
Antiproliferative and Apoptosis-Inducing Activities of Benchalokawichian Remedy Against Doxorubicin-Sensitive and -Resistant Erythromyelogenous Leukemic Cells
Wipob Suttana*, Chatubhong Singharachai, Rawiwan Charoensup, Narawadee Rujanapun, and Chutima SuyaPublished Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.056
Journal Issues : Number 3, July-September 2021
Abstract Chemotherapy can cause multidrug resistance in cancer cells and is cytotoxic to normal cells. Discovering natural bioactive compounds that are not cytotoxic to normal cells but inhibit proliferation and induce apoptosis in drug- sensitive and drug-resistant cancer cells could overcome these drawbacks of chemotherapy. This study investigated the antiproliferative effects of crude extracts of Benchalokawichian (BLW) remedy and its herbal components against drug-sensitive and drug-resistant cancer cells, cytotoxicity of the extracts toward normal cells, and their ability to induce apoptosis and cell cycle arrest in drug-sensitive and drug-resistant cancer cells. The extracts exhibited antiproliferative activity against doxorubicin-sensitive and doxorubicin-resistant erythromyelogenous leukemic cells (K562 and K562/adr). Tiliacora triandra root, BLW, and Harrisonia perforata root extracts displayed an IC50 of 77.00 ± 1.30, 79.33 ± 1.33, and 87.67 ± 0.67 µg/mL, respectively, against K562 cells. In contrast, Clerodendrum petasites, T. triandra, and H. perforata root extracts displayed the lowest IC50 against K562/adr cells (68.89 ± 0.75, 78.33 ± 0.69, and 86.78 ± 1.92 µg/mL, respectively). The resistance factor of the extracts was lower than that of doxorubicin, indicating that the extracts could overcome the multidrug resistance of cancer cells. Importantly, the extracts were negligibly cytotoxic to peripheral mononuclear cells, indicating minimal adverse effects in normal cells. In addition, these extracts induced apoptosis of K562 and K562/adr cells and caused cell cycle arrest at the G0/G1 phase in K562 cells.
Keywords: Antiproliferative, Apoptosis, Benchalokawichian, Cell cycle, Multidrug resistance
Funding: This research was supported by grants from Mae Fah Luang University.
Citation: Suttana, W., Singharachai, C., Charoensup, R., Rujanapun, N., and Suya, C. 2021. Antiproliferative and apoptosis-inducing activities of benchalokawichian remedy against doxorubicin- sensitive and -resistant erythromyelogenous leukemic cells. CMU J. Nat. Sci. 20(3): e2021056.
INTRODUCTION
Cancer is a severe disease and the most common cause of death among Thai people. The cancer mortality rate in Thailand has tended to increase every year (Strategy and Planning Division, 2020). Chemotherapy is a basic modality of cancer treatment. However, chemotherapy can result in multidrug resistance (MDR) of cancer cells and is cytotoxic to normal cells. These drawbacks can lead to treatment failure, adverse effects in patients, and high mortality rates. MDR of cancer cells is caused by persistent exposure to cytotoxic drugs, which alters the molecular mechanism in the cells and leads to inhibition of apoptosis (Synold, Dussault, & Forman, 2001).
Apoptosis is programmed cell death that occurs in physiological conditions without inducing cell damage and inflammation (Saraste & Pulkki, 2000). Induction of apoptosis is a potential chemotherapeutic strategy. Apoptosis is induced by several pathways, including cell cycle arrest (Zheng, Chiang, & Lin, 2005). Therefore, discovering bioactive compounds that are not cytotoxic to normal cells but inhibit cell proliferation and induce apoptosis of both drug-sensitive and drug-resistant cancer cells is a promising strategy to overcome these drawbacks of chemotherapy. Bioactive compounds from plants, especially polyphenols and flavonoids, are known to possess these desired properties (Kothan et al., 2004; Dechsupa et al., 2007; Suttana et al., 2010). These compounds can induce apoptosis in cancer cells by arresting cell cycle at the G0/G1 phase (Zheng et al., 2005; Thangapazham et al., 2007).
Benchalokawichian (BLW) is a traditional herbal remedy in Thailand. BLW has been used as an antipyretic drug for a long time and is included in the Herbal Products list of the Thailand National List of Essential Medicines A.D. 2006. BLW comprises the roots of five medicinal plants, including Clerodendrum petasites, Harrisonia perforata, Tiliacora triandra, Capparis micracantha, and Ficus racemosa (National Drug Committee, 2009). BLW and its herbal components consist of bioactive compounds, including alkaloids, flavonoids, and polyphenols (Brimson, Onlamoon, Tencomnao, & Thitilertdecha, 2019; Juckmeta et al., 2019; Noysang & Pummarin, 2019). Biological activities of BLW and its herbal components include anti-inflammatory, antioxidant, antimutation (Singharachai, Palanuvej, Kiyohara, Yamada, & Ruangrungsi, 2011), and antiproliferative activity against lung cancer cell lines (Juckmeta et al., 2019). However, the antiproliferative and apoptosis-inducing activities of BLW and its herbal components against drug-sensitive and drug-resistant cancer cells are unclear.
