
Trans- Free Fat Spread Produced from Rice Bran Oil and Rice Bran Oil Shortening Blends
Pravit Santiwattana and Sirirak Siramard*Published Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.038
Journal Issues : Number 2, April-June 2021
Abstract The objective of this study was to produce trans-free fat spread from rice bran oil and rice bran oil shortening blends to replace partially hydrogenated fats which contain high levels of trans fatty acids. The W/O emulsion of rice bran oil spread was prepared from blending of rice bran oil and rice bran oil shortening with the mass ratio of 40: 60 using PGPR as an emulsifier. Physicochemical properties, fatty acid compositions, thermal behaviors, micronutrients, sensory attributes and oxidative stability of rice bran oil spread were investigated and compared with commercial spread products. Results showed that physicochemical properties of the rice bran oil spread were similar to the commercial spread (B2). Trans fatty acids contents of the rice bran oil spread (0.2% TFAs) were much lower than the commercial spread (F2) produced from partially hydrogenated fat (4.9% TFAs). Thermal behaviors and SFC profile indicated good physical properties and spreadability of the rice bran oil spread which were comparable to the commercial spreads. Micronutrients in the rice bran oil spread were greater than those of commercial spread products. The rice bran oil spread had the highest overall preference scores compared to the two commercial fat spreads. In addition, the rice bran oil spread exhibited high oxidative stability. This study demonstrated that rice bran oil and rice bran oil shortening blends can be used as an alternative source of partially and fully hydrogenated fats as well as tropical oils to produce trans-free fat spreads with desirable properties.
Keywords: DSC, Fatty acid composition, Micronutrients, Oxidative stability, Rice bran oil, Rice bran oil shortening, Rice bran oil spread, SFC, Trans-free
Funding: The authors gratefully acknowledged Thai Edible Oil Co., Ltd. for the supports.
Citation: Santiwattana, P. and Siramard, S. 2021. Trans- free fat spread produced from rice bran oil and rice bran oil shortening blends. CMUJ. Nat. Sci. 20(2): e2021038.
INTRODUCTION
Fat spreads are products resembling margarine but contain less than 80% fat (Chryson, 2005). Fat spreads are water-in-oil (W/O) emulsions, which consist of an aqueous phase dispersed as fine droplets in liquid oil stabilized within a network of solid fat crystals (hardstock). A proper ratio of liquid oil and solid fat is needed to obtain a product with desirable physical and textural properties such as spreadability and in- mouth melting properties as well as stability (Arellano et al., 2015). The source of solid fats are tropical oils which are generally high in saturated fat, fully hydrogenated oils, or partially hydrogenated oils (PHOs) which contain trans fatty acids (TFAs) (Garsetti et al., 2016).
Awareness about PHOs with high TFAs was first raised in the 1990s, according to the reports that consumption of TFAs was associated with an increased risk of coronary heart disease (CHD) due to increased serum LDL cholesterol and decreased HDL cholesterol levels (Willett et al., 1993; Ascherio and Willett, 1997). In 2015, the United States Food & Drug Administration released the final determination that PHOs are not generally recognized as safe (GRAS) for any use in human food (FDA, 2015) and all food manufacturers cannot add PHOs to foods after Jan 1, 2021 (FDA, 2018). The Ministry of Public Health of Thailand also prescribed the prohibition of PHOs to be produced, imported, or sold in 2018 with an enforcement on Jan 9, 2019 (Minister of Public Health, 2018). Therefore, fat spreads producers had to modify the process in order to achieve the desired properties of products without PHOs.
The fractionation and interesterification processes are alternative methods to modify the melting point of fats without using hydrogenation to reduce TFAs in fat spreads (Arellano et al., 2015). The production of trans-free margarine from liquid oils such as canola oil or rice bran oil and solid fats such as coconut oil, palm kernel oil or palm stearin using fractionation or interesterification processes had been studied (Kim et al., 2008; Adhikari et al., 2010; Ornla-ied et al., 2016; Podchong et al., 2018). However, the blending of rice bran oil and rice bran oil shortening for the trans-free fat spreads production has not yet been studied.
Rice bran oil, a well-recognized healthy edible oil extracted from the outer bran layer of the rice grain, provides positive health effects, especially for cardiovascular diseases (Most et al., 2005). The health benefits of naturally occurring bioactive phytochemicals in the rice bran oil such as gamma-oryzanol, tocopherols, tocotrienols and phytosterols were reported for their antioxidant and cholesterol-lowering properties (Cicero and Derosa, 2005; Bumrungpert et al., 2019). However, as the oil is liquid at room temperature due to a high content of unsaturated fatty acids, it cannot be used as a sole oil ingredient in many margarines and spreads because the products would be too soft with low melting temperature (Ornla-ied et al., 2016).
