
Effect of 2,4-D and BAP on Morphological Characters and Genetic Stability of Kaffir Lime (Citrus hystrix DC.) Callus Cultures Among Generations
Woro Anindito Sri Tunjung*, Asti Fitri Widyasari, Agnes Iskandar, Alisa Julia Nurulita, Aries Bagus Sasongko, Ari Indrianto, Endang Semiarti, and MaryaniPublished Date : 2021-04-26
DOI : https://doi.org/10.12982/CMUJNS.2021.067
Journal Issues : Number 3, July-September 2021
Abstract Kaffir lime has medicinal properties for the treatment of many diseases. We used in vitro culture techniques to maintain quality and increase the production of active compounds in kaffir lime. The objective of this study is to determine the phenotype and genotype of kaffir lime callus cultures grown on media with the addition of 2,4-Dichlorophenoxyacetic (2,4-D) and Benzylaminopurine (BAP) on three generations (G0, G1, and G2). The callus was induced and subcultured on Murashige and Skoog medium added with 2,4-D: BAP at concentrations of 1:0.5; 2:0 and 5:0. Results showed no significant difference in callus initiation time in all treatment groups. Morphological characteristics, including color, texture, and biomass, varied among growth regulator concentrations and generation level. A high level of callus generation corresponds to a more friable texture and a more yellowish callus. Callus grown with the addition of 2,4-D and BAP 2:0 and 5:0 showed a more friable structure and yellowish color than 1:0.5. Growth regulator concentrations in each generation did not affect the callus growth curves, but the length of each phase between generations was different. The exponential phases of G1 and G2 were faster than that of G0. Despite slight differences in phenotype, the DNA profiles of callus suggest the same pattern between treatment groups, thereby indicating that our kaffir lime callus has genetic stability and that the callus can be used as raw material for medicinal purposes.
Keywords: ISSR markers, Kaffir lime callus, Generations, Genotype, Growth factor, Phenotype
Funding: This research was supported by Rekognisi Tugas Akhir grants 2020 funded by Universitas Gadjah Mada and the Penelitian Tesis Magister grant 2020 by the Ministry of Education and Culture of the Republic of Indonesia.
Citation: Tunjung, W.A.S., Widyasari, A.F., Iskandar, A., Nurulita, A.J., Sasongko, A.B., Indrianto, A., Semiarti, E., and Maryani 2021. Effect of 2,4-D and BAP on morphological characters and genetic stability of kaffir lime (Citrus hystrix DC.) callus cultures among generations. CMUJ. Nat. Sci. 20(3): e2021067
INTRODUCTION
Kaffir lime or Citrus hystrix DC. is distributed throughout tropical areas such as Southeast Asia (Wongpornchai, 2012 in Budiarto et al., 2019). The kaffir lime plant is used as a flavoring and spice in many foods. Kaffir lime is also used to maintain healthy teeth and gums, and to treat scurvy (Abirami et al., 2014 in Anuchapreeda et al., 2020). Kaffir lime contains secondary metabolite compounds in the form of alkaloids, flavonoids, terpenoids, tannins, and saponins, which have antibacterial, antioxidant, and anti-inflammatory functions and prevents free radicals. Previous studies also proved that kaffir lime has antiproliferative properties in epidermal carcinoma and murine leukemia cells (P388). Kaffir lime also has a cytotoxic effect against HL60 (promyelocytic leukemia), K562 (chronic myelocytic leukemia), Molt4 (lymphoblastic leukemia), and U937 (monocytic leukemia). (Siripongvutikorn et al., 2005; Hutadilok et al., 2006; Lertsatitthanakorn et al., 2006; Manosroi et al., 2006; Chueahongthong et al., 2011 in Tunjung et al., 2015). The anticancer activity of kaffir lime is influenced by secondary metabolite compounds.
Secondary metabolites in kaffir lime leaves are used as antibacterial agents (Tunjung et al., 2018), and secondary metabolites of kaffir lime are a potential resource to be used on a large scale in the pharmaceutical industry (Tunjung et al., 2020). However, the production of secondary metabolites with natural raw materials is less effective because many samples are required and the process is dependent on natural conditions. An effective method is needed to produce secondary metabolites on a large scale.
This study used plant tissue culture studies because it can produce disease-free plants, ensure rapid plant propagation, transform the plant genome, and produce secondary metabolites constantly (Debnarh et al., 2006; Altpeter et al., 2016; in Espinosa-Leal et al., 2018). In vitro culture techniques were used to scale up the production of secondary metabolites. Previously, we established a callus induction method for kaffir lime from seed explant. However, this study was conducted on generation 0 (G0) only (Tunjung et al., 2020, Tunjung et al., 2018). Kaffir lime callus can be used as raw material to produce cell suspension. Cell suspension culture in plants is conducted as bioproduction to produce high-value bioactive compound products in several plant species (Siva, 2012; Satdive, 2015; in Naik & Al-Khayri, 2018). The produced bioactive compounds can be higher than the parental plant due to cell suspension culture. This process is supported by the optimization of culture conditions, strain selection that is capable of producing high bioactive compounds, and the addition of precursors (Vanisree et al., 2004; in Balbuena et al., 2009) so that callus and elicited cell suspension can be used as a valuable strategy to produce secondary metabolites. Thus, obtaining a suitable callus for a particular purpose is necessary, such as for continuous growth, long-term storage, or for use as raw material for the production of cell suspension.
