
Sterile Tissue Preparation and Callus Induction of Curcuma longa Linn.
Warunya Kaewthip, Srisulak Dheeranupattana, Pornchai Junta, and Lalida Shank*Published Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.062
Journal Issues : Number 3, July-September 2021
Abstract Curcuma longa Linn. (family Zingiberaceae), commonly known as ‘turmeric’, is native to Southeast Asia. Turmeric has been used for color, flavor as a spice in cuisine and employed for treatment of various diseases. The major component in yellow-pigmented fraction of turmeric is curcuminoids. Curcuminoid production in callus of C. longa Linn. is our focus of study. Sterile techniques to obtain germ-free of C. longa Linn. explants were investigated and the results showed that immersing rhizome buds in 70% ethanol for 5 min, followed by 0.10% HgCl2 for 10 min offered approximately 66% survival rate. Multiple shoots were generated from the aseptic rhizome explants cultured on Murashige and Skoog (MS) agar medium fortified with 3.00 µM of 6-Benzylaminopurine (BA) and 0.50 µM of 1-Naphthaleneacetic acid (NAA) at 25 ± 2°C under a photoperiod of 16 h light and 8 h dark. The sterile leaf sheath and root were subsequently used for callus induction which produced various responses when cultured on MS agar medium fortified with different concentrations of 2,4-dichlorophenoxy acetic acid (2, 4-D), Thidiazuron (TDZ) and BA. The highest induction yields of friable callus were obtained from leaf sheath segments cultured on MS agar medium fortified with 0.50 mg/l 2, 4-D which are the conditions proposed for successful production of callus culture of C. longa Linn.
Keywords: Callus induction, Curcuma longa Linn., Turmeric, Plant tissue culture
Funding: The authors are grateful to Department of Chemistry and Center of Excellence for Innovation in Chemistry (PERCH-CIC), Faculty of Science, Chiang Mai University for project funding.
Citation: Kaewthip, W., Dheeranupattana, S., Junta, P., and Shank, L. 2021 Sterile Tissue Preparation and Callus Induction of Curcuma longa Linn. CMUJ. Nat. Sci. 20(3): e2021062.
INTRODUCTION
C. longa Linn., known as turmeric, is native to Southeast Asia and extensively cultivated in India and Southeast Asian countries. Turmeric has been used as a spice for color and flavor in food preparation (Ravindran et al., 2007; Nair, 2013) and mentioned in traditional literature to be valuable for treatment of various diseases (Goel et al., 2008; Rahman et al., 2015; El-Kenawy et al., 2019). The major component in yellow-pigmented fraction of turmeric is curcuminoids which consist of a mixture of curcumin (75–80%), demethoxycurcumin (15–20%), and bisdemethoxycurcumin (3–5%) (Hamaguchi et al., 2010).
Curcumin, the main component of curcuminoids has been shown to be a potent scavenger of a variety of reactive oxygen species and inhibit lipid peroxidation in different animal models (Maheshwari et al., 2006). In addition, its anti-inflammatory activity has been demonstrated by in vitro test system in human promyelocytic leukemia cell line (Lantz et al., 2003). Moreover, it has been shown to possess sortase A inhibitory activity which helps reduce infection by Stapphylococcus aureus (Park et al., 2005). Hence, curcumin can be applied in pharmaceutical industry. In recent years, curcumin has also been popularly included in plant based cosmetics as an alternative to synthetic compounds for its skin anti-inflammatory and anti-irritant properties (Draelos et al., 2018; Sawicka et al. 2021). This adds to the demand for curcumin.
Plant tissue culture system has served as an alternative mean to produce plant secondary metabolites (Bourgaud et al., 2001). In addition, plant tissue culture is now widely employed in commercial or industrial scale production of target compounds at the highest yield using optimal conditions (Kieran et al., 1997; Gerolino et al., 2015; Chandran et al., 2020). Elicitation is one of several ways to enhance secondary metabolite production in callus and suspension culture (Rao and Ravishankar, 2002; Zhao et al., 2005). Since we are interested in production of curcuminoids via callus culture, we have attempted to follow the method used by Srirat et al. (2009) for tissue sterilization using ethanol, followed by sodium hypochlorite. However, the method used still produced tissue with high rate of contamination which may have resulted from bacteria that are resistant to sodium hypochlorite (Fu et al. 2017). After an attempt to modify the concentration and time of exposure to sodium hypochlorite, we had a question whether the potency of chemical was sufficient to eliminate bacterial and fungal contaminants on the rhizomes. Hence, we have explored new sterilization steps with a hypothesis that sodium hypochlorite could be replaced by mercuric chloride for better sterilization and thus propose the procedure for a more successful outcome. With higher rate of survival, callus induction was subsequently performed. The optimal conditions obtained from this study are currently used in our curcuminoid production via callus culture of C. longa Linn.
