
A Panel of Four Anti-HSPG Monoclonal Antibodies Benefits in Increasing the Specificity in Detection of Colorectal Cancer
Ei Khaing Mon, Rujurek Chaiwongsa, Phennapha Klangsinsirikul, and Preeyanat Vongchan*Published Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.055
Journal Issues : Number 3, July-September 2021
Abstract Colorectal cancer (CRC) is the second leading with main cause of death is liver and lung metastasis. Using of a combination of genetic and epigenetic markers are addressed but the results have not been approved in clinical practice. A set of serum biomarkers has been proposed to increase accuracy in early diagnosis of CRC. In addition, non-invasive as well as the best prognostic panel of biomarkers and define predictive biomarkers for treatment of CRC are all aims of future research. HSPGs is an important biomolecule involving in cancer cell proliferation, differentiation, and migration. Membrane HSPGs shed into blood circulation and matrix in particular circumstance can be used as a specific biomarker for some cancer cells. In order to evaluate the benefit of a panel of anti-HSPGs monoclonal antibodies in increasing specificity to detect CRC, four clones of anti-HSPGs were studied for its specific reaction on various tumor cell lines by indirect immunofluorescent technique and analyzed by flow cytometer compared to normal white blood cells. A combination of two or more clones were focused. The results showed that all four clones presented a variation in reaction to all solid tumor cell lines tested but negative to normal white blood cells from different ABO blood groups. Interestingly, amongst those cells tested, HT29, a colorectal cancer cell lines were significantly reacted with all four monoclonal antibodies. Taken together, we proposed a panel of four anti-HSPGs monoclonal antibodies to be applied in various detection platforms to increase the specificity in screening of CRC.
Keywords: Cancer biomarkers, Colorectal cancer, HSPG
Funding: This research project was partly supported by Faculty of Associated Medical Sciences, Chiang Mai University.
Citation: Mon, E.K., Chaiwongsa, R., Klangsinsirikul, P., and Vongchan, P. 2021. A panel four anti-HSPGs monoclonal antibodies benefits in increasing the specificity in detection of colorectal cancer. CMUJ. Nat. Sci. 20(3): e2021055.
INTRODUCTION
There were 18.1 million new cases and 9.5 million cancer-related deaths worldwide in 2018. The number of new cancer cases per year is expected to rise to 29.5 million and the number of cancer-related deaths to 16.4 million by 2040 (https://www.cancer.gov/about-cancer/statistics). Amongst those, colorectal cancer (CRC) is the second leading with main cause of death is liver and lung metastasis (De Mattos-Arruda et al., 2011; Jemal et al., 2011). Significantly improvement of advances in treatment in recent years results in overall survival of patients with metastatic CRC with median of 10 months to 20 months (Wolpin and Mayer, 2008). Based on well understanding the mechanism of colorectal carcinogenesis, two monoclonal antibodies specific to epidermal growth factor receptor (EGFR), cetuximab (Erbitux) and to vascular endothelial growth factor (VEGF), bevacizumab (Avastin) have been targeted. In order to facilitate the selection of the best personalized treatment of metastatic CRC, predictive and prognostic biomarker are also increasingly focused.
Tumor markers or biomarkers refer to substances which mainly are proteins or glycolipids but can be DNA, RNA, microRNA (miRNA), changes in epigenetics, and antibodies. They can be detected in blood, body fluids, and tissues and is a sign of normal or abnormal process, condition or disease (Langan et al., 2013). CRC derived DNA, RNA, and other biomolecules in stool and blood have been investigated extensively and commercially available such as KRAS, TP53, adenomatous polyposis coli (APC) and microsatellite instability (MSI) (Traverso et al., 2002; Osborn and Ahlquist, 2005; Bosch et al., 2011; Lech, Slotwinski et al., 2016) Using of a combination of genetic and epigenetic markers are also addressed but the results have not approved in clinical practice. More interestingly, screening of biomarkers in blood provides obvious advantages compared to those from stool.
Amongst those protein biomarkers, carcinoembryonic antigen (CEA) has been used for more than 50 years (Gold and Freedman, 1965). However, CEA is not specific only for CRC. It can be detected in other gastric and pancreatic cancers as well as inflammatory conditions. Plasma concentration of CEA does not differentiate benign and malignant polyps. Therefore, CEA alone is not recommended for a screening test of CRC (Locker et al., 2006; Labianca et al., 2010; Duffy et al., 2014). Cancer antigen 19-1 or CA19-9 is another biomarker specific to CRC but sensitivity is much less compared to CEA. Therefore, elevation of plasma CA19-9 was agreed as a poor prognostic factor (Carpelan-Holmstrom et al., 2004; Locker et al., 2006; Nicolini et al., 2010; Labianca et al., 2010; Lumachi et al., 2012). Other biomarkers for CRC are under investigated and studied by many research groups and some results has not yet been published (Lech et al., 2016). Moreover, a combination of serum biomarkers has been proposed to increase accuracy in early diagnosis of CRC. Some example is the combination of fecal occult blood and tissue inhibitor of metalloproteinase-1 (TIMP-1) and S100A12 (Karl et al., 2008). Taken together, identification of biomarkers providing a non-invasive and cost-effective diagnosis as well as the recognition of the best prognostic panel of biomarkers and define predictive biomarkers for treatment of CRC are all aims of future research.
Proteoglycans (PGs) are complex biomolecules consisting of protein core covalently linked with one or more glycosaminoglycan chain (GAGs). GAGs are classified into 4 types including heparan sulfate (HS), chondroitin sulfate (CS), dermatan sulfate (DS), and keratan sulfate (KS). In addition to biochemical structure, PGs can be classified according to cell location (cell surface, intracellular, pericellular, and extracellular), gene and protein homology, and specific protein modules in protein cores (Iozzo and Schaefer, 2015). A single cell type can produce many different PGs types as well as varying in expression level in various cell stages (Johnson et al., 2007; Sasaki et al., 2008; Kraushaar et al., 2010; Kraushaar et al., 2013) PGs involves in a proper structural development, organization, hydration, and functional effects in normal cells and tissues by the interaction with other matrix molecules, cells, and cellular mediators (Theocharis et al., 2010). As a result, PGs contribute to a several processes that are essential for homeostasis, differentiation, and tissue morphogenesis, participating in various normal and pathological processes, such as wound repair, inflammation, and tumor development (Frantz et al., 2010; Lu et al., 2011). Notably, several PGs and GAGs are mis-expressed in cancer. Expression of modified PGs on tumor and stromal cell membranes play roles in cancer cell signaling, resulting in growth, migration, and angiogenesis that facilitating tumorigenesis via PGs functional modulation (Theocharis et al., 2010; Nikitovic et al., 2018).
