
Improvement of Thanaka Powder by Gamma Radiation: Microbial and Chemical Properties
Wachiraporn Pewlong*, Surasak Sajjabut, Sirilak Chookaew, Jarurattana Eamsiri, Khemruji Khemthong, and Lamai MaikaewPublished Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.041
Journal Issues : Number 2, April-June 2021
Abstract Thanaka powder is made from the stem bark of the Hesperethusa crenulata plant. Most Burmese women use it on the skin of the face as sun protection, a moisturizing agent and acne treatment. For a hygiene purpose, gamma radiation is currently used to control microorganisms and insect contamination in many products. In this experiment, the the effects of gamma radiation on the microbial numeration, total phenolic content, antioxidant activity, and chemical constituent alterations in Thanaka powder were investigated. Gamma irradiation was applied to powder samples at 5, 10, 15 and 20 kGy. The total bacterial count and total yeast and mold count were decreased with increasing irradiation dose. Gamma irradiation at a dose of 5 kGy was sufficient to reduce the microbial load in the powder to meet the standards of microbial quality for cosmetic products in Thailand. No significant change in the DPPH activity of the Thanaka powder was observed after irradiation. Additionally, the radiation process caused significant increases in total phenolic content, FRAP value and arbutin content. At a dose of 20 kGy, the total phenolic content, FRAP value and arbutin content were significantly higher than the non-irradiated Thanaka powder at 7.45, 9.59 and 16.04%, respectively.
Keywords: Antioxidant activity, Arbutin, Gamma irradiation, Thanaka
Citation: Pewlong, W., Sajjabut, S., Chookaew, S., Eamsiri, J.,Khemthong, K., and Maikaew, L.2021. Improvement of Thanaka Powder by Gamma Radiation: Microbial and Chemical Properties. CMUJ. Nat. Sci. 20(2): e2021041.
INTRODUCTION
Hesperethusa crenulata, syn. Naringi crenulata and Limonia acidissima, is a tropical plant that generally grows in Southeast Asia and the Indian subcontinent, especially in Myanmar and Thailand. It is commonly known as Thanaka, Wood apple and Threetree. Thanaka powder is made from the stem bark of these trees. Most Myanmar women have been using Thanaka powder as a cosmetic product for a long time. There are many purposes of using Thanaka powder in cosmetic products, such as for sun protection, acne healing and prevention, blemish treatment and skin whitening. (Lindsay et al., 1998; Lourith et al., 2010: Seiverling and Ahrns, 2013; Goldsberry et al., 2014). Sun protection was found to be the most important reason for applying Thanaka, which is a result of the presence of marmesin substance (Seiverling et al., 2017). Marmesin is a chemical compound which is extracted from root bark. It could filter ultraviolet light and lead to skin cell protection from sunlight (Joo et al., 2004). In addition, arbutin and coumarin are chemical substances that have also been found in Thanaka (Nayar and Bhan, 1972). Both of them exhibit different activities. Arbutin is a whitening agent and has anti-inflammatory properties, whereas coumarin is a fragrant substance (Lee and Kim, 2012; Migas and Miroslawa, 2015). A previous study found that Thanaka had many activities, such as antioxidant and tyrosinase inhibitory activity as well as anti-bacterial and anti-inflammatory properties (Wanthong et al., 2010). Nowadays, Thanaka powder is used in many cosmetic industries in Thailand. Therefore, the increase in its demand has raised concern regarding the safety of using Thanaka products. The safety and efficacy of Thanaka directly affects its quality. One of the important factors contributing to the poor quality of Thanaka is its contamination with heavy metals and its microbial load. For instance, Nimrat et al. (2014) analyzed the amount of total aerobic bacteria in all the Thanaka products that were imported from Myanmar. The results showed that the level of microorganisms exceeded the maximum level allowed which meets the standard of microbial quality (<103 CFU/g) for cosmetic products of Thailand (Notification of the Ministry of Public Health No.40 A.D. 2005). Consequently, microbial decontamination of the raw materials used in cosmetic products like Thanaka powder needs to be efficient without loss of bioactive ingredients (Haleem et al., 2015).
