
Effect of Sucrose on Microtuber Induction and Inulin Accumulation in Jerusalem Artichoke (Helianthus tuberosus L.)
Nalinee Homsuwan, Kajorn Mapiyaphun, and Budsaraporn Ngampanya*Published Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.063
Journal Issues : Number 3, July-September 2021
Abstract The effect of sucrose concentrations and photoperiod applying on microtuber induction and inulin accumulation of Jerusalem artichoke (Helianthus tuberosus L.) have conducted under in vitro condition. Numbers, lengths and weights of microtubers induced from the single node explants with 0.50 cm above stem node- and stem node- cutting was not significant difference. Concentration of sucrose (51.70, 60, 80, 100 and 108.20 g/l) containing in microtuber induction medium (MST) and photoperiod applying (10.30/13.70, 12/12, 16/8, 20/4 and 21.60/2.40 h light/dark) significant effected to numbers of microtubers (P ≤ 0.05). The optimized sucrose concentration and photoperiod applying for highest numbers of microtubers was 100 g/l and 20/4 h light/dark, respectively. The significant difference of inulin content (P ≤ 0.05) in microtuber induced from various conditions was determined. The microtubers induced on MST medium supplemented with 80 g/l sucrose under 16/8 h light/dark accumulated highest inulin content (324.84 ± 40.78 mg/ g dry weight) when compared with others. Data suggested that sucrose and light duration played role in microtuber induction and inulin accumulation of Jerusalem artichoke.
Keywords: Inulin, Jerusalem artichoke, Microtuber, Photoperiod, Sucrose
Funding: The authors are grateful for the research funding provided by Department of Biotechnology, Faculty of Engineering and Industrial Technology, Silpakorn University, Nakornpathom, Thailand.
Citation: Homsuwan, N., Mapiyaphun, K., and Ngampanya, B. 2021. Effect of sucrose on microtuber induction and inulin accumulation in jerusalem artichoke (Helianthus tuberosus L.). CMUJ. Nat. Sci. 20(3): e2021063.
INTRODUCTION
Jerusalem artichoke (Helianthus tuberosus L.) is one of important tuberous crops. It accumulates high amount of inulin in tuber as a reserve carbohydrate (Judprasong et al., 2018). Inulin is a fructan which consists of linear chains of β - 2, 1 - linked D - fructofuranose molecules terminated by a glucose residue through a sucrose-type linkage at the reducing end (Chi et al., 2011). Approximately 50% inulin is accumulated in the dried materials of the tubers (Pandey et al., 1999; Judprasong et al., 2011; Judprasong et al., 2018). It is currently used in food and pharmaceutical industry owing to its numerous benefits to human health and low caloric values when compared to other carbohydrates (Takeachi and Nagashima, 2011). In addition, it also has prebiotic property which can decrease the level of pathogenic bacteria and stimulate the growth of probiotic bacteria in the intestine. Jerusalem artichoke is therefore a choice of plants for inulin production. This plant can grow and yield in various conditions including cold and drought areas (Chi et al., 2011; Takeachi and Nagashima, 2011; Tanjor et al., 2012). Tubers from field- grown plant are major material for inulin production. However, those of field- grown tubers took at least four months for high yielding and may face plant disease and pest harmful. An alternative approach for tuber production of plant without abiotic and biotic interference is plant tissue culture technique. In vitro tuberization of plants such as potato (Solanum tuberosum L.) (Estrada et al., 1986; Dobránski et al., 2008; Mokshin et al., 2008; Nistor et al., 2010; Motallebi-Azar and Kazemiani, 2012; Fufa and Diro, 2014; Al-Hussaini et al., 2015; Rahman et al., 2015; Hossain et al., 2017; Li et al., 2020), steroid yam (Dioscorea composite) (Alizadeh et al., 1998) and Jerusalem artichoke (Helianthus tuberosus L.) (Gamburg et al., 1999; Polsa and Ngampanya, 2015) has been reported. In vitro developing tubers called microtubers of those plants were induced and taken their advantage as disease-free germplasm for vegetative micrpropagation and experimental tools in basic research. Tuberization under in vitro condition is a complex process