
Tropical Wetland Earthworm Vermifluid Promotes Mitotic Activities and Root Growth in Allium cepa at low concentrations
Ebenezer Olasunkanmi Dada*, Tirmizhi Abdulganiy, Stephen Olugbemiga Owa, Yusuf Olamilekan Balogun, Emmanuel Olorunleke Oludipe, Modupe Olatunde AkinolaPublished Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.064
Journal Issues : Number 3, July-September 2021
Abstract It is well established that earthworms and their products promote plant growth and improve crop yield, but more information about their mechanisms of action, especially at cellular level, is required. This study assessed the enzyme and bacterial activities of vermifluid secreted by a tropical wetland earthworm, Alma millsoni. The effects of the vermifluid on the mitotic activities and root growth of Allium cepa (onions) were evaluated. Vermifluid enzyme and bacterial activities were assessed using standard procedures. Mitotic activities were evaluated using the Allium assay squash technique. Root lengths in onions grown in graduated dilutions of vermifluid were measured using a metre rule. Phosphatase had the highest activity (39.00 ± 3.70 units/ml/min) in the vermifluid, followed by urease (4.90 ± 0.18 units/ml/min), protease (3.20 ± 0.28 units/ml/min), amylase (1.40 ± 0.09 units/ml/min) and lipase (0.001 ± 0.00 units/ml/min). The total microbial count (TBC) and total fungi count (TFC) in the vermifluid were 6.0 × 104 CFU/ml and 2.4 × 105 CFU/ml, respectively. Results also indicated that the lower the vermifluid concentrations, the higher the number of dividing cells and mitotic index in onion roots. Onion roots grown in 10% vermifluid had the highest significant (P < 0.05) root growth of 2.65 ± 0.30 cm, 4.01 ± 0.61 cm, and 4.54 ± 0.62 cm, at 12-hour, 24-hour, and 72-hour exposure period, respectively. The inverse relationships between onion mitotic activities, root growth and vermifluid concentrations imply that the growth-promoting enzymes and other products in vermifluid stimulate cellular root growth better in small concentrations.
Keywords: Alma millsoni, earthworm fluid, enzymes, microbial activities, mitosis, phosphatase.
Citation: Dada, E.O., Abdulganiy, T., Owa, S.O., Balogun, Y.O., Oludipe, E.O., Akinola,M.O., 2021. Tropical wetland earthworm vermifluid promotes mitotic activities and root growth in allium cepa at low concentrations. CMUJ. Nat. Sci. 20(3): e2021064.
INTRODUCTION
It has been well established that earthworms promote plant growth, improve crop yield, and renew soil fertility. Charles Darwin literarily described earthworms as the unheralded soldiers of mankind and farmers’ friends, working day and night under the soil (Sinha et al., 2010). Earthworms promote plant growth and crop productivity. They renew and restore soil fertility by continuously burrowing, ingesting, turning, mixing, aerating, and improving drainage of soil. They can increase soil fertility and plant growth by between 30 and 200% (Owa et al., 2010; Sinha et al., 2010; Dada et al., 2017). They have been successfully introduced into reclaimed soils to restore soil fertility (Butt, 1999).
The physical mechanisms of action of earthworms in improving plant growth and crop productivity are easily comprehensible and well documented. These mainly involve burrow movement, burrow creation, soil ingestion and milling in the gut of earthworms, and vermicast egestion. Burrowing leads to improved circulation and diffusion of soil gases including oxygen, efficient moisture drainage and dispersal, enhanced organic turnover and nutrients flow (Dada et al., 2021). Beyond, but related to these physical mechanisms, earthworms are known to harbour a vast array of microorganisms and enzymes in their guts, some of which directly and indirectly promote plant growth. Some enzymes and group of enzymes that have been associated with earthworms are amylases, proteases, lipases and cellulases. These are digestive enzymes which enhance biodegradation and mineralisation of organic matter (Aira et al., 2006; Ravindran et al., 2015). Earthworms, in association with microbes, have also been reported to secrete plant hormones which promote plant growth and increase bioavailability of nutrients (Sinha et al., 2011; Ravindran et al., 2015). Earthworm guts have been shown to host a diverse kind of microorganisms including species and strains of the genuses Bacillus, Pseudomonas, Enterobacters, Azobacters, Klebsiella, Proteus, Streptococcus (Singleton et al., 2003; Valle-Molinares et al., 2007; Brito-Vega and Espinosa-Victoria, 2009; Owa et al., 2013; Bamidele et al., 2014). Many of these bacteria have been described as plant growth promoters, free-living nitrogen fixers and phosphate stabilizers (Ravindran et al., 2015). These earthworm-associated microbes, enzymes, and hormones have also been confirmed to be richly present in earthworm cast (vermicast), mucus, surrounding soil and water. Hence, vermicasts (vermicompost), vermiwash or earthworm fluids are potential plant growth promoters (Suthar et al., 2005; Zarei et al., 2018).