Ionizing radiation is one of the processes used to disinfect herbs and foodstuffs. This treatment can restrain the growth of microorganisms and motivate molecular conformation modification (Krisko and Radman, 2013). Gamma radiation is a technology which is widely used in the postharvest elimination of microbial and insect infestation. Compare to other decontamination methods, irradiation methods are faster, safer, more convenient and more eco-friendly (Rahayu et al., 2016). The purpose of this study was to investigate the microbial contamination and chemical properties of Thanaka powder samples after gamma irradiation.
MATERIALS AND METHODS
Materials
Thanaka powder was purchased from a distribution company based in Bangkok and Nakhon Nayok province. Each sample was weighed (250 g) and was packed in aluminum foil (size 6x9 inch), sealed and labeled with its respective radiation dose. All the samples were stored at ambient temperature (28 ± 2 °C) until usage.
Gamma irradiation
The powder samples were irradiated at the Thailand Institute of Nuclear Technology (Public Organization) using a gamma chamber 5000 (BRIT, India) at a dose rate of 2.96 kGy/h for total doses of 5, 10, 15 and 20 kGy. Non-irradiated samples were also prepared as a control.
Microbial numeration
Non-irradiated and irradiated Thanaka samples were evaluated for the total viable bacteria counts and total yeast and mold counts. Microbiological assays were performed using the AOAC standard protocol (AOAC, 1990). Each sample (2 g) was mixed in 18 mL of 0.1% normal saline (10-1 dilution). A total of 1 mL was transferred to tubes with 9 mL of sterile water and serial dilution was performed until the dilution reached a level of 10-5. A fraction of 0.1 mL of each tube, in triplicate, was placed in petri dish containing plate count agar for the bacterial count and potato dextrose agar for the yeast and mold count. PCA plate samples were incubated at 35°C and PDA plate samples were incubated at 25 °C, and colony numbers were counted for 2-3 days. The results were expressed as colony forming units per gram of Thanaka powder (CFU/g). All the samples were processed in triplicate.
Antimicrobial activity
The antibacterial activity of irradiated and non-irradiated Thanaka extracts were analyzed using Gram-positive and Gram-negative bacterial strains (Staphylococcus aureus and Escherichia coli). The bacterial strains were kindly provided by Nopparatrajathanee Hospital, Bangkok, Thailand. Each bacterial strain was cultured overnight at 35 °C in Tryptic Soy Broth. The bacterial growth was harvested and diluted to attain a viable cell count of 107 CFU/mL using a spectrophotometer at a wavelength of 550 nm. The agar disc diffusion method was used to evaluate the antimicrobial activity of Thanaka extracts. Approximately 100 mg of each extract was redissolved in
1.0 mL of dimethylsulfoxide (DMSO) and sterilized through a Millipore filter (0.22 μm). Finally, a 20 μL drop of each extract was put on sterilized filter paper discs (6 mm in diameter). Mueller-Hilton agar medium (20 mL) was poured into sterile petri dishes that had been previously inoculated with a bacterial suspension. Sterilized filter paper discs inoculated with irradiated and non-irradiated Thanaka extract at a concentration of 100 mg/mL were placed on the top of the Mueller-Hilton agar plates. The plates were incubated at 35 °C for 24-48 h. The presence of inhibition zones was measured by using Vernier calipers, recorded and considered for indication of antimicrobial activity.
Determination of total phenolic content
Total phenolic content in the Thanaka powder samples was determined by using Folin–Ciocalteu reagent in the developed method of Velioglu et al. (1998) against gallic acid as a standard. Thanaka extracts (100 µL) were added to 0.75 mL of 10-fold diluted Folin–Ciocalteu reagent. The mixtures were mixed and allowed to stand at room temperature for 5 min. Then 0.75 mL of 6% Na2CO3 was added to each mixture, which was then left to stand for 90 min. Absorbance was read at 725 nm using a spectrophotometer and compared to gallic acid calibration curves. Gallic acid was used as the standard and distilled water as the blank. The total phenolic content was calculated using the equation of a straight line obtained from the gallic acid standard curve (20-100 mg/mL). The results were expressed as mg gallic acid equivalent/g dry sample (mgGAE/g).
