
Isolation of Corncob Xylan-Degrading Fungi and Its Application in Xylobiose Production
Tantry Febrinasari, Hasegawa Tae, Nakanishi Riki, Akkharapimon Yotsombat, Takata Goro*, Muhammad Nur Cahyanto, and Chartchai KhanongnuchPublished Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.039
Journal Issues : Number 2, April-June 2021
Abstract In the present study, a potential corncob xylan degradation fungi was isolated and screened from soil to produce xylanase, and was identified as Fusarium oxysporum. The production of xylanase by F. oxysporum under solid state fermentation using corncob powder as the solid substrate reached the maximum xylanase activity when using particle size of substrate of 60 mesh, water content ratio of 2 mL/g substrate, incubation temperature of 30°C, initial pH of 6.0, size of inoculum of 5x107 spore/3 g substrate, and incubation time of 2 days. The xylanase activity increased about 4 times up to 7.92 U/mL after optimization. The potential application of xylanase of F. oxysporum in hydrolyzing alkali-treated corncob xylan to produce xylobiose was also demonstrated. Hydrolysis of 6% of corncob xylan using 100 U/g substrate of enzyme loading under optimum pH and temperature conditions (pH 5.5 and 50°C, respectively) achieved the yield of xylobiose up to 28.7 g/100 g pure xylan after 12 h incubation. The purification of hydrolysate could retain 91.1% of xylobiose. Further separation step using activated charcoal column chromatography was able to get a pure xylobiose, but could only recover 59.3% of xylobiose.
Keywords: Corncob, Fusarium oxysporum, Solid state fermentation, Xylanase, Xylobiose
Funding: This research was supported by Kagawa University, Kagawa, Japan and Japan Student Services Organization (JASSO). This research was also supported in part by KAKENHI number 26450097, 22580088 (Grant-in-Aid for Scientific Research (C)).
Citation: Febrinasari, T., Tae, H., Riki, N., Yotsombat, A., Goro, T., Cahyanto, M.N., and Khanongnuch, C. 2021. Isolation of corncob xylan-degrading fungi and its application in xylobiose production. CMUJ. Nat. Sci. 20(2): e2021039.
INTRODUCTION
Over the past decades, xylooligosaccharides (XOS) gained considerable attention as nutraceutical ingredients, and their demands in food and health industries are rapidly increasing in recent years (Yang et al., 2015). In food industries, XOS are used as additives in a variety of functional and health food products or as active components of symbiotic preparations (Moure et al., 2006). XOS were found to possess various beneficial effects for human health, particularly as prebiotic to work against several gastrointestinal disorders (Aachary and Prapulla, 2011). As the emerging prebiotic, XOS can stimulate an increased levels of Bifidobacteria in a greater extent than other prebiotic oligosaccharides (Rycroft et al., 2001; Hsu et al., 2004). These compounds also exhibit temperature and acidity stability in a higher range than inulin and FOS (fructooligosaccharides) (Courtin et al., 2009).
Xylobiose is the dimer of XOS and considered to have the most active performance among XOS types owing to the highest prebiotic activity in promoting the proliferation of Bifidobacteria which play an important role in the maintenance of the gut health (Okazaki et al., 1990; Manisseri and Gudipati, 2012; Moura et al., 2007). The properties of xylobiose are also known to be the most suitable and favorable for industrial application (Jiang et al., 2017). Those potential properties of xylobiose have ignited an interest in enhancing the efficacy in the production of xylobiose. Xylobiose can be generated from an abundant, renewable, cheap, and susceptible lignocellulosic biomass, such as corncob (Tan et al., 2008; Chapla et al., 2012). During the past decades, Indonesia’s corn production tended to annually increase, reaching 28.9 million tons/year in 2017. Along with that, the increasing amount of corncob was also unavoidable, yet only a small percentage of millions tons of those was utilized, while most of them were only left to rot or burned in the field. Bioconversion of corncob will provide an economic benefit by recovery of high value added compounds instead of disposal of the wastes (Pandey et al., 2000). The use of agricultural waste as alternative bio-substrate for production of xylobiose is also a crucial priority for cost reduction. The lignocellulose component of corncob consists of cellulose (27–35%), hemicellulose (30–39%), and lignin (9–14%) (Yang et al., 2005; Samanta et al., 2012). A high hemicellulose content of corncob, which is mainly composed of xylan, promotes its potential utilization as a substrate for xylobiose production through hydrolysis process. Xylanase (endo-β-1,4-D-xylanase, EC 3.2.1.8) is a hemicellulotic enzyme that catalyzes the hydrolysis of β-1,4-xylan into xylose and xylooligosaccharides. Some species of filamentous fungi are known to be lucrative producers of xylanases in terms of industrial point of view since they are, in general, able to secret extracellular xylanase with higher level and productivity than bacteria or yeast and also have an activity in a wide range of temperatures and pH (Polizali et al., 2005; Bakri et al., 2008).
For years, a wide diversity of fungal species in nature has been screened to find a new potential source of xylanase with constructive and novel characteristics. Therefore, the main objectives of this study were focused on isolating and screening a potential corncob xylan degrading fungal strain from soil to produce xylanase with appreciable activity, and then evaluating a promising application of the isolated fungal xylanase in hydrolyzing alkali-treated corncob xylan to produce xylobiose. Purified xylobiose, compared to crude form, is more potential and effective when considering the prebiotic effect (Manisseri and Gudipati, 2012). Hence, several steps of purification and separation process of xylobiose was also demonstrated.
MATERIALS AND METHODS
Materials
Corncob was obtained from corn processing industry in Medan, Indonesia. Soil samples were collected randomly around Faculty of Agriculture, Kagawa University, 10–15 cm beneath the surface using spatula, packed in sterile polyethylene bags, and stored at 4 °C until used. All the reagents, media and chemicals used under study were of analytical grade (Nacalai Tesque, Kyoto, Japan). Xylan from beechwood was obtained from Serva, Germany. Standards for HPLC analysis (D-xylose, D-glucose, L-arabinose, xylobiose, xylotriose) were purchased from Wako-Chemical, Osaka, Japan.