There have been no standard in names or terms used to describe fluids obtained from earthworms’ body, products, and processes. Names like vermiwash, vermitea, vermicompost tea, veriwash, earthworm urine, earthworm fluid have been variously used by authors, sometimes in a conflicting manner. For instance, vermiwash may refer to the liquid that drains off vermicomposting bed (Zarei et al., 2018), or the light yellow fluid that results when earthworms are directly soaked in water to release mucus and exudates (Suthar et al., 2005). In this study, we have adopted the name ‘vermifluid’ to describe the light yellow fluid obtained when gut-voided earthworms are agitated in lukewarm distilled water for few minutes to secrete mucus and gastrointestinal fluid (exudates).
Though, earthworm fluids (vermiwash, vermitea) have been confirmed to stimulate plant growth and mitotic activities, the active compounds in the earthworm fluid and the mechanisms of growth stimulation at cellular level require more and wider research attention. Growth in plant at cellular level involves cell multiplication (mitosis), cell maturation and cell elongation in the apical meristems (root and shoot). Hence, the current research aimed to present additional information on the influence of vermifluid on plant growth at cellular level by assessing the effects of Alma millsoni vermifluid on mitotic activities and root growth of Allium cepa (onions). A. millsoni is a tropical earthworm species that inhabits marshy environments.
MATERIALS AND METHODS
Collection of earthworms and preparation of vermifluid
The adult A. millsoni used for this study were collected at the main campus of the University of Lagos, Akoka, Yaba, Lagos, Nigeria. Earthworm collection was by digging and hand sorting. Care was taken to minimise exposure of the earthworms to light, especially direct sunlight. To prepare vermifluid, harvested earthworms were put in dechlorinated tap water for about two hours to void their gut. Gut-voided earthworms weighing 400 g were put in a sterilised plastic bowl containing 200 ml lukewarm distilled water (37°C – 40°C). The earthworms were agitated for 5 minutes by swirling the container gently, causing them to secrete exudates (mucus, body fluids and gastrointestinal fluids). The earthworms were then transferred to pre-sterilised beaker containing 200 ml distilled water at room temperature, to wash them of adhering exudates. The contents of the plastic container and glass beaker were mixed together (Suthar et al., 2005); this mixture is the A. millsoni vermifluid. Four dilutions of the vermifluid (100%, 50%, 20%, 10%) were prepared from that stock by diluting with appropriate volumes of distilled water, where necessary. After vermifluid preparation, the earthworms were returned to their site of collection to mix with other members of their population. Vermifluid was stored at < 4°C when not in use, to avoid degradation.
Analysis of enzymes in vermifluid
A. millsoni vermifluid was screened for enzymes (phosphatase, urease, amylase, lipase, and protease) activities using the methods adapted from Anson (1938); Cupp-Enyard (2008).
Enumeration and isolation of heterotrophic bacteria and fungi in vermifluid
The vermifluid used in this study was analysed for total heterotrophic bacteria and fungi population in colony forming unit per millilitre (CFU/ml), using the ‘standard pour plate technique’ as described by Collins et al. (2008); Dubey and Maheshwari (2014). The vermifluid sample that had earlier been refrigerated at <4°C prior to analysis, was taken out and allowed to attain ambient temperature (26-31°C). One millilitre (1 ml) of vermifluid was taken and diluted serially in 9 ml of sterile water into eight folds (10- 1, to 10-8ml). One hundred microlitre (100 µl) of three different diluted samples were inoculated into sterile petri dishes in duplicates with the aid of micropipette fitted with sterile tips. Sterile molten agar and potato dextrose agar (supplemented with 1 mg per ml of chloramphenicol, to inhibit bacterial contaminants) were poured into the inoculated plates, swirled to ensure even distribution of the inoculum, and left to solidify. The plates were then incubated aerobically at 37°C for 24 hours (bacteria) and 28°C for 3-5 days (fungi). The colonies that developed were counted in duplicates using colony counter. The average colonies of the dilutions that meet up with the standard pour plate technique were taken and multiplied by the corresponding dilution factors, to give the total number of bacteria and fungi population per millilitre of the vermifluid sample analysed, as illustrated by the following equation.
