it can be concluded that the values of the Lipinski rule, GI absorption, toxicity, and binding affinity tests show that the main compound in mistletoes (S. atropurpurea and D. pentandra) is the proper combination in hypertension treatment and most recommended compounds as potential inhibitors of ACE. The results obtained in this research could be recommended for further studies such as molecular dynamics simulation, in vitro and in vivo to explore the ACE inhibitory potential of these ten compounds as a promising candidate for the new anti-hypertension agents
ACKNOWLEDGEMENT
The authors would like to thank Universitas Islam Malang and INBIO Research Institute for supporting this study.
AUTHOR CONTRIBUTIONS
Nour Athiroh Abdoes Sjakoer a assisted in conducting the experiments, performed the statistical analysis and data visualization and wrote the manuscript. Nurul Jadid Mubarakati and Ahmad Taufiq designed and conducted all of the experiments and wrote the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Attique, S.A., Hassan, M., Usman, M., Atif, R.M., Mahboob, S., Al-Ghanim, K.A, Bilal, M., and Nawaz, M.Z. 2019. A Molecular Docking Approach to Evaluate the Pharmacological Properties of Natural and Synthetic Treatment Candidates for Use against Hypertension. Int. J. Environ. Res. Public Health. 16: 923.
Athiroh, N., Permatasari, N., Sargowo, D., and Widodo MA. 2014 (a). Antioxidative and blood pressure-lowering effects of Scurrula atropurpurea on deoxycorticosterone acetate–salt hypertensive rats. Biomarkers and genomic medicine. 6: 32-36.
Athiroh, N. and Sulistyowati, E. 2015 (a). Evaluation of Methanolic Extract of Scurrula atropurpurea (Bl.) Dans Sub-Chronic Exposure on Wistar Rat Liver. Advances in Environmental Biology. 9: 245-250.
Athiroh, N. and Sulistyowati, E. 2015 (b). Scurrula atropurpurea increases nitric oxide and decreases malondialdehyde in hypertensive rats. Universa Medicina. 32: 44-50.
Athiroh, N., Permatasari, N., Sargowo, D., and Widodo, M.A. 2014 (b). Effect of Scurrula atropurpurea on nitric oxide, endothelial damage, and endothelial progenitor cells of DOCA-salt hypertensive rats. Iranian journal of basic medical sciences. 17: 622-625.
Athiroh, N. and Wahyuningsih, D. 2017. Study of Superoxide Dismutase and Malondialdehyde Concentrations in Mice After Administration of Methanolic Extract of Scurrula atropurpurea (BL.). Indonesian Journal of Veterinary Sciences. 11: 19-22.
Chander, S., Tang, C.R., Al-Maqtari, H.M., Jamalis, J., Penta A, Hadda, T.B., Sirat HM, Zheng YT, and Sankaranarayanan M. 2017. Synthesis and study of anti-HIV-1 RT activity of 5-benzoyl-4-methyl-1,3,4,5-tetrahydro-2H-1,5-benzodiazepin-2-one derivatives. Bioorg Chem. 72: 74-79
Chang, S.S., Lee, V.S.Y., Tseng, Y.L., Chang, K.C., Chen, K.B., and Chen, Y.L. 2012. Gallic acid attenuates platelet activation and platelet‐leukocyte aggregation: involving pathways of Akt and GSK3 beta. Evid Based Complement Alternat Med. 1– 8.
Daina, A., Blatter, M-C, Gerritsen, V.B., Palagi, P.M., Marek, D., Xenarios, I., Schwede, T., Michielin, O., and Zoete, V. 2017. Drug design workshop: A web-based educational tool to introduce computer-aided drug design to the general public. J Chem Educ. 94: 335-344.
Daina, A., Michielin, O., and Zoete, V. 2017. SwissADME: a free web tool to evaluate pharmacokinetics, drug likeness, and medicinal chemistry friendliness of small molecules. Sci Rep. 7: 42717.