Isolation and screening of a potential corncob-xylan degradation fungi
A 2.5 g of each soil sample was placed into 50 mL of enrichment MS medium
(0.2% KH2PO4, 0.12% NH4)2SO4, 0.05% MgSO4•7H2O, 0.01% CaCl2, 0.02% yeast extract, 0.05% Tween 80, 0.5% corncob in sterile deionized water). Cultures were incubated in an orbital shaker (TAITEC, Tokyo, Japan) (150 rpm) at 30°C for 3 days, then each culture was transferred into a fresh isolation medium and further incubated for 3 days. The enrichment cultures were diluted serially from 10−1 to 10−3 in sterile saline (0.9% NaCl). Diluted enrichment samples (10−2 and 10−3) were plated on potato dextrose agar (PDA) medium to which chloramphenicol (150 mg/L) and beechwood xylan (0.1%) were added. Cultures were incubated at 30°C for 5 days. Morphologically different fungal colonies were isolated by sub culturing on new PDA medium and incubated at 30°C for 5 days. For screening, two 0.5 cm agar discs of 3 days PDA plate cultures of each isolate were firstly inoculated into the sterile 20 mL of MS medium and incubated in an orbital shaking (150 rpm) at 30°C for 5 days. The substrate and fungal biomass were removed by centrifugation (12,000 rpm; 15 min). Crude enzyme was spotted on double layer agar (pH 6.0) in which the upper agar layer was supplemented with 0.5% beechwood xylan, then incubated at 30°C for 1 day prior to the Congo red assay. Six fungi selected based on Congo red assay were further assessed for their xylanase activities by reducing sugar production using Somogyi- Nelson method. A selected fungal strain with the highest xylanase activity was maintained at 4°C in slant PDA medium.
Identification of a potential corncob xylan degradading fungi
Selected fungal strain was identified based on morphological and molecular characteristics. Colony appearances were macroscopically observed after 3–7 days of incubation on PDA plate, while microscopic identification was carried out under optical inverted microscope by previously preparing slide culture and staining using lactophenol cotton blue solution. Molecular identification was done by amplifying the partial sequences of internal transcribed spacer/ITS gene by PCR. The sequences obtained were compiled and searched against the GenBank database using the BLAST program.
Preparation of corncob powder and xylan extraction by alkaline treatment
The agro-residue of corncob was washed thoroughly with deionized water to remove surface dust particles, dried in an oven at 50°C, chopped and ground into small pieces, and then sieved to get corncob powder with approximate particle size of 30–60 mesh and ≥60 mesh. For extractives removal, corncob powders were blended in deionized water with a solid-to-liquid ratio of 1:8 (g/mL) at 80°C for 1 h two times to remove the hydrophilic substance and soaked in 95% ethanol (v/v) with a solid-to- liquid ratio of 1:5 (g/mL) at 60°C for 1 h two times to remove the liposoluble substance. Alkaline treatment was performed using 10% NaOH (w/v) with a solid-to-liquid ratio of 1:10 (g/mL) and incubated at 65°C for 8 h in a shaker incubator (150 rpm). Alkali-treated material was then subjected to hydrothermal treatment by autoclaving at 121°C, 15 psi (1.0 atm) for 1 h. The mixture was filtered to separate liquor from residue by manual pressing and the residue was washed with deionized water. The liquor and washing were mixed and centrifuged (12,000 rpm; 10 min) to obtain a clear supernatant. The alkali-solubilized xylan was adjusted to pH 5.0 with glacial acetic acid followed by precipitation with 95% ice-cold ethanol (alkaline solution : alcohol at 1:1 ratio; v/v) and allowed to stand overnight. The precipitate was collected by centrifugation (12,000 rpm; 10 min), washed with water, dried in a forced hot air oven at 50°C, powdered, and stored at room temperature.
Optimization of xylanase production by solid state fermentation
The corncob powder with the certain particle size (30-60 and >60 mesh) in the flask was moistened using different power/50 mM buffer ratios (1:1, 1:2, 1:3, 1:4; w/v) as MS medium at various pHs (4.0, 5.0, 6.0, 7.0), prior to autoclaving. The sterilized preparations were inoculated with 1 mL of spore suspension (1x107, 5x107, 1x108, 2x108) per 3 g of substrate and incubated at different temperatures (25, 30, 40, 50°C) for varying time (1, 2, 3, 4, 5, 6, 7 days). After incubation, the whole content of the flasks was extracted in 10 volumes of 50 mM sodium citrate buffer (pH 6.0). The mixture was agitated at 200 rpm for 2 h at 30°C, centrifuged (12,000 rpm; 15 min; 4°C) two times and the supernatant was used to analyze the xylanase activity.
Partial purification of xylanase by ammonium sulfate precipitation
Crude xylanase was added by solid ammonium sulfate to achieve initially 30% saturation with constant stirring at 4°C for 30 min. After centrifugation (10,000 rpm; 20 min; 4°C), the precipitate was discarded and the supernatant was subsequently adjusted to 70% saturation. After being centrifuged, the precipitates were dissolved in a small volume of 50 mM sodium citrate buffer (pH 6.0). Enzyme solution was subjected to dialysis for about 18–24 h at 4°C against 100 volumes of 50 mM sodium citrate buffer (pH 6.0), with three intermittent changes of buffer.
Xylanase activity and protein assays
Xylanase activity is determined by measuring the production of reducing sugars according to Somogyi-Nelson method. One unit of xylanase is defined as the amount of enzyme that liberates 1 µmol of xylose equivalents per min under the assay conditions. Protein content is determined by the method of Bradford using bovine serum albumin (BSA) as the standard.
Characterization of xylanase
Substrate Specificity. Various substrates (beechwood xylan, oat spelt xylan, CM cellulose, p-nitrophenyl xylopyranoside, and p-nitrophenyl arabinofuranoside) with a concentration of 0.5% were dissolved in 50 mM citrate buffer (pH 5.5) and incubated at 50°C for 30 min. The amount of reducing sugar liberated was determined by the Somogyi-Nelson method using xylose as a standard for beechwood xylan and oat spelt xylan, glucose for CM cellulose, and p-nitrophenol for p-nitrophenyl xylopyranoside and p-nitrophenyl arabinofuranoside.
Effect of pH and temperature on xylanase activity and stability. The optimal pH was determined by testing xylanase activity using 0.5% beechwood xylan as substrate prepared in the respective buffer system at pH conditions ranging from 3.0 to 9.0 at 50°C. The pH stability was determined by diluting enzyme in the corresponding buffer system at the ratio of 1:10 and thereafter incubated at the selected pH range at 50°C for 1 h. Xylanase at optimum pH (pH 5.5) was assayed at different temperatures ranging from 30 to 80°C to determine the optimum temperature for enzymatic activity. Thermostability of xylanase was tested after incubation for 1 h at a temperature range of 30–80°C.