relied on many factors such as environmental factors (light duration, light quality and temperature) (Dobránski et al., 2008; Al-Hussaini et al., 2015; Li et al., 2020), composition of medium (sucrose, nitrogen, gelling agents) (Dobránski et al., 2008; Motallebi-Azar and Kazemiani, 2012; Al-Hussaini et al., 2015; Hossain et al., 2017), genotype and explants used for tuberization (Estrada et al., 1986; Dobránski et al., 2008; Nistor et al., 2010; Fufa and Diro, 2014). There has reported that photoperiod and storage temperature influenced on microtuber induction and inulin accumulation in in vitro microtuber of Jerusalem artichoke (Polsa and Ngampanya, 2015). Apart from light and temperature, medium composition particularly sucrose has also reported as an important factor playing role in tuberization of potato under in vitro condition (Dobranski et al., 2008; Nistor et al., 2010; Fufa and Diro, 2014; Al-Hussaini et al., 2015; Hossain et al., 2017). Sucrose is a carbon source that serves as energy for in vitro plant growth and development. There has evident that it can stimulate tuber formation and numbers in potato (Nistor et al., 2010; Fufa and Diro, 2014; Al-Hussaini et al., 2015; Hossain et al., 2017). Like potato, Jerusalem artichoke is in a group of tuberous crop and has a same pattern of development. Therefore, it may be possible to stimulate tuber formation via sucrose signaling as previously reported in potato (Dobranski et al., 2008; Nistor et al., 2010; Al-Hussaini et al., 2015; Hossain et al., 2017). Microtuber of potato did not induce and form when cultured on medium without sucrose supplementation. Minimum concentration of sucrose for microtuber formation in potato was 8% (Hossain et al., 2017). Additionally, light duration and quality is also reported as an environmental factor effected to tuber formation of potato (Dobranski et al., 2008; Al-Hussaini et al., 2015; Li et al., 2020). This research aimed to evaluate effects of sucrose concentration and photoperiod applying on the formation and numbers of microtubers of Jerusalem artichoke under in vitro condition. Additionally, inulin production and accumulation were also determined. The successful of Jerusalem artichoke microtuber induction under in vitro condition together with its high accumulation of inulin would be an alternative way for inulin production instead of inulin production relied on field- grown tuber.
MATERIALS AND METHODS
Effect of explant cutting on microtuber induction
Microtuber induction from single node explants (the 2nd- 4th stem node) with different cutting types (0.50 cm above stem node- and stem node- cutting) were compared. The 1.50 - 2 cm long nodal segments with axillary bud were excised from one-month old sterile plant and aseptically transferred to bottle containing tuber induction medium (MST; MS basal salts + MS vitamins + 5.00 mg/l benzyladenine (BA) + 500 mg/l chlorochlorine chloride (CCC) + 0.40 mg/l Thiamine HCl + 1% activated charcoal + 80 g/l sucrose, pH 5.60) (Polsa and Ngampanya, 2015). The cultures were cultivated under fluorescent light with long day photoperiod (16/8 h light/dark) at 25 ± 2°C for 45 days. Formation time, numbers, lengths and weights of microtubers were recorded. Three pieces of explants were cultured in one culture bottle. At least three bottles with 9 explants in total were investigated per treatment. Experiments were conducted three times with three replications (bottles).
Effect of sucrose on microtuber induction and inulin accumulation The 2 cm long nodal segments with axillary bud (0.50 cm above stem node- cutting) were excised from sterile plant and aseptically transferred to bottle containing MST medium (Polsa and Ngampanya, 2015) supplemented with 60, 80 or 120 g/l sucrose. The cultures were cultivated under fluorescent light with long day photoperiod (16/8 h light/dark) at 25 ± 2°C for 45 days. Ten pieces of explants were cultured per treatment. Experiments were conducted three times, and each experiment contained 10 explants). After 45 days of culturing, the formation and number of microtubers were recorded. The determination of fresh weight and the production of inulin was also recorded.