Amongst those, heparan sulfate proteoglycans (HSPGs) is most emphasized since it has crucial roles in normal growth and development. Perlecan, agrin and collagen XVIII are HSPGs found in ECM and two major families including syndecans and GPI-anchored glypicans are transmembrane HSPGs (Bernfield et al., 1999). Alteration in HSPG synthesis results in phenotypic changes from abnormal cell growth differentiation, organogenesis, bone formation and various pathophysiological process including cancer (Bernfield et al., 1999; Gallagher, 2001). Since modified PGs expression is markedly altered in carcinogenic tumor and stromal cell membranes, PGs are constituting prognostic markers and contributing to tumor progression through the modulation of virtually all hallmarks of cancer (Ibrahim et al., 2014).
Cell surface HSPGs play roles in cancer pathogenesis is conditional on tissue origin and is used as a biomarker and therapeutic target feasible. For example, Syndecan-1, a single transmembrane PGs is expressed in normal liver tissues (Tatrai et al., 2010) but significantly reduced in poorly differentiated HCC and extrahepatic metastasis (Matsumoto et al., 1997). Positive syndecan-1 in HCC was associated with good differentiation and no extrahepatic metastasis (Li et al., 2005). Glypican-3, one of six members of glypican family which is the PGs that bind to plasma membrane via its C-terminal glycosylphosphatidylinositol (GPI) anchor. It is highly expressed in HCC but not normal liver (Okabe et al., 2001) or benign, so it has a potential as a biomarker for diagnosis of early stage HCC (Zhu et al., 2001; Capurro et al., 2003; Libbrecht et al., 2006). Overexpression of glypican-3 was significantly related to poor prognosis of patients with HCC (Shirakawa et al., 2009; Kaseb et al., 2016; Liu et al., 2018; Zhang et al., 2018). Glypican-3 is not used only as a serum biomarker. It has been applied for an immune-specific target for cancer immunotherapy as well as a developing of cancer vaccine (Ishiguro et al., 2008; Nakano et al., 2009; Zhu et al., 2013; Ikeda et al., 2014).
Ectodomain of several membrane PGs can undergo controlled enzymatic cleavage by a so-called sheddases resulting in shedding of soluble intact ectodomain (Nam and Park, 2012). For instance, shedding of syndecan-1 by a cleavage of various matrix metalloproteinase (MMPs) including MMP-7 (Ding et al., 2005). Glypican-3 can also be detected in serum as cleavage by a hydrolase enzyme Notum (Traister et al., 2008). Serum glypican-3 was detected in 40% of HCC patients but not cirrhosis (Nakatsura et al., 2003). Moreover, one-third of HCC patients with negative AFP and des-gamma- carboxy prothrombin (DCP) were positive for serum glypican-3.
Human liver HSPG was isolated by strong anion exchange and monoclonal anti- HSPG antibodies was raised (Vongchan et al., 2005). One of those, 1E4-1D9 have been fully characterized and proved to be anti-glypican-3 (Vongchan and Linhardt, 2017). Another one, 1E4-1C2 was found to be positive by indirect immunofluorescent technique on various solid tumor cell lines including HT29, colorectal cancer. The study in HCC, hepatocellular carcinoma cell, demonstrated that 1E4-1C2 could significantly inhibit HCC cell proliferation in dose dependent manner both in vitro and animal model (Vongchan et al., 2011). However, there are a number of anti-HSPG have not yet been identified including 1E2-2B8, 1E2-2C9 and 1E2-1D11. Antigens specific to these monoclonal antibodies have not yet been characterized.
As previously reviewed, a combination of tumor markers and/or monoclonal antibodies specific to individual tumor cell may benefit to increase sensitivity and accuracy in screening of early phase cancer. HSPGs itself can also be cleavage and identified in matrix and serum. In addition, different expression level of HSPGs on each cell type depending also vary in phase of maturation. Moreover, some particular cells express more than one type of HSPGs. Thus, detection of a set of soluble HSPGs in serum sample at a time using a panel of anti-HSPGs may help increase sensitivity and/or specificity for early detection of specific tumor.
Therefore, we proposed that using a panel of monoclonal antibodies specific to HSPGs may help increase specificity in detection of individual tumor. In the present study, four monoclonal antibodies specific to HSPGs isolated from human liver were individually screened for its specific to react on various solid tumor cell lines compared to normal white blood cells collected from healthy subjects with various ABO blood group system. Upon analysis, it was demonstrated that four monoclonal anti-HSPGs antibodies could react specifically to colorectal cancer cell lines, HT29 while some or none in reacting to other cell lines. A panel of four monoclonal antibodies is proposed to be a candidate of choice for serum screening of early detection of colorectal cancer. Moreover, this is the first report of using anti-HSPGs in detection of its antigen specific on this implicated cancer.
MATERIALS AND METHODS
Cell lines and materials
HT29 (colorectal adenocarcinoma) was a kind gift from Prof. Dr. Pa-thai Yenchitsomanus, Faculty of Medicine Siriraj Hospital, Mahidol University, HepG2 (hepatocellular carcinoma) was given from Assoc. Prof. Dr. Ariyaphong Wongnoppavich, Department of Biochemistry, Faculty of Medicine, Chiang Mai University. Other cell lines including SW620 (colorectal adenocarcinoma metastatic, lymph node), SW1353 (chondrosarcoma), KB (mouth epidermal carcinoma) and MCF7 (breast adenocarcinoma) were gifts from Assoc. Prof. Dr. Weera Wongkum, Department of Biology, Faculty of Sciences, Chiang Mai University. All solid tumor cell lines were grown in DMEM high glucose supplemented with 10% Fetal calf serum (Gibco, Life Technologies, NY, USA). IsoStrip for antibody isotyping was purchased from Roche (IN, USA). Other common reagents used in this studies were purchased from local reputable companies including PCL Holdings (Thailand) and Pacific Sciences (Thailand).