A compound of 2,4-Dichlorophenoxyacetic (2,4-D) is an auxin commonly applied to induce callus growth. This compound can revert cells into a dedifferentiated state and then start to divide. Meanwhile, Benzylaminopurine (BAP) is a synthetic cytokinin that is widely used in plant growth (George et al., 2008). According to Sarker et al. (2002) in Kumlay & Ercisli (2015), 6-Benzylaminopurine (BAP) has a good influence on the formation of callus (callogenesis) and of organs in potato plants. When this compound is used in moderate concentrations, it can also increase the induction of root propagation. 2,4-D played a role in producing somatic embryos (Zouine & El Hadrami, 2007). However, the use of 2,4-D at high concentrations influenced caulogenesis induction and accelerated tissue browning. The role of 2,4-D in the induction and maintenance of embryo growth depends on each type of explant, which has functions for cell division. On the basis of research conducted by Harahap et al. (2019), the treatment of growth regulators 2,4-D and BAP could produce two types of callus textures, namely, compact and friable callus. In 2,4-D treatment, the texture of the callus was usually more fragile with dark treatment. Furthermore, the use of 2,4-D by itself produced a callus with a friable structure (Amoo & Aysire, 2005). Our study also showed that the addition of 2,4-D without BAP will produce a very friable callus, which is suitable as raw material for cell suspension (Damayanti et al., 2020). Hence, in this study, we used a basal medium with three different concentrations and combinations of growth regulators, namely, 2,4-D:BAP (2:0 ppm), 2,4-D:BAP (5:0 ppm), and 2,4-D:BAP (1:0.5 ppm).
Callus subculture is important to maintain the growth and development of callus. Nakasha et al. (2016) conducted callus subculture of the Musli plant every four weeks for four cycles in the 2,4-D variation. The result was a significant increase in callus weight from the first to fourth subcultures. Callus gross weight increased along with the number of subcultures; the highest callus gross weight was achieved in the fourth subculture.
Callus subcultured for generations could produce somaclonal variations such as morphology, cytology, and molecular variations (Larkin et al., 1989; Larkin & Scowcroft, 1981). This somaclonal variation could be caused by genetic mutations (Kaeppler et al., 2000), which result in the emergence of genetic variations, known as genetic polymorphisms. Genetic polymorphisms could allow bioactive compounds to be expressed differently (Forrester et al., 2015) so that they might be able to interfere with the quality of callus production. Producing callus with genetic stability in each generation is important.
In this study, genetic stability analysis was carried out using the inter simple sequence repeats (ISSR) method with several primers referring to research by Munankarmi et al. (2018). ISSR uses genetic markers that will stick to repeating nucleotide regions that have microsatellite motifs (Tautz & Renz, 1984). ISSR has also been proven to be able to detect and analyze genetic diversity in plants whose genome sequences are unknown (Motley et al., 2006). Therefore, the objective of this study is to determine the phenotype and genotype of kaffir lime callus grown on a medium with a combination of growth regulators 2,4-D and BAP among three generations.
MATERIALS AND METHODS
Sampling
Kaffir lime (Citrus hystrix DC.) fruits were picked in Kaliduren Village, Borobudur District, Magelang, Central Java, Indonesia. Only fresh fruits with a diameter of approximately 5–6 cm and the seeds diameter of 1–2 cm were used in this study.
Callus induction
We used solid Murashige and Skoog (MS) medium. The composition of MS medium includes macronutrients (NH4NO3, KNO3, CaCl2.2H2O, MgSO4.7H2O, and KH2PO4), iron (Na2EDTA and FeSO4.7H2O), micronutrients (MnSO4.H2O, ZnSO4.4H2O, H3BO3, KI, NaMoO4.2H2O, CuSO4.5H2O, and CoCl2.6H2O), vitamins (glycine, nicotinic acid, pyridoxine HCl, and thiamine HCl), myo-inositol, sucrose, and agar. Medium pH was adjusted to 5.8 (Murashige & Skoog, 1962). This basal medium was added with a combination of various concentrations of 2,4-Dichlorophenoxyacetic (Sigma-Aldrich):BAP (Benzylaminopurine) (Sigma-Aldrich) hormones. The concentration of growth regulators was based on previous studies, in which we tried several concentrations of 2,4-D and BAP (Tunjung et al., 2020; Tunjung et al., 2018) and several concentrations of 2,4-D (Damayanti et al., 2020). Hence, in this study, we focused only on three concentrations of 2,4-D: BAP namely 1:0.5, 2:0 and 5:0 ppm. Also, we revealed that without the addition of growth regulators (MS medium only), kaffir lime seeds will germinate and callus did not form (Tunjung et al., 2020). Thus, in this study, we did not culture callus without growth regulators. The seeds were sterilized by soaking them in 5.25% NaClO (Sigma-Aldrich) while being shaken gently for 5 minutes under aseptic conditions so that the seeds did not become contaminated (Tunjung et al., 2020). The seeds were sliced to create a wound on the surface and then sowed in a culture bottle that contained solid MS medium. Next, the cultures were stored in an incubation room under dark condition at a temperature of 25°C and approximately 50% humidity.
Observation of callus growth parameters
The explants were observed with the following callus growth parameter variables:
Callus induction time
Callus induction is characterized by the growth of cell mass on the surface of the injured seed. The time of appearance of the cell mass is recorded as callus induction time. Callus was observed macroscopically and microscopically every day. We used nine replicates, and the obtained data were processed using SPSS software with ANOVA analysis and continued with the DMRT test at 95% if a significant difference was observed. The results were displayed in tables.
Growth curves
Calluses were weighed every five days to measure gross and dry weight until they became completely brown and dying (approximately on day 50). Then, the data were plotted to obtain the callus’s growth pattern on the experimental medium, which represents lag, exponential, and stationary phase. Each group underwent three times of nine replications.
Callus morphology
Callus morphology was observed through changes in texture, color, and shape of the callus. The texture level of callus friability was examined by tipping the callus by using a forceps. Callus color was confirmed by using the Royal Horticultural Society color chart. We also captured the callus image to represent the shape of the callus during growth, because the callus had an irregular structure affected by an irregular pattern of cell differentiation. Each group underwent three times of nine replications.
Subculture
In accordance with the callus growth curve data, we subcultured the G0 callus in the exponential phase (day 25). The medium used for subculture was the same as that used during callus induction, namely, solid MS medium with the addition of 2,4-D: BAP 1:0.5, 2:0, 5:0. After the G0 callus reached the exponential phase, we subcultured it to G1 (day 20). Callus without BAP is already very friable and fragile. Thus, it should be we cultured the callus only until the third generation (G2). Each group underwent at least three times of nine replications.