Optimization of xylobiose production
Enzymatic hydrolysis was performed using xylanase at pH 5.5 and 50°C for 12 h. Four different extracted corncob-xylan concentrations (2, 4, 6, and 8%) were employed at an enzyme loading of 100 U/g of substrate to investigate the effect of solid loading on the enzymatic hydrolysis, while four enzyme loading levels (100, 200, 300, and 400 U/g of substrate) were used in the process at a solid loading of 6% to explore the effect of enzyme doses. Time course study of xylose, xylobiose, and xylotriose production from hydrolysis process at optimum conditions was done for different hydrolysis time (2, 4, 6, 8, 10, 12, 18, 24, and 48 h). After enzymatic hydrolysis, the hydrolysate suspensions were heated to 100°C for 5 min to stop the enzyme activity, centrifuged (12,000 rpm; 5 min), and the supernatants were analyzed by Somogyi-Nelson method to measure the reducing sugar content and by HPLC to quantify xylose, xylobiose, and xylotriose.
Purification of xylobiose
Firstly, precipitation by 1% (v/v) poly aluminium chloride (PAC) was carried out. The mixture was mixed and settled for 30 min and then centrifuged (15,000 rpm; 5 min) to remove the precipitate. Deionization process was performed by using combination of DIAION SK-1B cation-exchange resin (Mitsubishi-Chemical, Tokyo, Japan) and AMBERLITE IRA-411 anion-exchange resin (Organo, Tokyo, Japan). Each resin slurry was added alternately while stirred and maintained to pH 7.0 until the conductivity of hydrolysate reached ≤ 0.5 mS/m as measured by a conductivity meter. The hydrolysate was then filtrated by vacuum filter using cellulose acetate membrane stepwise with the pore size having the descending order, including 0.8, 0.6, 0.45, to 0.2 µm. The purified hydrolysate was concentrated by vacuum evaporation using a rotary evaporator (35°C; 18-20 psi). Activated charcoal was loaded into a column glass with a 40 mm i.d. x 110 mm –length column. Before fractionation, column was washed with pure water several times. The purified xylan hydrolysate was then loaded into the column and pure water was passed through the column to remove monosaccharides or non-adsorbed sugars. The xylobiose were then recovered by eluting 10% ethanol solution. After being washed with pure water to remove the ethanol, the column was re-loaded by the first separated xylobiose fraction to thoroughly get rid of any remain monosaccharides. The separated xylobiose and non-adsorbed sugars solution were analyzed by HPLC.
Analysis of sugar component by HPLC
The component of sugars were determined by HPLC using a Gelpack GL-C611 column (Hitachi-Kasei, Tokyo, Japan) with a refractive index detector (Shimadzu, Kyoto, Japan). Column temperature was 60°C with 0.1 mM NaOH as mobile phase at a flow rate of 1.0 mL/min.
RESULTS
Isolation and screening of a corncob xylan degradation fungi
A total of eleven morphologically different fungal strains were isolated from soil samples. For screening, each strain was further cultured in submerged medium comprising 1% corncob as main carbon source to investigate their potential in utilizing corncob xylan to produce xylanase. Determination of xylanase activity was qualitatively observed by Congo red assay to make an initial evaluation. Isolates that are capable of producing xylanase are characterized by the formation of clear zones around the enzyme spot after staining by Congo red solution. The larger and more vivid diameter of clear zone shows the increasing number of xylan degraded by xylanase, indicating the higher xylanase activity generated by the isolate. Six fungal strains, D21, D22, D23, D25, D26 and E32, comparatively displayed the largest clear zone. For further confirmation, the xylanase activity of those strains were further subjected to quantitative analysis by reducing sugar assay using Somogyi-Nelson method. The activity of each isolate varied from 0.02 U/mL to the highest of 3.25 U/mL, which was obtained from D26 strain.
Identification of a corncob xylan degradation fungi
Based on the macroscopic morphology, D26 strain was classified as filamentous fungi. The mycelia did not spread rapidly and were white in surface color and white- pinkish (sometimes becoming dark-purple) in reverse color. They had velvet-like texture, irregular form with no radial line and concentric circle. Microscopically, the strain had septate hyphae and hyaline filament in the aerial mycelium, as well as the microconidia that was produced in false heads on short, single, and lateral monophialides conidiophores. The result of molecular identification showed that the sequences of the fungal isolate D26 was found to be identical to that of Fusarium oxysporum.
Characterization of corncob and corncob xylan
The lignocellulosic composition of corncob used in this study was composed of 37.0 ± 0.33% cellulose, 36.7 ± 0.84% hemicellulose (xylan existed as much as 25.7%), and 11.6 ± 1.27% lignin and ash. The combination of pre-treatment using 10% alkali (NaOH) and steam application using an autoclave (121°C; 15 psi) helped to provide a high recovery of xylan up to 94%. The extracted corncob xylan was composed of 1.56% of glucose, 65.5% of xylose (57.6% pure xylan) and 6.52% of arabinose.
Production of F. oxsporum xylanase
Microbial metabolic activity and enzyme production are extremely sensitive to environmental factors. Therefore, some parameters affecting xylanase production under solid state fermentation (SSF), such as particle size of substrate, water content ratio, incubation temperature, initial pH, amount of inoculum/spore suspension, and incubation time were optimized to obtain the xylanase with the maximum activity. As shown in Figure 1a, the maximum xylanase production was observed when the smaller particle size of substrate (>60 mesh) was used, plausibly due to increased surface area per particle and reduced degree of crystallinity of substrate. The increasing surface area could facilitate easier penetration of fungal aerial hyphae into the matrix of substrate to break down xylan by producing xylanase, and also provided a favourable environment for the heat transfer and exchange of oxygen and carbon dioxide between air and the surface of the solid substrate, which significantly affected fungal growth. The moisture content determines the growth rate and other physiological activities of fungi. An increased moisture increases aerial mycelial growth, lowers oxygen transfer, and decreases substrate porosity. On the other hand, low moisture reduces nutrients and protein solubility owing to reduced surface area of the substrate. As seen in Figure 1b, a higher initial moisture content was not suitable for xylanase production of F. oxysporum. The highest xylanase activity was obtained at water content ratio of 1-2 mL/g substrate with the slightly highest at the ratio of 2 mL/g substrate. The incubation temperature is one of regulatory parameters affecting production of enzyme during fermentation. Figure 1C shows that substantial xylanase activity was observed between 25 and 30°C with the highest activity at 30°C (4.70 U/ml). Meanwhile, the drastic reduction in enzyme production was obtained at 40 and 50 °C since higher temperature more likely evaporated the water in the medium, hence without any moisture control in the system, the medium became dry during the incubation period and the growth of the fungus was inhibited, causing a decrease in the synthesis of the enzymes. Changes in medium pH affected the permeability of microbial cells, enzyme stability, and also caused the denaturation of secreted enzymes. As shown in Figure 1d, xylanase production was significantly higher at pHs 6.0 and 7.0, in which the highest activity was found at pH 6.0 (7.83 U/ml). Incubation below pH 6.0 caused the reduction in xylanase activity. The result suggested that a fairly acidic-to-neutral pH range was suitable for xylanase production by F. oxysporum. Figure 1e shows that lower xylanase activity was observed at lower concentration of inoculum since a small inoculum size might not be adequate for growth initiation, and delayed the lag phase as well as enzyme synthesis. An increase of inoculum size leads to an increase of activity due to the shortening of lag phase that will boost the growth of isolate and intensify the utilization of substrate. However, the result revealed that the use of inoculum size above 5x107 spores/3 g substrate did not give a significant change on xylanase production. This might be due to a possible competition for limited nutrient and a decrease in the specific velocity of oxygen consumption as the result of overcrowded growth of the organism per unit substrate. Hence, the optimum amount of inoculum was found at 5x107 spore/3 g substrate. Time course of xylanase production (Figure 1f) indicates that the second day of incubation time was the peak period for enzyme production, and thereafter the activity decreased gradually with increasing time until 7 days of incubation. The decrease in xylanase production probably occurred due to the non-optimal growth of fungi, lack of moisture, macro and micronutrient deficiencies in the cultivation medium, enzyme inhibition by the end product, or a change in pH during the cultivation process. The xylanase activity increased about 4 times up to 7.92 U/mL after optimization.