Optimization of sucrose concentration and photoperiod applying on microtuber induction and inulin accumulation
To obtain high microtuber numbers, the experimental treatment of sucrose concentration and photoperiods treatments was optimized by central composite design (CCD). The cultures were cultivated at 25 ± 2°C for 45 days. Ten pieces of explants were cultured per treatment. Experiments were conducted three times, and each experiment contained 10 explants). After 45 days of culturing, the formation and number of microtubers were recorded. The determination of dry weight and the production of inulin was also recorded.
Determination of inulin content by HPLC
Inulin content was determined according to HPLC analysis procedure described by Bampensin et al (2019). The obtained microtubers from each treatment were extracted by distilled water. The ratio of microtubers to distilled water was 1 : 2 (w/v). The homogenate was extracted three times for 10 min at room temperature. The supernatant was collected by centrifugation and pooled. Inulin contents were analyzed by HPLC. The types and contents of inulin were analyzed by comparison to HPLC chromatograms of the known inulin.
Statistical analysis
The obtained data were statistically analyzed using One - way ANOVA by SPSS program version 17.0. Significant difference was assessed at 5% level of probability (P ≤ 0.05).
RESULTS
Effect of different explant cutting on microtuber induction
Formation of microtubers from the single node explants with 0.50 cm above stem node- and stem node- cutting cultured on MST supplemented 80 g/l sucrose was observed after 15 days of induction and size of those microtubers was larger upon time course of culturing until 45 days as shown in Figure 1.
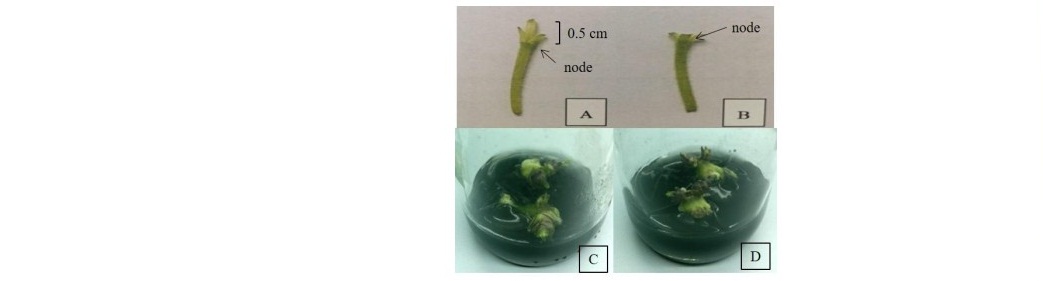
Figure 1. The single node explant with 0.50 cm above stem node- cutting (A) cultured on MST medium supplemented with 80 g/l sucrose under long day photoperiod (16/8 h light/dark) for 45 days (C) and the single node explant with the stem node- cutting (B) cultured on MST medium supplemented with 80 g/l sucrose under long day photoperiod (16/8 h light/dark) for 45 days (D).
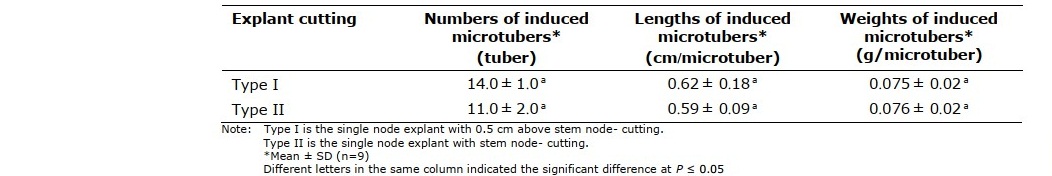
Table 1 indicated numbers, lengths and weights of microtubers induced from the single node explant with 0.50 cm above stem node- (type I) and stem node- cutting (type II) was not significant difference. The numbers of microtubers obtained from that of the type I cutting was higher than type II, therefore the type I cutting explant was selected for further experiments because of its numbers of microtubers was higher than those recorded in type II cutting explant.