MATERIALS AND METHODS
Materials
The fresh rhizomes of C. longa Linn. were purchased from a local supplier in Chiang Mai Province, Thailand and all chemicals used in this study were tissue culture grade.
Establishment of aseptic explants
Rhizome bud surface sterilization methods
The rhizomes were rinsed thoroughly with tap water, peeled and cut in to small pieces. The buds of rhizome were used as explants in five different sterilization methods as shown in Table 1 with steps in the order from left to right. The buds were excised (1.00 cm2) and inoculated on sterile MS agar medium, pH 5.80 containing 30 g/l sucrose and 0.20% (w/v) gelrite™. The cultures were incubated under a photoperiod of 16 h light and 8 h dark at room temperature (25 ± 2°C). The contamination, necrotic and survival percentage of culture were calculated using the following formula (Sundram et al., 2012).
![]()
Shoot bud surface sterilization methods
The rhizomes were rinsed thoroughly with tap water, air dried and placed in paper boxes to allow shoots to sprout to about 1 to 2 cm in length at room temperature (25 ± 2°C) in the dark. The sprouted shoots were used as explants in two different sterilization methods as shown in Table 2 with steps in the order from left to right prior to inoculation on sterile MS agar medium. The contamination, necrotic and survival percentage of culture were calculated using the same formula (Sundram et al., 2012).
Shoot initiation
The sterile explants were cultured on MS agar medium fortified with 3.00 µM BA and 0.50 µM NAA. The cultures were incubated under a photoperiod of 16 h light and 8 h dark at room temperature (25 ± 2°C) (Shahinozzaman et al., 2013a).
Callus induction
Leaf sheath segments (0.5 cm long) and root segments (1.0 cm long) from about 6 weeks old in vitro plantlets were cut and inoculated on MS agar medium (Zhang et al., 2011) fortified with different concentrations and combinations of 2,4-D (0.50, 1.00 and 2.00 mg/l), TDZ (0.20 and 0.50 mg/l) and BA (0.20 and 0.50 mg/l) for callus induction. The cultures were incubated under a photoperiod of 16 h light and 8 h dark at room temperature (25 ± 2°C). The number of explants forming calli was recorded as the induction frequency and calculated using following formula. The fresh weight of callus was also recorded after 8 weeks of culturing.
![]()
Statistical Analysis
Statistical analysis for this work was performed using One Way Analysis of Variance (ANOVA) from SPSS software version 17.0. The analysis was carried out using total number of at least 10 samples for each treatment.
Table 1. Rhizome bud surface sterilization methods.
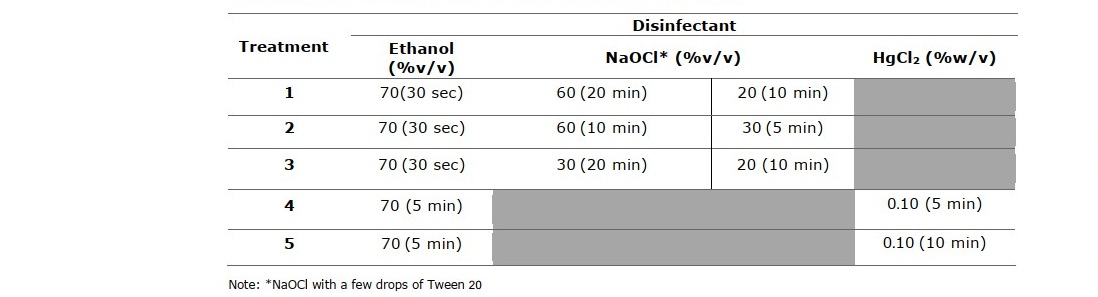
Table 2. Shoot bud surface sterilization methods.
