
Evaluation of the Antioxidant Activity of Bignay (Antidesma bunius (Linn.) Spreng var. Kalabaw) Flesh and Seeds as Affected by Maturity and Processing Method
Kristel June Sartagoda*, Ma. Cristina Ilano, Lloyd Earl Flandez, and Katherine Ann Castillo-IsraelPublished Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.042
Journal Issues : Number 2, April-June 2021
Abstract This study aimed to determine the influence of maturity stages and processing methods (blanching and steaming) on the antioxidant profile and in vitro antioxidant activities of bignay (Antidesma bunius (Linn.) Spreng var. Kalabaw) flesh and seeds. Bignay fruits of three maturities (unripe, half ripe, and fully ripe) were collected from Laguna, Philippines. Each maturity stage was subdivided into three lots. One lot underwent blanching at 90 ± 5°C for 2 minutes, and another underwent steaming at 105 ± 5°C for 5 minutes while the last did not undergo treatment. Seeds of the samples were then separated from the flesh. Both seeds and flesh were freeze-dried, extracted, and analyzed for antioxidant contents (total phenolic content, total flavonoid content, and total anthocyanin content) and antioxidant activity by DPPH, ABTS, and FRAP assays. Results show that both the maturity and processing methods significantly affect the antioxidant content and activity of the samples. Moreover, except for the FRAP assay done on flesh samples, all assays showed that there is significant interaction between the effect of maturity and processing method on the antioxidant contents and activity of bignay flesh and seeds. Results also show that fully ripe flesh and seeds yielded greater antioxidant content and antioxidant activity than their half-ripe and unripe counterparts; whereas, blanched flesh and seeds generally had higher antioxidant activities than their unprocessed and steamed counterparts.
Keywords: Antioxidant activity, Bignay, Blanching, Maturity stage, Steaming
Funding: We wish to thank the Philippine Council for Health Research and Development of the Department of Science and Technology (DOST-PCHRD) for providing financial support.
Citation: Sartagoda, K.J., Ilano, Ma.C., Flandez, L.E., and Castillo-Israel, K.A. 2021. Evaluation of the antioxidant activity of bignay (Antidesma bunius (Linn.) Spreng var. Kalabaw) flesh and seeds as affected by maturity and processing method. CMUJ. Nat. Sci. 20(2): e2021042.
INTRODUCTION
The association of nutrition and health had long been a topic of scientific interest. Substantial evidence to support their relationship had been collected over the years (Denardin et al., 2015). In the human diet, antioxidants comprise primarily of polyphenols (Scalbert et al., 2005). Albeit, the level of these compounds in berries is variable according to several factors, including fruit maturity and processing techniques (Lee and Kader, 2000; Tiwari and Cummins, 2013). Depending on the berry-type, antioxidant capacities may be higher or lower during the peak of maturity (Acosta- Montoya et al., 2010). Likewise, processing methods like blanching and steaming affect the antioxidant contents in fruits (Rickman et al., 2007). Blanching, a type of mild heat treatment, is used to inactivate enzymes in fruits before storage or further processing (Huang et al., 2016). Steaming, on the other hand, is a cooking method that utilizes steam by continuous boiling of water until it vaporizes (Lafarga et al., 2018).
The Philippines, as a tropical country, is richly endowed with a wide variety of wild edible but often underutilized fruits. Recent epidemiological studies report that these fruits have many beneficial health effects (Barcelo, 2015). Bignay (Antidesma bunius (Linn.) Spreng) is a fruit-bearing tree native to the Philippines. Its fruits are edible and can be eaten raw or processed into various products. In folk medicine, virtually all its parts are used to cure many ailments. It has been reported to exert anti-diabetic, antioxidant, antiradical, antiplatelet, anticoagulant, anti-dysenteric, antimicrobial, antihypertensive, anticancer effects, and sudorific activities (Islam et al., 2018). Most of the studies on bignay in the Philippines are limited to the quantification and characterization of its phytochemical contents, typically using fresh, ripe fruits. For example, Santiago et al. (2007) previously determined the antioxidant potential, total phenolic and total flavonoid contents, and flavonol and flavanol contents of fresh, mature bignay fruits. A survey on the phenolic and flavonoid contents of local fruits by Recuenco et al. (2016) also included semi-ripe to ripe bignay fruits. Similar studies have also been conducted by Barcelo (2015) and Barcelo et al. (2016). In vivo studies to assess the ameliorating effects of bignay, specifically on the biomarkers of obesity and associated co-morbidities such as dyslipidemia, inflammation, and oxidative stress, are lacking.