Determination of antioxidant activity by DPPH and FRAP assay
The scavenging effects of non-irradiated and irradiated Thanaka were determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay and ferric reducing antioxidant power (FRAP) assay. DPPH radicals were estimated according to the method of Khattak and Simpson (2008). A 100 µL sample of Thanaka was added to a 900 µL solution of 1, 1-diphenyl-2-picrylhydrazyl (DPPH) in methanol. The mixture was shaken vigorously and was allowed to stand for 15 min at room temperature in the dark. The absorbance of the resulting solution was measured at 517 nm with a UV- visible spectrophotometer (UV-1700, Shimadzu, Japan). The FRAP assay was evaluated according to the method of Benzie and Strain (1996). The FRAP reagent was prepared by mixing 16.7 mM FeCl3.6H2O and 8.3 mM 2,4,6-tripyridyl-s-triazine (TPTZ) with 250 mM acetate buffer; pH 3.6. A solution of 75 μL of 60% ethanolic extract and 225 μL of distilled water were added to 2.25 mL of freshly prepared FRAP reagent in a test tube. The mixture was incubated at room temperature for 30 min. The absorbance was read at 596 nm using a UV-visible spectrophotometer during the monitoring period. The antioxidant potential of the samples was determined based on a calibration curve plotted using FeSO4.7H2O at concentrations ranging between 400 and 2,000 μM.
Quantification of arbutin content
Arbutin was used as a standard substance in this experiment because it was found in an aerial part of Thanaka (Thongchai et al., 2007). Arbutin content was analyzed by using HPLC equipment (Waters e2695 Alliance, USA.). The chromatograms of samples were produced by using a Waters 2996 photodiode array detector at 280 nm with a C18 column (Sunfire 5µm, 150x4.6 mm). The mobile phase was acetonitrile and 3% acetic acid with a linear gradient solvent system: 10:90 at 0 min, 55:45 at 27 min, and held at 55:45. The amount of arbutin was calculated by using a calibration curve of standard arbutin (Sigma, USA).
Statistics
Analysis of variance (one-way ANOVA) of the experimental data was performed using the SPSS 21 software package. To separate treatment means within each measured parameter, Duncan’s multiple range test was performed at P ≤0.05.
RESULTS
Microbial decontamination
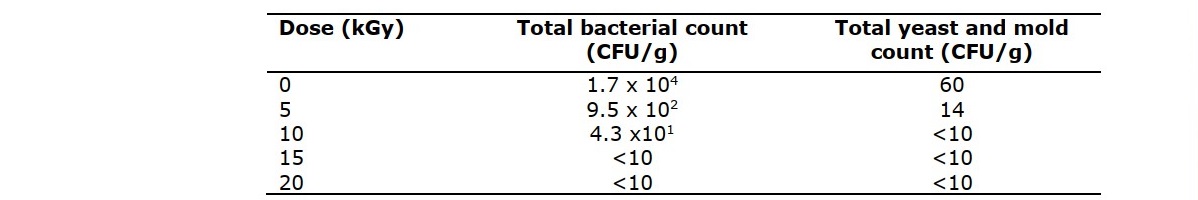
The effects of gamma radiation on the microbial population are shown in Table 1. The total bacterial count was 1.7x104 CFU/g for non-irradiated Thanaka, which did not meet the standard of microbial quality (<103 CFU/g) for cosmetic products of Thailand (Notification of the Ministry of Public Health No.40 A.D. 2005). When treated at dosages of more than 5 kGy, all irradiated Thanaka powder showed significant decreases in aerobic microbe population. Gamma radiation decreased the number of microbes to below 2 log CFU/g at a dose of 5 kGy. Microbial analysis revealed a yeast and mold population of 60 CFU/g for the non-irradiated sample. Gamma radiation at 10 kGy decreased yeast and mold counts to lower than 10 CFU/g. Both the total bacterial count and yeast and mold count of Thanaka treated at 5 kGy were significantly lower than those of the control sample.