Rice bran oil shortening is produced from a solid fraction of rice bran oil, called rice stearin, through a fractional crystallization process, without neither partial hydrogenation nor interesterification processes. It has balanced fatty acid compositions and is rich in bioactive compounds, such as gamma-oryzanol and phytosterols, similar to the rice bran oil (Santiwattana and Sirisukpornchai, 2012). The capability of rice stearin for improving the stability of W/O emulsions by forming a three-dimensional network of aggregated fat crystals in the oil phase that prevented droplet movement was studied (Prichapan et al., 2017). As a result, an optimum blending of rice bran oil shortening with rice bran oil may properly be used to improve the stability of the fat spreads.
To ensure stable W/O emulsion of fat spreads produced from rice bran oil and rice bran oil shortening blends, an appropriate emulsifier must be employed. Polyglycerol polyricinoleate (PGPR) has been reported to be the most effective emulsifier for application in low fat spreads with high water or protein content (Young and Wassell, 2008). It stabilizes W/O emulsions by forming a protective coating around the water droplets and by increasing the viscosity of the continuous phase (Wolf et al., 2012).
Overall, the objective of this study was to produce trans-free fat spreads from the healthy rice bran oil and rice bran oil shortening blends using PGPR as an emulsifier to replace the structured modification fats produced from the hydrogenation or interesterification processes. The physicochemical properties, fatty acid compositions, thermal behaviors, micronutrients, sensory attributes and oxidative stability of the rice bran oil spread were investigated to compare with the commercial spread products.
MATERIALS AND METHODS
Materials
Refined rice bran oil (iodine value 100.1; SFA 23.5%; MUFA 42.1%; PUFA 34.4%; TFAs 0.3%) and rice bran oil shortening (iodine value 76.5; SFA 36.7%; MUFA 35.1%; PUFA 28.2%; TFAs 0.4%) were products of Thai Edible Oil Co., Ltd. (Bangkok, Thailand). Milk protein (Ingredia S.A., France), butter flavor (Matrix Flavor’s & Fragrances Sdn. Bhd., Malaysia) and beta-carotene color (Chr. Hansen, Denmark) were obtained from Brenntag, Thailand. Polyglycerol polyricinoleate; PGPR (Lasenor Emul SL, Spain) and Potassium sorbate (Jinneng, China) were obtained from Maxway, Thailand.
Five brands of commercial fat spreads (F1: canola and fully hydrogenated palm oil based; F2: partially hydrogenated soybean oil based; F3: fully hydrogenated palm, olive and extra virgin olive oil based; F4: sunflower, canola and palm oil based; F5: palm oil based) and two brands of commercial blended fat spreads (B1: soybean, palm, rapeseed and butter oil based; B2: butter, rapeseed, palm and coconut oil based) were purchased from local supermarkets in Thailand. F5 and B1 are the products of Thailand, while F1, F2, F3, F4 are imported from Australia and B2 is imported from Denmark.
Preparation of rice bran oil spread
The formulation used in this study is shown in Table 1 (optimum formulation with the best texture, taste and spreadability among 6 formulas with different proportions of rice bran oil, rice bran oil shortening and PGPR obtained from preliminary study). Oil phase was prepared by dispersing PGPR and color in an oil phase containing rice bran oil and rice bran oil shortening blends, melted to 70oC. Water phase was prepared by dissolving milk protein, salt and potassium sorbate in water, heated to 50oC. The oil phase was cooled down to 50oC and homogenized with the water phase at the same temperature using a hand-held homogenizer (IKA-ULTRA-TURRAX® T 25 digital, Germany) at 12,000 rpm for 3 min, then flavor was added. The resulting W/O emulsion of rice bran oil spread was transferred to sample container, and then placed in an ice bath to cool down for 1 h. The sample was kept in a refrigerator (11±1oC) until tested.
Table 1. The formulation of rice bran oil spread.
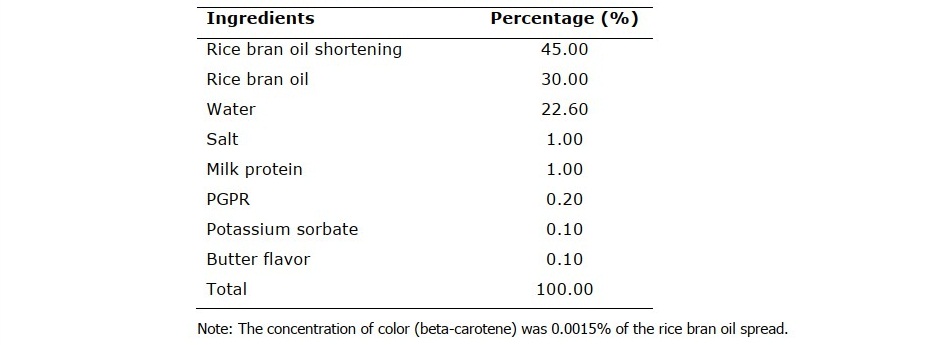
Physicochemical properties
The rice bran oil spread and commercial samples were analyzed for free fatty acid; FFA (Ca 5a-40), moisture content: MC (Ca 2c-25) and slip melting point; SMP (Cc 3-25) according to AOCS official methods.