![]()
Where: TBC = Total bacterial count
TFC = Total fungi count
Isolation of pure cultures of bacteria and fungi
Discrete colonies of bacteria and fungi were sub-cultured on sterile, dried, molten nutrient agar plates (using streaking techniques) and potato dextrose agar plates (using cork-borer), respectively. Both plates were then incubated at 37°C for 24 hours and room temperature for 3 days. The pure cultures were then selected for biochemical identification after preliminary examination through microscopy (Collins et al., 2008). The identity of the probable organisms was based on examination of cultural and cell morphological characteristics under the microscope (using 10x and 40x objective lenses).
Collection of onions and preparation for cytological assessment
The average sized (~ 6 cm diameter) bulbs of Allium cepa (onions) used for the study were purchased from Bariga market, Lagos, Nigeria (Latitude 6° 54’N and Longitude 3°39’E). The onion bulbs were sun-dried for two weeks after which the visibly healthy ones were selected for the test. The outer scales of the dried onion bulbs were removed and the dried roots were carefully scrapped to expose the primordial root. The onion bulbs were then grown in distilled water, at ambient temperature (26-31 °C), for 24 hours. Fifteen of the onions with the best root growth were selected, and the remaining ones were discarded. The selected onions bulbs were transferred to the prepared vermifluid treatments (10%, 20%, 50%, 100%). Onion bulbs grown in distilled water served as the control. Each treatment, including the control, was replicated thrice. The root length of each onion bulb was measured in centimetres (cm) using a metre rule after 24, 48, and 72 hours of exposure to test media (vermifluid and distilled water). The test media were changed daily. The mean root lengths of the onions grown in each treatment were determined by dividing the sum of root lengths by the number of roots measured in the root bundle. Since vermifluid is not a contaminant, and no visible root growth inhibition relative to control was observed, the percentage root length and root growth inhibition were not calculated as done in normal A. cepa genotoxicity assay.
Evaluation of mitotic activities in onion root cells
To evaluate mitotic activities in the root cells of onions grown in vermifluid, onion roots were harvested at 24, 48, and 72 hours of exposure to test media, and fixed in 1:3 acetic acid/ethanol (v/v). For slide preparation, harvested root was placed on a slide using forceps, and the root tip was cut for use. The other part of the root was discarded. The root tip was then hydrolysed in 1N HCl for 3 minutes. One root tip was squashed on each slide, and stained with lacto-aceto orcein for 20 to 25 minutes. Coverslips were carefully lowered onto the slide, avoiding air bubbles. Excess stain was removed using filter paper. Nail varnish was then used to seal the coverslip to the slide to prevent the slide from drying out. The slides were viewed under the 40x objective lens of a light microscope (Leica 2000 phase contrast microscope) to count the cells. The total number of cells and number of dividing cells were determined from 5 microscope fields for each of the treatments, including the control. These were used to calculate the mitotic index as in the equation below. No aberrant chromosome was observed. Photomicrographs of dividing cells were taken using a photomicroscope.
![]()
Where P, M, A, and T stand for prophase, metaphase, anaphase and telophase, respectively.
Statistical analysis of data
The data generated from the study were subjected to descriptive analysis using the General Linear Model (GLM) of IBM SPSS (version 25). Differences among the means were separated using Duncan Multiple Range Test (DMRT). Differences between means were considered significant at P < 0.05.
RESULTS
Enzyme activities in A. millsoni vermifluid
Analysis of enzyme activities in A. millsoni vermifluid as depicted in Figure 1 showed that phosphatase had the highest activity (39.00 ± 3.70 units/ml/min), followed by urease (4.90 ± 0.18 units/ml/min), protease (3.20 ± 0.28 units/ml/min), amylase (1.40 ± 0.09 units/ml/min) and lipase (0.001 ± 0.00 units/ml/min).