Table 1. Numbers, lengths and weights of microtubers induced from different cutting explants cultured on MST supplemented 80 g/l sucrose under long day photoperiod (16/8 h light/dark) for 45 days.
Effect of sucrose on microtuber induction and inulin accumulation Microtubers were successfully induced on the tuber induction medium (MST) supplemented all tested sucrose concentrations as shown in Figure 2. In case of explants cultured on MST with 60 g/l sucrose, shoot elongation and leaves development were clearly seen. In agreement with other reports (Gamburg et al., 1999; Polsa and Ngampanya, 2015), microtubers formation was observed on MST supplemented 80 g/l sucrose. The size of microtubers induced on MST supplemented with 80 g/l sucrose was larger than those induced on MST supplemented with 120 g/l sucrose.

Figure 2. Microtubers were induced on tuber induction medium supplemented with different sucrose concentrations; 60 (A), 80 (B) and 120 g/l (C) under long day photoperiod (16/8 h light/dark) for 45 days.
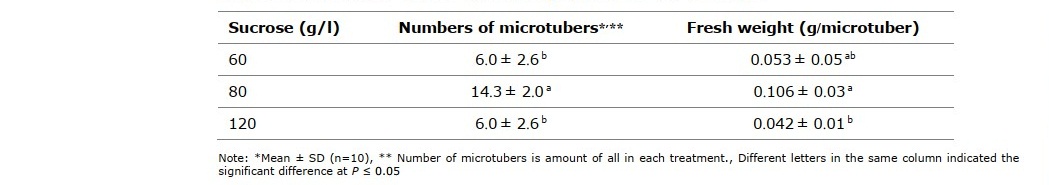
There was significant difference (P ≤ 0.05) of microtuber numbers and fresh weight as shown in Table 2. High numbers of microtubers was determined in the MST medium supplemented with 80 g/l sucrose while those induced on MST with 60 and 120 g/l sucrose gave less microtubers numbers and fresh weight.
Table 2. Numbers and fresh weight of microtubers induced on MST medium supplemented with 60, 80 and 120 g/l sucrose for 45 days.
After microtuber induction for 45 days, the obtained microtubers were extracted to determine the inulin content using HPLC technique by comparison to HPLC chromatograms of known inulin and other standard sugars. HPLC chromatograms revealed that peak pattern of microtuber extract was same as the peak pattern of extract from field- grown tuber as shown in Figure 3. The extract consisted of 6 carbohydrates which were inulin, nystose, 1-kestose, sucrose, glucose and fructose.
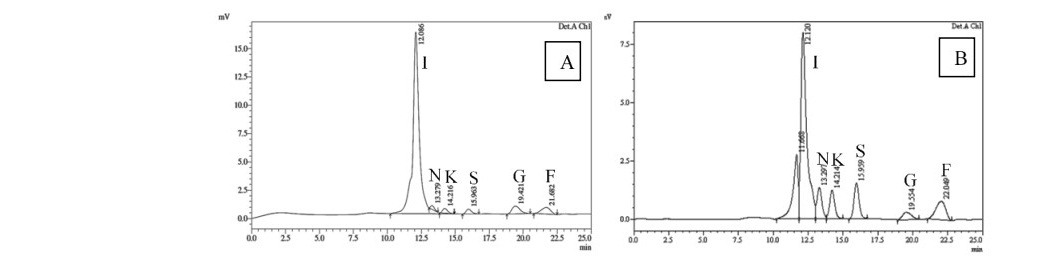
Figure 3. HPLC chromatograms of extract from field-grown tuber of Jerusalem artichoke (A) and microtubers induced on MST medium with 120 g/l sucrose (B) I = inulin, N = nystose, K = 1-kestose, S = sucrose, G = glucose and F = fructose.