Therefore, to gain a better understanding of the potential of bignay fruits as a functional food, an investigation on the influence of maturity stages and processing techniques (specifically blanching and steaming) on the antioxidant profile and in vitro antioxidant activities of bignay fruits were undertaken. The results of this investigation can evidence the health benefits of fruits indigenous to the Philippines and consequently be a scientific basis for the development of functional foods to address obesity and its associated metabolic disorders.
MATERIALS AND METHODS
Chemicals
All chemicals and reagents used in this study were of analytical grade to HPLC grade. Methanol, potassium chloride, and hydrochloric acid were purchased from Duksan Pure Chemicals Co., Ltd. (Ansan, South Korea). Aluminum chloride and sodium acetate were obtained from Techno Pharma Chem (Delhi, India). Folin-Ciocalteu reagent, quercetin, potassium persulfate, gallic acid, glacial acetic acid, (±)-6-hydroxy- 2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) were purchased from Sigma-Aldrich Corporation (Singapore).
Plant Material Preparation
Bignay (A. bunius (L.) Spreng var. Kalabaw) fruits were harvested in the province of Laguna, Philippines. The identity of bignay was authenticated by a curator at the Botanical Herbarium, Museum of Natural History, University of the Philippines Los Baños. The collected fruits were washed and cleaned with room temperature water and were divided into three maturity stages based on size and color (Figure 1). Each maturity stages were then subdivided into three lots. The first lot underwent blanching using a water bath at 90 ± 5°C for 2 minutes, the second lot underwent steaming carried out with a one-layer stainless-steel food steamer at 105 ± 5°C for 5 minutes, and the last lot was left unprocessed. All lots were then manually pulped to separate the seeds from the flesh and the skin. The separated seeds and flesh were freeze-dried using a freeze dryer fabricated for the Department of Science and Technology (DOST) by Gecar Machine Solutions, Inc. (Quezon, Philippines) and ground in a DV Tech® grinder Model 525 (Madrid, Spain). The resulting powder was then sieved with a commercially available fine-mesh (10 mm) sieve and stored at room temperature in clean, airtight, and coded containers protected from light.

Figure 1. Bignay (A. bunius (L.) Spreng var. Kalabaw) fruits at different stages of ripening (From left: unripe, half-ripe, and fully ripe).
Sample Extraction
The extraction procedure was done according to the method described by Larrauri et al. (1997) with modifications. Powdered freeze-dried samples (500mg) were extracted with 15mL of methanol: water: acetic acid (50:50:1, v: v: v) in a shaker for 60 minutes at room temperature. Each extracted sample was centrifuged for 15 minutes, and the supernatant was then collected and transferred in an ambered bottle glass. All samples were stored at 0-4°C. Necessary dilutions of the collected extract were done before each analysis.
Determination of Total Phenolics and Total Flavonoids
The total phenolic contents (TPC) of the samples were quantified using the Folin– Ciocalteu method described by Waterhouse (2002) with some modifications. A 0.3 ml portion of the diluted sample was mixed with 1.5 ml 10% Folin-Ciocalteu reagent. This was allowed to stand for 5 minutes at room temperature. After 5 minutes, 1.2 mL of 4% Na2CO3 was added. The solution was vortexed and was allowed to stand for 90 minutes. The absorbance readings were measured at 760 nm on a Chrom Tech spectrophotometer model CT-2600 (E-Chrom Tech Co., Ltd., Taipei, Taiwan. All further absorbance readings were performed using the same spectrophotometer.