Table 1. Microbial quality of Thanaka powder at various doses of gamma irradiation.
Antimicrobial activity
In the measurement of the antimicrobial activity of non-irradiated and irradiated Thanaka powder, the agar disk diffusion method was utilized. No inhibition zones of the Thanaka disks against Staphylococcus aureus and Escherichia coli were observed (data not shown). The lack of inhibition zones may be due to the impurity of the Thanaka samples (they were sometimes mixed with other ingredients), the solubility of the Thanaka samples and the indirect function with regard to S. aureus and E. coli (Seiverling et al., 2017).
Total phenolic content
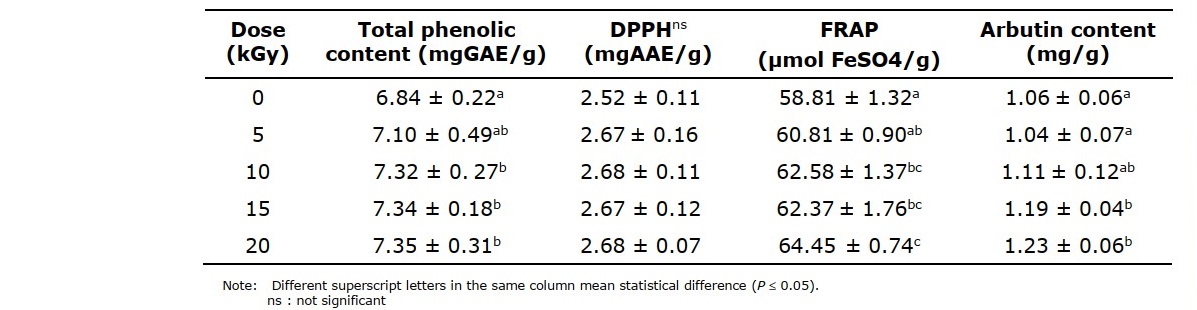
The quantification of phenolic compounds is shown in Table 2. The irradiated samples tended to have a higher antioxidant activity than the non-irradiated ones. Samples which had received gamma irradiation at a dose of more than 5 kGy had a significantly (P ≤0.05) increased phenolic content compared to non-irradiated ones. Thanaka powder samples irradiated at doses of 10, 15 and 20 kGy exhibited significant differences in total phenolic content compared to the control sample. The total phenolic content of the irradiated samples was increased with each increment of irradiation dose. The phenolic content of non-irradiated samples was 6.84 ± 0.22 mgGAE/g, whereas the phenolic content of irradiated samples was between 7.10 ± 0.49 and 7.35 ± 0.31 mgGAE/g.
Table 2. Total phenolic content, antioxidant activity and arbutin content of Thanaka powder at various doses of gamma irradiation.
Antioxidant activity
The scavenging effects of the Thanaka powder under investigation, with regard to DPPH radicals, are shown in Table 2. From the DPPH assay, it was found that there were no significant differences in the activity of DPPH in non-irradiated and irradiated samples. The values of all samples ranged from 2.52 ± 0.11 to 2.68 ± 0.07 mgAAE/g. In terms of FRAP, there was a significant difference in FRAP values between the irradiated and control samples. The highest FRAP value was found in irradiated Thanaka powder sample at 20 kGy. The lowest antioxidant activity according to the FRAP assay was obtained for the non-irradiated sample.
Arbutin content
The amount of arbutin, a major chemical substance found in the aerial part of Thanaka, was detected by HPLC. A significant difference (at P ≤0.05) in Thanaka powder irradiated at 10 kGy was observed, which represents higher levels of this bioactive compound in irradiated samples (Table 2). The content of arbutin in non-irradiated samples was 1.06 ± 0.06 mg/g of sample, and in irradiated samples, it ranged from 1.04 ± 0.07 to 1.23 ± 0.06 mg/g of sample. Significant differences in arbutin content were observed between irradiated and non-irradiated samples. Samples treated at 10, 15 and 20 kGy showed a significant increase in the amount of arbutin. The highest increase (16.04%) of this parameter was noted in the sample that was exposed to 20 kGy gamma irradiation. Therefore, it can be seen that 20 kGy irradiated samples showed the highest total phenolic content and FRAP value as well as highest arbutin content in these studies.