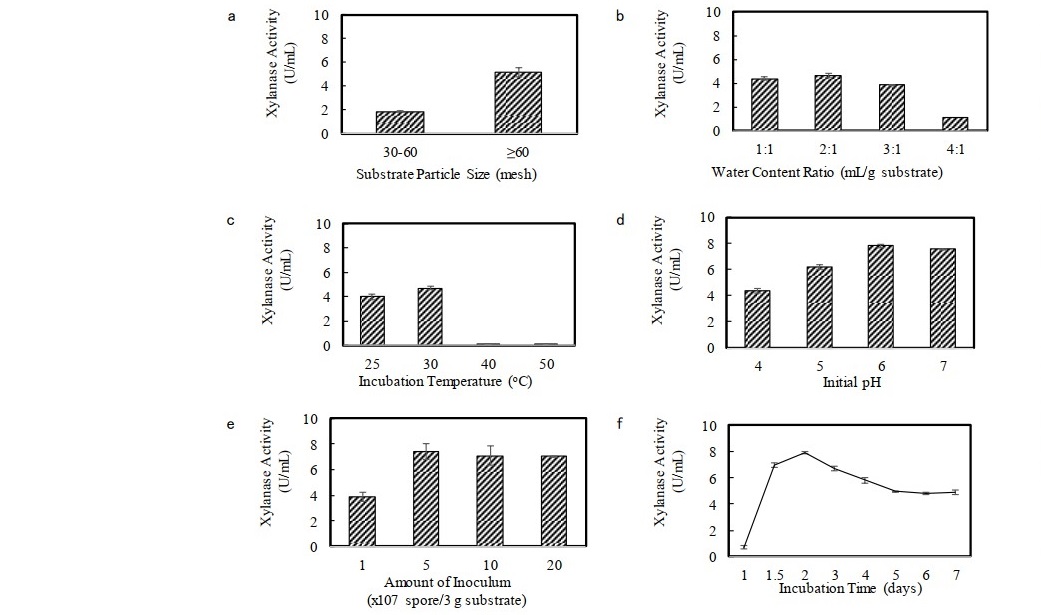
Figure 1. Xylanase activity at different parameter condition of xylanase production (a) substrate particle size, (b) water content ratio, (c) incubation temperature, (d) initial pH, (e) amount of inoculum/spore suspension, and (f) incubation time. Data points: mean values from two independent experiments. Error bars: standard deviations of duplicate independent experiments.
Characterization of F. oxsporum xylanase
The crude enzyme was partially purified using ammonium sulphate precipitation with saturation of 30-70% followed by dialysis. Crude xylanase was purified to 1.64 fold (9.55 U/mg) with the yield of 62.9%. The enzyme was then assayed for its activity on a variety of substrates (Table 1). The result showed that xylanase activities on commercial xylans (beechwood xylan and oat spelt xylan) were observed to be dominantly higher than that on alkali-treated corncob xylan. This was because the enzyme showed the highest affinity with beechwood xylan. The relative activity of enzyme towards alkali-treated corncob xylan was found to be lower (37.6%), compared to that of beechwood xylan. As presented in Figure 2a, F. oxysporum xylanase showed optimum activity in slightly acid region with the maximum activity at pH 5.5. Regarding the stability against pH conditions, the enzyme was incubated for 1 h in a pH range between 3.0 and 9.0 at 50°C. Figure 2b shows that pH 6.0 yielded the highest stability, in which the activity of 86.8% was retained. The enzyme activity was also tested at different temperatures (30 to 80°C). The enzyme activity gradually increased with increasing temperature and experienced the maximum activity at 50°C (Figure 3a). At temperatures above 50°C, significant reducing activity was attained. Thermo-stability of enzyme was tested by incubation of enzyme at temperatures between 30 and 80°C for 1 h. The enzyme was found to be the most stable at 30°C and retained 97.9% of activity (Figure 3b). The enzyme has poor stability at high temperatures as the residual activity substantially declined when temperature was increased. Incubation at optimum temperature (50°C) for 1 h caused the enzyme to denature, resulting in the lowered activity, with residual activity of 61.4%.
Table 1. Substrate specificity of partial purified xylanase.
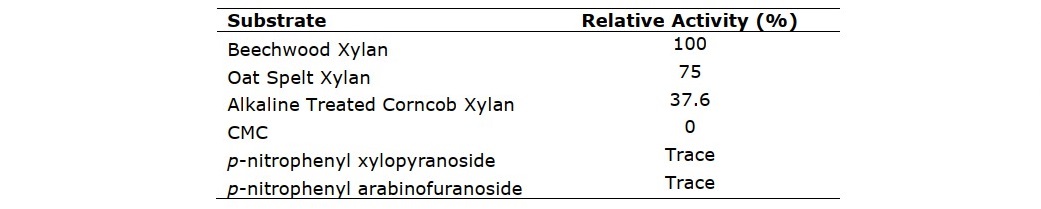
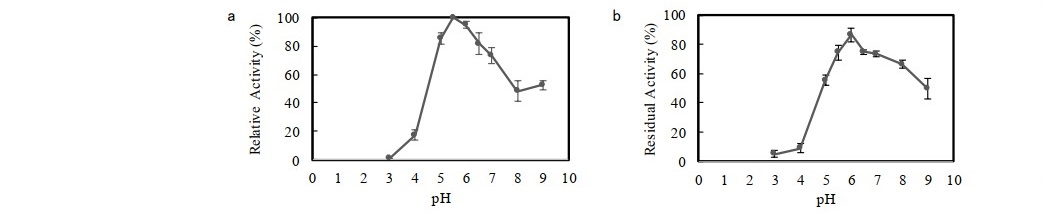
Figure 2. Effect of pH on (a) activity and (b) stability (after incubated at optimum pH, 5.5; 1 h) of F. oxysporum xylanase. Data points: mean values from two independent experiments. Error bars: standard deviations of duplicate independent experiments.