For the quantification of total flavonoid content (TFC) in the samples, a modified method of aluminum chloride assay by Luximon-Ramma et al. (2002) was used. Briefly, 2.5 ml of the diluted sample was mixed with 0.5 ml of 10% AlCl3 solution. The mixture was allowed to react for about 15 minutes at room temperature, and then absorbance readings were acquired at 367.5 nm.
Methanolic solutions of gallic acid and quercetin (3-100 µg mL-1) were used as standards for TPC and TFC, respectively. The results were expressed as milligrams standards per grams freeze-dried sample using Equation 1.
A = (cv/m)(1/1000) (Eq. 1)
where A is TPC or TFC, c is the concentration (µg/mL) of the standard established from the calibration curve; v is the volume (mL) of the extract solution, and m is the weight (g) of freeze dried sample, and 1/1000 is the conversion factor from µg to mg.
Determination of Total Anthocyanin Content
The total anthocyanin contents (TAC) of the samples were quantified using the pH differential method, as described by Lee et al. (2005). Each sample was diluted to a minimum dilution factor of 5 using potassium chloride buffer (0.025 M, pH 1.0) and sodium acetate buffer (0.40 M, pH 4.5). Dilution factors were chosen in such a way that the absorbance of the sample diluted with the pH 1.0 buffer at 520 nm is within
0.2 – 1.2 AU. The absorbance of the extracts diluted with the pH 1.0 buffer and pH 4.5 buffer were then read at 520 nm and 700 nm against a blank of distilled water. Total anthocyanin content (mg cyanidin-3-glucoside equivalents/L extract) was then computed using Equation 2.
TAC (mg/L) = A*MW*DF*(1000/(e*L)) (Eq.2)
where A is the absorbance = (A510 nm - A700 nm)pH 1.0 - (A510 nm - A700 nm) pH 4.5, MW is the molecular weight of cyanidin-3-glucoside (449.2 g/mol), DF is the dilution factor, e is the extinction coefficient of cyanidin 3-glucoside (26,900 L mol-1 cm-1) and L is the cell path length (1 cm). The computed total anthocyanin content (in mg cyanidin-3- glucoside equivalents/L extract) was then converted to and reported as milligrams cyanidin-3-glucoside equivalents per grams freeze-dried sample.
DPPH Radical Scavenging Activity Assay
The DPPH radical scavenging activity assay was performed according to the method of Pisoschi and Negulescu (2011) with modifications. A 1 mL portion of the diluted sample was mixed with 1 mL absolute methanol and 1 ml of freshly prepared DPPH solution. Methanolic solutions of Trolox (1-10 µg mL-1) were used as standards. The absorbance was then read at 517 nm against a blank of absolute methanol. Results were expressed as milligrams Trolox equivalents per gram freeze-dried sample.
Trolox Equivalent Antioxidant Capacity (TEAC) Assay
The TEAC assay conducted was a modified method adapted from Tomasina et al. (2012). The ABTS radical cation was prepared by reacting 0.25mM ABTS stock solution with 3.45 mM potassium persulfate, allowing the mixture to stand in the dark for 12- 16 hours at 4°C before use. After this, the ABTS radical cation solution was diluted with methanol until an absorbance of 0.70 ± 5 at 734 nm was achieved. Subsequently, 2.7 ml of ABTS radical cation solution was added to 0.3 ml of the diluted sample. The mixture was allowed to stand at room temperature in the dark for 15 minutes. Methanolic solutions of Trolox (2-40 µg mL-1) were used as standards. The absorbance of the solution was then measured at 734 nm. Results were expressed as mg Trolox equivalents per g freeze-dried sample.
Ferric Reducing Antioxidant Power (FRAP) Assay
The FRAP assay of the samples was conducted according to the method of Tomasina et al. (2012) with some modifications. Briefly, 2.7 mL of freshly prepared FRAP reagent (300mM acetate buffer (pH 3.6):10mM TPTZ (10mM):20mM FeCl3 solution; (10:1:1, v:v:v)) was added to 0.3 ml of the diluted sample. The reaction mixture was incubated for 5 minutes at 37°C, and its absorbance was read at 620 nm. Methanolic solutions of Trolox (3-100 µg mL-1) were used as standards. Results were reported as mg Trolox equivalents per g freeze-dried sample.