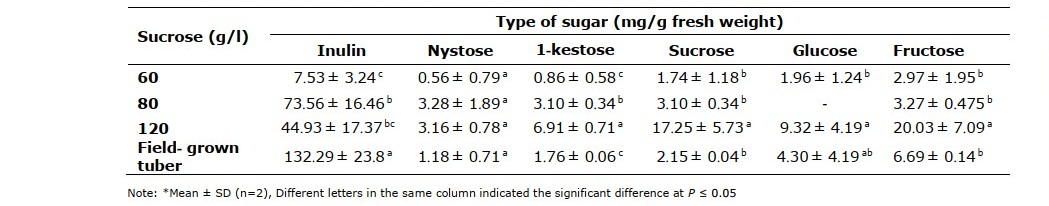
The inulin content in microtubers induced from MST medium with different concentrations of sucrose (60, 80 and 120 g/l) were significant difference (P ≤ 0.05) as shown in Table 3. The microtubers induced on MST medium supplemented 80 g/l sucrose accumulated higher inulin content (73.56 ± 16.46 mg/ g fresh weight) than those cultured on medium supplemented 120 (44.93 ± 23.80 mg/ g fresh weight) and 60 g/l sucrose (7.53 ± 3.24), respectively. Although, the inulin content in microtubers obtained from MST medium with 80 and 120 g/l were not significant difference (P ≤ 0.05) but the maximum yield of inulin content was obtained from induced microtubers cultured on MST medium supplemented with 80 g/l sucrose (Table 2). Additionally, this condition also gave higher values of fresh weight and number of obtained microtubers.
Table 3. Inulin, Fructo-oligosaccharides (Nystose and 1-kestose) and other sugars content of microtubers induced in different sucrose concentrations for 45 days.
Optimization of sucrose concentration and photoperiod applying on microtuber induction and inulin accumulation
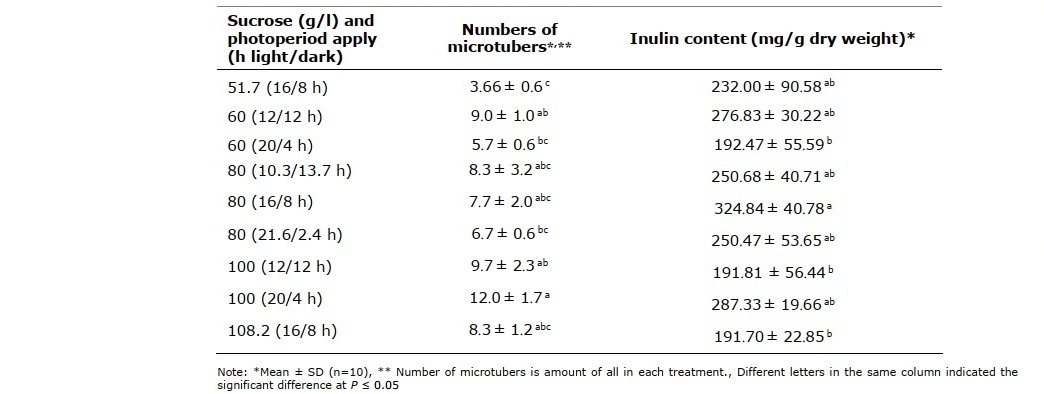
Sucrose concentration and photoperiod applying was optimized by central composite design (CCD). As shown in Table 4, sucrose concentrations in microtuber induction medium and photoperiod applying significantly affected to numbers of microtubers (P ≤ 0.05). The optimum condition for numbers of microtubers was 100 g/l sucrose with 20/4 h light/dark photoperiod while inulin content was highest in microtubers induced in 80 g/l sucrose with 16/8 h light/dark photoperiod.