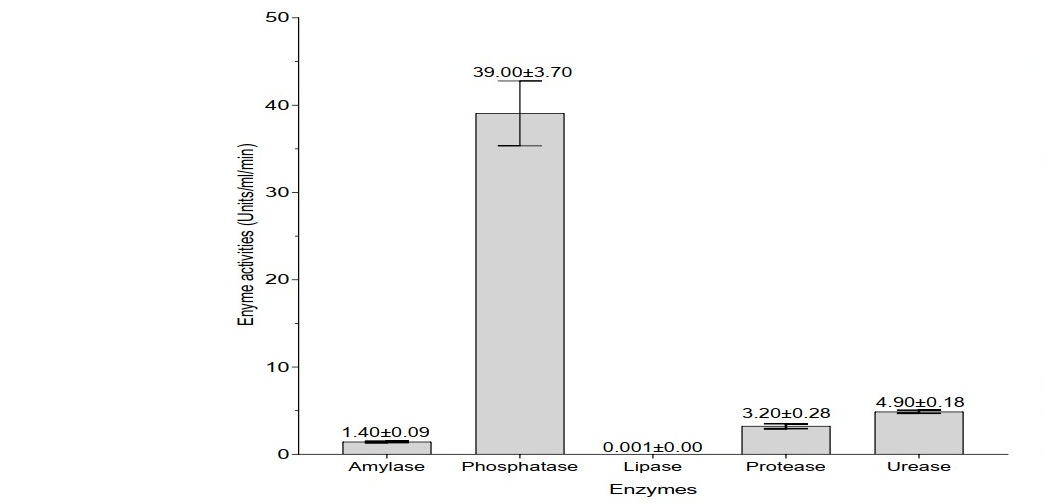
Figure 1. Enzyme activities in A. millsoni vermifluid
Microbial activities in A. millsoni vermifluid
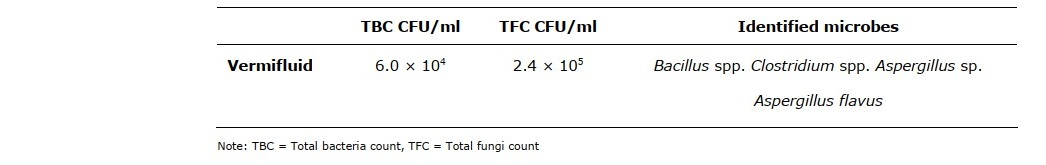
The total bacteria count, total fungi count, and identified microbes in A. millsoni vermifluid are shown in Table 1. The total bacteria count was 6.0 × 104 CFU/ml and the total fungi count was 2.4 × 105 CFU/ml. The organisms identified were Bacillus spp. Clostridium spp. Aspergillus flavus and another unidentified Aspergillus sp.
Table 1. Microbial activities in A. millsoni vermifluid
Effects of A. millsoni vermifluid treatments on A. cepa root growth
The mean root lengths (in cm) of onions grown in water (control), 10%, 20%, 50%, and 100% concentrations of A. millsoni vermifluid are presented in Figure 2. Onion roots of all the treatment groups showed continuous growth throughout the 72-hour experimental period. Root growth in onions showed an inverse relationship with vermifluid concentrations. Relative to onions grown in water and other vermifluid treatments, onion roots exposed to 10% vermifluid concentration had the highest and significant (P < 0.05) root length of 3.36 ± 0.34 cm, 4.01 ± 0.61 cm, and 4.54 ± 0.62 cm at 24-hour, 48-hour, and 72-hour of exposure, respectively. The roots of onions exposed to 100% vermifluid concentration had the lowest growth of 1.86 ± 0.51 cm, 2.25 ± 0.62 cm, and 2.33 ± 0.89 cm at 24-hour, 48-hour, and 72-hour of exposure, respectively.
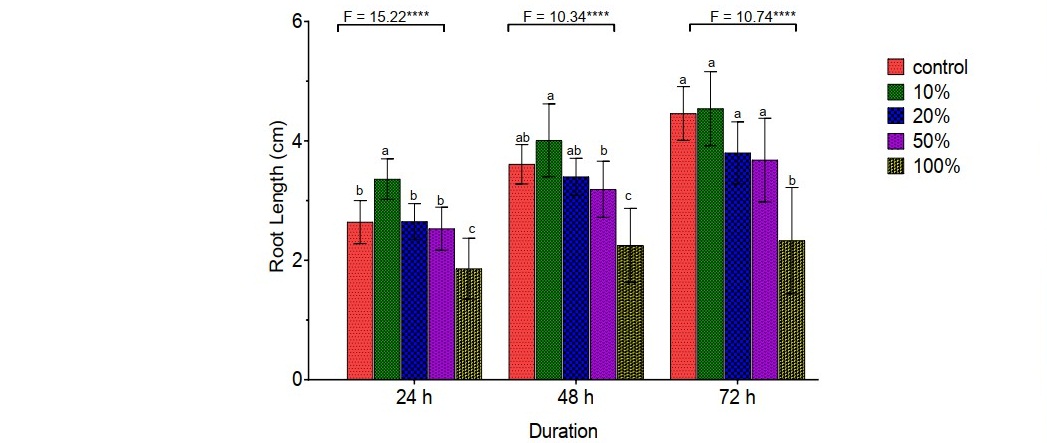
Figure 2. Effects of A. millsoni vermifluid treatments on A. cepa root growth
Mitotic activities in A. cepa roots exposed to different concentrations of vermifluid
Onion roots grown in water had significantly higher (P < 0.01) number of dividing cells and mitotic index, relative to those grown in A. millsoni vermifluid. The difference in number of dividing cells and mitotic index recorded for onions in different concentrations of vermifluid (10%, 20%, 50%, 100%) were generally not significant (P > 0.05). Mitotic index in onion roots increased with decreasing vermifluid concentrations, with onion roots exposed to 10% vermifluid recording the highest index of 12.85 ± 1.07, 13.03 ± 1.39, and 13.19 ± 2.35 at 24-hour, 48-hour, and 72-hour of exposure, respectively (Table 2). Representative photomicrographs of diving cells are shown in Figure 3(A, B, C, D).