Experimental Design and Statistical Analysis
The experiments were arranged in a 3 x 3 factorial experimental design in completely randomized design (CRD) with values presented as mean ± SEM computed from three values. The influence of the maturity stage and processing treatment on TFC, TPC, and TAC and antioxidant activities (ABTS, DPPH, and FRAP) of fruit seeds and flesh were analyzed using two-way ANOVA. Significant differences between mean values were assessed by Tukey's Honest Significant Difference (HSD) test. All the statistical tests were performed at the significance level of 0.05 using Minitab 19.0 for Windows (Minitab, 2019).
RESULTS
Effect of maturity and processing method on the TPC, TFC, and TAC of bignay seeds.
In general, a significant difference among maturity, processing, and their interaction was found for total phenolic content, total flavonoid content, and total anthocyanin content of bignay seeds (P <0.001, α=0.05). TPC, TFC, and TAC increased as fruits mature. Blanched seeds generally yielded greater TPC and TFC, followed by steamed seeds and unprocessed seeds. TAC on the other hand, was significantly greater in steamed fully ripe seeds than in blanched and unprocessed fully ripe seeds (Figure 2).
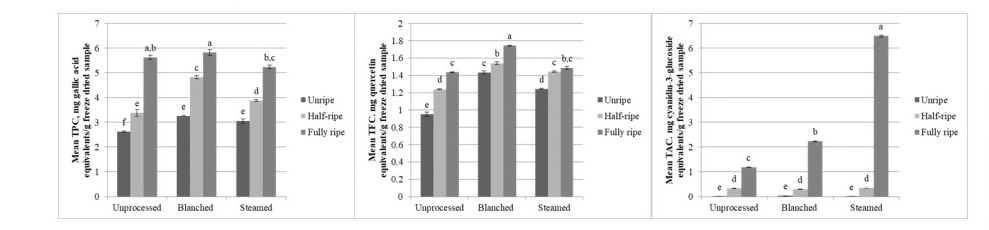
Figure 2. Total phenolic content, total flavonoid content, and total anthocyanin content (left to right) of bignay seeds as affected by maturity and processing method before freeze-drying (mean + SEM). Different lowercase letters denote a significant difference (P ≤0.05; Tukey’s test).
Effect of maturity and processing method on the TPC, TFC, and TAC of bignay flesh.
The difference among maturity, processing, and their interaction for total phenolic content, total flavonoid content, and total anthocyanin content of bignay flesh was found significant (P <0.001, α=0.05). Mature fruit flesh exhibited the highest TPC, TFC, and TAC values. Unprocessed fruit flesh generally yielded greater TFC and TPC, followed by blanched and steamed samples. The highest TAC was noted for steam-treated samples (Figure 3).
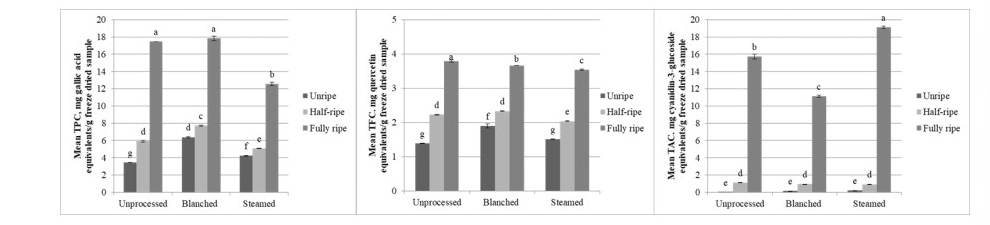
Figure 3. Total phenolic content, total flavonoid content, and total anthocyanin content (left to right) of bignay flesh as affected by maturity and processing method before freeze-drying (mean + SEM). Different lowercase letters denote a significant difference (P ≤ 0.05; Tukey’s test).