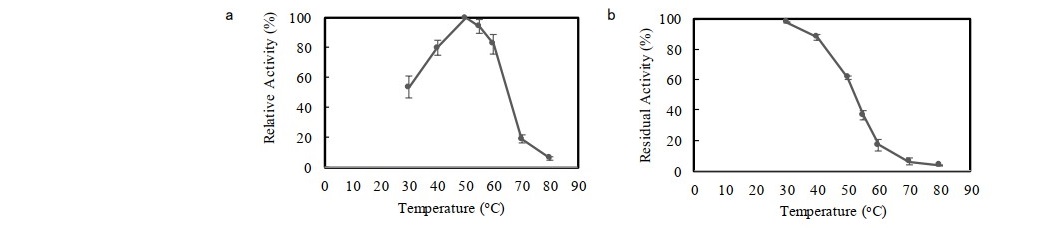
Figure 3. Effect of temparature on F. oxysporum xylanase's (a) activity and (b) stability (after incubated at optimum temperature, 50°C; 1 h). Data points: mean values from two independent experiments. Error bars: standard deviations of duplicate independent experiments
Enzymatic hydrolysis for production of xylobiose
Optimization of xylobiose production with different xylan concentrations using enzyme loading of 100 U/g substrate and incubated for 12 h achieved the highest xylobiose yield when using 6% xylan, in which xylobiose of 29.6 g/100 g pure xylan could be produced (Figure 4). The higher xylan concentration than 6% decreased the rate of xylobiose production due to the reduction of water content in the medium, of which enzyme availability was decreased with an increase in viscosity and density of the reaction mixture (Akpinar et al., 2010). The reducing sugar increased as xylan concentration increased and declined when the concentration was 8%. In addition, the xylan concentration higher than 6% could only increase the xylose yield. Therefore, using higher substrate concentration (>6%) was not advantageous as it reduced the efficiency of hydrolysis for xylobiose production. Figure 5 depicts the production of reducing sugar, xylobiose, and xylose from hydrolysis of 6% corncob xylan using varying loadings of xylanase for 12 h incubation. The result showed that an increase in enzyme loading more than 100 U/g substrate did not render any substantial increase in xylobiose yield, while the amount of reducing sugar and xylose yields continued to increase as the enzyme loading increased. Release of reducing sugar increased due to the breakdown of xylobiose and xylotriose, which led to the accumulation of more xylose. Therefore, using the higher dose of enzyme was avoided since liberating xylose could be increased, which might hinder the production of xylobiose. Use of 100 U/g substrate of enzyme loading was chosen as the best condition for production of xylobiose with the yield was up to 29.0 g/100 g pure xylan. Figure 6 shows time course study of xylose, xylobiose, and xylotriose production from hydrolysis process of 6% extracted corncob xylan using 100 U/g substrate of partially purified xylanase from F. oxysporum under optimum condition (pH 5.5, 50°C). The result showed that xylobiose was formed as the major product. The percentage of xylobiose in the hydrolysate was found to increase as the reaction time increased. During 12 h of hydrolysis, xylobiose production reached 28.7 ± 0.42 g/100 g pure xylan or 67.5 ± 0.91% of total sugars (4.89 ± 0.07 mg/mL). Upon the prolonged incubation after 12 h, increasing yield of xylobiose was found but not substantial. This might be due to the decrease of xylanase activity since the xylanase had low stability at 50°C, resulting in denaturation of enzyme during longer reaction. Moreover, with possible end product inhibition, loss of xylanase’s catalytic activity could take place (Wan Azelee et al., 2016). The production of xylose also positively increased as the incubation time increased as the result of residual β-xylosidase activity. In addition, there was a decline in the production of xylotriose after 12 h, indicating that the further hydrolysis caused hydrolysis of xylotriose into xylobiose and most became xylose. Thus, in order to maintain a high yield of xylobiose with more economical value and avoid a greater production of xylose, 12 h was chosen as the optimum hydrolysis time. However, considering the result of thermostability in this study, of which the xylanase was unstable at higher temperature, hydrolysis process performed at lower temperature than 50°C may possibly provide a higher yield of xylobiose. Though the xylanase activity at lower temperature was not as high as that at 50°C, the stability of the enzyme could still retain more than 80% activity for 1 h incubation, which is more effective for xylobiose production.
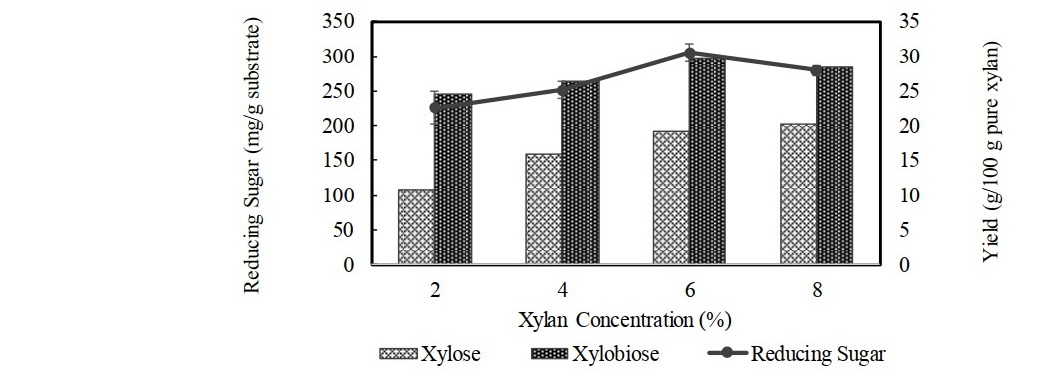
Figure 4. Effect of corncob xylan concentration on total reducing sugar and production of xylose and xylobiose using 100 U/g substrate of partial purified xylanase after 12 h of reaction time at pH 5.5 and temperature 50°C. Data points: mean values from two independent experiments. Error bars: standard deviations of duplicate independent experiments
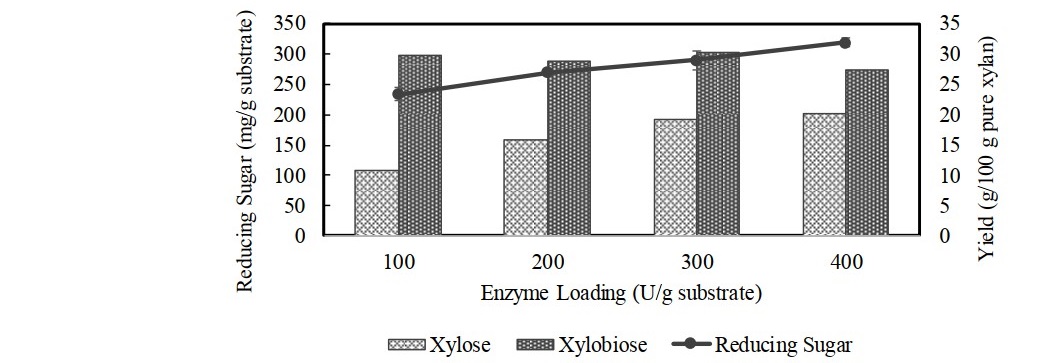
Figure 5. Effect of enzyme loading on total reducing sugar and production of xylose and xylobiose using 6% of extracted corncob xylan after 12 h of reaction time at pH 5.5 and temperature 50°C. Data points: mean values from two independent experiments. Error bars: standard deviations of duplicate independent experiments.