Table 4. Numbers and inulin content of microtubers induced from different sucrose concentrations and photoperiod applying
DISCUSSION
In vitro tuber is a complex process relied on many factors such as environmental factors (light and temperature), composition of medium (sucrose, nitrogen, gelling agents), genotype and explants used for tuberization (Dobránski et al., 2008; Motallebi- Azar and Kazemiani, 2012; Fufa and Diro, 2014; Al-Hussaini et al., 2015; Hossain et al., 2017; Li et al., 2020). In potato, microtubers were induced from over 50 different genotypes. The induced microtubers of potato were morphologically similar to field-grown tuber. However, different microtuber numbers and weight among genotypes tested were observed (Estrada et al., 1986; Nistor et al., 2010; Fufa and Diro, 2014; Hossain et al., 2017). Explant used for tuberization is an important factor. The physiological age, type of explants and explant density effected to tuber development. Stolons or axillary buds has documented to use as explant for tuber formation. Nodal segments were suitable explant for tuberization of plant under in vitro condition and explants with one node is induced and initiated higher microtubers than that explant with two-five nodes (Dobránski et al., 2008). One bud cutting was successful for microtuber induction in potato (Mokshin et al., 2008; Motallebi-Azar and Kazemiani, 2012; Fufa and Diro, 2014) and Jerusalem artichoke (Gamburg et al., 1999; Polsa and Ngampanya, 2015). To control hormonal balance, the single node from the 2nd -4th stem node was selected as explant for tuberization in this study. Both cutting type was not significant difference in microtuber formation and size. It may suggest that the hormone level at axillary bud of both explants was not difference and bud dormancy was terminated leading to tuber development. Apart from effect of explant used for tuberization, carbon source in medium also strongly influenced on tuber formation. In this study, microtubers were successfully induced on medium with exogenously supplemented plant growth regulators (BA and CCC as cytokinin) and sucrose at 60, 80 and 120 g/l. In case of explants cultured on MST with 60 g/l sucrose, shoot elongation and leaves development were clearly seen. It dues to sucrose concentration is not high enough for extra organs formation. Hossiain et al. (2017) has reported that stem segments of potato cultured on medium without sucrose supplementation did not form microtuber. Sucrose concentration at 8% was minimum level for microtuber formation. In current study, microtubers formation of Jerusalem artichoke was also observed on MST supplemented 80 g/l sucrose. This result was in agreement with other reports of microtubers induction in Jerusalem artichoke (Gamburg et al., 1999; Polsa and Ngampanya, 2015). The size of microtubers induced on MST supplemented with 80 g/l sucrose was larger than those induced on MST supplemented with 120 g/l sucrose. As generally known that sucrose serves as energy source for in vitro plant growth and development, but high level of soluble sugars can cause the formation of extra organ in different plant species such as potato (Gibson, 2005). Although high sucrose concentration can induce microtuber but higher level may effect to size of microtuber as well. There has reported in potato tuberization that increasing of sucrose concentration up to 12% caused a delay in tuber initiation and resulted in smaller tuber (Dobránski et al., 2008; Hossiain et al., 2017). In this study, number and size of microtubers obtaining from medium supplemented 120 g/l sucrose was also less than those induced on 80 g/l sucrose supplementation. In order to optimize sucrose concentration for microtuber production, sucrose concentrations at 51.70, 60, 80 and 100 g/l also tested in this study. The results indicated that 80-100 g/l sucrose is optimal for microtuber production. Additionally, microtuber numbers induced under long or short day photoperiod was not significant difference when 80-100 g/l sucrose was supplemented while short day photoperiod (12/12 h light/dark) effected to number of microtubers induced on medium supplemented 60 g/l sucrose. In potato, light duration significantly affected to tuber initiation only in high carbon source concentration (Al-Hussaini et al., 2015). Other sugars such as glucose, fructose and alcohol sugars (mannitol and sorbitol) has also reported to use for tuber induction of potato (Mokshin et al., 2008; Motallebi-Azar and Kazemiani, 2012). Among carbon sources used for tuberization, sucrose is a sugar normally used in plant tissue culture medium. It plays role in plant development and morphogenesis. It is essential for in vitro plant as energy source or osmotic potent agent. Additionally, it also serves as a signal for in vitro tuberization of plant. There has reported that high sucrose concentration (8%) in medium without plant growth regulators supplementation can induce microtuber of potato (Alix et al., 2001; Dobránski et al., 2008; Al-Hussaini et al., 2015; Hossiain et al., 2017). For inulin accumulation, results indicated that microtubers of Jerusalem artichoke induced in this study can produce same carbohydrates of field- grown tuber. The optimum condition for high inulin accumulation is 80 g/l sucrose supplementation. All data obtained from this study could be a choice for inulin production by means of organ culture techniques. Microtuber production is an efficient method for obtaining a healthy material that can produce same valuable substances as found in natural grown plant. In the same time the microtubers are important because they could be produced in any period of the year and they are easy to be transported and stored (Nistor et al., 2010). However, cost of high sucrose supplemented in culture medium for microtuber and inulin production should be further feasibility study.