Fatty acid compositions
Fatty acid compositions of the samples were determined as the methyl esters of fatty acids (FAMEs) which were prepared by esterifying with 14% Boron trifluoride- methanol reagent. FA compositions were analyzed according to the AOCS official method Ce 1e-89 by gas chromatography. A GC-2010 plus (Shimadzu, Japan) equipped with a flame ionization detector and a fused-silica capillary column (HP-88, 100 m x 0.25 mm x 0.20 µm, Agilent, USA) was used for GC analysis of the methyl esters. The FAME was injected into the GC system. The injection port and detector temperatures were both set at 250°C. The column was initially held at 140°C for 5 min, followed by temperature programming to 240°C at rate of 4oC/min, then held at 240°C for 15 min. The flow rate of carrier gas (helium) was 19 ml/ min. The split ratio was 1: 20. The peaks were identified based on their retention times with those of authentic standards. FA levels were quantified as relative proportions of the total composition and reported on a wet basis.
Thermal properties
Crystallization and melting behaviors of the samples were studied using a differential scanning calorimeter (DSC 3 STARe System, Mettler-Toledo, Switzerland) based on the method described by Hwang et al. (2013) with some modifications. The samples were prepared by heating in an oven at 65°C for 1 h to separate fat in order to prevent water interference. Fat layer of the samples (10-11 mg) were packed in aluminum pans. Dry nitrogen was used as a purge gas with a flow rate of 50 ml/min. The samples were equilibrated at -60°C for 20 min, heated to 90°C with heating rate of 5°C/min and cooled down to -60°C with cooling rate of 5oC/min.
Solid fat contents (SFC) of the samples were estimated using DSC melting thermogram at temperature range of -60 to 90°C. The percentages of liquid at various temperatures were obtained directly from the DSC 3 STARe software. The SFC were calculated from the percentages of liquid, and melting profiles were drawn by plotting SFC against temperature (Mayamol et al., 2004; Reddy and Jeyarani 2001).
Tocopherol and tocotrienol contents
The contents of tocopherols and tocotrienols in samples were determined by high- performance liquid chromatography according to the AOCS official method Ce 8-89. The samples were prepared by extraction in hexane with anhydrous MgSO4 added to remove water (Ye et al., 1998). The HPLC system was equipped with a high-pressure pump (Waters 1525 binary HPLC pump, USA) and a fluorescence detector (Waters 2475 Multi λ, USA) with the excitation and emission wavelength set at 290 and 330 nm, respectively. The silica-based HPLC column (5µm i.d., 250 x 4 mm) (VertiSepTM, Vertical, Thailand) was used in the normal phase with the solvent system n-hexane/ isopropanol (98.5: 5, v/v) at a flow rate of 1 ml/min. The tocopherol and tocotrienol contents of samples were quantified by calculation on a wet basis.
Gamma-oryzanol content
The gamma-oryzanol determination of separated fat in the samples was performed using UV-Visible spectrophotometer (UV-1800, Shimadzu, Japan) at 315 nm according to the method described by CODEX STAN 210-1999. The gamma-oryzanol content of samples was quantified by calculation on a wet basis.
Sensory evaluation
The rice bran oil spread and two brands of commercial fat spreads (F1 and F2; two highest sensory scores in preliminary study) were evaluated for sensory attributes (appearance, color, odor, taste, texture, spreadability and overall acceptability) using a liking score on a nine-point hedonic scale (dislike extremely to like extremely) by thirty untrained panelists.
Oxidative stability
The oxidative stability of rice bran oil spread and commercial fat spread (F1; the highest sensory scores in preliminary study) were studied during one-year storage in refrigerator (11 ± 1°C). Peroxide values of the samples were analyzed according to the AOCS official method Cd 8-53 every two months.
Statistical analysis
Statistically significant differences (P <0.05) of the mean values were evaluated by one-way analysis of variance (ANOVA).
RESULTS
Physicochemical properties
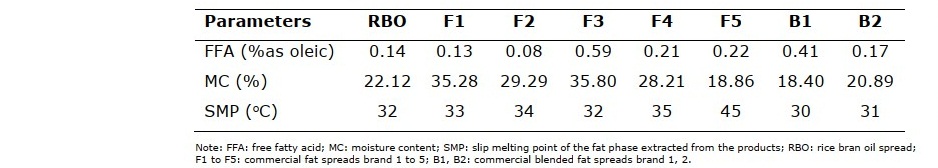
Physicochemical properties of rice bran oil spread and commercial samples are presented in Table 2. FFA, MC and SMP of the rice bran oil spread were 0.14%, 22.12% and 32°C, respectively. The physicochemical properties of rice bran oil spread were more closely to B2. The highest FFA and MC were found in F3, while F5 had the highest SMP.