Table 2. Mitotic activities in A. cepa roots exposed to different concentrations of vermifluid.
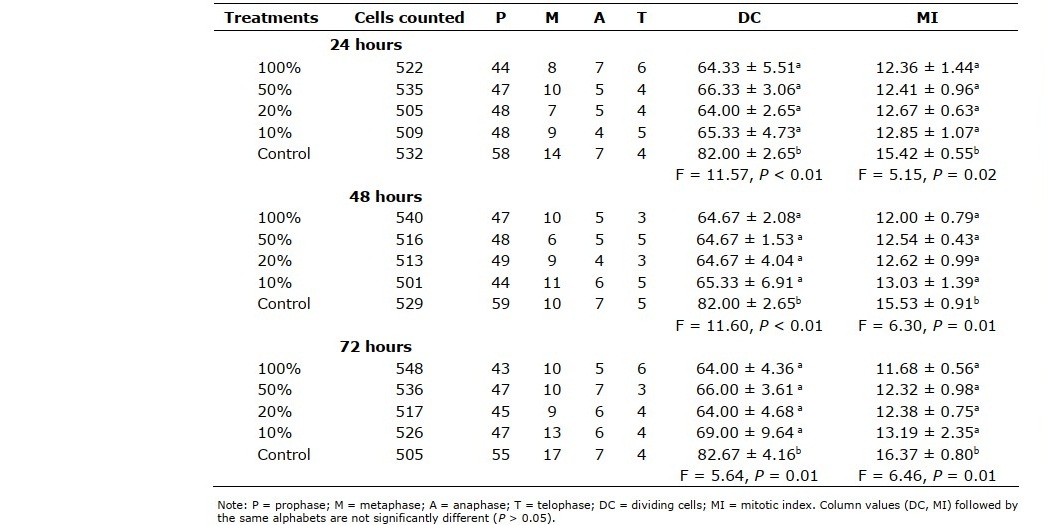
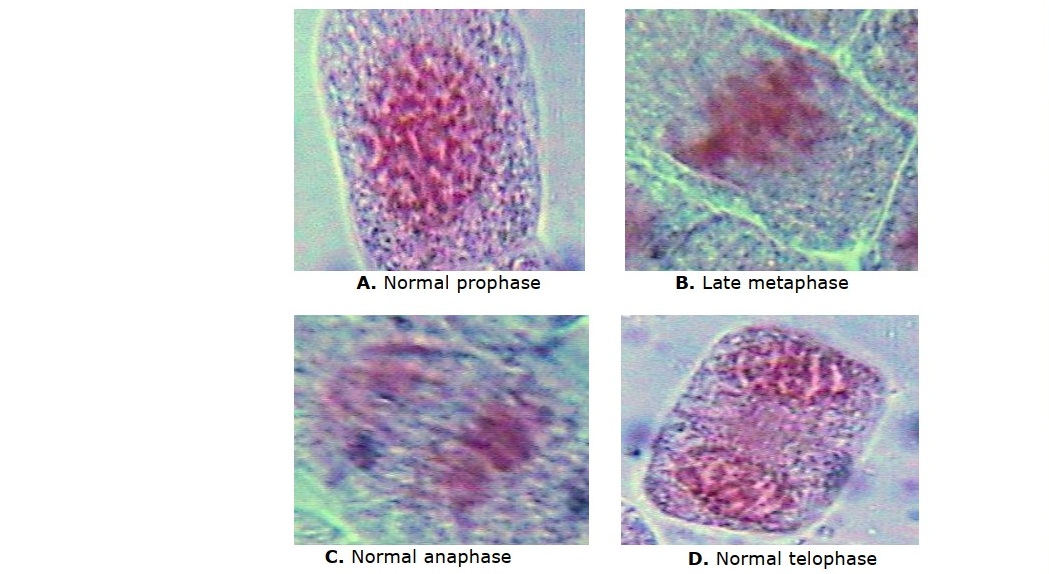
Figure 3. (A,B,C,D). Representative photomicrographs of diving cells of A. cepa roots grown in vermifluid
DISCUSSION
In this study, A. millsoni vermifluid was found to contain relatively high concentration of phosphatase, followed by moderate to low concentrations of urease, protease, amylase, and lipase. The vermifluid was active in microbial activities, with bacteria having higher count relative to fungi. The presence phosphatase, urease, protease and amylase in the vermifluid is in agreement with Zambare et al. (2008); Kizilkaya et al. (2010). While Kizilkaya et al. (2010) worked on vermiwash obtained from Eisenia fetida vermicomposting process, Zambare et al. (2008) worked on exudates secreted directly by an unnamed earthworm species. The presence of these enzymes in vermifliud is an indirect substantiation of earthworm-soil-plant relationships because the enzymes are important plant-growth promoters, naturally present in soil, and produced in plants. These enzymes play major roles in hydrolysing polymerised, complexed soil nutrients into useable plant nutrients (Ali and Elozeiri, 2017). Phosphatases, which occurred in highest concentrations, is an essential group of enzymes that increases phosphorus bioavailability to plant. Phosphorus is a plant macronutrient that functions in plant root and leaf development. It is abundantly present in soil, but mostly in complex, inaccessible form, until hydrolysed by phosphatases (Ali and Elozeiri, 2017). Proteases play important roles in nitrogen mineralisation (a process that regulates the amount of available nitrogen for plants), and also indirectly promote plant growth by digesting the cell wall of growth-impeding plant pathogens (Khare and Yadav, 2017). Ureases play an important role in nitrogen cycle by catalysing the hydrolysis of urea (a nitrogenous raw material) to ammonia and carbon dioxide (Cao et al., 2010). The occurrence of microorganisms including Bacillus spp. Clostridium spp. and Aspergillus spp. in the vermifluid is complementary to enzymes’ presence in same. Some of these microorganisms themselves produce plant growth-promoting enzymes. For instance, Bacillus spp. produce amylases, lipases, ureases and proteases; Aspergillus spp. produce amylase and protease (Turan et al., 2017). In essence, A. millsoni vermifluid, and likely vermifluid from other earthworm species, are a potential rich source of plant growth-promoting microorganisms and enzymes.