This study investigated the effects of crude extracts of BLW and its herbal components on the proliferation, apoptosis, and cell cycle of drug-sensitive and drug- resistant cancer cells, and the cytotoxicity of the extracts toward normal cells. The results will provide preliminary information for in vivo and clinical studies leading to anticancer drug development.
MATERIALS AND METHODS
Plant material
Roots of five medicinal plants, including C. petasites, H. perforata, T. triandra, C. micracantha, and F. racemosa, were collected from Nakorn Pathom province, Thailand. Voucher specimens were deposited at the Medicinal Plants Innovation Center of Mae Fah Luang University, Chiang Rai, Thailand, with a herbarium number of MPIC134, MPIC0132, MPIC0131, MPIC0133, and MPIC0090, respectively.
Chemicals and reagents
RPMI 1640 medium, penicillin/streptomycin, and 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Fetal calf serum (FCS) and phosphate-buffered saline (PBS) were purchased from Hyclone Laboratories Inc. (Logan, UT, USA). Lymphoprep™ solution was acquired from Axis-Shield Diagnostics (Dundee, UK). Dimethyl sulfoxide (DMSO) and isopropanol were acquired from RCI Labscan Limited (Bangkok, Thailand). Hydrochloric acid (HCl) was purchased from Sigma-Aldrich Chemical Company (St Louis, MO, USA). MuseTM Annexin V and Dead Cell and MuseTM Cell Cycle reagents were purchased from Luminex Corporation (Austin, TX, USA).
Extract preparation
BLW and each herbal component were ground and soaked in ethanol for 3 days. The liquid extracts were filtered through filter paper and the solvent was removed using a rotary evaporator. The powders of all crude extracts were kept in a desiccator until use. Before the experiments, the extracts were individually dissolved in DMSO and sterilized by passage through a 0.2-μm syringe filter.
Cell models
Cancer cells: Human erythromyelogenous leukemic cell lines from the American Type Culture Collection were generously provided by Dr. Sawitree Chiampanichayakul, Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University. The cells included doxorubicin-sensitive cells (K562) and doxorubicin- resistant cells (K562/adr) (CMU-IBC MTA01).
Normal cells: Human peripheral blood mononuclear cells (PBMCs) were separated from the buffy coat of healthy blood donors who displayed negative results for infectious markers at Chiangrai Prachanukroh Hospital. The procedure was approved by the hospital’s ethical committee (CR0032.102/RES/EC314).
Cell culture and preparation
Cancer cells were cultured in RPMI 1640 supplemented with 10% FCS and 1% penicillin/streptomycin at 37°C in a 95% humidified atmosphere of 5% CO2. Subculturing was performed every 3 days. PBMCs were isolated from the buffy coat of healthy blood donors using density gradient centrifugation. The buffy coat was diluted with PBS and overlayered on Lymphoprep™ solution. After centrifugation at 400 g for 30 min at room temperature, PBMCs were collected, washed twice with PBS, and centrifuged at 200 g for 10 min at room temperature. The cells were used immediately for cytotoxicity assay.
Cytotoxicity assay
Cancer cells (104 cells) were added to 100 µL of RPMI 1640 supplemented with 10% FCS and 1% penicillin/streptomycin at 37°C in a 95% humidified atmosphere of 5% CO2 in 96-well plates. Untreated samples served as controls and the test samples received 12.5, 25, 50, 100, or 200 µg/mL of the extracts. The samples were incubated for 72 h. After incubation, MTT (0.45 mg/mL solution) was added, and the samples were incubated for 4 h. The formazan crystals formed by viable cells were dissolved by the addition of 0.04 N HCl in isopropanol. The absorbance at 570 nm (Abs570nm) of each treated and control well was determined using an EPOCHTM2 microplate reader (BioTek Instruments Inc., Winooski, VT, USA). In addition, cells (104) were also added to the MTT solution immediately to measure the absorbance of formazan produced by the cells before incubation (Abs570nm before incubation). The concentration of compound required to inhibit proliferation by 50% (IC50) was determined by plotting the percentage of proliferation inhibition (% proliferation inhibition) versus the extract concentration. The percentage of cell growth inhibition was calculated as: % Proliferation inhibition = (Abs570nm of control well − Abs570nm of treated well) × 100 / (Abs570nm of control well − Abs570nm before incubation). The percentage cell proliferation (%Proliferation) was calculated as: % Proliferation = (Abs570nm of treated well - Abs570nm before incubation)× 100 / (Abs570nm of control well - Abs570nm before incubation). The resistance factor (RF) was calculated as the IC50 of the extract against drug-resistant cancer cells / IC50 of the extract against drug-sensitive cancer cells.
PBMCs (104) were seeded in 100 µL of RPMI 1640 supplemented with 10% FCS and 1% penicillin/streptomycin and incubated at 37°C in a 95% humidified atmosphere of 5% CO2 in 96-well plates. Untreated samples served as controls and the test samples received 12.5, 25, 50, 100, or 200 µg/mL of the extracts. After 72 h, the samples were treated with MTT and the absorbance measured as described above for the cancer cells. The percentage of cell viability (%Viability) was calculated according to the formula: % Viability = [(Abs570nm of treated well / Abs570nm of the control well)] × 100.