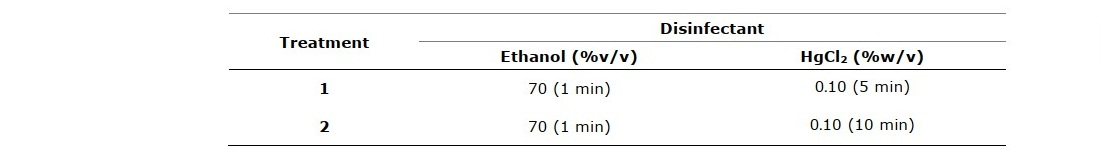
RESULTS
Surface sterilization and shoot induction
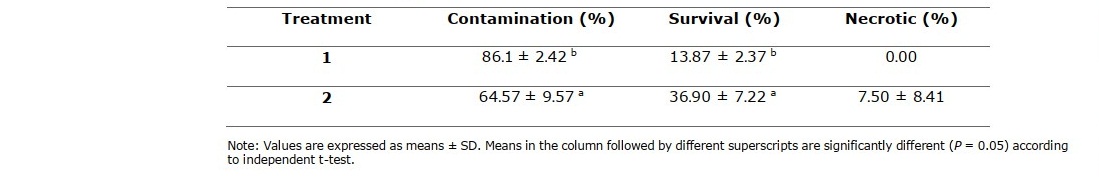
Representatives of rhizome buds and shoot buds are shown in Figure 1A and Figure 2A, respectively. Rhizome bud surface sterilization by immersing in ethanol, followed by sodium hypochlorite (NaOCl) solution in this study gave low percentage of contamination but did not yield any surviving explants (Table 1). Sodium hypochlorite is a common sterilizing agent for explants including leaves and stems at lower concentrations (3-5 %v/v) without tissue damage (Yildiz et al., 2012). However, in the case of rhizomes and roots that are exposed to soil and microorganism higher concentrations may be required. Plant tissue exposed to high concentrations of sodium hypochlorite could become damaged to the point where the explants are sterile, but cell growth cannot proceed and subsequently leading to cell death, a condition called necrotic. In this work surface sterilization by immersing explants in ethanol, followed by mercuric chloride (HgCl2) solution provided high rates of survival. Rhizome buds were able to withstand treatment by mercuric chloride better than shoot buds considering survival at 44.46% (Table 3, treatment 4), 66.67% (Table 3, treatment 5), 13.87% (Table 4, treatment 1) and 36.90% (Table 4, treatment 2). Out of the seven surface sterilization treatments carried out, treatment of rhizome buds with 70% ethanol (v/v) for 5 min, followed by 0.10% (w/v) HgCl2 for 10 min was the most effective technique.
Table 3. Effect of different treatments in rhizome bud surface sterilization of C. longa Linn. after culturing for 2 weeks.
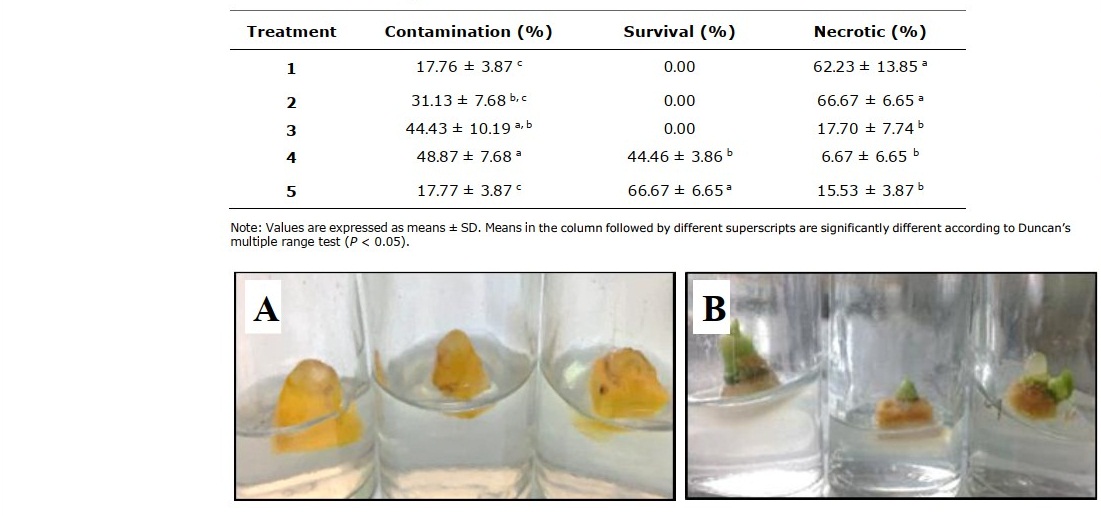
Figure 1. Rhizome buds from C. longa Linn. (A) and shoot formation from rhizome buds after culturing for 2 weeks (B).