Effect of maturity and processing method on antioxidant activity (ABTS, DPPH, and FRAP) of bignay seeds.
The difference among maturity, processing, and their interaction was found to be significant for the antioxidant activity of bignay seeds using ABTS, DPPH, and FRAP assays (P <0.001, α=0.05). All three assays indicate that the antioxidant activity of bignay seeds increased with maturity. In terms of the effect of processing, blanching yielded the highest antioxidant activity across the three methods used (Figure 4).
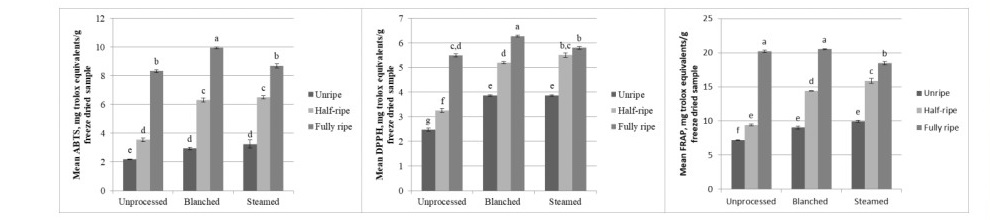
Figure 4. Antioxidant activities of bignay seeds expressed as mg Trolox equivalents per g freeze-dried sample determined using ABTS, DPPH, and FRAP assays (left to right) as affected by maturity and processing method before freeze-drying (mean + SEM). Different lowercase letters denote a significant difference (P ≤ 0.05; Tukey’s test).
Effect of maturity and processing method on antioxidant activities (ABTS, DPPH, and FRAP) of bignay flesh.
A significant difference among maturity, processing, and their interaction was observed in the antioxidant activity bignay flesh using both DPPH and ABTS (P <0.001, α=0.05). The results for FRAP, on the other hand, indicated that it was associated with maturity and processing but not with the interaction of the two factors. A similar trend of increasing antioxidant activity with maturity was noted. Among all the samples tested, blanched fruit flesh showed the greatest antioxidant activities using ABTS and FRAP assays. Steamed fully ripe bignay flesh extracts, on the other hand, showed the most considerable DPPH radical scavenging activity, although this is not significantly different from that of the unprocessed and blanched bignay flesh extracts (Figure 5).
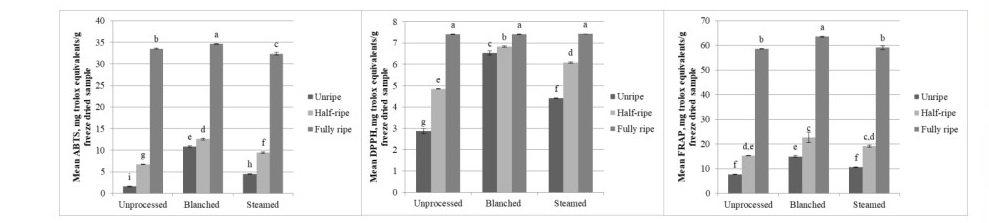
Figure 5. Antioxidant activities of bignay flesh expressed as mg Trolox equivalents per g freeze-dried sample determined using ABTS, DPPH, and FRAP assays (left to right) as affected by maturity and processing method before freeze-drying (mean + SEM). Different lowercase letters denote a significant difference (P ≤0.05; Tukey’s test).
DISCUSSION
This study was undertaken to determine the influence of maturity stages and processing methods (blanching and steaming) on the antioxidant profile and in vitro antioxidant activities of bignay fruits. The results of this investigation can provide shreds of evidence for the utilization of bignay as a functional food.