DNA extraction and PCR amplification
Callus on the exponential phase of every generation was subjected to ISSR method, namely, G0 (day 25), G1 (day 20), and G2 (day 20). DNA isolation was carried out using the Promega kit following the protocol from Promega Corp with minor modification. We isolated DNA from nine calluses of each group as replication. The concentration of extracted DNA was first equalized to 70 ng/µL. DNA was amplified using five markers: UBC 810, UBC 812, UBC 836, UBC 842, and UBC 857 (Munankarmi et al., 2018). According to Munakarmi et al. (2018) these five primers have the highest percent of polymorphism and polymorphism information content rates among 21 primers used to analyze polymorphism in 60 cultivars of Citrus aurantifolia. Although we used different species, on the basis of a study by Penjor et al. (2013), our sample, Citrus hystrix, has the closest relation with Citrus aurantifolia. Hence, we used these five primers to examine genetic stability in callus. Furthermore, we used trnL-F as housekeeping gene. The PCR program involved denaturation for 1 cycle at 95°C for 2 minutes, a second round of denaturation at 95°C for 30 seconds, annealing at a certain temperature depending on the primer used for 30 seconds, elongation at a temperature of 72°C for approximately 1 minute and 30 seconds, and a second round of elongation at a temperature of 72°C for 5 minutes. DNA fragments were separated on 1.5% agarose gel by using ethidium bromide staining at a current strength of 100 volts for approximately 28 minutes. Finally, DNA band visualization was performed using a UV transilluminator, then the DNA band was scaled based on the presence or absence of DNA bands in each sample. The DNA band that was formed was considered as 1 character, which represents 1 DNA locus. DNA bands with the same migration rate were assumed to be homologous loci.
Table 1. Sequences and annealing temperature of the primers.
|
Primer Code |
Primer Sequences (5’-3’) |
Primer length |
Primer Annealing Temperature (°C) |
|
UBC 810 |
GAGAGAGAGAGAGAGAT |
17 |
44.6 |
|
UBC 812 |
GAGAGAGAGAGAGAGAA |
18 |
47.6 |
|
UBC 836 |
AGAGAGAGAGAGAGAGYA |
18 |
43.6 |
|
UBC 842 |
GAGAGAGAGAGAGAGAYG |
18 |
42.1 |
|
UBC 857 |
ACACACACACACACACYG |
17 |
42.1 |
|
Trnl-F |
Forward: 5 'CGAAATCGGTAGACGCTACG Reverse: 5' ATTTGAACTGGTGACACGAG |
|
58 |
RESULTS
Callus initiation time
The time when callus began to appear was recorded as the callus initiation time (Table 2).
Table 2. Callus initiation time of three variations of growth regulator concentration in MS medium.
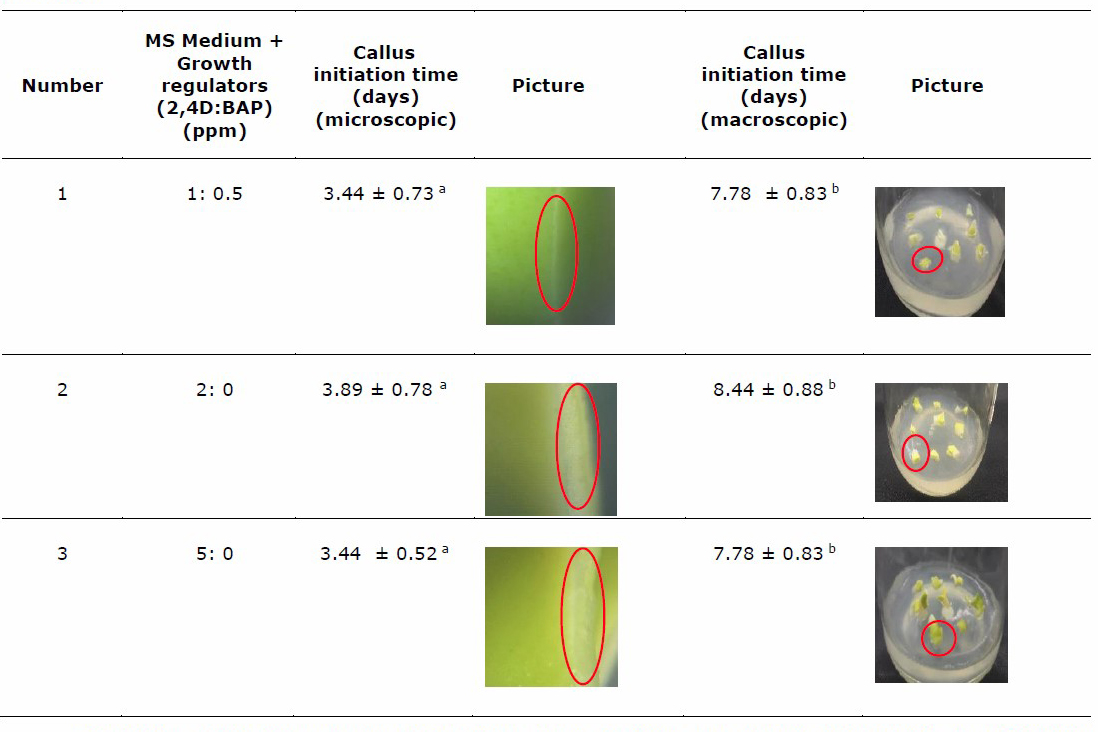
Note: Numbers followed by the same letter notation in different columns show that no significant results exist based on the one-way ANOVA test at the significance level α < 0.05. n = 9 callus. Red circle shows callus initiation.