Preparation of monoclonal anti-HSPG antibodies
Four hybrid clones producing anti-HSPGs (1E4-1C2, 1E2-2B8, 1E2-2C9, and 1E2- 1D11) were grown in DMEM high glucose (Gibco, Life Technologies, NY, USA) supplemented with OPI (Sigma-Aldrich (St. Louis, MO, USA).) and 10% fetal bovine serum (Gibco, Life Technologies, NY, USA) in 37 °C, 5%CO2, and 5% humidification. Isotype of monoclonal antibodies was identified using IsoStrip as according to the manufacturer’s instructions. Briefly, cell culture supernatant from hybrid anti-HSPGs was firstly 1:10 diluted with 1%BSA in PBS pH 7.2. Diluted sample (150 μL) was then applied onto an isotyping strip and incubated for 1 minute. Finally, the strip was removed and let air dried for 10 minutes and the reaction was read. Monoclonal anti-HSPG antibodies were purified from cell culture supernatant using Protein L affinity agarose beads (InvivoGen, Life Science Research Products, Califonia, USA). In brief, 4 mL of 50% slurry Protein L beads are packed into the column and washed with equilibrate/wash buffer (10 mM sodium phosphate, 150 mM sodium chloride, pH 7.2). Cell culture supernatant was applied and allowed to flow completely into the resin. Column was washed with equilibrate/wash before eluting with 5 mL of elution buffer (0.1M glycine, pH 2.0). Fractions of 1.0 mL were collected and adjusted to physiologic pH with 40 µL of neutralization buffer (1M Tris, pH 7.5). Fractions were then measured the absorbance at 280 nm by UV spectrophometer (EON Biotek, Scientific Instruments, Vermont, USA). Purified monoclonal antibody was dialyzed against PBS pH 7.2 and concentrated.
Expression of antigen specific to monoclonal anti-HPGs antibodies on various cell lines
Indirect immunofluorescent staining was performed to determine the expression of antigen specific to monoclonal antibody clone 1E4-1C2, 1E2-2B8, 1E2-2C9, and 1E2- 1D1 on various solid tumor cell lines. Fifty µL of cells (1 x 107 cells/mL) was incubated with heat inactivated normal human AB serum on ice at a final concentration of 10% for 30 min to block Fc receptor. Monoclonal antibody was individually added 50 µL to a final concentration of 20 µg/mL and incubated on ice for another 30 min. One percent BSA in phosphate buffered saline (PBS) pH 7.2, 0.02% sodium azide were used as conjugate control. After 3 washes with 1%BSA in PBS pH 7.2, 0.02% sodium azide, cells were resuspended to 50 µL with 1%BSA in phosphate buffered saline and added with 50 µL of FITC conjugated rabbit anti-mouse Igs, 1:25 (DAKO, Glostrup, Denmark). The reactions were extended incubation on ice for 30 minutes and washed out with 4 changes of 1%BSA in PBS pH 7.2, 0.02% sodium azide. Finally, stained cells were suspended with 500 µL of sheath fluid (Becton Dickinson, CA, USA) and analyzed by flow cytometer (Coulter, MA, USA).
Expression of antigen specific to anti-HPGs monoclonal antibodies on peripheral blood cells
Heparinized whole blood was collected from ten normal healthy subjects with different ABO blood group system (Human Ethic approval No. AMSEC-63EX-023). Buffy coat was separated after centrifugation at 250 xg for 10 minutes. Buffy coat was washed three times and adjusted to 1x107 cells/mL with cold 1%BSA in PBS pH 7.2, 0.02% sodium azide. To block non-specific FcR-mediated binding of antibody, cells was incubated for 30 minutes on ice with heat inactivated normal human AB serum in a final concentration of 10%. A reaction of 50 µL of treated cell suspension was individually added with the monoclonal anti-HSPGs antibody in a final concentration of 20 µg/mL and incubated on ice for 30 minutes. One percent BSA in phosphate buffered saline (PBS) pH 7.2, 0.02% sodium azide were used as conjugate control. After 3 washes with 1%BSA in PBS pH 7.2, 0.02% sodium azide, cells were resuspended to 50 µL with 1%BSA in phosphate buffered saline and added with 50 µL of FITC conjugated rabbit anti-mouse Igs, 1:25 (DAKO, Glostrup, Denmark). The reactions were extended incubation on ice for 30 minutes and washed out with 4 changes of 1%BSA in PBS pH 7.2, 0.02% sodium azide. Finally, stained cells were suspended with 500 µL of sheath fluid (Becton Dickinson, CA, USA) and analyzed by flow cytometer (Coulter, MA, USA).
RESULTS
Expression of antigens specific to four monoclonal anti-HSPGs antibodies on various solid tumor cell lines.
Each monoclonal anti-HSPGs antibody was identified for its isotype by commercial kit. The result indicated that 1E4-1C2 was IgG1, kappa while the other three clones were IgA, kappa (data not shown). Based on indirect immunofluorescent technique, monoclonal antibodies specific to HSPGs isolated from human liver including 1E4-1C2, 1E2-2B8, 1E2-2C9, and 1E2-1D11 was individually studied for the expression of its specific antigen on various solid tumor cell lines including HT29, KB, MCF7, SW620, SW1353, and HepG2 and analyzed by flow cytometer. It was found that all clones demonstrated different patterns in the reaction amongst cell lines tested varied from negative to weakly and strong positive. Clone 1E4-1C2 showed various strongly positive to all cell lines tested after conjugate control subtraction but weakly positive with KB as shown in Figure 1.
Expression of a panel of four monoclonal anti-HSPGs antibodies to its specific antigen on HT29
Clone 1E2-2B8 was strongly reacted to HT29 but weakly positive to SW1353. Another clone, 1E2-2C9 was also strongly bound to its specific antigen on HT29 but weakly reacted to only SW620. Clone 1E2-1D11 showed negative reaction to almost cell lines tested except HT29 (Table 1). Interestingly, while those solid tumor cell lines demonstrated a different individually reacted with each monoclonal antibody varying from negative and weakly to strongly positive, HT29 was shown to be strongly reacted with all four clones tested (Figure 2).