Eff, A.R.Y., Rahayu, S.T, Mahayasih, P.G, and Januarko, M.U. 2020. Standardization of Indonesian Traditional Antihypertensive Medicines (Jamu) through the ACE Inhibitor Mechanism. Pharmacogn J. 12: 422-429.
Eun, M.C. 2012. Protective effect of quercitrin against hydrogen peroxide-induced dysfunction in osteoblastic MC3T3-E1 cells. Exp. Toxicol. Pathol. 64: 211–216.
Haidara, K., Zamir, L., Wen, S.Q., and Batist, G. 2006. The flavonoid Casticin has multiple mechanisms of tumor cytotoxicity action. Cancer Letters. 242: 180-90.
Hussain, T., Tan, B., Yin, Y., Blachier, F., Tossou, M.C.B., and Rahu, N. 2016.Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Medicine and Cellular Longevity.
Kumar, S., Sharma, U.K., Sharma, A.K., and Pandey, A.K. 2012. Protective efficacy of Solanum xanthocarpum root extracts against free radical damage: phytochemical analysis and antioxidant effect. Cellular and Molecular Biology. 58: 174–181.
Karmaka, B., Talukdar, P., and Talapatra, S.N. 2019. An in-silico study for two anti-inflammatory flavonoids of Nerium oleander on proinflammatory receptors. Research Journal of Life Sciences, Bioinformatics, Pharmaceuticals and Chemical Sciences. 5: 582-596.
Khaki, A., Azad, F.F., Nouri, M., and Khaki, A.A. 2011. Effects of basil, Ocimum basilicum on spermatogenesis in rats. Journal of medicinal plant research. 5.
Kumar, R., Kumar, A., Långström, B. et al. Discovery of novel choline acetyltransferase inhibitors using structure-based virtual screening. Sci Rep 7, 16287 (2017).
Li, X.C., Liu, J.J., Lin, J., Wang, T.T., Huang, J.Y., Lin, Y.Q., and Chen, D.F. 2016. Protective effects of dihydromyricetin against OH-induced mesenchymal stem cells damage and mechanistic chemistry. Molecules. 21: 604.
Mustarichie R., Warya S, Saptarini N.M., and Musfiroh I. 2016. Acute and subchronic toxicities of indonesian mistletoes Dendrophthoe pentandra L. (Miq.) ethanol extract. Journal of Applied Pharmaceutical Science. 6: 109-11.
Mustarichie, R., Runadi, D., and Ramdhani, D. 2017. The Antioxidant Activity and Phytochemical Screening of ethanol extract, fractions of water, ethyl acetate, and n-hexane from Mistletoe tea (Scurrula atropurpurea BL. Dans). Asian J Pharm Clin Res. 10: 1-5.
Mendis, S., Puska, P., and Norrving B. 2011. World Health Organization. Global Atlas on Cardiovascular Disease Prevention and Control. World Health Organization: Geneva (Switzerland).
Mubarakati, N.J., Puspitarini, O.R., Rahayu T., and Maulidiyah, A. 2019. In silico exploration the phenolic compound of olive leaves as acetylcholinesterase enzyme (AChE) inhibitor for Alzheimer’s disease therapy. Berkala Penelitian Hayati Journal of Biological Researches. 24: 84-89.
Ohashi, K., Winarno, H., Mukai, M., Inoue, M., Prana, M.S., Simanjuntak, P,, and Shibuya, H. 2003. Indonesian Medicinal Plants. XXV. Cancer Cell Invasion Inhibitory Effects of Chemical Constituents in The Parasitic Plant Scurrula atropurpurea (Loranthaceae). Chem. & Pharm. Bulletin. 51: 343—345.
Oh, W.J., Endale, M., Park, S‐C, Cho, J.Y., Rhee, M.H. 2012. Dual roles of quercetin in platelets: phosphoinositide‐3‐Kinase and MAP kinases inhibition, and cAMP‐dependent vasodilator‐stimulated phosphoprotein stimulation. Evid Based Complement Alternat Med. 1– 10.