Table 2. Physicochemical characteristics of rice bran oil spread compared with commercial spread products.
Fatty acid compositions
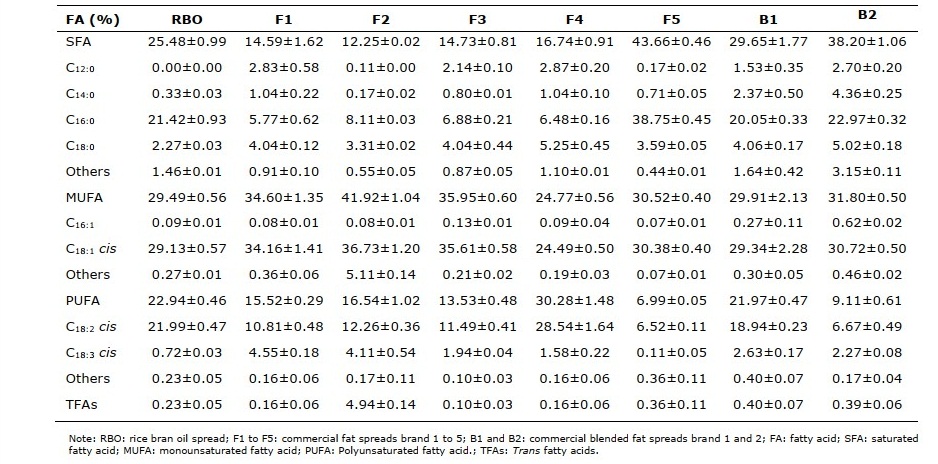
FA compositions of rice bran oil spread and commercial samples are shown in Table 3. The predominant fatty acids of the rice bran oil spread were MUFA (29.49%), followed by SFA (25.48%) and PUFA (22.94%). The majority FA of commercial samples (F1, F2, F3 and B1) were also MUFA (29-42%). TFAs in the rice bran oil spread were 0.23%, similar to those of commercial spreads which TFAs were lower than 0.50%, excepted only F2 containing highest TFAs (4.94%) from the partially hydrogenated soybean oil.
Table 3. FA compositions (%fatty acids, g/ 100g sample) in rice bran oil spread compared with commercial spread products.
Thermal properties
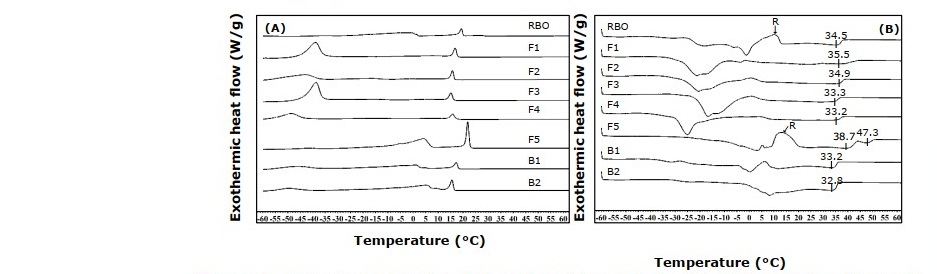
Figure 1. DSC crystallization (A) and melting (B) thermograms of rice bran oil spread (RBO) compared with commercial spread products (F1, F2, F3, F4, F5, B1 and B2).
Thermal profiles of rice bran oil spread and commercial samples are shown in Figure 1. Crystallization profile of rice bran oil spread exhibited two distinct peaks with a sharp peak at 19°C and a broader peak at -2°C. The melting profile exhibited two broad melting regions. The first melting region was located from -43 to 10°C and the second region was located from 10 to 39°C corresponding to the transitions of lower- and higher-melting TAGs as described by Podchong et al. (2018). The second melting region is associated with spread or margarine’s semisolid characteristics at ambient temperature (Podchong et al., 2018; Tan and Che Man, 2000). The second melting region of the rice bran oil spread (Tp 34.5°C) was similar to those commercial spread products (excepted F5) which exhibited comparable physical characteristics to the commercial spreads. The exothermic recrystallization peaks (R) between first and second melting regions of the rice bran oil spread and the F5 produced from palm oil were similar to the results of Jennings and Akoh (2010) and Litwinenko et al. (2002) which studied the thermal behaviors of shortening prepared with palm stearin and rice bran oil structured lipid and palm oil based-shortening, respectively. The recrystallization probably occurred when the α polymorph, unstable form, transitioned to the β’ form, stable and desired form, due to an effect of high cooling rate (5°C/min) during crystallization and melting by DSC analysis (Litwinenko et al., 2002).