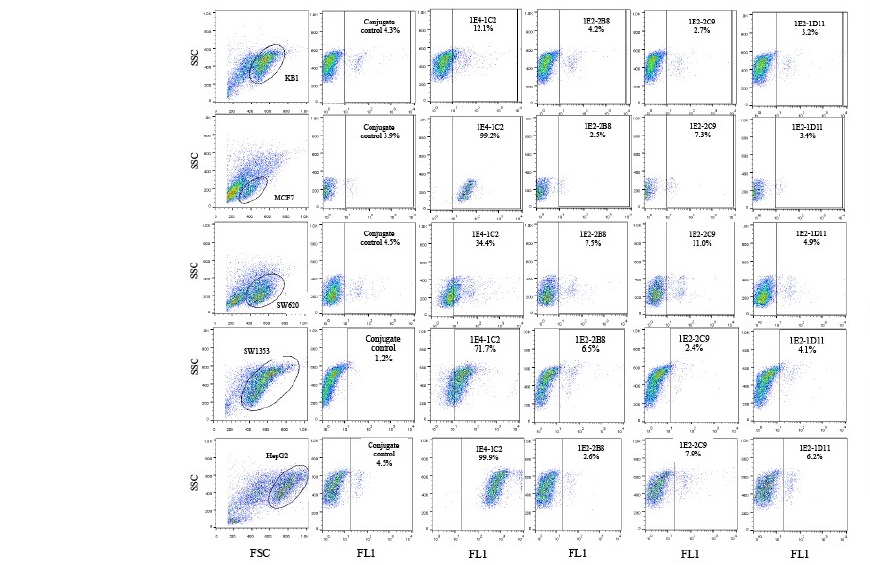
Figure 1. Expression of antigens specific to four monoclonal anti-HSPGs antibodies on various solid tumor cell lines. Fifty µL of human AB serum treated cells (1 x 107 cells/mL) was individually reacted with monoclonal anti-HSPGs clone 1E4- 1C2, 1E2-2B8, 1E2-2C9, and 1E2-1D11 at a final concentration of 20 µg/mL. After 30 minutes incubation on ice following with three washes with 1%BSA in PBS pH 7.2, 0.02% sodium azide, cells were resuspended to 50 µL of the same buffer. Fifty µL of FITC conjugated rabbit anti-mouse Igs (1:25) was applied. The reaction was incubated for another 30 minutes before 4 washes. Finally, stained cells were suspended with 500 µL of sheath fluid and analyzed by flow cytometer.
Table 1. Patterns in the reaction of four anti-HSPG monoclonal antibodies to various solid tumor cell lines.
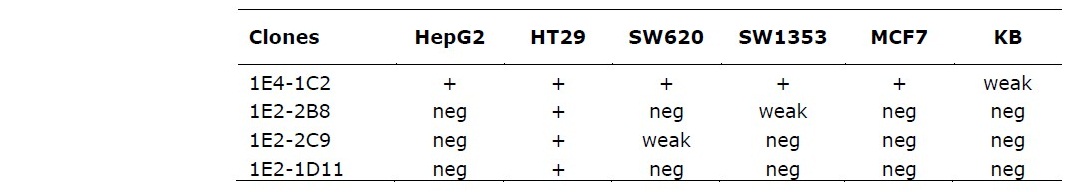
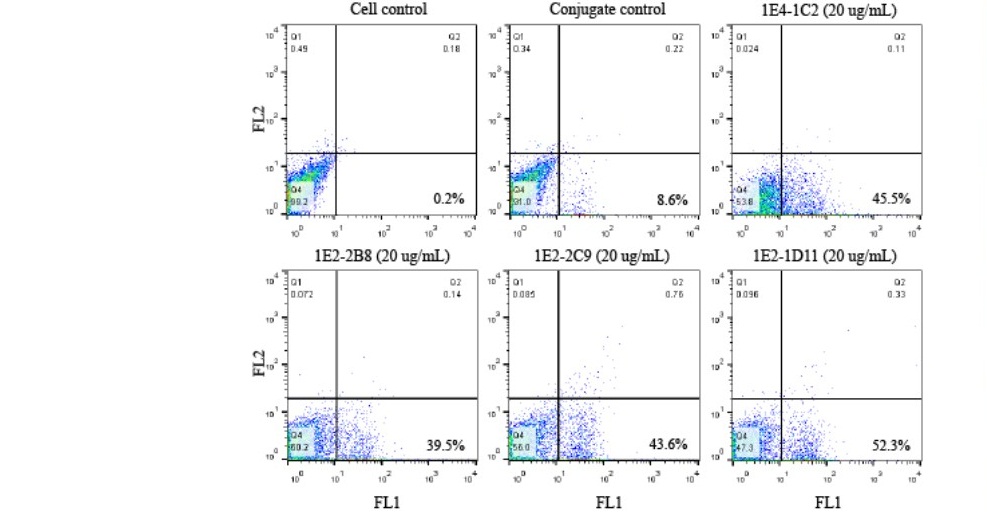
Figure 2. A panel of four monoclonal anti-HSPGs antibodies were strongly reacted to its specific antigen on HT29. Fifty µL of human AB serum treated HT29 (1 x 107 cells/mL) was individually reacted with monoclonal anti-HSPGs clone 1E4-1C2, 1E2-2B8, 1E2-2C9, and 1E2-1D11 at a final concentration of 20 µg/mL. After 30 minutes incubation on ice following with three washes with 1%BSA in PBS pH 7.2, 0.02% sodium azide, cells were resuspended to 50 µL of the same buffer. Fifty µL of FITC conjugated rabbit anti-mouse Igs (1:25) was applied. The reaction was incubated for another 30 minutes before 4 washes. Finally, stained cells were suspended with 500 µL of sheath fluid and analyzed by flow cytometer.
No expression of antigens specific to four monoclonal anti-HSPGs antibodies on normal white blood cells.