Apoptosis assay
Cancer cells (105 cells) were added to 24-wells plates containing 500 µL of RPMI 1640 supplemented with 10% FCS and 1% penicillin/streptomycin. The samples were incubated at 37°C in a 95% humidified atmosphere of 5% CO2. The samples were either not treated (control) or treated with 50 or 100 µg/mL of the extracts (test). After 24 h, the cells were collected, washed with PBS, and centrifuged at 200 g for 10 min at 4°C. The cells from each well were suspended in 100 µL of PBS, stained with 100 µL of MuseTM Annexin V and Dead Cell reagent, and incubated for 20 min in the dark at room temperature. The stained cells (5 × 103 cells) were analyzed using a Guava™ Muse™ Cell Analyzer (Luminex Corporation). Dot plots were generated using Flowing software 2.5.1 (Turku Bioscience, Turku, Finland). The results were expressed as percentages of living cells (AnnV–, 7-AAD–), early apoptotic cells (AnnV+, 7-AAD-), late apoptotic cells (AnnV+, 7-AAD+), and necrotic cells (AnnV-, 7-AAD+).
Cell cycle analysis
Cancer cells (2 × 105 cells) were seeded in 24-well plates containing 500 µL of RPMI 1640 supplemented with 10% FCS and 1% penicillin/streptomycin at 37°C in a 95% humidified atmosphere of 5% CO2. The samples were incubated with 50 or 100 µg/mL of the extracts (test) or without the extracts (control) for 24 h. The cells were collected, washed with PBS, and centrifuged at 200 g for 10 min at 4°C. The cells were fixed with 1 mL of ice-cold 70% ethanol and kept at 20°C for at least 3 h before staining. The fixed cells were collected and stained with 200 µL of MuseTM Cell Cycle Reagent and incubated for 30 min in the dark at room temperature. The stained cells (5 × 103) were analyzed using a Guava™ Muse™ Cell Analyzer (Luminex Corporation). The results were expressed as percentage of cells in each phase of the cell cycle from the histogram using Flowing software 2.5.1 (Turku Bioscience, Turku, Finland).
Statistical analysis
The results from the experiments are expressed as mean ± standard error of the mean (SEM) from three independent experiments. Statistically significant differences between the values of treated and control cells were analyzed using the Wilcoxon signed ranks test at P < 0.01 and 0.05.
RESULTS
Effect of BLW and its herbal component extracts on proliferation of drug-sensitive and drug-resistant cancer cells
BLW and its herbal component extracts inhibited the proliferation of doxorubicin- sensitive (K5562) and doxorubicin-resistant (K562/adr) erythromyelogenous leukemic cells. T. triandra root, BLW, and H. perforata root extracts displayed the lowest IC50 values against K562 cells (77.00 ± 1.30, 79.33 ± 1.33, and 87.67 ± 0.67 µg/mL, respectively). C. petasites, T. triandra, and H. perforata root extracts displayed the lowest IC50 values against K562/adr cells (68.89 ± 0.75, 78.33 ± 0.69, and 86.78 ± 1.92 µg/mL, respectively) (Table 1). Based on their potent antiproliferative property, these extracts were selected to further investigate their influence on the induction of apoptosis and their effect on cell cycle. In addition, the RF of the extracts was lower than that of doxorubicin, which is an established chemotherapy drug. In particular, C. petasites, H. perforata, and T. triandra root extracts had an RF ≤ 1 (Table 2). These results indicate that the extracts inhibited the proliferation of doxorubicin-resistant cells to a similar or greater extent as compared to inhibition of doxorubicin-sensitive cells. These enhanced activities could overcome the MDR of cancer cells. The antiproliferative activities of BLW and its herbal component extracts with K562 and K562/adr cells are depicted in Figure 1.
Table 1. IC50 of BLW, BLW herbal component extracts, and doxorubicin in doxorubicin- sensitive (K562) and doxorubicin-resistant (K562/adr) erythromyelogenous leukemic cells after 72 h of incubation.
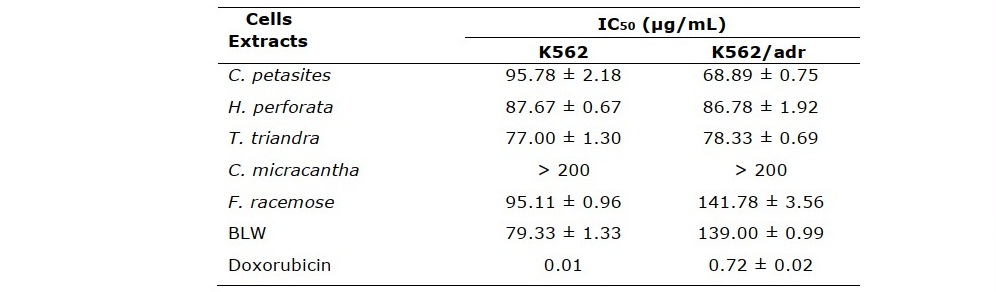
Table 2. Resistance factor (RF) of BLW, BLW herbal component extracts, and doxorubicin in erythromyelogenous leukemic cells.