DISCUSSION
Herbs are valued for their physical and chemical characteristics such as aroma, color, flavor and bioactive substances. Drying of herbs after harvest, especially by traditional methods reveals them to contaminate by microorganism. Consequently, dried herbs may exist in high levels of different groups of microorganisms including pathogenic bacteria and toxigenic fungi (Beyzaie, 2018). The basic principle methods for diminish the initial load of microbial in dried herb products is thermal process. However, thermal treatment would have some negative impacts on sensorial and nutritional characteristics of the dried herb products. Gamma irradiation (non-thermal methods) is a good choice for using instead of thermal process because it is not involve any increase in temperature. Thus, this method is appropriate for reducing the amount of microbial in dried herb products. The radiation resistance of microorganisms has been correlated with their chemical and physical structure, and their ability to recover from radiation injury (Krisko and Radman, 2013). Gamma radiation could destroy the microorganisms by damaging DNA structure and the reproductive system of them (Belbe and Tofana, 2010). As a result, the total viable count and total yeast count of Thanaka powder decreased with increasing irradiation dose.
Antioxidant activity plays an important role in ensuring plant growth and promoting health properties in the human diet (Colonna et al., 2016). The results from this experiment showed that gamma radiation affected both total phenolic content and FRAP values, whereas DPPH activity was not changed. The obtained results suggested that phenolic compounds are related to the FRAP value. These phenomena could be explained by the various mechanisms of antioxidant activity and difference in testing methods. The total phenolic content method was used to detect phenolic and non- phenolic compounds. However, the FRAP assay did not measure compounds that perform radical quenching (H transfer) as thiol antioxidants. In the case of DPPH, it could interfere with carotenoids at 513 nm (Balogh et al., 2010). Besides, the reactions of antioxidant activity depend on the number and location of hydroxyl radicals in the aromatic ring, as well as their correlated positions (Sommer et al., 2009).
The results obtained from this report correlated with those of Behgar et al. (2011) who showed that gamma irradiation at a dose of 8 kGy significantly increased phenolic content in pistachio hull. Similarly, Pereira et al. (2013) reported the reliability of the antioxidant activity on the increase of total phenolic content in irradiated borututu (Cochlospermum angolensis Welw.). Borututu is a medicinal plant that is commonly used for liver diseases and malaria prophylaxis treatment in Angola. Likewise, Khawory et al. (2020) reported that the amount of total phenolic compounds was higher in irradiated medicinal plants (6 –12 kGy) when compared to the control. These significant increases may be concerned with the degradation of tannin (Variyar et al, 1998) and the alteration of molecule conformation (Topuz and Ozdemir, 2004). The reduction of tannin in samples irradiated by ionizing radiation has been related to the increase of total phenolic compounds (Stajner et al., 2007; Harrison and Were, 2007; Kim et al., 2008) and a rise in the gallic acid content (Variyar et al., 1998). In contrast, some studies have mentioned a reduction in the total phenolic content of samples treated with radiation. Schindler et al. (2005) showed degradation of phenolic compounds in tomatoes had been observed at 2, 4 and 6 kGy of gamma irradiation. Kortei et al. (2014) also revealed that phenolic acid degradation after gamma irradiation at doses of 2 kGy in dried Pleurotus ostreatus mushroom. In the same way, Mahmoud et al. (2016) reported a reduction in tannin content and phenolic compounds in millet grains after irradiation at doses of 2 kGy. The significant reduction of phenolic compounds may be attributed to radiation-induced product degradation (Sajilata and Singhal, 2006). In other studies, radiation did not result in any significant difference regarding total phenolic content compared with the control. For example, Chatterjee et al. (2009) reported no significant difference in the total phenolic content of irradiated turmeric (Curcuma longa) and fenugreek (Trigonella foneum) samples when compared to non- irradiated samples. The effect of ionizing radiation on the alteration of phenolic compound content may be partly due to the higher extractability of these compounds in irradiated samples as a result of alternations in cellular compounds and release of bound or insoluble phenolics, especially at high doses of irradiation (Guerreiro et al., 2016).