To verify their specificity only on tumor cells, all four monoclonal antibodies were also tested on normal white blood cells. Heparinized whole blood from 10 normal healthy volunteers with various ABO blood groups were collected. Buffy coat was individually reacted by indirect immunofluorescent technique and analyzed by flow cytometer. The result reveled that all four monoclonal antibodies did not react to all population of normal white blood cells and with no different between ABO blood group system. Figure 3 demonstrated a represent of one blood sample showing a negatively reacted with all four monoclonal anti-HSPGs antibodies. The results of all ten blood samples with various ABO blood groups were aligned overlapping and shown in Figure 4. It was revealed that all monoclonal anti-HSPGs antibodies were not reacted to normal white blood cells. They are specific to only some particular cancer cells. Taken together, the results demonstrated that HT29, colorectal cancer was significantly positive with all four anti- HSPG monoclonal antibodies. These four anti-HSPGs monoclonal antibodies can be applied as a set or panel of biomarker for screening of colorectal cancer.
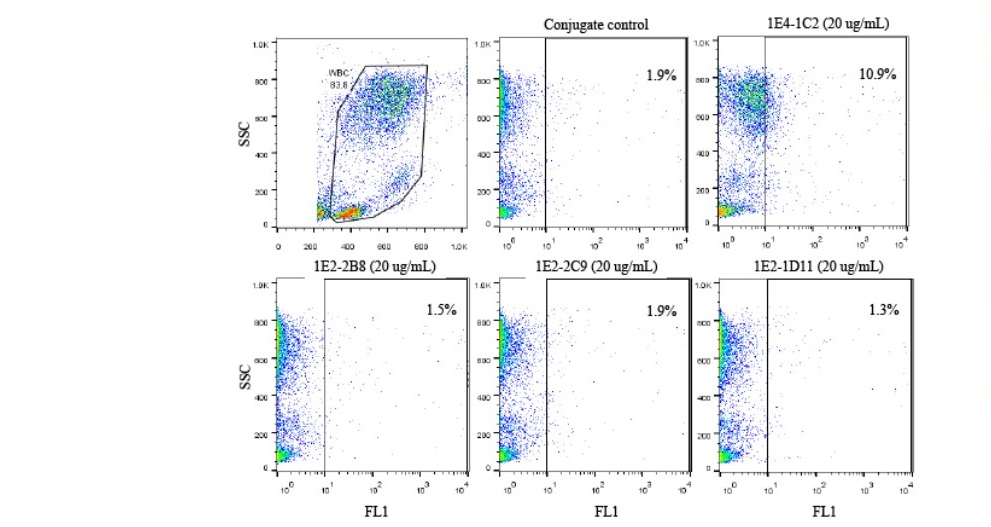
Figure 3. Four monoclonal anti-HSPGs antibodies did not react to normal white blood cells. Heparinized whole blood was collected. Washed buffy coat (1x107 cells/mL) treated with heat inactivated normal AB serum to block non-specific FcR- mediated binding of antibody site for 30 minutes on ice. A reaction of 50 µL of treated cell suspension was individually added with the monoclonal anti-HSPGs antibody in a final concentration of 20 µg/mL and incubated on ice for another 30 minutes before three washes with 1%BSA in PBS pH 7.2, 0.02% sodium azide. Cells were resuspended to 50 µL with the same buffer and added with 50 µL of FITC conjugated rabbit anti- mouse Igs (1:25). The reaction was incubated for another 30 minutes before 4 washes. Finally, stained cells were suspended with 500 µL of sheath fluid and analyzed by flow cytometer.
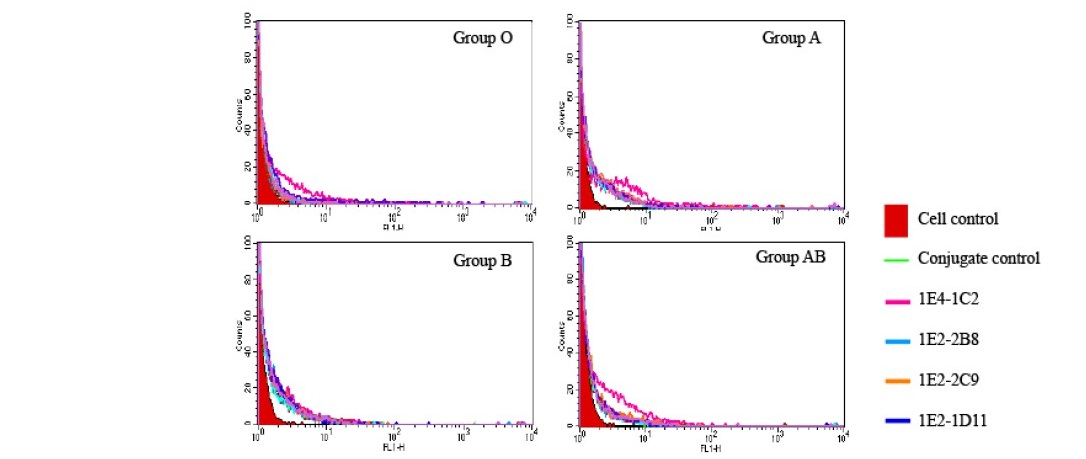
Figure 4. Negative reaction four monoclonal anti-HSPGs antibodies on all normal white blood cells of different ABO blood groups. Ten samples of heparinized whole blood with different ABO blood groups were collected. Washed buffy coat (1x107 cells/mL) treated with heat inactivated normal AB serum to block non- specific FcR-mediated binding of antibody for 30 minutes on ice. A reaction of 50 µL of treated cell suspension was individually added with the monoclonal anti-HSPGs antibody in a final concentration of 20 µg/mL and incubated on ice for another 30 minutes before three washes with 1%BSA in PBS pH 7.2, 0.02% sodium azide. Cells were resuspended to 50 µL with the same buffer and added with 50 µL of FITC conjugated rabbit anti- mouse Igs (1:25). The reaction was incubated for another 30 minutes before 4 washes. Finally, stained cells were suspended with 500 µL of sheath fluid and analyzed by flow cytometer. The data from ten results were aligned overlapping.