Table 4. Effect of different treatments in shoot bud surface sterilization of C. longa Linn. after culturing for 2 weeks.
The in vitro shoot initiation was successfully established by rhizome buds cultured on MS agar medium (pH 5.80) containing 3% (w/v) sucrose and 0.20% (w/v) Gelrite™ supplemented with 3.00 µM BA and 0.50 µM NAA under a photoperiod of 16 h light and 8 h dark at room temperature (25 ± 2°C). At 6 weeks after culturing, the shoots and roots were healthy and used for callus induction (Figure 3).
Callus induction
Callus induction from leaf sheath segments of C. longa Linn.
The leaf sheath segments (Figure 4A) from in vitro plantlet were used as the starting material. Various concentrations of auxin (2,4-D) in combination with cytokinin (TDZ and BA) used in this study produced different callus induction percentages and callus fresh weights (Table 5). MS medium supplemented with 0.5 mg/l 2,4-D was the most effective medium for friable callus induction at 73% with 518.56 mg of fresh weight (Figure 4B). Increasing concentration of 2,4-D in combination with TDZ and BA resulted in significantly (P < 0.05) reduced percentages of callus producing explants and callus with lower fresh weights. The combination of 1.0 mg/l 2,4-D with 0.50 mg/l TDZ and 0.20 mg/l BA gave the highest callus induction percentage of all the combinations, 36% (Figure 4C).
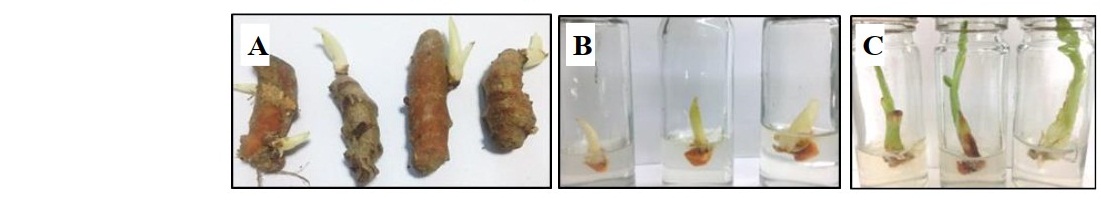
Figure 2. Shoot buds from C. long Linn. (A and B) and shoot elongation after culturing for 2 weeks (C).

Figure 3. Shoot formation derived from rhizome buds of C. longa Linn. after culturing for 4 weeks (A) and 6 weeks (B).
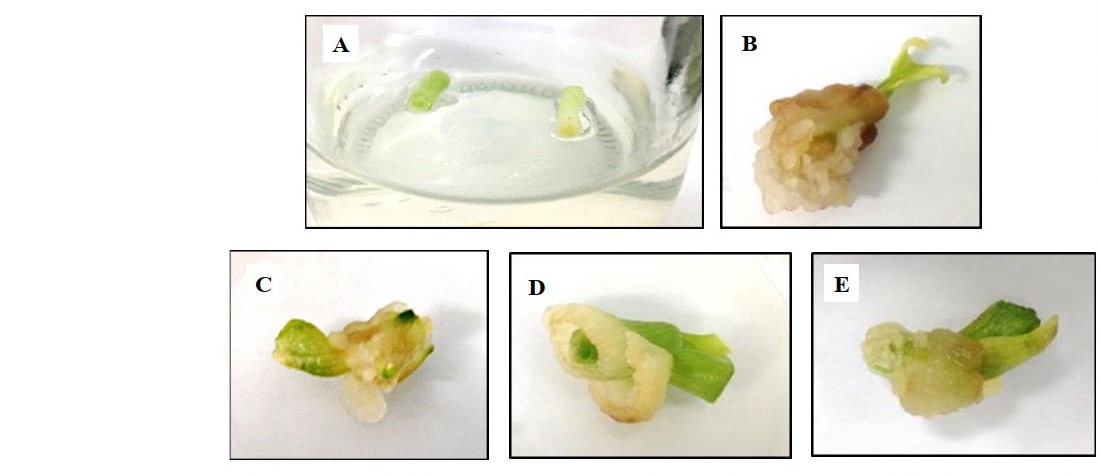
Figure 4. Leaf sheath segments used for callus induction (A), induction of callus on MS medium fortified with 0.50 mg/l 2,4-D (B), 0.10 mg/l 2,4-D + 0.50 mg/l TDZ and 0.20 mg/l BA (C), 0.50 mg/l 2,4-D + 0.20 mg/l TDZ and 0.50 mg/l BA (D) and 0.50 mg/l 2,4-D + 0.50 mg/l TDZ and 0.20 mg/l BA (E) after 8 weeks.