The foregoing fact notwithstanding, onion root growth in this study was observed to be inversely proportional to vermifluid concentrations. In the same vein, the number of dividing cells and mitotic index in onion roots increased with decreasing vermifuid concentrations. While this result contrasts some other findings (Sivasubramanian and Ganeshkumar, 2004; Ansari, 2008; Suthar, 2010; Jandaik et al., 2015), it corroborates the findings of Pandurangan et al. (2016); Senthilmurugan et al. (2018) who found vermifuid to promote increased growth at lower concentrations. Three developmental regions can be recognised in a root: (i) meristematic region (root tip) containing actively dividing cells, (ii) region of cell maturation and elongation where cells elongate rapidly, (iii) region of cell differentiation where cells become specialised. The pattern and extent of growth at any of these regions may be influenced by many factors including microorganisms (bacteria and fungi), enzymes and hormones (especially auxins and cytokinins). Depending on their concentrations, beneficial enzymes and bacteria may enhance shoot growth but inhibit primary root growth; and within the primary root, meristematic cell division may be affected either positively or negatively. In the root tip, high concentration of some enzymes and hormones may induce cell division but limit differentiation (Verbon and Liberman, 2016). Therefore, the fact that the least vermifluid concentration (10%) in this study yielded the highest root growth, relative to other concentrations and control (water), suggests that the microorganisms, enzymes and hormones present in the test vermifluid potentially promote root growth better at low concentrations. Lower vermifluid concentrations than those assayed in this study might have yielded even longer roots, and increased mitotic index. Hence, if A. millsoni vermifluid, and possibly other vermifluids, will be used as a plant growth supplement, it will be necessary to carry out a preliminary baseline test to determine the optimum concentration that will best impact growth.
CONCLUSION
Vermifluid was obtained from a tropical wetland earthworm, A. millsoni, by putting field-sourced, gut-voided earthworms in distilled water, and allowing them to secrete exudates. The vermifluid contained potentially growth-promoting enzymes and microorganisms. The lowest vermifluid concentration assayed in the study induced the highest growth and mitotic index in A. cepa roots, relative to other vermifluid of higher concentrations. Thus, suggesting that the enzymes and microorganisms in the vermifluid potentially promote root growth better at low concentrations. Therefore, if vermifluid will be used as a plant growth supplement, it will be necessary to carry out a preliminary baseline test to determine the optimum concentration that will best impact growth.