PubChem National Library of Medicine. Chain A, Crystal Structure of Human Angiotensin Converting Enzyme in Complex with Lisinopril. 2017 April 15th [accessed 2020 21 August]. https://pubchem.ncbi.nlm.nih.gov.
PubChem National Library of Medicine. Chain A. [accessed 2020 21 August]. https://pubchem.ncbi.nlm.nih.gov.
Park, J.Y., Kim, C.S., Park, K.M., and Chang, P.S. 2019. Inhibitory characteristics of favonol-3-O-glycosides from Polygonum aviculare L. (common knotgrass) against porcine pancreatic lipase. Scientific Reports. 9:18080.
RSC Protein Data Bank. Crystal Structure of Human Angiotensin Converting Enzyme in complex with lisinopril; 2003 February 07 [accessed 2020 August 21st]. https://www.rcsb.org/structure/1O86.
Sarkar, B., Alam, S., Rajib, TH., Islam, S.S., Araf, Y., and dan Ullah, M.D.A. 2021. Identification of the most potent acetylcholinesterase inhibitors from plants for possible treatment of Alzheimer’s disease: a computational approach. Journal of Medical Human Genetics. 22: 2-20.
Sliwosky, G., Kothiwale, S., Meiler, J., Lowe, E.J.Jr. 2014. Computational methods in drug discovery. pharmacol Rev, 66 :334-395.
Tripathi, P., Ghosh, S., and Talapatra, S.N. 2019. Bioavailability prediction of phytochemicals present in Calotropis procera (Aiton) R. Br. by using Swiss-ADME tool. World Scientific News. 131: 147-163.
Winata, D.K. 2019. Control Hypertension with Lifestyle Changes. [accessed 2020 August 20]. https://mediaindonesia.com/read/detail/236222-kendalikan-hipertensi-dengan-perubahan-gaya-hidup.
Way2Drug Understanding Chemical-Biological Interaction. [accessed 2020 21 August]. http://www.pharmaexpert.ru/passonline.
Way2Drug Understanding Chemical-Biological Interaction. [accessed 2020 21 August]. http://pharmaexpert.ru/PASSonline/index.php.
World Health Organization (WHO). 2019. Hypertension. [accessed 2021 28 February]. https://www.who.int/health-topics/hypertension/#tab=tab_1
Yang, H.M., Ham, Y.M., Yoon ,W.J., Seong W.R., Jeon, Y., Tatsuya, O., Kang, S.M., Kang, MC, Kim, EA, and Kim, DY. 2012. Quercitrin protects against ultraviolet B-induced cell death in vitro and in vivo zebrafish model. J. Photochem. Photobiol. B. 114: 126–131.
Zhu, M.X., Li, J.K., Wang, K., Hao, X.L., Ge, R., and Li, Q.S. 2016. Isoquercitrin inhibits hydrogen peroxide-induced apoptosis of EA.hy926 Cells via the PI3K/Akt/GSK3β signaling pathway. Molecules. 21: 356.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Nour Athiroh Abdoes Sjakoer1,*, Nurul Jadid Mubarakati1, and Ahmad Taufiq2
1 Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Islam Malang, Jalan Mayjen Haryono No.193, Dinoyo, Lowokwaru, Malang 65144, East Java, Indonesia
2 Department of Physics, Faculty of Mathematics and Natural Sciences, Universitas Negeri Malang, Jalan Semarang No.5, Sumbersari, Lowokwaru, Malang, Indonesia
Corresponding author: Nour Athiroh Abdoes Sjakoer, E-mail: nour.athiroh@unisma.ac.id
Total Article Views
Editor: Korakot Nganvongpanit, Chiang Mai University, Thailand
Article history:
Received: February 9, 2021;
Revised: April 3, 2021;
Accepted: April 9, 2021;
Published online: April 28, 2021