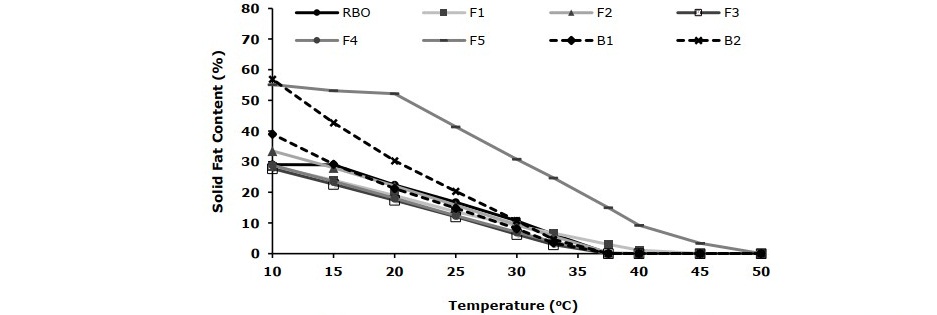
Figure 2. Solid fat content profiles of rice bran oil spread (RBO) compared with commercial spread products (F1, F2, F3, F4, F5, B1 and B2).
The SFC of rice bran oil spread and commercial samples calculated from DSC data are revealed in Figure 2. The rice bran oil spread had SFC content of 29, 22 and 0% at 10, 20 and 37°C, respectively. The results indicated good physical properties of the rice bran oil spread according to the desired attributes of fat-based foods described by Teles dos Santos et al. (2014) which stated that the SFC value at 10°C should not be greater than 32% for good spreadability and the SFC at 20°C should not be less than 10% to prevent oiling off. Moreover, at body temperature (37°C) the spread should melt completely for less or no waxy mouthfeel.
Micronutrients
The results reveal higher micronutrients in rice bran oil spread compared to those of commercial spread products (Table 4). The rich of bioactive phytochemicals in rice bran oil and rice bran oil shortening provided higher micronutrients in the rice bran oil spread which total tocopherols, tocotrienols and gamma-oryzanol contents were 164, 349 and 2,200 ppm, respectively.
Table 4. Micronutrients (based on wet basis) of rice bran oil spread compared with commercial spread products.

Sensory evaluation
The results show comparable sensory attributes between rice bran oil spread and two commercial fat spread products (Table 5). Although appearance and color scores of the rice bran oil spread were lower than those two commercial spreads because of stronger yellow color, the other sensory scores of the rice bran oil spread were similar or higher. Moreover, the rice bran oil spread had the highest overall acceptability scores (P >0.05)
Table 5. Sensory scores of rice bran oil spread compared with two commercial fat spread products.

Oxidative stability
The oxidative stability of rice bran oil spread and F1 during one-year storage in refrigerator (11 ± 1°C) are presented in Figure 3. The initial peroxide values of rice bran oil spread and F1 were 0.37 and 0.33 meq O2/kg sample, respectively. The increasing of peroxide values during storage were found in the samples. However, after one-year storage the rice bran oil spread had much lower peroxide values compared with the F1 (POV 0.98 and 5.49 meq O2/kg sample, respectively). The results revealed more oxidative stability of the rice bran oil spread corresponding to higher antioxidative compounds (tocopherols, tocotrienols and gamma-oryzanol) found in the sample.
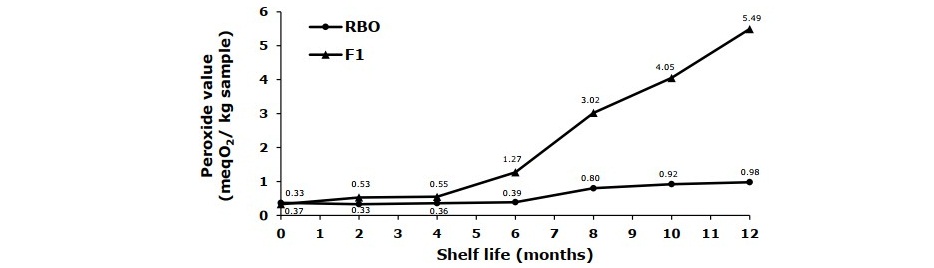
Figure 3. Peroxide values of rice bran oil spread (RBO) compared with commercial fat spread (F1) during one-year storage in refrigerator (11 ± 1°C).