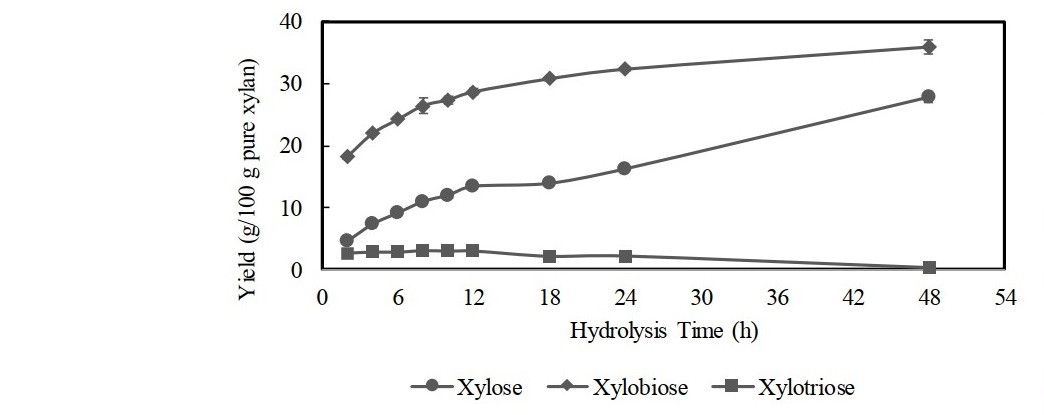
Figure 6. Time course study of xylose, xylobiose, and xylotriose production at optimal experimental conditions (pH: 5.5, temperature: 50°C, xylan concentration: 6%, and enzyme loading: 100 U/g substrate). Data points: mean values from two independent experiments. Error bars: standard deviations of duplicate independent experiments.
Purification of hydrolysate
Precipitation by adding electrolyte such as PAC was aimed to get rid of dissolved organic matters or colloidal particles, such as polysaccharides, macro-lignin particles, cell wall-proteins and lipids that can lead to high turbidity of the resulting hydrolysate. However, small molecules such as mineral salt ions cannot be precipitated by PAC and still remained as impurities in the hydrolysate. Therefore, the mixture of cation-anion exchange resins (SK-1B and IRA411, respectively) was used for the removal of soluble ionized substances from hydrolysate. Cation-exchange resin is able to remove the additive cations such as Na+ which mainly comes from the alkali added during xylan extraction process, meanwhile anion-exchange resin can remove anions such as the molecule of lignin derivatives possessing the negative charges on their surfaces (Alexandratos et al., 2009). The reducing conductivity of hydrolysate up to 0.121 mS/m from 367 mS/m was observed in hydrolysate after deionization. Membrane filtration based on microfiltration technique was done to remove the traces of impurities, which still existed in the hydrolysate. After passing through three steps of purification, the xylobiose was recovered up to 91.1%. However, these purification steps still could not remove monomeric sugars that were also liberated during hydrolysis. Based on HPLC result, the chromatogram of hydrolysate after purification shows the presence of monomeric sugars such as xylose, glucose, and arabinose. Therefore, further separation step was required to separate xylobiose from sugar mixtures and obtain a pure xylobiose.
Separation of xylobiose
The separation of xylobiose from hydrolysate was accomplished by adsorption- separation processes carried out in a fixed-bed column containing granular activated charcoal. Activated charcoal is the most commonly used adsorbent for purification of sugar liquors due to its low price and availability. Adsorption by activated charcoal was found to be the most efficient and suitable method for separation of oligosaccharides due to its large surface area and pore volume, associated with a good sorption capacity, the simplicity of the system and its regeneration, the capacity to separate large amounts of oligosaccharides using only a single small column, and the low cost of the process. The main separation process of xylobiose was carried out in three steps: (1) adsorption of sugars onto the activated charcoal column; (2) elution of monosaccharides/ nonadsorbed sugars with water; and (3) desorption of xylobiose with 10% ethanol. Based on chromatogram results (Figure 7), separation of sugar mixtures in hydrolysate using activated charcoal column chromatography was able to remove monomeric sugars, such as xylose, glucose, and arabinose, and pure xylobiose was attained. Finally, the final yield of xylobiose from alkaline treated corncob xylan was 59.3% recovered after separation procedure.
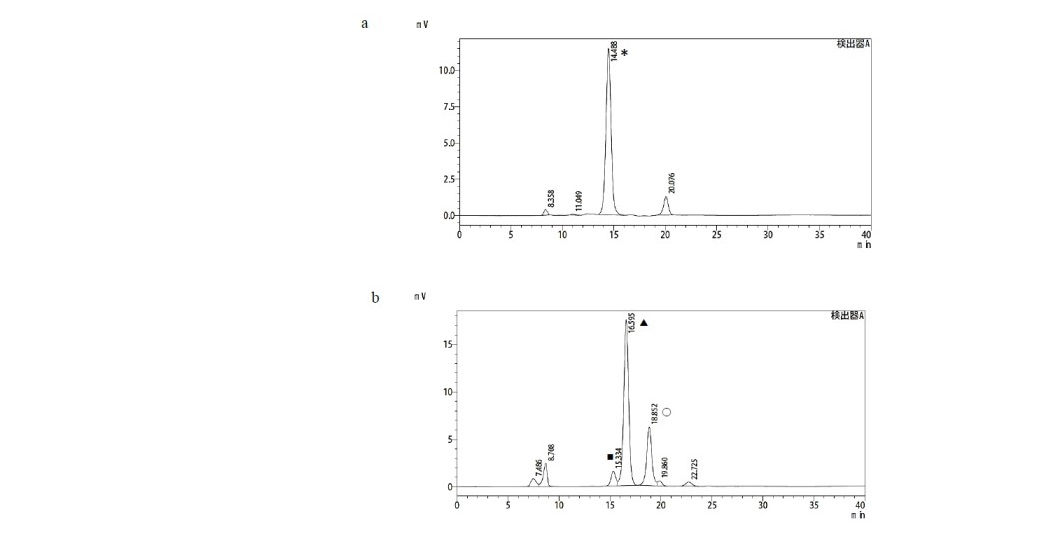
Figure 7. Chromatograms of (a) purified xylobiose and (b) nonadsobed sugars.