DISCUSSION
CRC is the third most common cancer and third cause of cancer death (https://www.cancer.gov/about-cancer/statistics). The effective early screening can reduce the death. In addition, research in detection with non-invasive technique is in focus since early CRC is asymptomatic. Therefore, development of simple non-invasive sensitive technique for CRC would be beneficiary for patients. Non-invasive method is acceptable for patients and simple in procedures. Biomarkers play an important clinical role in early diagnosis, treatment, and prognosis (McKeown et al., 2014) and a combination of biomarkers is proved to increase sensitivity in detection of CRC (Grady and Pritchard, 2014; Luo and Xu, 2014; Lech et al., 2016). HSPGs are remarked to relate to many cancer types in all aspects including pathogenesis and prognosis. In addition, some monoclonal antibody specific to membrane HSPGs has been applied in target immunotherapy such as anti-glypican 3 (Ishiguro et al., 2008; Nakano et al., 2009; Zhu et al., 2013; Ikeda et al., 2014). Interestingly, particular cells can express one or more type of HSPG and even in different part of cell (Tanaka et al., 2018). The present study also supports that particular cell type may express one or more types of HSPGs as demonstrated in HT29, colorectal cancer cell lines. Antigens specific to those 4 clones investigated are not characterized yet, However, the patterns of expression on other cancer cells were also demonstrated the difference (Figure 1).
Moreover, membrane HSPGs can be dissociated from tumor cells in a particular circumstance by specific proteolytic cleavage and can be detected in blood circulation (Traister et al., 2008; Nam and Park, 2012; Piperigkou et al., 2016). The aim of this study was therefore, to study and identify the possibility of using a panel of anti-HSPGs monoclonal antibodies in increasing the specificity of screening for particular cancer cell. In the present study, a set of four monoclonal antibodies raised against HSPGs isolated from human liver (Vongchan et al., 2005) were in vitro studied for the specific reaction on various solid tumor cell lines by indirect immunofluorescent technique in order to evaluate their specificity to screen particular solid cancer cell lines. Four clones of anti- HSPGs were individually reacted to various solid tumor cancer cell lines and the result was then analyzed to match a couple or more for more specific detection of particular cancer cell type. The results revealed that each antibody demonstrated a specific pattern in reaction to each cancer cell lines tested. The reaction was varied from negative to weakly and strong positive. Monoclonal antibody clone 1E4-1C2 showed varied special characteristics in reacting to all cancer cell lines tested including HT29, KB, MCF7, SW620, SW1353, and HepG2. From the result, it was revealed that, clone 1E4-1C2 alone could be used as a biomarker for all cancer cell lines tested. However, consideration according to particular cell line, the result demonstrated that SW620 could be more specifically detected when testing with a couple of monoclonal antibodies of 1E4-1C2 and 1E2-2C9. In addition, more specific in detection of SW1353 should be obtained when a couple of two anti-HSPGs monoclonal antibodies including 1E4-1C2 and 1E2-2B8 were used as summarized in Table 1. It was noticed that two colon cancer cell lines used in the present study demonstrated different patterns of expression when reacted with those four monoclonal antibodies. It can be elucidated according to the source of original cell (Ahmed et al., 2013). Moreover, SW620 is a lymph node metastatic form that may express different number and types of HSPGs compared to HT29, a primary tumor. The most interestingly, only HT29 was strongly specifically reacted with all four anti-HSPGs monoclonal antibodies. To verify that a panel of four monoclonal antibodies could be used to specify only the cancer cells, normal white blood cells were also tested as a model. The results showed that all antibodies were not bound onto normal white blood cells. The antigens specific to those monoclonal antibodies have not yet been characterized, however, as a difference in reaction to various cell lines tested, its specific antigen might be different to each other. In addition, Further study in these antigens would be help increase understanding of its role(s) in particular cancer cell.
Membrane HSPGs can be enzymatic cleavage and shed into matrix and serum i.e., glypican-3 and syndecan-1 which are applied for the detection and monitoring of particular cancer cell. To detect these antigens in matrix and/or serum is a platform of choice helps in diagnosis of cancer instead of other invasive technique. The author proposed that those membrane antigens specific to the four monoclonal antibodies specific to colorectal cancer cell line mentioned in this study may be also secreted. Therefore, demonstration of those antigens shedding in cell culture supernatant of HT29 cultivation is essential to support our hypothesis. Combination of those four antigens can be investigated and applied as a panel of CRC specific markers based on double sandwich ELISA platform. The panel of specific antigens may help increase the sensitivity of detection compared to one marker. However, furthermore studies based on ELISA platform is suggested to verify the sensitivity and specificity in early detection of CRC compared to serum samples from normal healthy individuals and other liver non- cancer pathologies.
Taken together, the present study is the first report demonstrating the utilization of a panel of four anti-HSPGs monoclonal antibodies to be a candidate set of antibodies in specific early detection of human colorectal cancer (CRC).
ACKNOWLEDGEMENTS
The author would like to thank Ms. Yupanun Wutti-In, a PhD candidate, Faculty of Medicine, Siriraj Hospital, Mahidol University for her kind help in data retrieval and analysis.
REFERENCES
Ahmed, D., Eide, P.W., Eilertsen, I.A., Danielsen, S. A., Eknaes, M., Hektoen, M., and Lothe, R.A. 2013. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2: e71.
Bernfield, M., Gotte, M., Park, P.W., Reizes, O., Fitzgerald, M.L., Lincecum, J., and Zako, M. 1999. Functions of cell surface heparan sulfate proteoglycans. Annual Review of Biochemistry. 68: 729-777.
Bosch, L.J., Carvalho, B., Fijneman, R.J., Jimenez, C.R., Pinedo, H.M., van Engeland, M., and Meijer, G.A. 2011. Molecular tests for colorectal cancer screening. Clinical Colorectal Cancer. 10: 8-23.
Capurro, M., Wanless, I.R., Sherman, M., Deboer, G., Shi, W., Miyoshi, E., and Filmus, J. 2003. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 125: 89-97. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12851874
Carpelan-Holmstrom, M., Louhimo, J., Stenman, U.H., Alfthan, H., Jarvinen, H., and Haglund, C. 2004. CEA, CA 242, CA 19-9, CA 72-4 and hCGbeta in the diagnosis of recurrent colorectal cancer. Tumor Biology. 25: 228-234.
De Mattos-Arruda, L., Dienstmann, R., and Tabernero, J. 2011. Development of molecular biomarkers in individualized treatment of colorectal cancer. Clinical Colorectal Cancer. 10: 279-289.
Ding, K., Lopez-Burks, M., Sanchez-Duran, J.A., Korc, M., and Lander, A.D. 2005. Growth factor-induced shedding of syndecan-1 confers glypican-1 dependence on mitogenic responses of cancer cells. Journal of Cell Biology. 171:729-738.