DISCUSSION
The properties of rice bran oil spread produced from rice bran oil and rice bran oil shortening blends were studied to compare with commercial spreads produced from various fat and oil sources. The physicochemical properties of the rice bran oil spread were comparable to the commercial spreads, especially B2. FFA of the rice bran oil spread (0.14%) was closely to other commercial products, however F3 showed the highest FFA (0.59%) which caused by high FFA containing in an extra virgin olive oil (Tarhan et al., 2017). Higher MC of F1, F2, F3 and F4 (28-36%) compared with the rice bran oil spread (22.12%) were found according to their lower fat content (60-66%). The SMP of rice bran oil spread (32oC) was similar to the commercial spreads (30-35°C), excepted F2 (45°C) which was produced from palm oil.
FA compositions of the rice bran oil spread (25.5% SFA; 29.5% MUFA; 22.9% PUFA; 0.2% TFAs) were corresponded to the proportion of rice bran oil and rice bran oil shortening blends. MUFA were the majority FA of rice bran oil spread, F1, F2, F3 and B1, according to their oil-based ingredients which were rice bran oil, canola oil, PHO soybean oil, olive oil and soybean oil, respectively. While SFA were the main FA of F5 and B2 produced from palm oil and butter oil, respectively. Unlike other spreads, most of FA in F4 were PUFA which mainly derived from sunflower oil. TFAs contents in rice bran oil spread and most commercial spread products were less than 0.5%, excepted F2 which contained high levels of TFAs (4.9% TFAs) from the PHO soybean oil (Karabulat et al., 2003).
Crystallization and melting profiles of rice bran oil spread exhibited each two regions corresponding to the blending of rice bran oil and rice bran oil shortening. The first regions appeared at low temperature related to lower- melting TAGs of olein fractions, whereas the second regions appeared at high temperature associated with higher- melting TAGs of stearin fractions (Prichapan et al., 2017; Podchong et al., 2018). Similar thermal profiles were found in commercial spread products with some differences depending on their oil-based TAGs. Comparable second melting peak which related to spread’s characteristics at ambient temperature (Podchong et al., 2018; Tan and Che Man, 2000) of the rice bran oil spread and most commercial spreads were also discovered. Unlike other spreads, the thermal profiles of F5 exhibited higher melting temperature which related to higher-melting TAGs derived from palm oil.
The SFC profiles of rice bran oil spread exhibited good physical properties and spreadability according to the report of Teles dos Santos et al. (2014). Additionally, the similar SFC profiles between rice bran oil spread and the commercial spreads (F2 and B1) indicated comparable physical characteristics. In contrast, F5 and B2 exhibited higher SFC profiles compared to other spreads corresponding to the higher SFA contents obtained from palm oil and butter oil, respectively. Moreover, more than 14% SFC at body temperature (37°C) was found in the F5 indicated that it would cause waxy mouthfeel (Chrysan, 2005).
The rice bran oil spread had the highest micronutrients compared with commercial spread products corresponded to the bioactive phytochemicals derived from rice bran oil (Singanusong and Garba, 2019) and rice bran oil shortening (Santiwattana and Sirisukpornchai, 2012). The result revealed that only rice bran oil spread contained gamma-oryzanol (2,200 ppm) which is one of the most effective antioxidative compounds rich in rice bran oil (Rao, 2000). Moreover, the highest total tocopherols and tocotrienols contents were detected in rice bran oil spread, followed by B1, F5, F4, F2, B2, F3 and F1, respectively. The total tocopherols and tocotrienols of commercial spreads were related to their oil-based ingredients as well as manufacturing processes which resulted in the remaining of micronutrients in the products. Beside those studied micronutrients, the spread products may contains other micronutrients such as phytosterols rich in rice bran oil (Van Hoed et al., 2006; Sawadikiat et al., 2014), or polyphenols and squalene rich in an extra virgin olive oil (Silva et al., 2010; Tuberoso et al., 2007), depending on the proportions and types of oil used in formulations.
The sensory attributes of rice bran oil spread compared with two commercial fat spreads (F1 and F2) were evaluated. The lower appearance and color scores of the rice bran oil spread compared to the commercial spreads were obtained as a result of stronger yellow color. However, the highest odor and taste scores of the rice bran oil spread were found (P ≤0.05). Texture scores were comparable in all samples. Spreadability scores of rice bran oil spread were the highest (P >0.05) corresponding to the desirable physical properties which was shown in the SFC profiles. Moreover, rice bran oil spread had the highest overall acceptability scores (P >0.05) indicated good sensory characteristics of the rice bran oil spread produced from rice bran oil and rice bran oil shortening blends.
In addition, the higher oxidative stability of the rice bran oil spread during one- year storage in refrigerator (11 ± 1°C) was found compared with the commercial fat spread (F1). The result associated with the higher antioxidative compounds detected in the sample. Therefore, the blending of rice bran oil and rice bran oil shortening can be potentially used to produce trans-free fat spread with desirable physical, nutritional and sensory properties as well as stability.