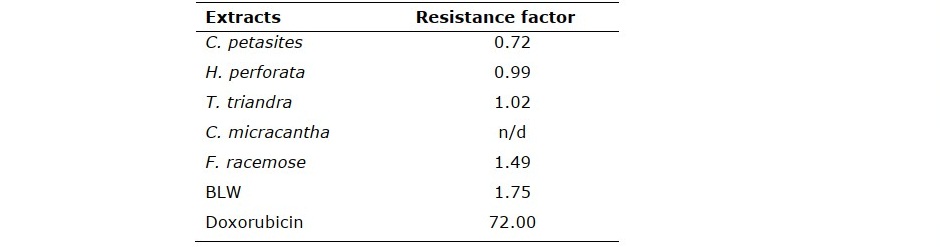

Figure 1. Percentage proliferation after treatment with various concentration of BLW and its herbal component extracts for 72 h in (A) doxorubicin-sensitive erythromyelogenous leukemic cells (K562) and (B) doxorubicin-resistant cells (K562/adr).
Cytotoxicity of extracts toward normal cells
The extracts were negligibly cytotoxic to normal cells (PBMCs). The highest concentration tested reduced cell viability by less than 20% (Figure 2).
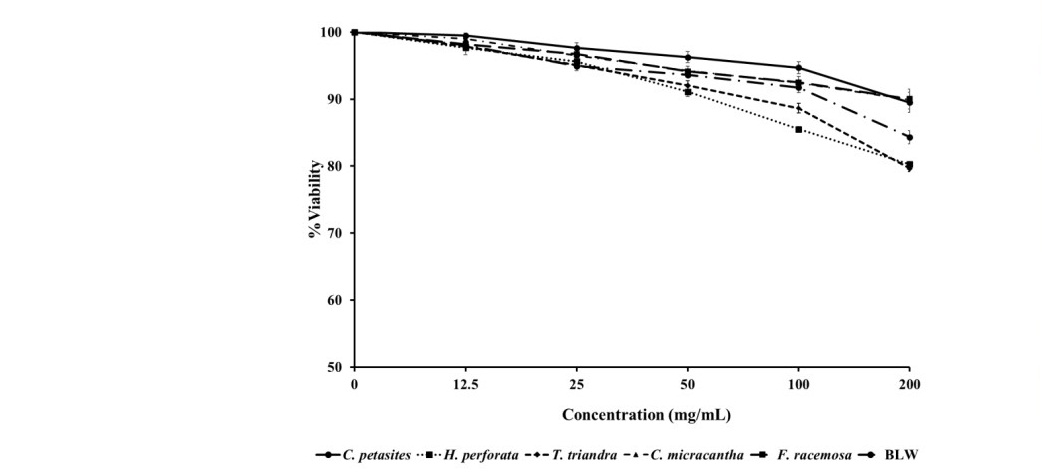
Figure 2. Percentage viability after treatment of peripheral blood mononuclear cells (PBMCs) with various concentration of BLW and its herbal component extracts for 72 h.
Induction of apoptosis by selected extracts in K562 and K562/adr cancer cells
H. perforata and T. triandra root extracts, and BLW were selected for investigating apoptosis induction in K562 cells. C. petasites, H. perforata, and T. triandra root extracts were selected for investigating apoptosis induction in K562/adr cells. The percentage of K562 and K562/adr cell viability significantly decreased, while the percentage of early and late apoptotic cells, and cell necrosis significantly increased after incubation with 50 and 100 µg/mL of the extracts for 24 h compared to the control (P < 0.01, P < 0.05), as shown in Figures 3 and 4. These findings indicate the induction of apoptosis by the extracts in doxorubicin-sensitive and doxorubicin-resistant cancer cells.
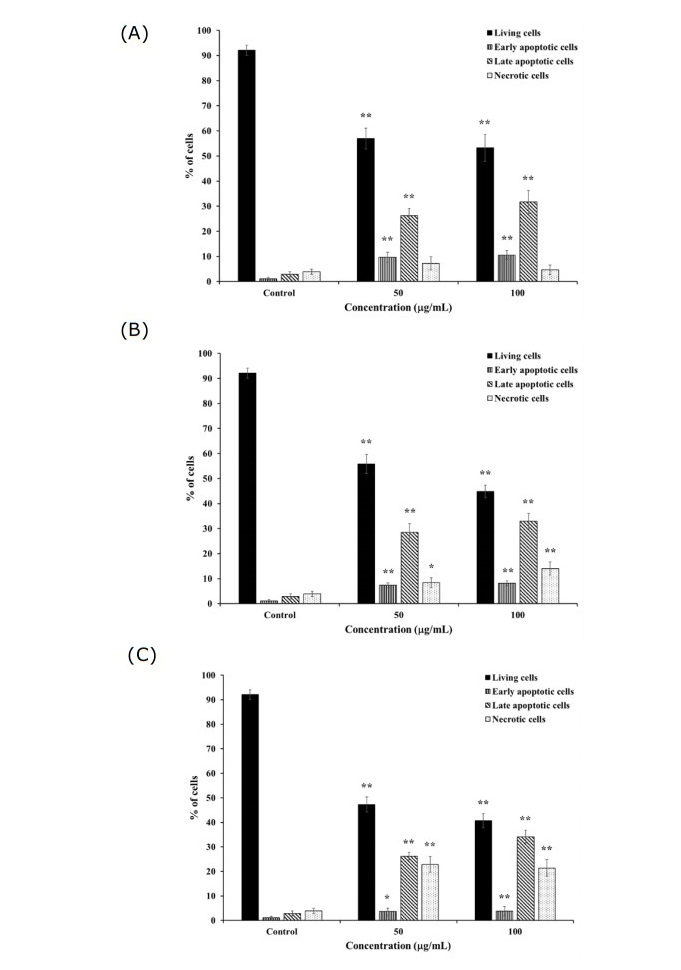
Figure 3. Percentage of apoptotic K562 and K562/adr cells after treatment with 50 and 100 μg/mL of (A) H. perforata, (B) T. triandra root, and (C) BLW extracts for 24 h. Statistically significant differences compared to control are indicated at *p < 0.05 and **p < 0.01.