Both maturity and processing are significantly associated with the antioxidant contents (TPC, TFC, TAC) and antioxidant activities as measured by DPPH, FRAP, and ABTS assay of bignay flesh and seeds. Moreover, with the exception of the FRAP assay done on flesh samples, a significant interaction between processing methods and maturity stage was found on all assays done on flesh and seeds. This interaction is possibly due to the changes in the ratio of phenolic compounds in the flesh and seeds during ripening. Siriwoharn et al. (2004) showed that the concentration of some anthocyanins and polyphenols of Marion and Evergreen blackberries increase during ripening while some of them decrease during ripening. Moreover, concentrations of simple phenols such as gallic acid, ellagic acid, and caffeic acid in cactus berries significantly differ at different maturity stages (Herrera-Hernández et al., 2011). Since different phenolic compounds respond to processing differently (N'Dri et al., 2012; Fischer et al., 2011), the interaction observed is reasonable.
Still, it is consistent that the total phenolic content, total flavonoid content, and total anthocyanin content of both the flesh and seed increase as maturity increases. Similarly, the antioxidant activity of the flesh and seeds, as measured by ABTS, FRAP, DPPH assays, also increases with increasing maturity. Arrayan berry (Luma apiculata) also exhibits the same general increasing trend of TPC, TFC, and antioxidant activity by DPPH and FRAP assays with increasing maturity (Fuentes et al., 2016). Moreover, natal plum (Carissa macrocarpa) also exhibit the same general increasing trend of TAC and antioxidant activity by DPPH and FRAP assays by increasing maturity (Ndou et al., 2019). This signals that to maximize the antioxidant potential of bignay fruit and seeds, they should be harvested at the fully ripe stage.
With regard to the effect of the processing method, results are more variable. Processing methods affect bignay flesh and seeds of different maturities in different ways, with some processing methods giving greater antioxidant activities and contents at one maturity stage and not on another. Still, a few general trends were observed. First, blanched samples generally have greater total phenolic content and antioxidant activities as measured by FRAP, ABTS, and DPPH assays than their unblanched counterparts. The higher phenolic content of blanched samples coincides with the study of Heras-Ramírez et al. (2012). In this study, blanched apple pomace was found to have higher total phenolic content than their unblanched counterparts. The authors speculated that this might be due to the release of bound polyphenolics from tissues broken by blanching.
Moreover, Rossi et al. (2003) observed that the radical scavenging activity of juice from frozen highbush blueberries blanched before processing is greater than that of the juice from unblanched frozen highbush berries. The authors attributed this result to the higher recovery of phenolic compounds in the berry juice due to blanching. Similarly, in this study, the higher antioxidant activity of the blanched bignay seeds and flesh can be attributed to the release of bound polyphenols from the fruit, making it easier to be extracted from the freeze-dried sample. This, in turn, resulted in a higher antioxidant activity observed.
Second, TAC was observed to be considerably higher in steamed fully ripe flesh and seeds than that of their unprocessed and blanched counterparts. This may be because anthocyanins in fruits were released more during steam treatment as a consequence of swelling and eventual rupture of plant cells (Wang and Weller, 2006). Another possible reason is that anthocyanins in bignay are more prone to leaching out to the processing water. Leaching out of anthocyanin in processing water has already been reported in cauliflowers (Volden et al., 2009) and purple waxy corn (Harakotr et al., 2014). This can also explain why fully ripe blanched flesh and skin had the least anthocyanin content among its unprocessed and steamed counterparts. Even so, since the antioxidant activity is still the greatest for blanched samples, it can be inferred that anthocyanins are not the main contributors to antioxidant activity in bignay flesh and seeds. Thus, based on these results, blanching can enhance the antioxidant potential of bignay flesh and seeds.
It should be noted that bignay fruits in this study are of the Kalabaw variety and were sampled in one province, which was Laguna, Philippines. Moreover, the processing methods employed in this study were only blanching and steaming. Future studies should look into the antioxidant activities and contents of bignay fruits of different varieties grown under different conditions. Moreover, the effect of other processing methods on the antioxidant activity of bignay can also be a topic for future studies.