CONCLUSION
Rice bran oil and rice bran oil shortening blends can be utilized to produce trans- free fat spreads with desirable properties. TFAs contents of the rice bran oil spread were lower than the commercial spread produced from partially hydrogenated fat, and comparable to the spreads produced from non-partially hydrogenated fats. The DSC and SFC profiles exhibited good physical properties and spreadability of the rice bran oil spread. The highest micronutrients and overall sensory scores were also discovered. Moreover, the rice bran oil spread exhibited high oxidative stability. Therefore, rice bran oil and rice bran oil shortening blends can be effectively used as a replacement of partially and fully hydrogenated fats as well as tropical oils to produce trans-free fat spreads. Further studies on the optimum formulation of reduced fat or low-fat spread from rice bran oil and rice bran oil shortening blends should be continued to produce the healthier fat spreads.
ACKNOWLEDGEMENTS
The authors would like to thank department of research and development and quality assurance of Thai Edible Oil Co., Ltd. for technical works.
REFERENCES
Adhikari, P., Shin, J.A., Lee, J.H., Hu, J.N., Zhu, X.M., Akoh, C.C., and Lee, K.T. 2010. Production of trans-free margarine stock by enzymatic interesterification of rice bran oil, palm stearin and coconut oil. Journal of the Science of Food and Agriculture. 90: 703-711.
AOCS. 1997. AOCS Official Method Cd 8-53 and Ce 1c-89. Official Methods and Recommended Practices of the American Oil Chemist’s Society, 5th edn., Washington D.C.
AOCS. 2017. AOCS Official Method Ca 2c-25, Ca 5a-40, Cc 3-25 and Ce 8-89. Official Methods and Recommended Practices of the American Oil Chemist’s Society, 7th edn., Washington D.C.
Arellano, M., Norton, I.T., and Smith, P. 2015. Specialty oils and fats in margarines and low-fat spreads. In: Talbot, G. (ed) Specialty Oils and Fats in Food and Nutrition. Woodhead Publishing, Cambridge. p. 241-270.
Ascherio, A. and Willett, W.C. 1997. Health effects of trans fatty acids. Journal of American Oil Chemists’ Society. 66: 1006s-1010s.
Bumrungpert, A., Chongsuwat, R., Phosat, C., and Butacnum, A. 2019. Rice Bran Oil Containing Gamma-Oryzanol Improves Lipid Profiles and Antioxidant Status in Hyperlipidemic Subjects: A Randomized Double-Blind Controlled Trial. The Journal of Alternative and Complementary Medicine. 25: 353-358.
Chrysan, M.M. 2005. Margarines and spreads. In: Shahidi, F. (ed) Bailey’s industrial oil and fat products. John Wiley & Sons, New Jersey. p. 33-82.
Cicero, A.F.G. and Derosa, G. 2005. Rice bran and its main components: Potential role in the management of coronary risk factors. Current Topics in Nutraceutical Research. 3: 29-46.
CX-STAN. 1999. Codex standard for named vegetable oils. CODEX STAN 210-1999. FDA. 2015. Final determination regarding partially hydrogenated oils. Federal Register. 80: 34650-34670.
FDA. 2018. Final determination regarding partially hydrogenated oils. Federal Register. 83: 23358-23359.
Garsettia, M., Balentine, D.A., Zock, P.L., Blom, W.A.M., and Wanders, A.J. 2016. Fat composition of vegetable oil spreads and margarines in the USA in 2013: a national marketplace analysis. International Journal of Food Sciences and Nutrition. 67: 372-382.
Hwang, H-S., Singh, M, Bakota, E.L., Winkler-Moser, J.K., Kim, S., and Liu, S.X. 2013. Margarine from organogels of plant wax and soybean oil. Journal of American Oil Chemists’ Society. 90: 1705-1712.
Jennings, B.H. and Akoh, C.C. 2010. Trans-free plastic shortenings prepared with palm stearin and rice bran oil structured lipid. Journal of American Oil Chemists’ Society. 87: 411-417.
Karabulut, I., Kayahan, M., and Yaprak, S. 2003. Determination of changes in some physical and chemical properties of soybean oil during hydrogenation. Food Chemistry. 81: 453-456.
Kim, B.H., Lumor, S.E., and Akoh, C.C. 2008. Trans-Free margarines prepared with canola oil/palm stearin/palm kernel oil-based structured lipids. Journal of Agricultural and Food Chemistry. 56: 8195-8205.
Litwinenko, J.W., Rojas, A.M., Gerschenson, L.N., and Marangoni, A.G. 2002. Relationship between crystallization behavior, microstructure, and mechanical properties in a palm oil-based shortening. Journal of American Oil Chemists’ Society. 79: 647-654.