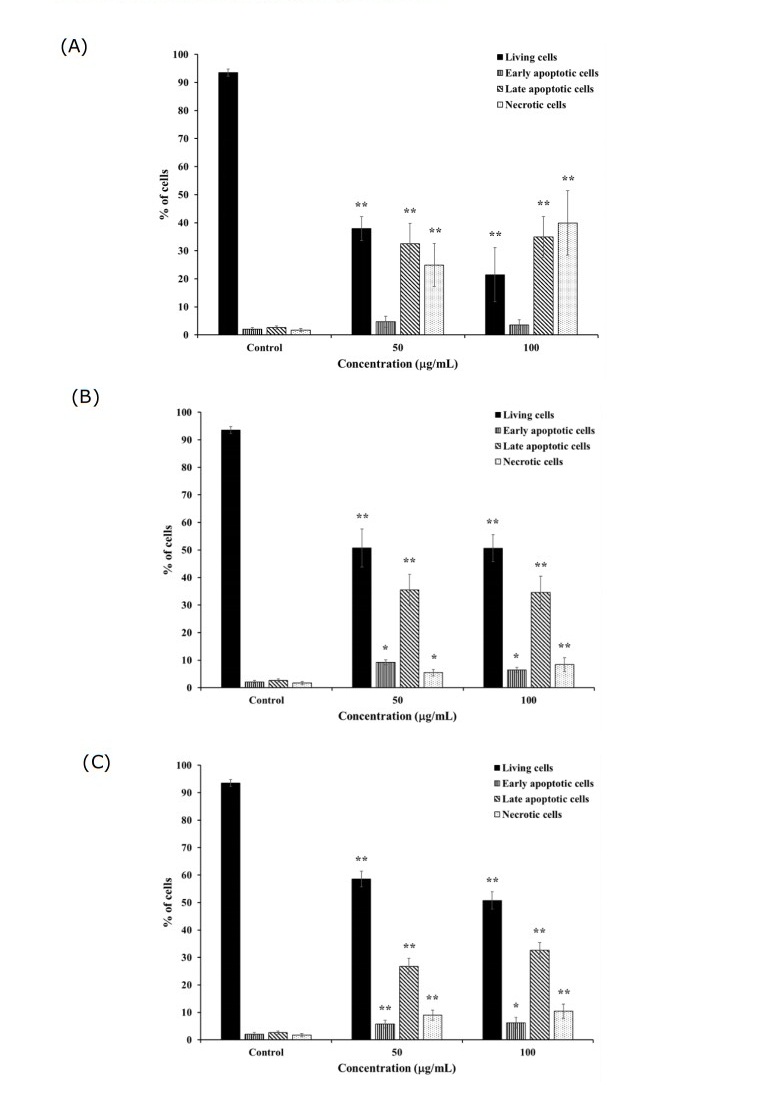
Figure 4. Percentage of apoptotic K562 and K562/adr cells after treatment with 50 and 100 μg/mL of (A) C. petasites, (B) H. perforata, and (C) T. triandra root extracts for 24 h. Statistically significant differences compared to control are indicated at *p < 0.05 and **p < 0.01.
Effect of selected extracts on K562 and K562/adr cell cycle progression
Similar selected extracts as used in the apoptosis assay were used to investigate their effects on cell cycle progression of the drug-sensitive and drug-resistant cancer cells. After incubation with 50 and 100 µg/mL of the extracts for 24 h, the percentage of K562 cells in the G0/G1 phase significantly increased and that in the G2/M phase significantly decreased compared to the control (P < 0.01, P < 0.05) (Figure 5). The findings indicated that the extracts induced cell cycle arrest at the G0/G1 phase. In addition, incubation with 50 and 100 µg/mL of the extracts for 24 h produced a non- significant decrease in the percentage of K562/adr cells at the G0/G1 phase and increase in cells at the G2/M phase compared to the control (P > 0.05) (Figure 6).