CONCLUSION
High sucrose concentrations significantly affected to microtubers numbers of Jerusalem artichoke (P ≤ 0.05). The high numbers of microtuber was obtained when 80- 100 g/l sucrose was supplemented to MST medium. Microtubers can produce inulin that was similar to that was found in field- grown tuber. In conclusion, MST medium supplemented 80-100 g/l sucrose was optimal for microtuber induction and inulin accumulation.
ACKNOWLEDGMENTS
Financial support from Silpakorn University Research, Innovation and Creativity Administration Office (SURIC) and Department of Biotechnology, Faculty of Engineering and Industrial Technology are gratefully acknowledged.
AUTHOR CONTRIBUTIONS
Nalinee Homsuwan conducted all of the experiments excepted the part of optimization of sucrose concentration and photoperiod applying on microtuber induction and inulin accumulation, performed the statistical and wrote the manuscript. Kajorn Mapiyaphun conducted the experiments in the part of optimization of sucrose concentration and photoperiod applying on microtuber induction and inulin accumulation, performed the statistical. Budsaraporn Ngampanya assisted in designed and gave the advices in all of the experiments, wrote and improve the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
Al-Hussaini, Z.A., Yousif, SH.A., and Al-Ajeely, S.A. 2015. The role of sucrose and light duration on in vitro tuberization for two cultivars of potato Solanum tuberosum L. International Journal of Current Microbiology and Applied Sciences 4: 277-283.
Alizadeh, S., Mantell, S.H., and Viana, A.M. 1998. In vitro shoot culture and microtuber induction in the steroid yam Dioscorea composita Hemsl. Plant Cell, Tissue and Organ Culture 53: 107–112.
Bampensin, T., Homsuwan, N., and Ngampanya, B. 2019. Effect of amino acids on biomass and inulin production of suspension culture in Kaentawan (Helianthus tuberosus L.). p.143-149. In proceeding of the 3rd ISAT 2019 and 17th ISBB 2019 harmonization of smart and sustainable agriculture, 2-5 July 2019. Krabi Resort, Krabi province, Thailand.
Chi, Z., Zhang, T., Cao, T., Liu, X., Cui, W., and Zhao, C. 2011. Biotechnology potential of inulin for bioprocesses. Bioresource Technology 102: 4295-4303.
Dobránszki, J., Magyar-Tábori, K., and Hudák, I. 2008. In vitro tuberization in hormone- free systems on solidified medium and dormancy of potato microtuber. Fruit, Vegetable and Cereal Science and Biotechnology (Special Issue 1): 82-94.
Estrada, R., Tovar, P., and Dodds, J.H. 1986. Induction of in vitro tubers in a broad range of potato genotypes. Plant Cell, Tissue and Organ Culture 7: 3–10.
Fufa, M., and Diro, M. 2014. Microtuber induction of two potato (Solanum tuberosum L.) varieties. Advances in Crop Science and Technology 2.