REFERENCES
Aira, M., Monroy, F. and Dominguez, J. 2006. Eisenia fetida Oligochaeta, Lumbricidae) activates fungal growth, triggering cellulose decomposition during vermicomposting. Microbial Ecology. 52: 738-746.
Ali, A.S and Elozeiri, A.A. 2017. Metabolic Processes During Seed Germination. 10.5772/intechopen.70653. DOI: 10.5772/intechopen.70653 http://dx.doi.org/10.5772/intechopen.70653
Ansari, A.A. 2008. Effect of vermicompost and vermiwash on the productivity of Spinach (Spinacia oleracea), Onion (Allium cepa) and Potato (Solanum tuberosum). World Journal of Agricultural Sciences. 4: 554-557.
Anson, M.L. 1938. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. The Journal of General Physiology. 22: 79–89.
Bamidele, J. A., Idowu, A. B., Adewunmi, B., Ademolu, K.O. and Atayese, A.O. 2014. Microbial diversity and digestive enzyme activities in the gut of earthworms found in sawmill industries in Abeokuta, Nigeria.
Brito-Vega, H. and Espinosa-Victoria, D. 2009. Bacterial diversity in the digestive tract of earthworms (Oligochaeta). Journal of Biological Sciences. 9: 192-199
Butt, K.R. 1999. Inculcation of earthworms into reclaimed soils: The U.K. experience. Journal of Land Degradation and Development. 10: 565-575.
Cao, F., Werner, A.K., Dahncke, K., Romeis, T., Liu, L. and Witte C. 2010. Identification and characterization of proteins involved in rice urea and arginine catabolism. Plant Physiology. 154: 98-108.
Collins, S.L., Sinsabaugh, R.L., Crenshaw, Green, C.L., Porras-Alfaro, A., Stursova, M. and H. Zeglin, L. 2008. Pulse dynamics and microbial processes in aridland ecosystems. Journal of Ecology. 96:413–420.
Cupp-Enyard, C. 2008. Sigma’s Non-specific Protease Activity Assay - Casein as a Substrate. Journal of Visualized Experiments. 19 : 1-3.
Dada, E.O., Njoku, K.L., Osuntoki, A.A. and Akinola, M.O. 2017. Effects of pot types and loamy soil enriched with different proportions of cow dung on the breeding performance of a wetland earthworm. Nigerian Journal of Ecology. 16(1): 14-21.
Dada, E.O., Akinola, M.O., Owa, S.O., Dedeke, G.A., Aladesida, A.A., Owagboriaye, F.O. and Oludipe, E.O. 2021. Efficacy of vermiremediation to remove contaminants from soil. Journal of Health and Pollution. 11: 210302.
Dubey, R.C. and Maheswari, D.K. 2014. A Textbook of Biotechnology. S. Chand and Co. Ltd., India. 702 pp.
Jandaik, S., Kumar, V. and Thakur, P. 2015. Vermiwash: Plant growth enhancer and antifungal agent. International Journal of Extensive Research. 2:38-41.
Khare, E. and Yadav, A. 2017. The role of microbial enzyme systems in plant growth promotion. Climate Change and Environmental Sustainability. 5: 122–145.
Kizilkaya, R., Hepsen Turkay, F.S., Bayrakli, B., Askin, T., Turkmen, C., Akca, I. and Ceyhen, V. 2010. ‘Enzyme and earthworm activities during vermicomposting in sewage sludge’. In: Kizilkaya, R., Gulser, C. and Dengiz, O. (eds.). Proceedings of the International Soil Science Congress on Management of Natural Resources to Sustain Soil Health and Quality. Ondokuz Mayis University, Samsun, Turkey. pp. 1047-1054.
Owa, S.O., Dedeke, G.A., Moreyibi, O.H. Morafa, S.O.A., Senjobi, B.A. and Aladesida, A. A. (2010). Partitioning of chemical effects of earthworms on growth performance of the vegetable Amaranthus. Australian Journal of Basic and Applied Sciences 4: 3755-3761.
Owa, S.O., Olowoparija, S.F., Aladesida, A.A., Dedeke, G.A. 2013. Enteric bacteria and fungi of the Eudrilid earthworm Libyodrilus violaceus. African Journal of Agricultural Research. 8: 1760-1766.
Pandurangan, P., Prasad, U., Sunkar, S., Saikrishna, N. M., Atul, A., Kumar, A. (2016). Formulation of vermiwash and humic Acid and its application on Allium cepa. Biosciences Biotechnology Research Asia. 13: 523-529.