Moreover, the combination of 0.50 mg/l 2,4-D with different concentrations of TDZ and BA did not give significant (P < 0.05) difference in callus induction percentage, but resulted in significant weight difference (Figure 4D and 4E).
Callus induction from root segments of C. longa Linn.
For callus induction from root segments (Figure 5A), MS medium with 0.5 mg/l 2, 4-D gave the highest callus induction percentage at 63% (Figure 5B), but gave lower fresh weight of callus than that from the combination of 2,4-D with 0.50 mg/l TDZ and 0.20 mg/l BA (Figure 5C) (46.23 mg and 114.02 mg, respectively).
Table 5. Effect of 2,4-D, TDZ and BA on callus induction from leaf sheath segments for 8 weeks.
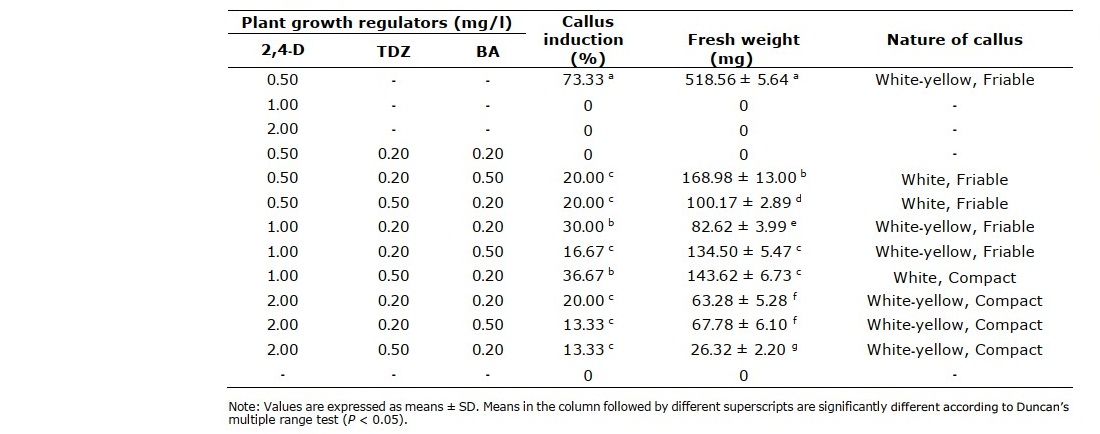
Table 6. Effect of 2,4-D, TDZ and BA on callus induction from root segments for 8 weeks.