Gamburg, K.Z., Vysotskaya, E.F., and Gamanets, L.V. 1999. Microtuber formation in micropropagated Jerusalem artichoke (Helianthus tuberosus). Plant Cell, Tissue and Organ Culture 55: 115-118.
Gibson, S.I. 2005. Control of plant development and gene expression by sugar signaling. Current Opinion in Plant Biology 8: 93-102.
Hossain, Md.S., Hossain, M.M., Hossain, T., Haque, M.M., Zakaria, M., and Sarkar, Md.D. 2017. Varietal performance of potato on induction and development of microtuber in response to sucrose. Annals of Agricultural Science 62: 75-81.
Judprasong, K., Tanjor, S., Puwastien, P., and Sungpuag, P. 2011. Investigation of Thai plants for potential sources of inulin-type fructans. Journal of Food Composition and Analysis 24: 642-649.
Judprasong, K., Archeepsudcharit, N., Chantapiriyapoon, K., Tanaviyutpakdee, P., and Temviriyanukul, P. 2018. Nuttrients and natural toxic substances in commonly consumed Jerusalem artichoke (Helianthus tuberosus L.) tuber. Food Chemistry 238: 173-170.
Li, R., You, J., Kong, L., Long, J., Yan, Y., Xu, Z., and Liu, X. 2020. Monochromatic lights regulate the formation, growth, and dormancy of in vitro- grown Solanum tuberosum L. microtubers. Scientia Horticuturae 261:108947.
Mokshin, E.V., Lukatkin, A.S., and Teixeira da Silva, J.A. 2008. Induction of microtuberization for one-bud potato cuttings in vitro. Fruit, Vegetable and Cereal Science and Biotechnology 2 (Special Issue): 118-124.
Motallebi-Azar, A., and Kazemiani, S. 2012. Effect of alcohol sugars on in vitro potato microtuberization. South Western Journal of Horticulture, Biology and Environment 3: 73-83.
Nistor, A., Campeanu, G., Atanasiu, N., Chiru, N., and Karácsonyi, D. 2010. Influence of potato genotypes on “in vitro” production of microtubers. Romanian Biotechnological Letters 15: 5317-5324.
Pandey, A., Soccol, C.R., Selvakumar, P., Soccol, V.T., Krieger, N., and Jose, D. 1999. Recent developments in microbial inulinases, its production, properties and industrial applications. Applied Biochemistry and Biotechnology 81: 35-52.
Polsa, S., and Ngampanya, B. 2015. Effect of photoperiod and storage temperature on inulin and fructo-oligosaccharides accumulation in In vitro microtubers of kaentawan (Helianthus tuberosus L.). Journal of Food Science and Agricultural Technology 1: 89-92.
Rahman, Md. Z., Shahinul Islam, S. M., Chowdhury, A.N., and Subramaniam, S. 2015. Efficient microtuber production of potato in modified nutrient spray bioreactor system. Scientia Horticulturae 192: 369-374.
Takeuchi, J., and Nagashima, T. 2011. Preparation of dried chips from Jerusalem artichoke (Helianthus tuberosus L.) tubers and analysis of their functional properties. Food Chemistry 126: 922-926.
Tanjor, S., Judprasong, K., Chaito, C., and Jogloy, S. 2012. Inulin and fructooligosacharides in different varieties of Jerusalem artichoke (Helianthus tuberosus L.). KKU Research Journal 17: 25-34.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Nalinee Homsuwan, Kajorn Mapiyaphun, and Budsaraporn Ngampanya*
Department of Biotechnology, Faculty of Engineering and Industrial Technology, Silpakorn University, Muang, Nakornpathom 73000, Thailand
Corresponding author: Budsaraporn Ngampanya, E-mail: ngampanya_b@silpakorn.edu
Total Article Views
Editor: Wasu Pathom-aree, Chiang Mai University, Thailand
Article history:
Received: December 25, 2020;
Revised: February 19, 2021;
Accepted: March 3, 2021;
Published online: March 25, 2021