All variations of growth regulators could induce callus by 100%, with no significant difference in callus induction time. This result is identical to our previous result (Tunjung et al., 2020), namely, the addition of 2,4-D and BAP can 100% induce callus from seed 100%, whereas the seed will undergo germination without growth regulator. Table 2 shows that callus was detected microscopically on day 3.44, 3.89, and 3.44 for the group of MS with 2,4 D:BAP 1:0.5, 2:0, and 5:0 ppm, respectively. Callus was detected macroscopically (aided vision was not needed) on days 7.78, 8.44, and 7.78 for the group of 1:0.5; 2:0, and 5:0, respectively. Callus of group 2,4-D:BAP 5:0 ppm and 2,4-D BAP 1:0.5 shows the same initiation time possibly because the higher concentration of 2,4-D will quickly initiate the formation of callus. The appropriate ratio of 2,4-D and BAP can also accelerate the initiation of callus formation. The presence of cytokinins affects the callogenesis process by reducing cell wall lignification and assists callus initiation and callus growth in vitro. After being observed, callus proliferation usually starts from the cut surface of the explant until it covers the entire explant (Kumlay & Ercisli, 2015).
Callus growth curves
The gross and dry weight were measured to create the growth curve until the 50th day for several generations, namely, G0; G1 or generation 1, which comes from subculturing G0; and G2 or generation 2, which comes from subculturing G1. Each generation was inoculated in MS medium with a combination of growth regulators 2,4-D:BAP at concentrations of 1:0.5, 2:0, and 5:0.
Table 3 Growth curve of three generations of kaffir lime callus culture based on gross and dry weight.
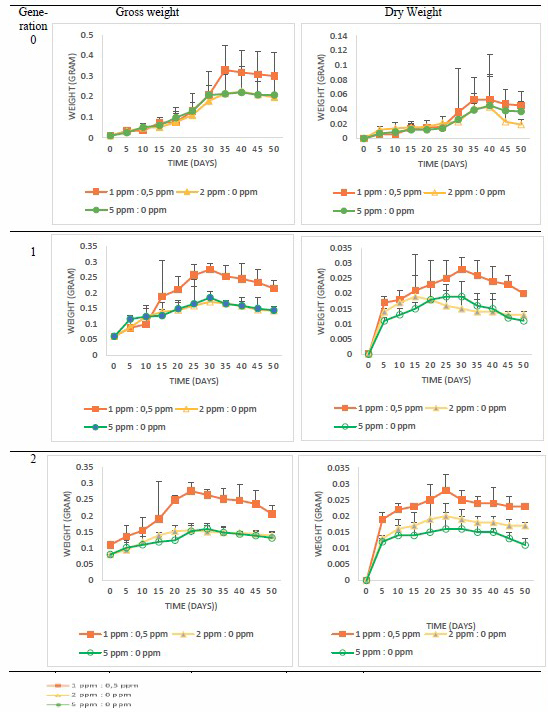
In accordance with the growth curve data, we summarize the growth phase in Table 4.
Table 4. Growth phase among generations and concentrations of growth regulators.
|
Growth phase |
Generation 0 |
Generation 1 |
Generation 2 |
||||||
|
1:0.5* |
2:0 |
5:0 |
1:0.5 |
2:0 |
5:0 |
1:0.5 |
2:0 |
5:0 |
|
|
Lag Phase(Day–) |
0–10 |
0–10 |
0–10 |
0–10 |
0–10 |
0–10 |
0–10 |
0–10 |
0–10 |
|
Exponential Phase(Day–) |
10–35 |
10–35 |
10–35 |
10–30 |
10–30 |
10–30 |
10–30 |
10–30 |
10–30 |
|
Stationary Phase (Day–) |
35–50 |
35–50 |
35–50 |
30–50 |
30–50 |
30–50 |
30–50 |
30–50 |
30–50 |
Note: *Ratio of growth regulator 2,4-D: BAP
According to growth curves, the variation of growth regulator concentration in one generation did not show the time difference in each phase. However, the time difference was observed in the varied generations. The exponential phases of G1 and G2 were faster than that of G0. Moreover, the stationary phase of G1 and G2 callus was earlier than that of G0.
Callus growth and development can also be seen through its biomass. Callus biomass contains several cell aggregate sizes, where the aggregate cells size can produce bioactive compounds that affect callus biomass. The cell aggregate is obtained from cell suspension. Haida et al. (2019) found that an increase in aggregate cells size can cause an increase in callus biomass. Table 3 shows that the gross and dry weight of callus with BAP addition was higher than that without BAP addition. According to Criado et al. (2009), BAP can also increase protein levels in older leaves by preserving protein synthesis and suppressing the decrease in protein levels even though the plant is under stress. The use of BAP also causes a decrease in cell wall lignification so that it results in callogenesis and helps in callus initiation and growth in vitro (Kumlay & Ercisli, 2015).
Callus morphology
The morphological parameters, including color and texture, of the callus are shown in Table 5.
Callus inoculated on medium enriched with 2,4-D: BAP 1:0.5 showed a friable texture and was larger than that without BAP addition. This type is more suitable for growth (continuous subculture) or long-term storage because the callus texture is more compact. Without BAP, the callus is very friable/fragile and easy to separate, thereby making it suitable as a raw material of cell suspension because not much cell debris remains. The junction of each cell is also tenuous. At a higher concentration of auxin, the callus that is formed also more strongly exhibits a friable texture. This finding is supported by Harahap et al., (2019), who stated that the callus with 2,4-D treatment (auxin group) had a more friable texture.
Table 5. Callus morphology of three generations of kaffir lime.