(*: xylobiose; ■: D-glucose, ▲: D-xylose, Ο: L-arabinose)
DISCUSSION
The fungal isolate of D26 was selected to be the xylanase producer in this study as it showed the highest capacity in producing xylanase. The result of molecular identification showed that the sequences of the fungal isolate D26 was identified as F. oxysporum. F. oxysporum is a soil inhabitant and a major crop plant-pathogenic ascomycete whose genome encodes a complete xylanolytic degradative system. Some studies have been reported the ability of F. oxysporum in producing xylanases. Their high activity and overall efficiency in degrading plant cell-wall components make F. oxysporum xylanase a candidate of strategic potential for the lignocellulose-based industries.
We succeeded to obtained 57.6% pure xylan from corncob. As the main constituent of hemicellulose, xylan is integrated with cellulose and lignin–polysaccharide matrix via covalent and non-covalent interactions, resulting in the resistance to hydrolysis. Therefore, various pretreatments have been used for the complete extraction of hemicellulose to make xylan more accessible to enzymatic reactions. In this study, finely ground corncob was pre-treated by alkali and hydrothermal treatment to extract the xylan before being subjected to the enzymatic hydrolysis. Alkali treatment may cause swelling, leading to the increase in internal surface areas, decrease in the degree of polymerization and crystallinity, separation of structural linkages between lignin and carbohydrates, and disruption of lignin. Thus, xylan could be recovered from lignocellulosic substrates to a higher extent. Furthermore, Singh, et al. (2018) suggested that reaction in high temperature plays a critical role in maximising xylan extraction.
The growth conditions of SSF for enzyme production were optimized. The maximum xylanase activity when using particle size of substrate of 60 mesh, water content ratio of 2 mL/g substrate, incubation temperature of 30°C, initial pH of 6.0, size of inoculum of 5x107 spore/3 g substrate, and incubation time of 2 days. The xylanase activity increased about 4 times up to 7.92 U/mL after optimization. F. oxysporum was grown under optimized conditions and the extracellular enzyme was prepared. The enzyme was then purified and characterized. The enzyme showed lower activity against alkaline treated corncob xylan than beechwood xylan. This might be due to the presence of side-chain residues that may retard the hydrolysis rate of xylan structure. Different xylan preparations can differ both in the types and degrees of substitutions and this can result in a significant variation of the activity detected (Nawel et al., 2011). In addition, the alkali-treated corncob xylan still contains lignin. Lignin and hemicellulose are linked via multiple bonds to form lignin-carbohydrate-complex that limited the complete removal of lignin in the treated xylan, and lignin is considered to be inhibitory of the affinity between enzyme and substrate (Westbye et al., 2007; Kim et al., 2018). The enzyme did not exhibit activity on cellulase. This coincided with no activity when tested using CMC as a substrate. While, slight activity on p-nitrophenyl xylopyranoside and p-nitrophenyl arabinofuranoside was detected. This indicates that partial purification using ammonium sulphate precipitation could remove β-xylosidase, which played a role in releasing xylose during saccharification. Comparatively, an extracellular endo-l,4-β- xylanase from F. oxysporum exhibited the optimum pH and temperature at pH 5.0 and 50°C, respectively (Alconada and Martinez, 1994). In accordance with Jorge, et al. (2005), xylanase from F. oxysporum had an optimum pH at 5.5 and retained more than 70% activity within the pH range of 4.5–6.5. The optimum temperature was 55°C and more than 90% of activity was retained within a temperature range of 45–55°C. The enzyme showed similar results with these enzymes on pH and temperature properties.
Xylobiose from alkaline treated corncob xylan was produced under optimum conditions. During 12 h of hydrolysis, xylobiose production reached 28.7 ± 0.42 g/100 g pure xylan or 67.5 ± 0.91% of total sugars (4.89 ± 0.07 mg/mL). Compared to similar study by Chapla et al. (2012), hydrolysis of 1% corncob xylan using 20 U of partially purified xylanase from Aspergillus foetidus MTCC at 45°C for 12 h incubation resulted in 1.2 ± 0.85 mg/mL yield of xylobiose. The separation of xylobiose from hydrolysate was accomplished by activated charcoal column chromatography. The xylobiose separation in hydrolysate using activated charcoal column chromatography was succeeded and pure xylobiose was attained. Tan et al. (2008) and Chapla et al. (2012) also separated hydrolysate containing XOS using activated charcoal column and found xylobiose as the major end product. Impurities such as monomeric sugars were removed from the enzymatic hydrolysate as they were not adsorbed on charcoal and hence were separated easily from the hydrolysate. Even though the clearer and colorless solution was obtained, only around 59.3% of xylobiose were recovered after separation procedure. This might be because two cycles of separation were implemented. As a consequence, xylobiose might be lost during the elution, especially with water. The further optimization of column condition, such as length and diameter of column, elution rate, and concentration of ethanol, was required to increase the efficiency of separation.
CONCLUSION
The present study established the potential of xylanolytic soil fungus, F. oxysporum, in production of xylanase and xylobiose from corncob. Maximum production of xylanase by F. oxysporum reached up to 7.92 U/mL under the optimized solid state fermentation conditions. A xylanase of F. oxysporum was successfully exploited for saccharification process of corncob xylan to produce xylobiose rich- xylooligosaccharides. Hydrolysis of 6% alkali-extracted corncob xylan using xylanase at 100 U/g substrate under the optimum conditions released 28.7 ± 0.42 g/100 g pure xylan of xylobiose in 12 h of incubation. However, using lower temperature was suggested to obtain higher yield of xylobiose due to the better stability of xylanase. The study also showed high recovery of xylobiose after purification and the separation of xylobiose using activated charcoal column chromatography should be further optimized.
REFERENCES
Aachary, A.A. and Prapulla, S.G. 2011. Xylooligosaccharides (XOS) as an emerging prebiotics: microbial synthesis, utilization, structural characterization, bioactive properties, and applications: comprehensive reviews in food science and food safety. Food Safety. 10: 1–15.
Akpinar, O., Erdogan, K., Bakir, U., and Yilmaz, L. 2010. Comparison of acid and enzymatic hydrolysis of tobacco stalk xylan for preparation of xylooligosaccharides. LWT - Food Science and Technology. 43: 119–125.