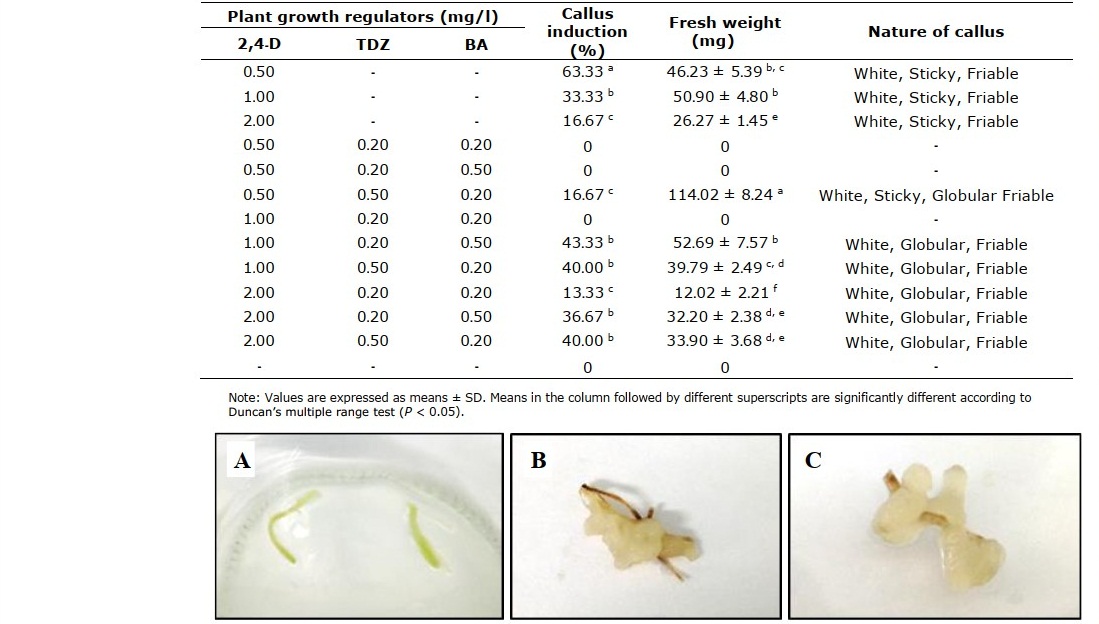
Figure 5. Root segments used for callus induction (A), induction of callus on MS medium fortified with 0.50 mg/l 2,4-D (B) and 0.50 mg/l 2,4-D + 0.50 mg/l TDZ and 0.20 mg/l BA (C) after 8 weeks.
DISCUSSION
In this study, the explants were prepared from underground rhizomes of C. longa Linn. and must be sterilized before culturing on medium. Surface sterilization of this plant material was achieved using ethanol and mercuric chloride (HgCl2) solution. These sterilizing agents have been reported as effective for many Zingiberaceae species (Mohanty et al., 2008; Kou et al., 2013; Solanky et al., 2013). Optimal concentration and period of exposure for each agent needed for disinfection of rhizome buds and shoot buds were investigated. It was found that treatment of rhizome buds with 70% ethanol (v/v) for 5 min, followed by 0.10% (w/v) HgCl2 for 10 min provided sterile and healthy explants of C. longa Linn.
Salvi et al. (2002) reported that the combination of 5.00 µM BA and 1.00 µM NAA was effective for shoot formation for C. longa Linn. Similar results were reported for C. zedoaria (Shahinozzaman et al., 2013b). However, we found that MS agar medium supplemented with 3.00 µM BA and 0.5 µM NAA was sufficient for initiation of shoot of C. longa Linn. in vitro. Our finding suggests that lower concentrations of BA and NAA can also be used for shoot induction.
Callus induction of C. longa Linn. by 0.50 mg/l 2,4-D gave the highest callus induction percentage and the combination of 2,4-D with TDZ and BA resulted in decreased percentages of explants producing callus. Accordingly, 2,4-D alone would be sufficient for callus induction in C. longa Linn.. 2,4-D at 1.00 mg/l was reported to be effective plant growth regulator that could produce rapid proliferating friable callus in C. var. Chattip (Jala, 2013) and at 0.50 - 2.00mg/l 2,4-D was effective for callus induction in C. amada Roxb. (Prakash et al., 2004). Another study by Sukontharat et al. (2015) used 1.00 mg/l dicamba in C. longa Linn.which induced callus production at 70%. Dicamba and 2,4-D are both synthetic auxins. We selected 2,4-D because it is commonly used as plant growth regulator in plant cell culture medium. With estimated 73% of callus induction 2, 4-D at the optimal concentration of 0.50 mg/l is suitable for C. longa callus culture preparation for curcuminoid production and elicitation study.
CONCLUSION
Our study indicated that C. longa Linn. rhizome surface sterilization was accomplished by immersing rhizome buds in 70 % ethanol for 5 min, followed by 0.10% HgCl2 for 10 min. In addition, in vitro shoot initiation was established by culturing rhizome buds on MS agar medium supplemented with 3.00 µM BA and 0.50 µM NAA under photoperiod of 16 h light and 8 h dark at room temperature (25 ± 2°C). The callus culture from in vitro leaf sheath segment on MS agar medium containing 0.5 mg/ml 2,4-D offered the highest callus induction percentage and fresh weight of callus at 8 weeks after culturing.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Department of Chemistry and Center of Excellence for Innovation in Chemistry (PERCH-CIC) and Plant Tissue Culture Research Laboratory, Faculty of Science, Chiang Mai University for providing financial support, facilities and equipment for the research work.