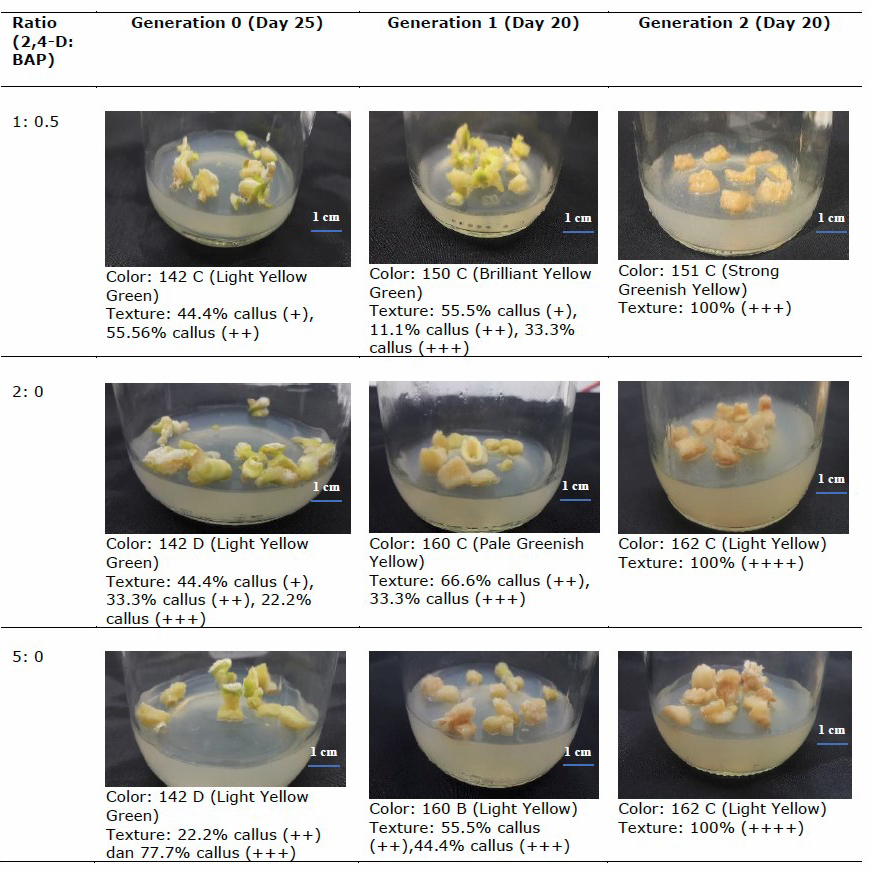
Note:
Scoring for texture:
-: no callus has appeared yet
+: the texture is compact
++: the callus spreads to all parts and the texture is less compact
+++: callus mass dominates explant mass and friable texture
++++: callus texture is very friable and fragile
Genetic stability
Five ISSR markers were used. Each marker resulted in a different number of DNA bands referred to different attachment sites of primer in DNA.
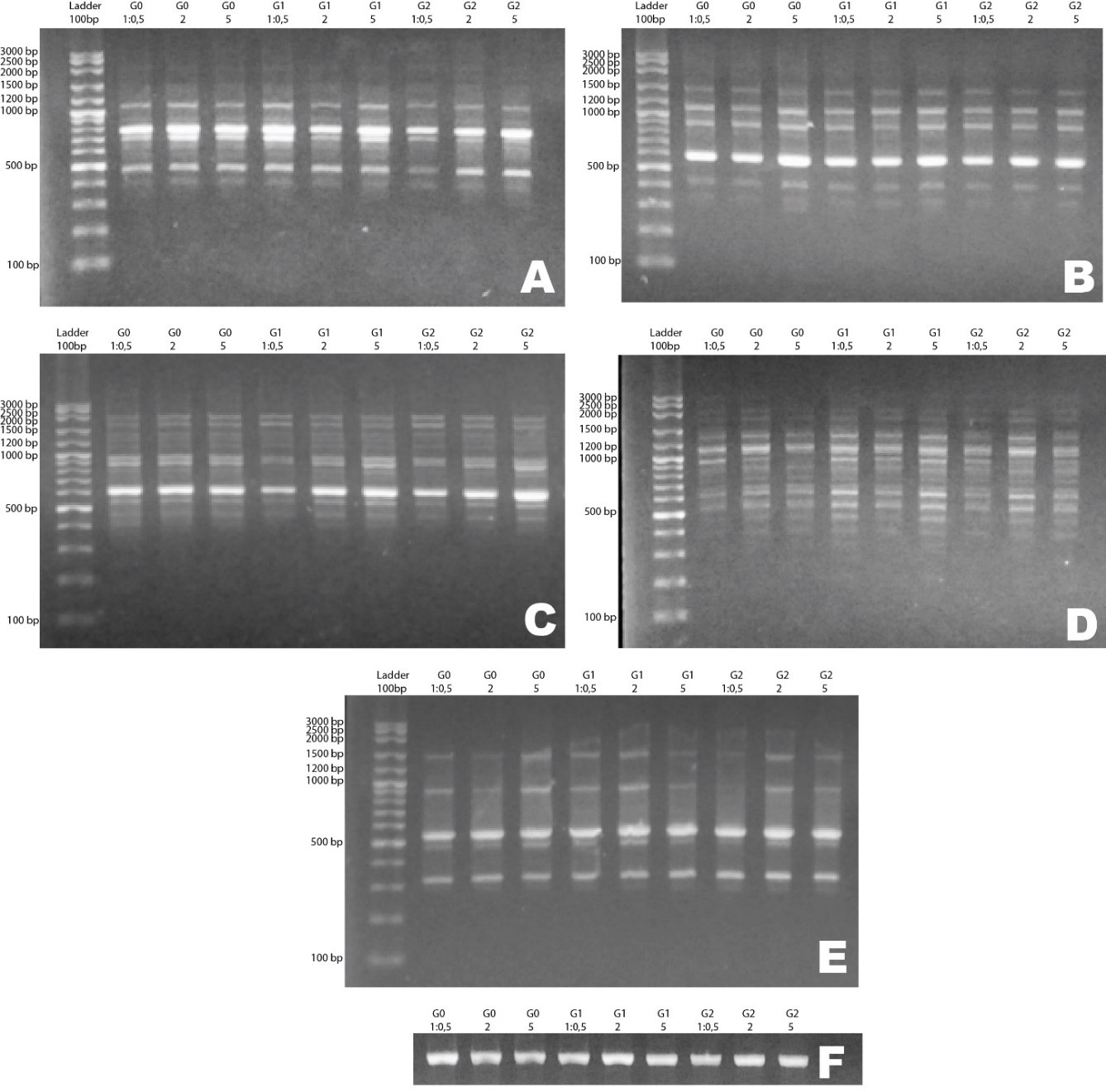
Figure 1. Electrophoresis of PCR products of kaffir lime callus culture from three generations and three variations of growth regulator concentration using primers A) UBC 810, B) UBC 857, C) UBC 842, D) UBC 836, E) UBC 812, and F) Trnl-F. (Note: G0: generation 0, G1: generation 1, G2: generation 2. 1:0.5; 2, and 5 show the concentration of growth regulators used on the medium.)
