
Production of Xyloligosaccharides from Rice Straw by Microwave-assisted Enzymatic Hydrolysis and Evaluation of Their Prebiotic Properties
Alisa Pattarapisitporn, Nonglak Thiangthong, Pakorn Inthajak, Pannapapol Jaichakan, Wantana Panpa, and Wannaporn Klangpetch*Published Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.037
Journal Issues : Number 2, April-June 2021
Abstract Rice straw (RS) is a by-product from rice production process. It is rich in cellulose, hemicellulose and lignin. RS hemicellulose mainly composes of arabinoxylan (AX). This research aimed to investigate the potential of microwave- pretreatment in AX extraction from RS and substrate to produce xylooligosaccharides (XOS) via enzymatic hydrolysis. The extractive-free RS was pretreated by microwave process at 160°C for 5-15 min, then the AX was extracted with 4% sodium hydroxide. The total sugar and reducing sugar content of AX exhibited that increasing microwave-pretreatment time increased the yield of AX. The highest AX content was found at 160°C for 10 min as 7.73%, reducing sugar content of 11.89 mg/g, and total sugar of 165.85 mg/g. The crude AX obtained by microwave-pretreatment was then used as a substrate for XOS production by two commercial xylanases of Pentopan mono BG (BG) and Ultraflo Max (UM), at the enzyme concentration of 50-300 U/g AX (50°C, pH 6.0) for 24 h. The reducing sugar content and sugar profiles were monitored by DNS assay, and thin layer chromatography (TLC) which revealed that BG 50 U/g at 12 h and UM 50 U/g at 24 h showed the promising reducing sugar of 16.4 and 25.44 mg/g, respectively. The composition of XOS derived from RS (RS-XOS) prepared by BG was xylobiose (X2), xylotriose (X3), xylotretraose (X4), and xylopentaose (X5) while by UM was xylobiose (X2), xylotriose (X3) and xylotretraose (X5). Moreover, XOS produced by BG contained very low amount of xylose (X1). In addition, the RS-XOS could the growth of Lactobacillus brevis greater than commercial XOS.
Keywords: Arabinoxylan, Microwave-assisted enzyme hydrolysis, Rice straw, Xylooligosaccharides
Funding: Authors would like to thank the Thailand research fund for supporting this research grant (Grant No. TRG6180290).
Citation: Pattarapisitporn, A., Thiangthong, N., Inthajak, P., Jaichakan, P., Panpa, W., and Klangpetch, 2021. Production of xyloligosaccharides from rice straw by microwave-assisted enzymatic hydrolysis and evaluation of their prebiotic properties. CMUJ. Nat. Sci. 20(2): e2021037.
INTRODUCTION
Rice is the main product of Thailand and very important for national economy. In the process of harvesting, it is found that there is abundant amount of rice straw (RS) obtained as by-product. RS is mostly used as fertilizer, animal feed or in the construction industry. In general, the RS price is approximately around 3,000 Baht/tons, showing low value.
RS mainly contains hemicellulose up to 26% (w/w). Hemicellulose is a non- digestible polysaccharide which consists of many types of sugar. RS hemicellulose consists of xylose as backbone, called xylan. Xylan is a group of hemicelluloses made up of β-1, 4-linked xylose residues with or without side branches of α-arabinofuranose and α-glucuronic acid. Arabinoxylan (AX) is one of the four main xylan families (Kulkarni and Shendye, 1999). AX can be converted into XOS that have been considered as functional foods due to their potential prebiotic properties. Prebiotics are a non-active food constituent that shifts to the colon and is then selectively fermented by normal flora. The benefit to the host is mediated during selective stimulation of the growth and/or activity of one or a limited number of bacteria (Gibson and Roberfroid, 1995)
In recent year, there are many methods of AX extraction. In traditional extractions, fermentation or solvent extraction can reduce the loss of important substances by heat but these methods take a long time and causing a number of chemical wastes (Azwanida, 2015; Handa et al., 2008). Whereas, using high temperature for long time may cause the loss of important substances and large amounts of monosaccharide. While microwave extraction may overcome those disadvantages, due to its short processing time, low amount of solvent (Aguilar- Reynosa et al., 2017). The conversion of AX into XOS by enzymatic hydrolysis has gained much interest due to the mild condition used and the specific products obtained. XOS production is usually performed by endo-xylanases that are negligible as the presence of β-xylosidase and exo-xylanase activities to prevent high xylose production. This end-product might affect the enzyme activity and overall efficiency of XOS production (Akpinar et al., 2009). Consequently, this research aims to investigate a potential approach for microwave-pretreatment of RS before extraction of AX, which was then used as the substrate for XOS production by the commercial xylanases. The prebiotics property of RS-XOS was also tested by the selected probiotic strains. The results of this study might be the valuable information for production and the application of XOS from the RS.