AUTHOR CONTRIBUTIONS
Warunya Kaewthip assisted in conducting the experiments, performed the statistical analysis, carried out data visualization and wrote the manuscript. Srisulak Dheeranupattana and Pornchai Junta provided technical supports. Lalida Shank designed, conducted all of the experiments, wrote and prepared the manuscript. All authors have read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare that they hold no competing interests.
REFERENCES
Bourgaud, F., Gravot, A., Milesi, S., and Gontier, E. 2001. Production of plant secondary metabolites: a historical perspective. Plant Science. 161: 839-851.
Chandran, H., Meena, M., Barupal, T., and Sharma, K. 2020. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnology Reports. 26: 1-10.
Draelos, Z.D. 2018. Updates in Medical Skin Care. Advances in Cosmetic Surgery. 1: 211–217.
EL-Kenawy, A.E.-M., Hassan, S.M.A., Mohamed, A.M.M., and Mohammed, H.M.A. 2019. Tumeric or Curcuma longa Linn. Nonvitamin and Nonmineral Nutritional Supplements. 447–453.
Fu, Y., Deering, A.J., Bhunia, A.K., and Yao, Y. 2017. Biofilm of Escherichia coli O157:H7 on cantaloupe surface is resistant to lauroyl arginate ethyl and sodium hypochlorite. International Journal of Food Microbiology. 260:11-15.
Gerolino, E.F., Chierrito, T.P.C., Filho, A.S., Souto, E.R., Goncalves, R.A.C., and Braz de Oliveira, A.J. 2015. Evaluation of limonoid production in suspension cell culture of Citrus sinensis. Revista Brasileira de Farmacognosia. 25: 455-46.
Goel, A., Kunnumakkara, A.B., and Aggarwal, B.B. 2008. Curcumin as “Curecumin”: From kitchen to clinic. Biochemical pharmacology. 75: 787-809.
Hamaguchi, T., Ono, K., and Yamada, M. 2010. Curcumin and Alzheimer’s disease. CNS Neuroscience and Therapeutics. 16: 285-297.
Jala A. 2013. The effect of the 2,4-dichlorophenoxy acetic acid, benzyl adenine and paclobutrazol, on vegetative tissue-derived somatic embryogenesis in turmeric (Curcuma var. Chattip). International Transaction Journal of Engineering, Management, and Applied Sciences and Technologies. 4: 105-110.
Kieran, P.M., MacLoughlin, P.F. and Malone, D.M. 1997. Plant cell suspension culture: some engineering considerations. Journal of Biotechnology. 59: 39-52.
Kou, Y., Ma, G., da Silva, J.A.T., and Liu, N. 2013. Callus induction and shoot organogenesis from anther cultures of Curcuma attenuate Wall. Plant Cell Tissue Organ Culture.
Lantz, R.C., Chen, G.J., Solyom, A.M., Jolad, S.D., and Timmermann, B.N. 2003. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine. 12: 445-452.
Maheshwari, R.K., Singh, A.K., Gaddipati, J., and Srimal, R.C. 2006. Multiple biological activities of curcumin: A short review. Life Sciences. 78: 2081-2087.
Mohanty, S., Panda, M.K., Subudhi, E., and Nayak, S. 2008. Plant regeneration from callus culture of Curcuma aromatica and in vitro detection of somaclonal variation through cytophotometric analysis. Biologia Plantarum. 52: 783-786.
Nair, K.P.P. 2013. The Agronomy and Economy of Turmeric and Ginger. Elsevier.
Park, B.S., Kim, J.G., Kim, M.R., Lee, S.E., Oh, G.R., Takeoka, K.B., and Kim, J.H. 2005. Curcuma longa L. constituents inhibit sortase A and Staphylococcus aureus cell adhesion to fibronectin. Journal of Agricultural and Food Chemistry. 53: 9005- 9009.