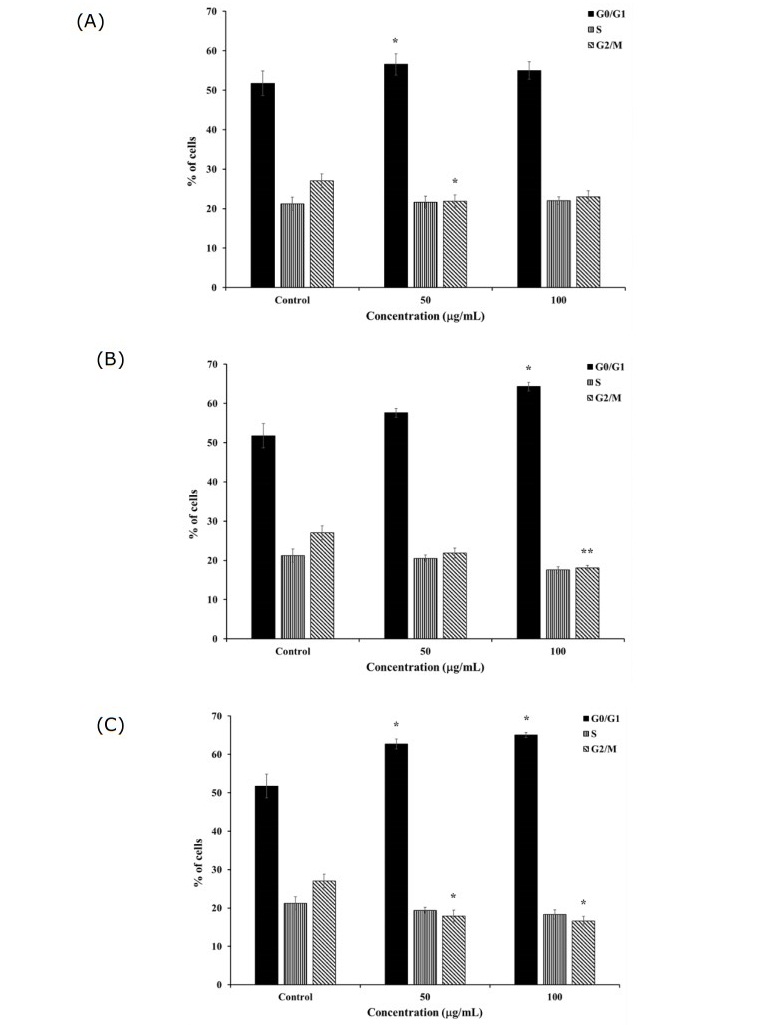
Figure 5. Cell cycle distribution after 24-h treatment of K562 and K562/adr cells with 50 and 100 μg/mL of (A) H. perforata, (B) T. triandra root, and (C) BLW extracts. Statistically significant differences compared to control are indicated at *p < 0.05 and **p < 0.01.
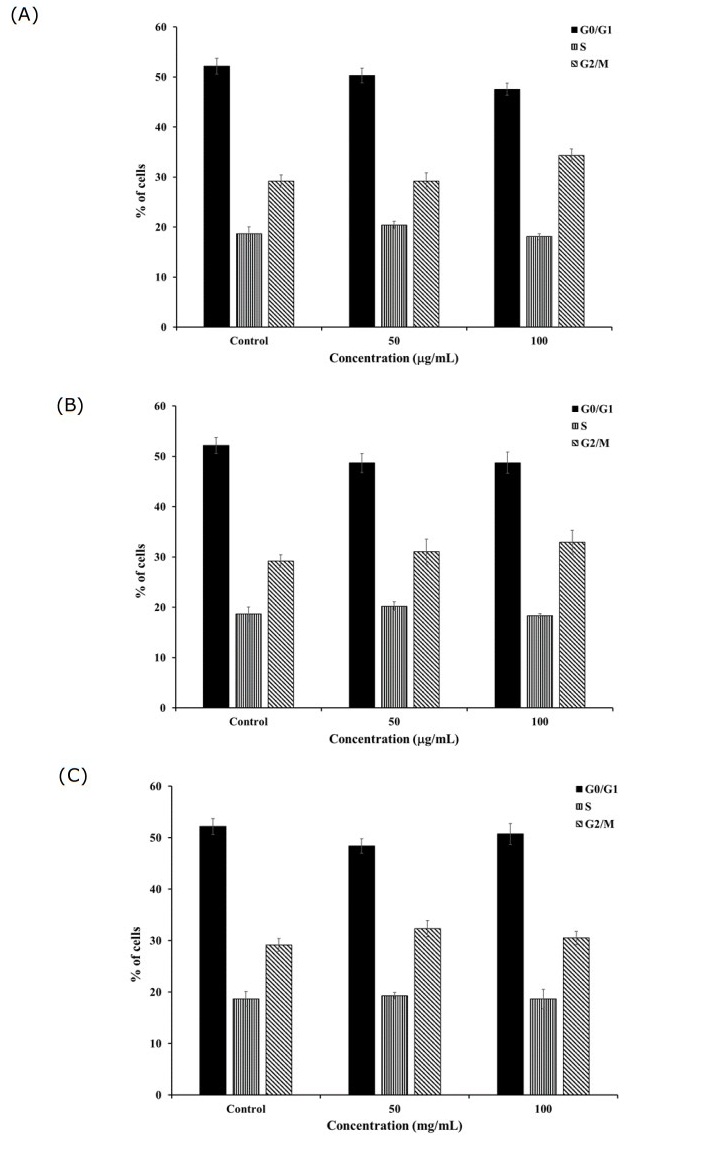
Figure 6. Cell cycle distribution after 24-h treatment of K562 and K562/adr cells with 50 and 100 μg/mL of (A) C. petasites, (B) H. perforata, and (C) T. triandra root extracts. Statistically significant differences compared to control are indicated at *p < 0.05 and **p < 0.01.