In terms of arbutin content, gamma radiation at doses more than 10 up to 20 kGy significantly affected these values. The increase in irradiation dose led to an increase in arbutin content. Pewlong et al. (2016) obtained a similar result; gamma irradiation at dose 15 and 20 kGy resulted in significant increases in glycyrrhizic acid, of 7.8 and 21.7 percent, respectively. The increase in arbutin values of irradiated samples is explained on the basis of the effect of irradiation on increasing the extractability of chemical compounds because of the changes in the cellular structure. Gamma irradiation makes the cell walls more permeable and open, which results an increased diffusion of solvent extraction and enhanced swelling of the cells. This enhanced swelling of the cell walls favors the efficient leaching and extraction of the constituents (Hussain et al., 2013). As a result, the increased arbutin content in the extracts was found due to the increased extractability of irradiated Thanaka powder. Therefore, irradiation treatment of Thanaka powder has proved beneficial in enhancing the release of bioactive compounds rather than causing any significant degradation.
CONCLUSION
In summary, it was demonstrated that gamma irradiation affected the microbial load, antioxidant activity and chemical properties of irradiated Thanaka powder. With regard to cosmetic product safety, the application of gamma radiation clearly diminished microbial contamination. The obtained data indicated that an irradiation dose of 5 kGy resulted in a principal impact with the benefit of reducing microbial load to below 2 log CFU/g. In terms of chemical efficacy, gamma irradiation more than 10 kGy significantly increased the phenolic content, the FRAP value and the arbutin content. These results suggest that gamma radiation treatments might be a useful non- chemical treatment for decontamination without inducing significant losses of chemical properties. In addition, gamma irradiation at a dose of 20 kGy was advantageous, especially as it increased total phenolic content, FRAP value and arbutin content by 7.45%, 9.59% and 16.04% respectively.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Mr. Alan Platt (Lecturer at Language Centre, Srinakharinwirot University) for proofreading through the manuscript.
REFERENCES
AOAC. 1990. Official methods of the association of official analytical chemists, Washington DC, 15th edition.
Balogh, E., Hegedüs, A., and Stefanovits-Banyai, E. 2010. Application of and correlation among antioxidant and antiradical assays for characterizing antioxidant capacity of berries. Scientia Horticulturae. 125: 332-336.
Behgar, M., Ghasemi, S., Naserian, A., Borzoie, A., and Fatollah, H. 2011. Gamma radiation effects on phenolics, antioxidants activity and in vitro digestion of pistachio (Pistachia vera) hull. Radiation Physics and Chemistry. 80: 963-967.
Belbe, T.H. and Tofana, Maria. 2010. Effect of ionizing radiation on microbiological contaminants of foods. Bulletin UASVM Agriculture. 67: 178-185.
Benzie, I. F. and Strain, J. J. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 239: 70-76.
Beyzaie, B. 2018. Efficient control of microbial load in dried herb. Journal of Food Processing and Technology. 9: 84.
Chatterjee, S., Variyar, P.S., and Sharma, A. 2009. Stability of lipid constituents in radiation processed fenugreek seeds and turmeric: role of phenolic antioxidants. Journal of Agriculture and Food Chemistry. 57: 9226-9233.
Colonna, E., Rouphael, Y., Barbieri, G., and De Pascale, S. 2016. Nutritional quality of ten leafy vegetables harvested at two light intensities. Food Chemistry. 199: 702- 710.
Goldsberry, A., Dinner, A., and Hanke, C.W. 2014. Thanaka: Traditional Burmese Sun Protection. Journal of Drugs and Dermatology. 13: 306-307.
Guerreiro, D., Madureira, J., Silva, T., Melo, R., Santos, P.M.P., Ferreira, A., Trigo, M.J., Falcao, A., Margaca, F.M.A., and Verde, S.C. 2016. Post-harvest treatment of cherry tomatoes by gamma radiation: Microbial and physicochemical parameters evaluation. Innovative Food Science and Emerging Technologies. 36: 1-9.