Table 6. Number of DNA bands of kaffir lime callus from three generations of all variations of growth regulator concentration.
|
Primer Code |
Number of DNA Bands |
Number of monomorphic bands |
Number of polymorphic bands |
Percentage of polymorphism |
||
|
G0 |
G1 |
G2 |
|
|||
|
UBC 810 |
8 |
8 |
8 |
8 |
0 |
0% |
|
UBC 812 |
6 |
6 |
6 |
6 |
0 |
0% |
|
UBC 836 |
12 |
12 |
12 |
12 |
0 |
0% |
|
UBC 842 |
12 |
12 |
12 |
12 |
0 |
0% |
|
UBC 857 |
7 |
7 |
7 |
7 |
0 |
0% |
Figure 1 shows the electrogram of the ISSR primer. Primer UBC 810 has an amplicon size range of 390–1200 bp, UBC 812 has a size of 300–1500 bp, UBC 836 has a size of 320–2500 bp, UBC 842 has a size of 400–2000 bp, and UBC 857 has a size of 300–1500 bp. Table 5 and Figure 1 show that all primers produce monomorphic bands and no polymorphic band is detected. The amplified DNA shows that the patterns and numbers of bands are the same in all generations and variations of growth regulator concentration treatments. DNA bands also showed the same locus in all treatments. This phenomenon indicates that the DNA bands of callus with different growth regulator concentrations showed genetic stability among generations.
DISCUSSION
The objective of this study is to obtain a suitable callus for a particular purpose, such as for continuous growth or long-term storage. Furthermore, a callus can be used as raw material for the production of cell suspension. The cell suspension can be elicited and subjected to traditional medicine or pharmaceutical uses.
The growth curve pattern of callus was different in varied generations. The exponential phases of G1 and G2 were much faster than that of G0. However, callus growth patterns did not differ in various concentration treatments. The morphology, texture, and color of callus were influenced by different generations. The color turned into yellow with the further generation of callus. During callus induction, different callus colors will appear in an in vitro culture. Callus color indicates a change in pigmentation, which contains bioactive compounds. Green callus contains high bioactive compounds, antioxidants, and cytotoxic potential (Ashokhan et al., 2019; in Ashokhan et al., 2020), whereas brown callus indicates exposure to a 2,4-D hormone that has changed into an auxinic herbicide. Brown callus indicates a stress condition on the callus and the ease of the morphogenic response (Mithila and Hall, 2005; Shibli et al., 2001; in Kiong et al., 2007). A white color indicates that the callus will die soon (bleaching) (Sulistiani et al., 2012). The yellow color of callus during the culture process indicates that red pigmentation is contained in the growth regulators used in the medium (Comia-Yebron et al., 2017). The color of the tested callus may vary from day to day because it corresponds to the response of the callus in a medium used for several days. The callus turned brown and died after about 30 days of testing (Cai et al., 2013). The generation also caused more friable callus than the previous generations. This condition could be caused by repeated subculturing on the same medium.
Callus size is influenced by variations in the growth regulators. Callus with BAP addition (2,4-D:BAP 1:0.5) had a bigger size than that without BAP addition (2,4-D:BAP 2:0 and 2,4 D:BAP 5:0) due to the presence of BAP. Skoog and Miller (1950) in Haberer & Kieber, (2002) revealed that the presence of auxin and cytokines in the medium could stimulate cells, namely, parenchymal tissue cells, to divide. Cytokinins have been known to play an important role in almost all aspects of plant growth and development, including cell division, initiation, and growth of shoots.
A higher concentration of auxin resulted in the callus becoming more friable. Auxin in the form of 2,4-D can increase osmotic pressure and cell permeability to water, reduce pressure on the cell wall, increase protein synthesis and plasticity, and develop cell walls (Robles-Martínez et al., 2016). Auxins play a role in cell wall loosening and cell expansion by modifying cell wall composition. Auxins activate gene expression, stimulating proton pumps so that the apoplasts become acidic. Auxins also activates the plasma membranes of H + -ATPases, which create an acidic apoplast environment. In this acidic environment, the active wall proteins become loose and large. This condition causes calcium to be active, thereby pumping calcium into the cell wall, increasing pH, and halting growth (Nishitani et al., 1981; Perrot et al., 2010; Ren et al., 2015; in Majda & Robert, (2018). The cell wall eases because a low pH activates an enzyme that breaks the bonds between the limiting polysaccharides in the cell wall, and then the cell will grow faster because of increased turgor pressure (Govinden-Soulange et al., 2009)
Hence, the variation of growth regulator concentration will be suitable for different purposes. Callus in 2,4-D:BAP 2:0 medium is best used for cell suspension because of the friable texture of the callus. The suspension was used for elicitation and can be used to increase the contents of secondary metabolites in kaffir lime culture. Callus in 2,4-D:BAP 1:0.5 medium is good for long-term storage because the callus texture is compact, the color is still green, and the callus size is larger than that in the BAP-free medium. A callus that was stored in the long term can be used as a stock. Our results are supported by Amoo & Ayisire, (2005), who revealed that the use of a combination of 2,4-D and BAP as a whole produces more friable callus, especially at the top of the callus, with a slightly compact structure at the bottom. Furthermore, the use of 2,4-D 0,4 mg/L solely in the callus induction of Parkia biglobosa (Jacq) Benth woody plants produced callus with a friable structure. These results show that the addition of BAP made the callus less friable compared with callus in medium without BAP (Amoo & Ayisire, 2005).