DISCUSSION
BLW and its herbal component extracts inhibited the proliferation of K562 and K562/adr cells. C. petasites, H. perforata, and T. triandra root extracts inhibited the proliferation of doxorubicin-resistant cells to a similar or greater extent as compared to inhibition of doxorubicin-sensitive cells. BLW and its herbal component extracts consist of bioactive compounds that include alkaloids, flavonoids, and polyphenols (Rattana, Cushnie, Taepongsorat, and Phadungkit, 2016; Brimson et al., 2019; Juckmeta et al., 2019; Noysang and Pummarin, 2019). Previous studies have described the antiproliferative activity of these compounds against K562 and K562/adr cells (Kothan et al., 2004; Suttana et al., 2010; Yao et al., 2016; Chamani et al., 2020) as well as against imatinib-resistant K562 cells (Zamani, Jusoh, Al-Jamal, Sul’ain, and Johan, 2016). The present observation of the very low cytotoxicity of the extracts to PBMCs is consistent with other descriptions of the very low toxicity of alkaloids, flavonoids, and polyphenols in PBMCs and other normal cell models (Menezes et al., 2016; Rossi et al., 2020)
The extracts induced apoptosis in both K562 and K562/adr cells. In K562 cells, this involved cell cycle arrest at the G0/G1 phase. On the contrary, another mechanism was evident for K562/adr cells. These actions might have been caused by alkaloids, flavonoids, and polyphenols, as previous studies demonstrated that these compounds can induce apoptosis in K562 cells through cell cycle arrest at G0/G1 and G2/M phases (Li et al., 2012; Mahbub et al., 2013; Asmaa, Ali, Farid, & Azman, 2015; Zamani et al., 2016)
CONCLUSION
BLW and its herbal component extracts inhibit proliferation of K562 and K562/adr cells. This inhibition by C. petasites, H. perforata, and T. triandra root extracts is similar to or greater in doxorubicin-resistant cells than in doxorubicin-sensitive cancer cells. The extracts are negligibly cytotoxic to PBMCs. The extracts induce apoptosis in K562 and K562/adr cells, involving cell cycle arrest at the G0/G1 phase in K562 cells. These findings provide preliminary information on the anticancer activities of the crude extracts of BLW and its herbal components, therefore, other investigations of their mechanisms of action are still needed. Isolation of pure bioactive compounds and investigation of their mechanism of action for anticancer activities are also needed. The anticancer activities of BLW and its herbal components in vivo and at clinical levels should be further investigated.
ACKNOWLEDGMENTS
The authors thank Associate Professor Dr. Sawitree Chiampanichayakul, Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, for providing the cancer cell lines.
REFERENCES
Asmaa, M.J., Ali, A.J., Farid, J.M., and Azman, S. 2015. Growth inhibitory effects of crude pomegranate peel extract on chronic myeloid leukemia, K562 cells. International Journal Applied and Basic Medical Research. 5: 100-105.
Brimson, J.M., Onlamoon, N., Tencomnao, T., and Thitilertdecha, P. 2019. Clerodendrum petasites S. Moore: The therapeutic potential of phytochemicals, hispidulin, vanillic acid, verbascoside, and apigenin. Biomedicine and Pharmacotherapy. 118: 109319.
Chamani, E., Ebrahimi, R., Khorsandi, K., Meshkini, A., Zarban, A., and Sharifzadeh, G. 2020. In vitro cytotoxicity of polyphenols from Datura innoxia aqueous leaf- extract on human leukemia K562 cells: DNA and nuclear proteins as targets. Drug and Chemical Toxicology. 43: 138-148.
Dechsupa, S., Kothan, S., Vergote, J., Leger, G., Martineau, A., Berangeo, S., Kosanlavit, R., Moretti, J.L., and Mankhetkorn, S. 2007. Quercetin, Siamois 1 and Siamois 2 induce apoptosis in human breast cancer MDA-mB-435 cells xenograft in vivo. Cancer Biology and Therapy. 6: 56-61.
Juckmeta, T., Pipatrattanaseree, W., Jaidee, W., Dechayont, B., Chunthorng-Orn, J., Andersen, R.J., and Itharat, A. 2019. Cytotoxicity to five cancer cell lines of the respiratory tract system and anti-inflammatory activity of Thai traditional remedy. Natural Product Communication. 14: 1-6.
Kothan, S., Dechsupa, S., Leger, G., Moretti, J.L., Vergote, J., and Mankhetkorn, S. 2004. Spontaneous mitochondrial membrane potential change during apoptotic induction by quercetin in K562 and K562/adr cells. Canadian Journal of Physiology and Pharmacology. 82: 1084-1090.