MATERIALS AND METHODS
Materials
The RS used in this research were purchased from a rice mill in Phitsanulok Province in Thailand. The commercial xylanases, UM (700 U/mLfrom Aspergillus oryzae and Trichoderma reesei), BG (2,500 U/g from Thermomyces lanuginosus) were purchased from Novozyme Co. Ltd., Denmark. All chemicals and solvents used in this research were of analytical grade.
Rice straw preparation and Carbohydrates compositional analysis Sun dried RS was crushed with sharp blade blender and sieved through a 40 µm screen. One gram of RS powder was soaked in 20 mL of acetone: ethanol mixture (1:2 v/v) at ambient temperature for 24 h. The RS residue was washed with boiling water and twice with distilled water, dried at 45°C in hot air oven to obtain extractive-free RS for the next step of microwave pretreatment of rice straw.
The determination of sugar composition of extractive-free RS was modified procedures of Jaichakan et al., (2019). Briefly, 0.4 g of extractive-free RS were pre-hydrolyzed with 4.5 mL of 72% sulfuric acid in mortar and mixed for 30 min. Upon completion of pre-hydrolysis, the slurry was diluted the acid to a 4% concentration by adding 84 mL distilled water and autoclaved for one hour at 121°C. After completion of the autoclave cycle, transferred an approximately 10 mL aliquot and neutralized to pH 5-6 with calcium carbonate. This sample will be used to determine carbohydrates by using High Performance Anion Exchange Chromatography with Dionex CarboPac PA-1 column (250 mm×4 mm) and a guard column (50 mm×4 mm) at flow rate 1.0 ml/min. The post-column pump has operated the flow rate at 0.5 ml/min of 300 mM NaOH. A stepwise linear gradient was applied over 20 min by 100% distilled water and was applied over 16 min by mixing solutions of 200 mM NaOH and 200 mM NaOAc in 170mM NaAc. Eluted oligosaccharides were monitored by PAD detection using an Au electrode. A series of calibration standards containing xylose (Merck, Germany), arabinose (Sigma, Germany), mannose (Merck, Germany), galactose (Sigma, Germany), and glucose (Sigma, Germany)
Microwave pretreatment of extractive-free RS
The experiments were carried out with microwave (Analytik Jena, Germany). Microwave irradiation power was applied to heat the suspension to 160°C and held for the desired time of 5, 10, and 15 min were set by a control panel. According to the experimental design, the extractive-free RS were suspended in distilled water with a solid to liquor ratio of 1:30 (w/v) in each run. After completion, the reactant was immediately cooled to ambient temperature and vacuum-filtered to separate microwave-pretreated RS residues fraction. The solid residue of microwave-pretreated RS was washed with 95% ethanol and twice with distilled water, then dried overnight at 45°C in hot air oven to obtain rice straw water-unextractable arabinoxylans (RS-WUAX) for further processing of the alkali-extractable arabinoxylans extraction.