Prakash, S., Elangomathavan, R., Seshadri, S., Kathiravan, K. and Ignacimuthu, S. 2004. Efficient regeneration of Curcuma amada Roxb. plantlets from rhizome and leaf sheath explants. Plant Cell, Tissue and Organ Culture. 78: 159–165.
Rahman, A.F.M.M., Angawi, R.F., and Kadi, A.A. 2015. Spatial localization of curcumin and rapid screening of the chemical compositions of turmeric rhizomes (Curcuma longa Linn.) using direct analysis in real time-mass spectrometry (DART-MS). Food Chemistry. 173: 489-494.
Rao, S.R. and Ravishankar, G.A. 2002. Plant cell cultures chemical factories of secondary metabolites. Biotechnology Advances. 20: 101-153.
Ravindran, P.N., Nirmal Babu, K., and Sivaraman, K. 2007. Turmeric: The Genus Curcuma. , CRC Press, Florida.
Salvi, N.D., George, L., and Eapen, S. 2002. Micropropagation and field evaluation of micropropagated plants of turmeric. Plant Cell, Tissue and Organ Culture. 68: 143–151.
Sawicka, B., Skiba, D., Umachandran, K., and Dickson, A. 2021. Preparation of Phytopharmaceuticals for the Management of Disorders. The Development of Nutraceuticals and Traditional Medicine. Chapter 27 - Alternative and new plants: 491-537.
Shahinozzaman, M., Ferdous, M.M., Faruq, M.O., Azad, M.A.K. and Amin, M.N. 2013a. Micropropagation of black turmeric (Curcuma caesia Roxb.) through in vitro culture of rhizome bud explants. Journal of Central European Agriculture. 14: 110-115.
Shahinozzaman, M., Faruq, M.O., Azad, M.A.K., and Amin, M.N. 2013b. Studies on in vitro propagation of an important medicinal plant - Curcuma zedoaria Roscoe using rhizome explants. Persian Gulf Crop Protection. 2: 1-6.
Solanky, R.U., Patel, S.R., and Patel, J.R. 2013. In vitro regeneration of ginger (Zingiber officinale Rosc.) through callus culture. AGRES – An International e-Journal. 2: 196-202.
Srirat, P., Sirisansaneeyakul, S., Parakulsuksatid, P., Prammanee, S., and Vanichsriratana, W. 2009. In vitro shoot propagation of Curcuma longa L. from rhizome bud explants. p.13. Proceeding of the 3rd International Conference on fermentation Technology for Value Added Agricultural Products, 26-28 Aug 2009. Khon Kaen, Thailand.
Sukontharat, P., Khawniam, T., and Te-chato, S. 2015. Sterile methods and callus induction from leaf sheath of Curcuma longa Linn. in vitro. Songklanakarin Journal of Plant Science. 2: 36-40.
Sundram, T.C.M., Suffian, M., Annuar, M., and Khalid, N. 2012. Optimization of culture condition for callus induction from shoot buds for establishment of rapid growing cell suspension cultures of mango ginger (Curcuma mangga). Australian Journal of Crop Science. 6: 1139-1146.
Yildiz, M., Özcan, S.F., Kahramanogullari, C.T., and Tuna, E. 2012. The effect of sodium hypochlorite solutions on the viability and in vitro regeneration capacity of the tissue. The Natural Products Journal. 2: 328-331.
Zhang, S., Liu, N., and Sheng, A. 2011. In vitro plant regeneration from organogenic callus of Curcuma Kwangsiensis Lindl. (Zingiberaceae). Plant Growth Regulation. 64: 141-145.
Zhao, J., Davis, L.C., and Verpoorte, R. 2005. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnology Advances. 23: 283- 333.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Warunya Kaewthip1, Srisulak Dheeranupattana2, Pornchai Junta3, and Lalida Shank3, *
1 Division of Biotechnology, Graduate School, Chiang Mai University, Chiang Mai 50200, Thailand
2 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
3 Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
Corresponding author: Lalida Shank, E-mail: lalida.shank@cmu.ac.th
Total Article Views
Editor:
1. Korakot Nganvongpanit, Chiang Mai University, Thailand
2. Wasu Pathom-aree, Chiang Mai University, Thailand
Article history:
Received: December 22, 2020;
Revised: February 18, 2021;
Accepted: March 3, 2021;
Published online: March 18, 2021