The production of secondary metabolite using plant explants requires standardized conditions. One of the conditions is that the quality of callus must be the same, especially its genetic characters. This feature is crucial because of the possibility that genetic characteristics will change due to the length of culture time. Moreover, chemical compound such as growth regulator can act as mutagen. Therefore, the genetic stability of explants needs to be determined in callus subculture in several generations and various growth regulator treatments. One objective of this study is to reveal that the callus used for the production of secondary metabolites is genetically stable. The ISSR primer is based on a study conducted by Munankarmi et al. (2018). The ISSR marker was also suggested by Shahsavar et al. (2007), who tested 33 genotypes of Citrus spp., and Fang and Roose (1997), who tested it using 68 cultivars of Citrus spp. In this study, we used kaffir lime seeds from the same cultivar and grown in the same location. The genetic stability of this microsatellite region of the callus could be characterized by using the ISSR method.
According to our data, growth regulators cause morphological differences, such as color, texture, and size, but not on a genetic level. Callus from a combination of auxin and cytokinins has a greener color. This finding is supported by a previous study that found that the presence of cytokinins can assist the formation of chlorophyll (George et al., (2008); in Sari et al., 2018). Furthermore, a medium with 2,4-D alone produces callus with a more friable texture than the combination medium because 2,4-D can increase the plasticity of the cell walls to become loose, which causes water to easily flow to the inner cells by osmosis so that the cells become elongated (Robbiani et al., 2010 in Sari et al., 2018). The genotype-level callus of kaffir lime seeds showed a stable characteristic in all treatments. Although we used synthetic growth hormone, the concentrations of the growth regulator that we used were not affected at the genetic level. The results also showed the genetic stability of callus until the third generation. The effect of growth regulator and generation on the production of bioactive compounds in callus needs to be elucidated and investigated further.
CONCLUSION
No significant difference in callus initiation time was found in all treatment groups. Morphological characteristics, including color, texture, and biomass, varied among growth regulator concentrations and generation level. With a higher level of callus generation, the texture became more friable and the callus became more yellowish. Callus grown with the addition of 2,4-D and BAP 2:0 and 5:0 showed a more friable structure and yellowish color than that grown with 1:0.5. Hence, adjusting the concentration or combination of the growth regulator will produce callus that meets our needs. Growth regulator concentrations in each generation did not affect the callus growth curves, but the length of each phase between generations was different. Despite slight differences in phenotype, kaffir lime calluses were genetically stable among generations and variations of growth regulator concentration treatments.
REFERENCES
Amoo, S.O. and Aysire, B.E. 2005. Induction of callus and somatic embryogenesis from cotyledon explants of Parkia biglobosa (Jacq.) Benth. African Journal of Biotechnology. 4: 68–71.
Anuchapreeda, S., Chueahongthong, F., Viriyaadhammaa, N., Panyajai, P., Anzawa, R., Tima, S., Ampasavate, C., Saiai, A., Rungrojsakul, M., Usuki, T., et al. 2020. Antileukemic cell proliferation of active compounds from kaffir lime (Citrus hystrix) leaves. Molecules. 25: 1–16.
Ashokhan, S., Othman, R., Rahim, M.H.A., Karsani, S.A., and Yaacob, J.S. 2020. Effect of plant growth regulators on coloured callus formation and accumulation of azadirachtin, an essential biopesticide in Azadirachta indica. Plants. 9.
Balbuena, T.S., Santa-Catarina, C., Silveira, V., Kato, M.J., and Floh, E.I.S. 2009. In vitro morphogenesis and cell suspension culture establishment in Piper solmsianum C.DC. (Piperaceae). Acta Botanica Brasilica. 23: 274–281.
Budiarto, R., Poerwanto, R., Santosa, E., Efendi, D., and Agusta, A. 2019. Production, post-harvest and marketing of kaffir lime (Citrus hystrix DC) in Tulungagung, Indonesia. Journal of Tropical Crop Science. 6: 138–143.
Cai, X.D., Wang, G.Y., and Cao, W.J. 2013. In vitro induction and proliferation of callus from immature cotyledons and embryos of Juglans regia cv. “xiangling.” Notulae Botanicae Horti Agrobotanici Cluj-Napoca.41: 378–384.
Comia-Yebron, R., Aspuria, E.T., and Bernardo, E.L. 2017. Callus induction in Amaranthus tricolor and Amaranthus spinosus. Journal of the International Society for Southeast Asian Agricultural Sciences. 23: 12–23.
Criado, M.V., Caputo, C., Roberts, I.N., Castro, M.A., and Barneix, A.J. 2009. Cytokinin-induced changes of nitrogen remobilization and chloroplast ultrastructure in wheat (Triticum aestivum). Journal of Plant Physiology. 166: 1775–1785.
Damayanti, F., Indrianto, A., Sasongko, A.B., Fajarina, S., Prabowo, B.H., Iskandar, A., Hidayati, L., and Sri Tunjung, W.A. 2020. Variation of 2,4-dichlorophenoxyacetic acid (2,4-D) concentration on kaffir lime callus growth as raw material for cell suspension. AIP Conference Proceedings, 2260(September).
Espinosa-Leal, C.A., Puente-Garza, C.A., and García-Lara, S. 2018. In vitro plant tissue culture: means for production of biological active compounds. Planta. 248: 1–18.
Fang, D.Q. and Roose, M.L. 1997. Identification of closely related citrus cultivars with inter-simple sequence repeats markers. Theoretical and Applied Genetics. 95: 408–417.
Forrester, J.V., Dick, A.D., McMenamin, P.G., Roberts, F., and Pearlman, E. 2015. The Eye : Basic Sciences in Practice (4th ed). Elsevier Health Sciences.
George, E.F., Hall, M.A., and De Klerk, G.J. 2008. Plant propagation by tissue culture 3rd edition (1st ed.). Springer.
Govinden-Soulange, J., Boodia, N., Dussooa, C., Gunowa, R., Deensah, S., Facknath, S., and Rajkomar, B. 2009. Vegetative propagation and tissue culture regeneration of hibiscus sabdariffa. January. World Journal of Agricultural Sciences. 5: 651–661.
Haberer, G., and Kieber, J.J. 2002. Cytokinins. New insights into a classic phytohormone. In Plant Physiology. 128: 354–362.
Haida, Z., Syahida, A., Ariff, S. M., Maziah, M., and Hakiman, M. 2019. Factors affecting cell biomass and flavonoid production of Ficus deltoidea var. kunstleri in cell suspension culture system. Scientific Reports, 9: 1–8.