Haleem, R.M., Salem, M.Y., FatahallahLaila, F.A., and Abdelfattah, E. 2015. Quality in the pharmaceutical industry –A literature review. Saudi Pharmaceutical Journal. 23: 463-469.
Harrison, K. and Were, L.M. 2007. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of almond skin extracts. Food Chemistry. 102: 932- 937.
Hussain, P.R., Chatterjee, S., Variyar, P.S., Sharma, A., Dar, M.A., and Wani, A.M. 2013. Bioactive compounds and antioxidant activity of gamma irradiated sun dried apricots (Prunus armeniaca L.). Journal of Food Composition and analysis. 30: 59-66.
Joo, S.H., Lee, S.C. and Kim, S.K. 2004. UV absorbent, marmesin, from the bark of Thanaka, Hesperethusa crenulata L. Journal of Plant Biology. 47: 163-165.
Khawory, M.H., Sain, A.A., Rosli, M.A.A., Ishak, M.S., Noordin M.I., and Wahab, H.A. 2020. Effects of gamma radiation treatment on three different medicinal plants: microbial limit test, total phenolic content, in vitro cytotoxicity effect and antioxidant assay. Applied Radiation and Isotopes. 157.109013. 1-7.
Khattak, K.F. and Simpson, T.J. 2008. Effect of gamma irradiation on the extraction yield, total phenolic content and free radical-scavenging activity of Nigella sativa seed. Food Chemistry. 110: 967-972.
Kim, J., Lee, B.C., Lee, J., Nam, K., and Lee, S., 2008. Effect of electron-beam irradiation on the antioxidant activity of extracts from Citrus unshiupomaces. Radiation Physics and Chemistry. 77: 87-91.
Kortei, N.K., Odamtten, G.T., Obodai, M., Appiah, V., Akuamoa, F., Adubo-bi, A.K., Annan, S.N.Y., Armah, J.N.O., and Acquah, S.A. 2014. Evaluating the effect of gamma radiation on the total phenolic content, flavonoids, and antioxidant activity of dried Pleurotus ostreatus ((Jacq. Ex. Fr.) Kummer) store in packaging materials. Advances in Pharmaceutics. 2014. Article ID 262807.
Krisko, A. and Radman, M. 2013. Biology of extreme radiation resistance: the way of Deinococcus radiodurans. Cold Spring Habor Perspectives in Biology. 5:a012765: 1-11.
Lee, H.J. and Kim, K.W. 2012. Anti-inflammatory effects of arbutin in lipopolysaccharide stimulated BV2 microglial cells. Agent and Action. 61: 817-825.
Lindsay, S.W., Ewald, J.A., Samung, Y., Apiwathnasorn, C., and Nosten, F. 1998. Thanaka (Limonia acidissima) and Deet (dimethyl benzamide) Mixture as a Mosquito Repellent for Use by Karen Women. Medical and Veterinary Entomology. 12: 295-301.
Lourith, N., Kanlayavattanakul, M., and Pongpunyayuen, S. 2010. Skin Lightening Agent from Naringi Crenulata. World Academy of Science Engineering and Technology. 46: 1022-1023.
Mahmoud, N.S., Awad, S.H., Madani, R.M.A., Osman, F.A., Elmanoun, K., and Hassan, A.B. 2016. Effect of γ radiation processing on fungal growth and quality characteristics of millet grains. Food Science and Nutrition. 4: 342-347.
Migas, P. and Miroslawa, K.B. 2015. The significance of arbutin and its derivatives in therapy and cosmetics. Phytochemistry Letters. 13:35-40.
Nayar, M. and Bhan, M. 1972. Coumarins and other constituents of Hesperethusa crenulata. Phytochemistry. 11: 3331- 3333.
Nimrat, S., Suksawad, T., and Vuthiphandchai, V. 2014. Evaluation of bacterial contamination in Thanaka products distributed in Republic of the Union of Myanmar. KKU Science Journal. 42: 781-791.