Mayanol, P.N., Samuel, T., Balachandran, C., Sundaresan, A., and Arumughan, C. 2004. Zero-trans shortening using palm stearin and rice bran oil. Journal of American Oil Chemists’ Society. 81: 407-413.
Minister of Public Health. 2018. Prescribed Prohibited Food to be Produced, Imported, or Sold. Notification of the Ministry of Public Health No. 388 B.E.2561 (2018).
Most, M.M., Tulley, R., Morales, S., and Lefevre, M. 2005. Rice bran oil, not fiber, lowers cholesterol in humans. Journal of American Oil Chemists’ Society 81: 64-8.
Podchong, P., Sonwai, S., and Rousseau, D. 2018. Margarines produced from rice bran oil and fractionated palm stearin and their characteristics during storage. Journal of American Oil Chemists’ Society.
Prichapan, N., McClements, D.J., and Klinkesorn, U. 2017. Influence of rice bran stearin on stability, properties and encapsulation efficiency of polyglycerol polyricinoleate (PGPR)-stabilized water-in-rice bran oil emulsions. Food Research International 93: 26-32.
Rao, B.N. 2000. Nutritive value of rice bran. Nutrition Foundation of India Bulletin. 21: 5–7.
Reddy, S.Y. and Jeyarani, T. 2001. Trans-free bakery shortening from mango kernel and mahua fats by fractionation and blending. Journal of American Oil Chemists’ Society. 78: 635-640.
Santiwattana, P. and Sirisukpornchai, S. 2012. Micronutrients and effect of rice bran oil shortening on baked products. The Fourth International Conference on Natural Products for Health and Beauty. 28-30 Nov 2012, Chiang Mai. p. 246.
Sawadikiat, P., Chaiseri, S., and Hongsprabhas, P. 2014. Phytochemicals in refined vegetable oils commercially available in Thailand. The 52th Kasetsart University Annual Conference. 4-7 Feb 2014, Bangkok. p.74-80.
Silva, L., Garcia, B., and Paiva-Martins, F. 2010. Oxidative stability of olive oil and its polyphenolic compounds after boiling vegetable process, LWT-Food Science and Technology. 43, 1336-1344.
Singanusong, R. and Garba, U. 2019. Micronutrients in rice bran oil. In: Cheong, L-Z. and Xu, X. (eds). Rice Bran and Rice Bran Oil. AOCS Press. p. 125-158.
Tan, C.P. and Che Man, Y.B. 2000. Differential scanning calorimetric analysis of edible oils: comparison of thermal properties and chemical composition. Journal of American Oil Chemists’ Society. 77: 143-155.
Tarhan, İ., Ismail, A.A., and Kara, H. 2017. Quantitative determination of free fatty acids in extra virgin olive oils by multivariate methods and Fourier transform infrared spectroscopy considering different absorption modes. International Journal of Food Properties. 20: S790-S797.
Teles dos Santos, M., Gerbaud, V., and Le Roux, G.A.C. 2014. Solid fat content of vegetable oils and simulation of interesterification reaction: predictions from thermodynamic approach. Journal of Food Engineering 126: 198-205.
Tuberoso, C.I.G., Kowalczyk, A., Sarritzu, E., and Cabras, P. 2007. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chemistry 103: 1494-1501.
Van Hoed, V., Depaemelaere, G., Vila Ayala, J., Santiwattana, P., Verhe, R., and De Greyt, W. 2006. Influence of chemical refining on the major and minor components of rice brain oil. Journal of American Oil Chemists’ Society. 83: 315- 321.
Willett, W.C., Stampfer, M.J., Manson, J.E., Colditz, G.A., Speizer, F.E., Rosner, B.A., Sampson, L.A., and Hennekens, C.H. 1993. Intake of trans fatty acids and risk of coronary heart disease among women. The Lancet 341: 581-585.
Wolf, F., Koehler, K., and Schuchmann, H.P. 2012. Stabilization of water droplets in oil with pgpr for use in oral and dermal applications. Journal of Food Process Engineering.
Ye, L., Landen Jr, W.O., Lee, J., and Eitenmiller, R.R. 1998. Vitamin E content of margarine and reduced fat products using a simplified extraction procedure and HPLC determination. Journal of Liquid Chromatography & Related Technologies 21: 1227-1238.
Young, N. and Wassell, P. 2008. Margarines and spreads. In: Hasenhuettl, G.L. and Hartel, R.W. (eds). Food Emulsifiers and Their Applications, Berlin. p. 307-326.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Pravit Santiwattana and Sirirak Siramard*
Research and Development Department, Thai Edible Oil Co., Ltd, Bangkok 10110, Thailand
Corresponding author: Sirirak Siramard, E-mail: sirirak.sir@kingriceoilgroup.com
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: May 12, 2020;
Revised: July 1, 2020;
Accepted: October 12, 2020