Harahap, F., Diningrat, D.S., Poerwanto, R., Nasution, N.E.A., and Hasibuan, R.F.M. 2019. In vitro callus induction of sipahutar pineapple (Ananas comosus L.) from North Sumatra Indonesia. Pakistan Journal of Biological Sciences. 22: 518–526.
Kaeppler, S.M., Kaeppler, H.F., and Rhee, Y. 2000. Epigenetic aspects of somaclonal variation in plants. Plant Molecular Biology. 43: 179–188
Kiong, A.L.P., Huan, H.H., and Hussein, S. 2007. Callus induction from leaf explants kiong, 2007. International Journal of Agricultural Research. 2: 227–237.
Kumlay, A.M. and Ercisli, S. 2015. Callus induction, shoot proliferation and root regeneration of potato (Solanum tuberosum L.) stem node and leaf explants under long-day conditions. Biotechnology and Biotechnological Equipment. 29: 1075–1084.
Larkin, P.J., Banks, P.M., Bhati, R., Brettell, R.I.S., Davies, P.A., Ryan, S.A., Scowcroft, W.R., Spindler, L.H., and Tanner, G.J. 1989. From somatic variation to variant plants: Mechanisms and applications. Genome. 31: 705–711.
Larkin, P.J. and Scowcroft, W.R. 1981. Somaclonal variation - a novel source of variability from cell cultures for plant improvement. In Theoretical and Applied Genetics. 60.
Majda, M. and Robert, S. 2018. The role of auxin in cell wall expansion. In International Journal of Molecular Science. s 19.
Motley, T.J., Zerega, N., and Cross, H. 2006. Darwin’s Harvest: New Approaches to the Origins, Evolution, and Conservation of Crops. Columbia University Press.
Munankarmi, N.N., Rana, N., Bhattarai, T., Shrestha, R.L., Joshi, B.K., Baral, B., and Shrestha, S. 2018. Characterization of the genetic diversity of acid lime (Citrus aurantifolia (Christm.) swingle) cultivars of eastern Nepal using inter-simple sequence repeat markers. Plants, 7: 1–14.
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 15: 473–497.
Naik, P.M. and Al-Khayri, J.M. 2018. Cell suspension culture as a means to produce polyphenols from date palm (Phoenix dactylifera L.). Ciencia e Agrotecnologia, 42: 464–473.
Nakasha, J.J., Sinniah, U.R., Kemat, N., and Mallappa, K.S. 2016. Induction, subculture cycle, and regeneration of callus in Safed musli (Chlorophytum borivilianum) using different types of phytohormones. Pharmacognosy Magazine. 12: S460–S464.
Penjor, T., Yamamoto, M., Uehara, M., Ide, M., Matsumoto, N., Matsumoto, R., and Nagano, Y. 2013. Phylogenetic relationships of citrus and its relatives based on matK gene sequences. PLoS ONE. 8: e62574.
Robles-Martínez, M., Barba-De la Rosa, A.P., Guéraud, F., Negre-Salvayre, A., Rossignol, M., and Santos-Díaz, M.D.S. 2016. Establishment of callus and cell suspensions of wild and domesticated Opuntia species: study on their potential as a source of metabolite production. Plant Cell, Tissue and Organ Culture. 124: 181–189.
Sari, Y. P., Kusumawati, E., Saleh, C., Kustiawan, W., and Sukartingsih, S. 2018. Effect of sucrose and plant growth regulators on callogenesis and preliminary secondary metabolic of different explant Myrmecodia tuberosa. Nusantara Bioscience. 10: 183–192.
Shahsavar A.R., Izadpanah, K., Tafazoli, E., and Sayed Tabatabaei, B.E. 2007. Characterization of citrus germplasm including unknown variants by inter-simple sequence repeat (ISSR) markers. Scientia Horticulturae. 112: 310–314
Sulistiani, E., Soelistyowati, D.T., Alimuddin, and Yani, S.A. 2012. Callus induction and filaments regeneration from callus of cottonii seaweed (Kappaphycus alvarezii (Doty) collected from natuna islands, riau islands province. Biotropica. 19: 103–114.
Tautz, D. and Renz, M. 1984. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Research. 12: 4127–4138.
Tunjung, W.A.S., Cinatl, J., Michaelis, M., and Smales, C.M. 2015. Anti-cancer effect of kaffir lime (Citrus Hystrix DC) leaf extract in cervical cancer and neuroblastoma cell lines. Procedia Chemistry. 14: 465–468.
Tunjung, W.A.S., Liana, D., and Hidayati, L., 2018. Antibacterial activity and composition of crude extracts of kaffir lime (Citrus hystrix DC.) leaves and callus. Proceedings of the Pakistan Academy of Sciences: B. Life and Environmental Sciences. 55: 45–53.
Tunjung, W.A.S., Fatonah, V., Christy, G.P., Triono, S., Hidayati, L., Priyanto, D., Purwestri, Y.A., Sasongko, A.B., Hennisa, Faizah, N., et al. 2020. Effect of growth factor in callus induction and bioactive compounds in seed explant of kaffir lime (Citrus hystrix DC.). Indonesian Journal of Pharmacy. 31: 61–68.
Zouine, J. and El Hadrami, I. 2007. Effect of 2,4-d, glutamine and BAP on embryogenic suspension culture of date palm (Phoenix dactylifera L.). Scientia Horticulturae. 112: 221–226.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand
https://cmuj.cmu.ac.th
Woro Anindito Sri Tunjung*, Asti Fitri Widyasari, Agnes Iskandar, Alisa Julia Nurulita, Aries Bagus Sasongko, Ari Indrianto, Endang Semiarti, and Maryani
Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia
Corresponding author: Woro Anindito Sri Tunjung, E-mail: wanindito@ugm.ac.id
Total Article Views
Editor: Korakot Nganvongpanit, Chiang Mai University, Thailand
Article history:
Received: November 15, 2020;
Revised: February 15, 2021;
Accepted: April 9, 2021;
Published online: April 26, 2021