Li, L., Dai, H.J., Ye, M., Wang, S.L., Xiao, X.J., Zheng, J., Chen, H.Y., Luo, Y.H., and Liu, J. 2012. Lycorine induces cell-cycle arrest in the G0/G1 phase in K562 cells via HDAC inhibition. Cancer Cell International. 12: 49.
Mahbub, A.A., Le Maitre, C.L., Haywood-Small, S.L., McDougall, G.J., Cross, N.A., and Jordan-Mahy, N. 2013. Differential effects of polyphenols on proliferation and apoptosis in human myeloid and lymphoid leukemia cell lines. Anti-Cancer Agents in Medicinal Chemistry. 13: 1601-1613.
Menezes, L.R.A., Sousa Costa, C.O.D., Rodrigues, A.C.B.C., Santo, F.R.E., Nepel, A., Dutra, L.M., Silva, F.M.A., Soares, M.B.P., Barison A. Costa, E.V., et al. 2016. Cytotoxic Alkaloids from the Stem of Xylopia laevigata. Molecules. 21: 890.
National Drug Committee. 2009. List of Herbal Medicine Products A.D. 2006. Nonthaburi: Agricultural Co-operative Federation of Thailand, Ltd.
Noysang, C., and Pummarin, T. 2019. Evaluation of phytochemicals and pharmacological activities of Ben-Cha-Lo-Ka-Wi-Chian remedy. Applied Mechanics and Materials. 891: 52-59.
Rossi, Y.E., Bohla, L.P., Vanden Braber, N.L., Ballatore, M.B., Escobar, F.M., Bodoira, R., Maestri, D.M., Porporatto, C., Cavaglieri, L.R., and Montenegro, M.A. 2020. Polyphenols of peanut (Arachis hypogaea L.) skin as bioprotectors of normal cells. Studies of cytotoxicity, cytoprotection and interaction with ROS. Journal of Functional Foods. 67: 103862.
Saraste, A., and Pulkki, K. 2000. Morphologic and biochemical hallmarks of apoptosis. Cardiovascular Research. 45: 528-537.
Singharachai, C., Palanuvej, C., Kiyohara, H., Yamada, H., and Ruangrungsi, N. 2011. Safety evaluation of Thai traditional medicine remedy: Ben Cha Lo Ka Wi Chian. Journal of Health Research. 25: 83-90.
Strategy and Planning Division, Ministry of Public Health. 2020. Public Health Statistics A.D.2018. Bangkok: Strategy and Planning Division, Ministry of Public Health.
Suttana, W., Mankhetkorn, S., Poompimon, W., Palagani, A., Zhokhov, S., Gerlo, S., Haegeman, G., and Berghe, W.V. 2010. Differential chemosensitization of P-glycoprotein overexpressing K562/Adr cells by withaferin A and Siamois polyphenols. Molecular Cancer. 9: 99.
Synold, T.W., Dussault, I., and Forman, B.M. 2001. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nature Medicine. 7: 584- 590.
Thangapazham, R.L., Singh, A.K., Sharma, A., Warren, J., Gaddipati, J.P., and Maheshwari, R.K. 2007. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Letter. 245: 232-241.
Yao, J., Jiao, R., Liu, C., Zhang, Y., Yu, W., Lu, Y., and Tan, R. 2016. Assessment of the cytotoxic and apoptotic effects of chaetominine in a human leukemia cell line. Biomolecules and Therapeutics. 24: 147-155.
Zamani, A., Jusoh, S.A.M., Al-Jamal, H.A.N., Sul’ain, M.D., and Johan, M.F. 2016. Anti- proliferative effects of Dendrophthoe pentandra methanol extract on BCR/ABL- positive and Imatinib-resistant leukemia cell lines. Asian Pacific Journal of Cancer Prevention. 17: 4857-4861.
Zheng, P.W., Chiang, L.C., and Lin, C.C. 2005. Apigenin induced apoptosis through p53-dependent pathway in human cervical carcinoma cells. Life Sciences. 76: 1367-1379.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Wipob Suttana1,*, Chatubhong Singharachai2, Rawiwan Charoensup3, 4, Narawadee Rujanapun4, and Chutima Suya5
1 Department of Biomedical Science, School of Health Science, Mae Fah Luang University, Chiang Rai 57100, Thailand
2 Department of Public Health, School of Health Science, Mae Fah Luang University, Chiang Rai 57100, Thailand
3 Department of Applied Thai Traditional Medicine, School of Integrative Medicine, Mae Fah Luang University, Chiang Rai 57100, Thailand
4 Medicinal Plants Innovation Center, Mae Fah Luang University, Chiang Rai 57100, Thailand
5 Department of Medical Technology, Chiangrai Prachanukroh Hospital, Chiang Rai 57000, Thailand
Corresponding author: Wipob Suttana, E-mail: wipob.sut@mfu.ac.th
Total Article Views
Editor: Korakot Nganvongpanit, Chiang Mai University, Thailand
Article history:
Received: December 11, 2020;
Revised: January 15, 2021;
Accepted: January 21, 2021;
Published online: March 15, 2021