Alconada, T.M., and Martinez. 1994. M.J., Purification and characterization of an extracellular endo-1, 4-beta-xylanase from Fusarium oxysporum f. sp. melonis. FEMS Microbiology Letters. 118: 305–310.
Alexandratos, S.D. 2009. Ion-exchange resins: a retrospective from industrial and engineering chemistry search. Industrial & Engineering Chemistry Research. 48: 388–398.
Bakri, Y., Jawhar, M., and Arabi, M.I.E. 2008. Improvement of xylanase production by Cochliobolus sativus in solid state fermentation. The Brazilian Journal of Microbiology. 39: 602–604.
Chapla, D., Pandit, P., and Shah, A. 2012. Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by probiotics. Bioresource Technology. 115: 215–221.
Courtin, C.M., Swennen, K., Verjans, P., and Delcour, J.A. 2009. Heat and pH stability of prebiotic arabinoxylooligosaccharides, xylooligosaccharides and fructooligosaccharides. Food Chemistry. 112: 831–837.
Hsu, C.K., Liao, J.W., Chung, Y.C., Hsieh, C., and Chan, Y.C. 2004. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. The Journal of Nutrition. 134: 1523–1528.
Jiang, S.X., Zhao, S., Lu, C.Y., Xue, J.L., Duan, C.J., and Feng, J.X. 2017. A combined process is used for efficient isolation and purification of xylobiose from xylanase- hydrolysed sugarcane bagasse xylan hydrolysate. Industrial Crops and Products. 109: 637–643.
Jorge, I., de la Rosa, O., Navas-Cortes, J.A., Jimenez-Diaz, R.M., and Tena, M. 2005. Extracellular xylanases from two pathogenic races of Fusarium oxysporum f. sp. ciceris: enzyme production in culture and purification and characterization of a major isoform as an alkaline endo-beta-(1, 4)-xylanase of low molecular weight, Antonie Van Leeuwenhoek. 88: 48–59.
Kim, C.K., Choi, H.S., Lee, S.J., Lee, J.H., Yoo, H.Y., Han, S.O., and Kim, S.W. 2018.
Production of xylanase from a novel engineered Pichia pastoris and application to enzymatic hydrolysis process for biorefinery. Process Biochemistry. 65: 130-135. Manisseri, C., and Gudipati. 2012. M., Prebiotic activity of purified xylobiose obtained from ragi (Eleusine coracana, Indaf-15) bran. Indian Journal of Microbiology. 52:
251–257.
Moure, A., Gullo´n, P., Domı´nguez, H., and Parajó, J.C. 2006. Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals, Process Biochemistry. 41: 1913–1923.
Moura, P., Barata, R., Carvalheiro, F., Gírio, F., Loureiro-Dias, M.C., and Esteves, M.P. 2007. In vitro fermentation of xylooligosaccharides from corncobs autohydrolysis by Bifidobacterium and Lactobacillus strains. LWT - Food Science and Technology. 40: 963–972.
Nawel, B., Said, B., Estelle, C., Hakim, H., and Duchiron, F. 2011. Production and partial characterization of xylanase produced by Jonesia denitrificans isolated in Algerian soil. Process Biochemistry. 46: 519-525.
Okazaki, M., Fujikawa, S., and Matsumoto, N. 1990. Effect of xylooligosaccharide on growth of Bifidobacterium. Journal of Japan Society of Nutrition and Food Sciences. 43: 395–401.
Pandey, A., Soccol, C.R., and Mitchell, D. 2000. New development in solid state fermentation: I—bioprocesses and products, Process Biochem. 35: 1153–1169.
Polizeli, M.L.T.M., Rizzatti, A.C.S, Monti, R., Terenzi, H.F., Jorge, J.A., and Amorim, D.S. 2005. Xylanases from fungi: properties and industrial applications. Applied Microbiology and Biotechnology. 67: 577–591.
Rycroft, C.E., Jones, M.R., Gibson, G.R., and Rastall, R.A. 2001.A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. Journal of Applied Microbiology. 91: 878–887.
Samanta, A.K., Senani, S., Kolte, A.P., Sridhar, M., Sampath, M.K.T., Jayapal, N., and Devi, A. 2012. Production and in vitro evaluation of xylooligosaccharides generated from corn cobs. Food and Bioproducts Processing. 90: 466–474.
Singh, R.D., Banerjee, J., Sasmal, S., Muir, J., and Arora, A. 2018 High xylan recovery using two stage alkali pre-treatment process from high lignin biomass and its valorisation to xylooligosaccharides of low degree of polymerization. Bioresource Technology. 256: 110–117.
Tan, S.S., Li, D.Y., Jiang, Z.Q., Zhu, Y.P., Shi, B., and Li, L.T. 2008. Production of xylobiose from the autohydrolysis explosion liquor of corncob using Thermotoga maritima xylanase B (Xyn B) immobilized on nickel-chelated Eupergit C. Bioresource Technology. 99: 200–204.
Wan Azelee, N.I., Jahim, Md J., Ismail, A.F., Fuzi, S.F.Z.M., Rahman, R.A., and Illias, Md R. 2016. High xylooligosaccharides (XOS) production from pretreated kenaf stem by enzyme mixture hydrolysis. Industrial Crops and Products. 81: 11–19.
Westbye, P., Köhnke, T., Glasser, W., and Gatenholm, P. 2007.The influence of lignin on the self-assembly behaviour of xylan rich fractions from birch (Betula pendula). Cellulose. 14: 603–613.
Yang, R., Xu, S., Wang, Z., and Yang, W. 2005. Aqueous extraction of corncob xylan and production of xylooligosaccharides. LWT - Food Science and Technology 38: 677–682.
Yang, Q., Gao, Y., Huang, Y., Xu, Q., Luo, X.M., Liu, J.L., and Feng, J.X. 2015. Identification of three important amino acid residues of xylanase AfxynA from Aspergillus fumigatus for enzyme activity and formation of xylobiose as the major product. Process Biochemistry. 50: 571–581.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Tantry Febrinasari1, Hasegawa Tae2, Nakanishi Riki2, Akkharapimon Yotsombat2, Takata Goro2,*, Muhammad Nur Cahyanto1, and Chartchai Khanongnuch3
1 Faculty of Agricultural Technology, Universitas Gadjah Mada, Special Region of Yogyakarta, Indonesia
2 Faculty of Agriculture, Kagawa University, Kagawa, Japan
3 Faculty of Agro-Industry, Chiang Mai Univertsity, Chiang Mai 50200, Thailand
Corresponding author: Takata Goro, E-mail: takata.goro@kagawa-u.ac.jp
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: May 12, 2020;
Revised: July 1, 2020;
Accepted: October 12, 2020