Ravindran, B., Contreras-Ramos, S.M. and Sekaran, G. 2015. Changes in earthworm gut associated enzymes and microbial diversity on the treatment of fermented tannery waste using epigeic earthworm Eudrilus euginae. Ecological Engineering. 74: 394-401.
Senthilmurugan, S., Sattanathan, G., Vijayan, P.P.K. and Tamizhazhagan, V. 2018. Evaluation of different concentration of vermiwash on seed germination and biochemical response in Abelmoschus esculentus (L.). International Journal of Biology Research. 3: 228-231.
Singleton, D.R., Hendrix, P.F., Coleman, D.C. and Whitman, W. B. 2003. Identification of uncultured bacteria tightly associated with the intestine of the earthworm Lumbricus rubellus (Lumbricidae: Oligochaeta). Soil Biology and Biochemistry. 35: 1547-1555.
Sinha, R.K., Chauhan, K., Valani, D., Chandran, V., Soni, B.K., and Patel, V. 2010. Earthworms: Charles Darwin's 'Unheralded Soldiers of Mankind': Protective and productive for man and environment. Journal of Environmental Protection. 1: 251- 260.
Sinha, R. K., Hahn, G., Singh, P. K., Suhane, G. and Anthony Reddy, R. K. (2011). Organic farming by vermiculture: producing safe, nutritive and protective foods by earthworms (Charles Darwin’s friends of farmers). American Journal of Experimental Agriculture. 1: 393-399.
Sivasubramanian, K. and Ganeshkumar, M. 2004. Influence of vermiwash on the biological productivity of Marigold. Madras Agricultural Journal. 91: 221-225.
Suthar, S. 2010. Evidence of plant hormone like substances in vermiwash: An ecologically safe option of synthetic chemicals for sustainable farming. Ecological Engineering. 36:1089-1092.
Suthar, S., Choyal, R.., Singh, R. and Sudesh, R. (2005). Stimulatory effect of earthworm body fluid (vermiwash) on seed germination and seedling growth of two legumes. Journal of Phytological Research. 18: 219-222.
Turan, M. , Nikerel, E., Kaya, K., Kitir, N., Gunes, A., Mokhtari, N. E. P., Tüfenkçi, Ş., Karaman, M. R. and Çimri̇n, K. M. 2017. Enzyme Dynamic in Plant Nutrition Uptake and Plant Nutrition. <https://www.intechopen.com/books /enzyme-inhibitors- and-activators/enzyme-dynamic-in-plant-nutrition-uptake-and-plant-nutrition>
Valle-Molinares, R., Borges, S. and Rios-Velazquez, C. 2007. Characterization of possible symbionts in Onychochaeta borincana (Annelida: Glossoscolecidae). European Journal of Soil Biology. 43: 14-18.
Verbon, E.H. and Liberman, L.M. 2016. Beneficial microbes affect endogenous mechanisms controlling root development. Trends in Plant Science. 21: 218-229.
Zambare, V.P., Padul, M.V., Yadav A.A. and Shete, T.B. 2008. Vermiwash: biochemical and microbiological approach as ecofriendly soil conditioner. Asian Research Publishing Network Asian Research Publishing Network. 3: 1-5.
Zarei, M., Abadi, V.A.J.M. and Moridi, A. 2018. Comparison of vermiwash and vermicompost tea properties produced from different organic beds under green house conditions. International Journal of Recycling of Organic Waste in Agriculture. 7: 25-32.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Ebenezer Olasunkanmi Dada1,*, Tirmizhi Abdulganiy1, Stephen Olugbemiga Owa2,
Yusuf Olamilekan Balogun1, Emmanuel Olorunleke Oludipe1,3, Modupe Olatunde Akinola1
1 Department of Cell Biology and Genetics, Environmental Biology Unit, Faculty of Science, University of Lagos, Akoka, Yaba, Lagos, Nigeria.
2 Applied Biology and Biotechnology Programme, Landmark University, Omu-Aran, Kwara State, Nigeria.
3 Department of Microbiology, College of Pure and Applied Science, Landmark University, Omu-Aran, Kwara State, Nigeria.
Corresponding author: Ebenezer Olasunkanmi Dada, E-mail: eodada@yahoo.com; eodada@unilag.edu.ng
Total Article Views
Editor: Wasu Pathom-aree, Chiang Mai University, Thailand
Article history:
Received: August 19, 2020;
Revised: February 26, 2021;
Accepted: March 3, 2021;
Published online: March 25, 2021