Pereiraa, C., Calhelhaa, R.C., Barrosa, L., and Ferreira, I.C.F.R. 2013. Antioxidant properties, anti-hepatocellular carcinoma activity and hepatotoxicity of artichoke, milk thistle and borututu. Industrial Crops and Products. 49: 61-65.
Pewlong, W., Sajjabut, S., Eamsiri, J., Chookaew, S., and Boonsirichai, K. 2016. Enhancement of glycyrrhizic acid and microbial decontamination of Glycyrrhiza glabra (licorice) by gamma radiation. Chiang Mai University Journal of Natural Sciences. 15: 181-188.
Rahayu, D.P., Saputri, F.C., and Darwis, D. 2016. The Effect of Gamma Radiation on Microbial Content and Curcuminoids of Curcuma amada Roxb. Rhizomes. Atom Indonesia. 42: 53-58.
Sajilata, M.G. and Singhal, R.S. 2006. Effect of irradiation and storage on the antioxidant activity of cashew nuts. Radiation Physics and Chemistry. 75: 297- 300.
Schindler, M., Solar, S., and Sontag, G. 2005. Phenolic compounds in tomatos. Natural variation and effect of gamma irradiation. European Food Research and Technology. 221: 439-445.
Seiverling, E.V. and Ahrns, H.T. 2013. Thanaka and its dermatologic uses in Myanmar (Burma). Journal of Investigative Dermatology. 133: S88-S103.
Seiverling, E.V., Trubiano, J.P., Williams, J.C., Ahrns, H.T., Craft, D.W., and England,
M.R. 2017. Analysis of the antimicrobial properties of thanaka, a Burmese powder used to treat acne. Journal of Bioscience and Medicines. 5: 1-6.
Sommer, I., Schwartz, S., Solar, S., and Sontag, G. 2009. Effect of γ-Irradiation on Agaritine, γ-Glutaminyl-4-hydroxybenzene (GHB), antioxidant capacity, and total phenolic content of mushrooms (Agaricus bisporus). Journal of Agriculture of Food Chemistry. 57: 5790-5794.
Stajner, D., Milosevic, M., and Popovic, B.M., 2007. Irradiation effects on phenolic content, lipid and protein oxidation and scavenger ability of soybean seeds. International Journal of Molecular Sciences. 8: 618-627.
Thongchai, W., Liawruangrath, B., and Liawruangrth, S. 2007.Hogh-performance liquid chromatography determination of aebutin in skin-whitening creams and medicinal plant extracts. Journal of Cosmetic Science. 58: 35-44.
Topuz, A. and Ozdemir, F., 2004. Influences of gamma irradiation and storage on the capsaicinoids of sun-dried and dehydrated paprika. Food Chemistry. 86: 509-515. Variyar, P.S., Bandyopadhyay, C., and Thomas, P. 1998. Effect of gamma-irradiation on the phenolic acids of some Indian spices. International Journal of Food Science
and Technology. 33: 533-537.
Velioglu, Y.S., Mazza, G., Gao, L., and Oomah, B.D. 1998. Antioxidant capacity and total phenolics in selected fruits, vegetable and grain products. Journal of Agricultural and Food Chemistry. 46: 4113-4117.
Wangthong, S., Palaga, T., Rengpipat, S., Wanichwecharungruang, S., Chanchaisak, P., and Heinrich, M. 2010. Biological activities and safety of Thanaka (Hesperethusa crenulata) stem bark. Journal of Ethnopharmacology. 132: 466-472.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Wachiraporn Pewlong*, Surasak Sajjabut, Sirilak Chookaew, Jarurattana Eamsiri, Khemruji Khemthong, and Lamai Maikaew
Research and Development Group, Thailand Institute of Nuclear Technology (Public Organization), Nakhon Nayok 26120, Thailand
Corresponding author: Wachiraporn Pewlong, E-mail: wachiraporn03@yahoo.com
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: May 12, 2020;
Revised: July 1, 2020;
Accepted: October 12, 2020