Duffy, M.J., Lamerz, R., Haglund, C., Nicolini, A., Kalousova, M., Holubec, L., and Sturgeon, C. 2014. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. International Journal of Cancer. 134: 2513-2522.
Frantz, C., Stewart, K.M., and Weaver, V.M. 2010. The extracellular matrix at a glance. Journal of Cell Science. 123: 4195-4200.
Gallagher, J.T. 2001. Heparan sulfate: growth control with a restricted sequence menu. Journal of Clinical Investigation. 108: 357-361. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&do pt=Citation&list_uids=11489926
Gold, P., and Freedman, S.O. 1965. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. Journal of Experimental Medicine. 121: 439-462.
Grady, W.M. and Pritchard, C.C. 2014. Molecular alterations and biomarkers in colorectal cancer. Toxicologic Pathology. 42: 124-139.
Ibrahim, S.A., Hassan, H., and Gotte, M. 2014. MicroRNA regulation of proteoglycan function in cancer. The FEBS Journal. 281: 5009-5022.
Ikeda, M., Ohkawa, S., Okusaka, T., Mitsunaga, S., Kobayashi, S., Morizane, C., Suzuki, I., Yamamoto, S., and Furuse, J. 2014. Japanese phase I study of GC33, a humanized antibody against glypican-3 for advanced hepatocellular carcinoma. Cancer Science. 105: 455-462.
Iozzo, R. V. and Schaefer, L. 2015. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biology. 42: 11-55.
Ishiguro, T., Sugimoto, M., Kinoshita, Y., Miyazaki, Y., Nakano, K., Tsunoda, H., Sugo, I., Ohizumi, I., Aburatani, H., Hamakubo, T. et al. 2008. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Research. 68: 9832-9838.
Jemal, A., Bray, F., Center, M.M., Ferlay, J., Ward, E., and Forman, D. 2011. Global cancer statistics. CA: A Cancer Journal for Clinicians. 61: 69-90.
Johnson, C.E., Crawford, B.E., Stavridis, M., Ten Dam, G., Wat, A.L., Rushton, G., Ward, C.M., Wilson, V., van Kuppevelt, T.H., Esko, J.D. et al. 2007. Essential alterations of heparan sulfate during the differentiation of embryonic stem cells to Sox1-enhanced green fluorescent protein-expressing neural progenitor cells. Stem Cells. 25: 1913-1923.
Karl, J., Wild, N., Tacke, M., Andres, H., Garczarek, U., Rollinger, W., and Zolg, W. 2008. Improved diagnosis of colorectal cancer using a combination of fecal occult blood and novel fecal protein markers. Clinical Gastroenterology and Hepatology. 6: 1122-1128.
Kaseb, A.O., Hassan, M., Lacin, S., Abdel-Wahab, R., Amin, H.M., Shalaby, A., Wolff, R.A., Yao, J., Rashid, A., Vennapusa, B. et al. 2016. Evaluating clinical and prognostic implications of Glypican-3 in hepatocellular carcinoma. Oncotarget. 7: 69916-69926.
Kraushaar, D.C., Dalton, S., and Wang, L. 2013. Heparan sulfate: a key regulator of embryonic stem cell fate. Journal of Biological Chemistry. 394: 741-751.
Kraushaar, D.C., Yamaguchi, Y., and Wang, L. 2010. Heparan sulfate is required for embryonic stem cells to exit from self-renewal. Journal of Biological Chemistry. 285: 5907-5916.
Labianca, R., Nordlinger, B., Beretta, G.D., Brouquet, A., Cervantes, A., and Group, E.G.W. 2010. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Annals of Oncology. 21: v70-77.
Langan, R.C., Mullinax, J.E., Raiji, M.T., Upham, T., Summers, T., Stojadinovic, A., and Avital, I. 2013. Colorectal cancer biomarkers and the potential role of cancer stem cells. Journal Cancer. 4: 241-250.
Lech, G., Slotwinski, R., Slodkowski, M., and Krasnodebski, I.W. 2016. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World Journal of Gastroenterology. 22: 1745-1755.
Li, H.G., Xie, D.R., Shen, X.M., Li, H.H., Zeng, H., and Zeng, Y.J. 2005. Clinicopathological significance of expression of paxillin, syndecan-1 and EMMPRIN in hepatocellular carcinoma. World Journal of Gastroenterology. 11: 1445-1451.
Libbrecht, L., Severi, T., Cassiman, D., Vander, B.S., Pirenne, J., Nevens, F., Verslype, C.V., Jos van, P., and Roskams, T. 2006. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. The American Journal of Surgical Pathology. 30: 1405-1411.
Liu, H., Yang, C., Lu, W., and Zeng, Y. 2018. Prognostic significance of glypican-3 expression in hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore), 97: e9702.
Locker, G.Y., Hamilton, S., Harris, J., Jessup, J.M., Kemeny, N., Macdonald, J.S., Somerfield, M.R., Hayes, D.F., Bast Jr, R.C., Asco. 2006. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. Journal of Clinical Oncology. 24: 5313-5327.
Lu, P., Takai, K., Weaver, V.M., and Werb, Z. 2011. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harbor Perspectives in Biology.3.
Lumachi, F., Marino, F., Orlando, R., Chiara, G.B., and Basso, S.M. 2012. Simultaneous multianalyte immunoassay measurement of five serum tumor markers in the detection of colorectal cancer. Anticancer Research. 32: 985-988. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22399621
Luo, H.Y. and Xu, R.H. 2014. Predictive and prognostic biomarkers with therapeutic targets in advanced colorectal cancer. World Journal of Gastroenterology. 20: 3858-3874.
Matsumoto, A., Ono, M., Fujimoto, Y., Gallo, R.L., Bernfield, M., and Kohgo, Y. 1997. Reduced expression of syndecan-1 in human hepatocellular carcinoma with high metastatic potential. International Journal of Cancer. 74: 482-491.
McKeown, E., Nelson, D. W., Johnson, E. K., Maykel, J. A., Stojadinovic, A., Nissan, A.,. . . Steele, S. R. (2014). Current approaches and challenges for monitoring treatment response in colon and rectal cancer. J Cancer, 5(1), 31-43. doi:10.7150/jca.7987
Nakano, K., Orita, T., Nezu, J., Yoshino, T., Ohizumi, I., Sugimoto, M., Furugaki, K., Kinoshita, Y., Ishiguro, T., Hamakubo, T. et al. 2009. Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochemical and Biophysical Research Communications. 378: 279-284.