CONCLUSION
The antioxidant content and activity of bignay flesh and seeds are affected by both maturity and processing methods (blanching and steaming). Moreover, with the exception of the antioxidant activity measured by the FRAP assay done on flesh samples, a significant interaction between the effect of maturity and processing methods on the antioxidant content and activities of bignay flesh and seeds was observed. In general, fully-ripe flesh and seeds yielded greater antioxidant content and antioxidant activity than their half-ripe and unripe counterparts. Moreover, blanched flesh and seeds generally had greater antioxidant activities than their unprocessed and steamed counterparts.
REFERENCES
Acosta-Montoya, Ó., Vaillant, F., Cozzano, S., Mertz, C, Pérez, A.M., and Castro, M.V. 2010. Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl.) during three edible maturity stages. Food Chemistry. 119: 1497–1501.
Barcelo, J.M., Nullar, A.R.M., Caranto, J.K.P., Gatchallan, A.M., and Aquino, I.J.B. 2016. Antioxidant and antimutagenic activities of ripe Bignay (Antidesma bunius) crude fruit extract. Philippine e-Journal for Applied Research and Development. 6: 32–43.
Barcelo, R. 2015. Phytochemical screening and antioxidant activity of edible wild fruits in benguet, cordillera administrative region, Philippines. Electronic Journal of Biology. 11:80-9.
Denardin, C.C., Hirsch, G.E., da Rocha, R.F., Vizzotto, M., Henriques, A.T., Moreira, J.C., Guma, F.T.C.R., and Emanuelli, T. 2015. Antioxidant capacity and bioactive compounds of four Brazilian native fruits. Journal of food and drug analysis. 23: 387-398.
Fischer, U.A., Dettmann, J.S., Carle, R., and Kammerer, D.R. 2011. Impact of processing and storage on the phenolic profiles and contents of pomegranate (Punica granatum L.) juices. European Food Research and Technology. 233: 797.
Fuentes, L., Valdenegro, M., Gómez, M.G., Ayala-Raso, A., Quiroga, E., Martínez, J.P., Vinet, J., Caballero, E., and Figueroa, C.R. 2016. Characterization of fruit development and potential health benefits of arrayan (Luma apiculata), a native berry of South America. Food chemistry. 196: 1239-1247.
Harakotr, B., Suriharn, B., Tangwongchai, R., Scott, M.P., and Lertrat, K. 2014. Anthocyanin, phenolics and antioxidant activity changes in purple waxy corn as affected by traditional cooking. Food chemistry. 164: 510-517.
Heras-Ramírez, M.E., Quintero-Ramos, A., Camacho-Dávila, A.A., Barnard, J., Talamás-Abbud, R., Torres-Muñoz, J.V., and Salas-Muñoz, E. 2012. Effect of blanching and drying temperature on polyphenolic compound stability and antioxidant capacity of apple pomace. Food and Bioprocess Technology. 5: 2201- 2210.
Herrera-Hernández, M.G., Guevara-Lara, F., Reynoso-Camacho, R., and Guzmán- Maldonado, S.H. 2011. Effects of maturity stage and storage on cactus berry (Myrtillocactus geometrizans) phenolics, vitamin C, betalains and their antioxidant properties. Food chemistry. 129: 1744-1750.
Huang, Y., Xiao, D., Burton-Freeman, B.M., and Edirisinghe, I. 2016. Chemical changes of bioactive phytochemicals during thermal processing. Reference Module in Food Science.
Islam, M.S., Ahammed, M.S., and Sukorno, F.I. 2018. A review on phytochemical and pharmacological potentials of Antidesma bunius. Journal of Analytical and Pharmaceutical Research. 7: 602-604.
Lafarga. T., Bobo, G. Viñas, I., Zudaire, L., Simo, J., and Aguilo-Aguayo, I. 2018. Steaming and sous-vide: Effects on antioxidant activity, vitamin C, and total phenolic content of Brassica vegetables. International Journal of Gastronomy and Food Science.
Larrauri, J.A., Ruperez, P., and Saura-Calixto, F. 1997. Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. Journal of Agricultural and Food Chemistry. 45: 1390-1393.
Lee, J., Durst, R.W., and Wrolstad, R.E. 2005. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. Journal of the AOAC International. 88: 1269-1278.