Nakatsura, T., Yoshitake, Y., Senju, S., Monji, M., Komori, H., Motomura, Y., Hosaka, S., Beppu, T., Ishiko, T., Kamohara, H. et al. 2003. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochemical and Biophysical Research Communications. 306: 16-25.
Nam, E.J. and Park, P.W. 2012. Shedding of cell membrane-bound proteoglycans. Methods in Molecular Biology. 836: 291-305.
Nicolini, A., Ferrari, P., Duffy, M.J., Antonelli, A., Rossi, G., Metelli, M.R., Fulceri, F., Anselmi, L., Conte, M., Berti, P. et al. 2010. Intensive risk-adjusted follow-up with the CEA, TPA, CA19.9, and CA72.4 tumor marker panel and abdominal ultrasonography to diagnose operable colorectal cancer recurrences: effect on survival. Archives of Surgery. 145: 1177-1183.
Nikitovic, D., Berdiaki, A., Spyridaki, I., Krasanakis, T., Tsatsakis, A., and Tzanakakis, N. 2018. Proteoglycans-Biomarkers and Targets in Cancer Therapy. Front Endocrinol (Lausanne), 9, 69. doi:10.3389/fendo.2018.00069
Okabe, H., Satoh, S., Kato, T., Kitahara, O., Yanagawa, R., Yamaoka, Y., Tsunoda, T., Furukawa, Y., Nakamura, Y. 2001. Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: identification of genes involved in viral carcinogenesis and tumor progression. Cancer Research. 61: 2129-2137.
Osborn, N.K. and Ahlquist, D.A. 2005. Stool screening for colorectal cancer: molecular approaches. Gastroenterology. 128: 192-206.
Piperigkou, Z., Mohr, B., Karamanos, N., and Gotte, M. 2016. Shed proteoglycans in tumor stroma. Cell and Tissue Research. 365: 643-655.
Sasaki, N., Okishio, K., Ui-Tei, K., Saigo, K., Kinoshita-Toyoda, A., Toyoda, H. Nishimura, T., Suda, Y., Hayasaka, M., Hanaoka, K. et al. (2008). Heparan sulfate regulates self-renewal and pluripotency of embryonic stem cells. Journal of Biological Chemistry. 283: 3594-3606.
Shirakawa, H., Suzuki, H., Shimomura, M., Kojima, M., Gotohda, N., Takahashi, S., Nakagohri, T., Konishi, M., Kobayashi, N., Kinoshita, T. 2009. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Science. 100: 1403-1407.
Tanaka, Y., Tateishi, R., and Koike, K. 2018. Proteoglycans Are Attractive Biomarkers and Therapeutic Targets in Hepatocellular Carcinoma. International Journal of Molecular Sciences. 19.
Tatrai, P., Egedi, K., Somoracz, A., van Kuppevelt, T.H., Ten Dam, G., Lyon, M., Deakin, J.A., Kiss, A., Schaff, Z., Kovalszky, I. 2010. Quantitative and qualitative alterations of heparan sulfate in fibrogenic liver diseases and hepatocellular cancer. Journal of Histochemistry and Cytochemistry. 58: 429-441.
Theocharis, A.D., Skandalis, S.S., Tzanakakis, G.N., and Karamanos, N.K. 2010. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS Journal. 277: 3904-3923.
Traister, A., Shi, W., and Filmus, J. 2008. Mammalian Notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochemical Journal. 410: 503-511.
Traverso, G., Shuber, A., Levin, B., Johnson, C., Olsson, L., Schoetz, D. J., Jr., Hamilton, S.R., Boynton, K., Kinzler, K.W. Vogelstein, B. 2002. Detection of APC mutations in fecal DNA from patients with colorectal tumors. The New England Journal of Medicine. 346: 311-320.
Vongchan, P., Kothan, S., Wutti-In, Y., & Linhardt, R.J. 2011. Inhibition of human tumor xenograft growth in nude mice by a novel monoclonal anti-HSPG isolated from human liver. Anticancer Research. 31: 4067-4074
Vongchan, P. and Linhardt, R.J. 2017. Characterization of a new monoclonal anti- glypican-3 antibody specific to the hepatocellular carcinoma cell line, HepG2. World Journal of Hepatology. 9: 368-384.
Vongchan, P., Warda, M., Toyoda, H., Toida, T., Marks, R.M., and Linhardt, R.J. 2005. Structural characterization of human liver heparan sulfate. Biochimica Et Biophysica Acta-General Subjects. 1721: 1-8.
Wolpin, B.M. and Mayer, R.J. 2008. Systemic treatment of colorectal cancer. Gastroenterology, 134: 1296-1310.
Zhang, J., Zhang, M., Ma, H., Song, X., He, L., Ye, X., & Li, X. 2018. Overexpression of glypican-3 is a predictor of poor prognosis in hepatocellular carcinoma: An updated meta-analysis. Medicine (Baltimore). 97: e11130.
Zhu, A.X., Gold, P.J., El-Khoueiry, A.B., Abrams, T.A., Morikawa, H., Ohishi, N., Ohtomo, T., and Philip, P.A. 2013. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clinical Cancer Research. 19: 920-928.
Zhu, Z.W., Friess, H., Wang, L., Abou-Shady, M., Zimmermann, A., Lander, A. D., Korc, M., Kleeff, J., and Buchler, M.W. 2001. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 48: 558-564.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Ei Khaing Mon1, 2, Rujurek Chaiwongsa1, Phennapha Klangsinsirikul1, and Preeyanat Vongchan1,*
1 Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai 50200, Thailand
2 Department of Medical Technology, University of Medical Technology, Mandalay, Myanmar
Corresponding author: Preeyanat Vongchan, E-mail: preeyanat.v@cmu.ac.th
Total Article Views
Editor: Korakot Nganvongpanit, Chiang Mai University, Thailand
Article history:
Received: December 11, 2020;
Revised: January 20, 2020;
Accepted: January 20, 2020;
Published online: February 1, 2021