Lee, S.K. and Kader, A.A. 2000. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest biology and technology. 20: 207-220. Luximon-Ramma A., Bahorun, T., Soobrattee M.A., and Arouma O.I. 2002. Antioxidant activities of phenolic, proanthocyanidin and flavonoid components in extracts of
Cassia fistula. Journal of Agricultural and Food Chemistry. 50: 5042-5047.
Minitab 19 Statistical Software. 2019. Windows version. State College, PA: Minitab, Inc. www.minitab.com
Ndou, A., Tinyani, P.P., Slabbert, R.M., Sultanbawa, Y., and Sivakumar, D. 2019. An integrated approach for harvesting Natal plum (Carissa macrocarpa) for quality and functional compounds related to maturity stages. Food chemistry. 293: 499- 510.
N'Dri, D., Mazzeo, T., Zaupa, M., Ferracane, R., Fogliano, V., and Pellegrini, N. 2013. Effect of cooking on the total antioxidant capacity and phenolic profile of some whole-meal African cereals. Journal of the Science of Food and Agriculture. 93: 29-36.
Pisoschi, A.M. and Negulescu, G.P. 2011. Methods for Total Antioxidant Activity Determination: A Review. Biochemistry & Analytical Biochemistry. 1:106.
Recuenco, M.C., Lacsamana, M.S. Hurtada, W.A., Sabularse, V.C. 2016. Total Phenolic and total Flavonoid Contents of Selected Philippine Fruits. The Philippine Journal of Science. 145:275-81.
Rickman, J.C., Barrett, D.M., and Bruhn, C.M. 2007. Nutritional comparison of fresh, frozen and canned fruits and vegetables. Part 1. Vitamins C and B and phenolic compounds. Journal of the Science of Food and Agriculture. 87: 930–944.
Rossi, M., Giussani, E., Morelli, R., Scalzo, R.L., Nani, R.C., and Torreggiani, D. 2003. Effect of fruit blanching on phenolics and radical scavenging activity of highbush blueberry juice. Food Research International. 36: 999-1005.
Santiago, D.M.O., Garcia, V.V., Dizon, E.I., and Merca, F.E. 2007. Antioxidant activities, flavonol and flavanol content of selected Southeast Asian indigenous fruits. Philippine Agricultural Scientist. 90: 123.
Scalbert, A., Johnson, I.T., and Saltmarsh, M. 2005. Polyphenols: antioxidants and beyond. The American Journal of Clinical Nutrition. 81: 215S-217S.
Siriwoharn, T., Wrolstad, R.E., Finn, C.E., & Pereira, C.B. 2004. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. Journal of Agricultural and Food Chemistry. 52: 8021-8030.
Tiwari, U. and Cummins, E. 2013. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations. Food Research International. 50: 497–506.
Tomasina, F., Carabio, C., Celano L., and Thomson, L. 2012. Analysis of two methods to evaluate antioxidants. Biochemistry and Molecular Biology Education. 40: 266- 270.
Volden, J., Borge, G.I.A., Hansen, M., Wicklund, T., and Bengtsson, G.B. 2009. Processing (blanching, boiling, steaming) effects on the content of glucosinolates and antioxidant-related parameters in cauliflower (Brassica oleracea L. ssp. botrytis). LWT-Food Science and Technology. 42: 63-73.
Wang, L. and Weller, C.L. 2006. Recent advances in extraction of nutraceuticals from plants. Trends in Food Science and Technology. 17(6): 300–312.
Waterhouse, A.L. 2002. Determination of total phenolics. Current protocols in food analytical chemistry. 6: I1-1.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Kristel June Sartagoda*, Ma. Cristina Ilano, Lloyd Earl Flandez, and Katherine Ann Castillo-Israel
Institute of Food Science and Technology, College of Agriculture and Food Science, University of the Philippines – Los Baños, Laguna, Philippines 4031
Corresponding author: Kristel June Sartagoda, E-mail: kdsartagoda1@up.edu.ph
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: May 12, 2020;
Revised: July 1, 2020;
Accepted: October 12, 2020