Extraction of alkali-extractable rice straw arabinoxylans (RS-AX)
One gram of RS-WUAX was suspended in 25 mL of 2% sodium hydroxide (w/v) at ambient temperature with continuous shaking at 180 rpm for 24 h of extraction time. Subsequently, the alkali rich-arabinoxylan solution was collected by centrifuged at 9056×g 25°C for 15 min. The liquor was adjusted pH to 6.0 with conc. HCl and then added 95% ethanol to a final ethanol concentration of 80% (v/v) to precipitate RS-AX. The precipitate was centrifuged at 9056×g, 4°C for 10 min, and washed against acetone. Finally, the precipitate was dried for 24 h at 45°C in a hot air oven to obtain crude RS-AX. Total sugar and reducing sugar contents were analyzed by using phenol- sulfuric method (Dubois et al., 1956) and DNS method (Miller, 1959).
Enzymatic xylooligosaccharides production from rice straw arabinoxylans
Hydrolysis of crude RS arabinoxylan (RS-AX) was performed with two commercial xylanases namely Ultraflo Max and Pentopan Mono BG. Briefly, dried RS-AX was suspended in 100 mM sodium phosphate buffer (pH 5.0) to a final substrate concentration of 2% (w/v). Then each xylanase was separately added at enzyme concentration 50, 150, and 300 U/g substrate, and incubated at 50°C in water bath shaker at 170 rpm for 24 h. The sample was periodically taken at the pre-set time. The reaction was stopped by boiling for 5 min and the total reducing sugar was inspected by DNS method. The XOS composition was qualitatively checked by thin layer chromatography according to the previous method (Jaichakan et al., 2019). In brief, Merck TLC silica gel 60 was used as stationary phase. The mobile phase consisted of n-butanol: acetic acid: water 2:1:1 by volume, once run. The TLC was being sprayed with 10% sulfuric acid in ethanol with 0.2% of orcinol and developed once with heating in hot air oven at 110°C. The mixed XOS (X1-X5) (Wako, Japan) was used as standard.
One Unit of xylanase activity (U) is defined as the amount of enzyme liberating one µmole of reducing sugar (as xylose) equivalents from 1% birch wood xylan (Megazyme, Ireland) per minute at 50°C.
Fermentability of RS–XOS
The bacterial strain used in this study was Lactobacillus brevis (TISTR860) which obtained from the Thailand Institute of Scientific and Technological Research Thai (TISTR) and kept at the culture collection of the Department of Biotechnology (Kasetsart University, Thailand). Before fermentation, the strain was pre-cultured in MRS broth under aerobic conditions. The modified medium used in fermentations was modified from that of Nakphaichit et al., 2011 as follows: peptone 1% (w/v), beef extract 1% (w/v), yeast extract 0.5% (w/v), dipotassium hydrogen phosphate 0.2% (w/v), sodium acetate 0.5% (w/v), ammonium monohydrogen citrate 0.2% (w/v), magnesium sulfate 0.01% (w/v), manganese sulfate 0.005% (w/v), and Tween 80 0.1% (v/v), the initial pH of medium was adjusted to 6.5 by 1 M NaOH or 1 M HCl. Two percentage reducing sugar content of RS-XOS was dissolved in a small volume of sterilized modified medium, filtered through 0.2 µm sterilized filter and then added back to the medium with the final concentration of 2%. The commercial XOS was used as positive control in this study. The suspension 1% bacterium culture was each inoculated in 200 µL of media on sterilized 96 wells plate and carried out at 37°C for 48 h. Bacterial growth was monitored at 600 nm using an automatic microplate reader (Drawell, DM-SM600, China). The growth of L. brevis used RS-XOS was compared with glucose, commercial XOS and modified medium without carbon source. The data was derived from 3 replications.
Statistical analysis
Data were analyzed by one-way Analysis of variance (ANOVA). Significant differences (P ≤0.05) between samples were evaluated using Duncan’s new multiple range test. Two replications were performed in the experiment.
RESULTS
Chemical and sugar composition of rice straw
As depicted in Table 1, rice straw (RS) contained the majority of carbohydrate (71.47% dry basis), due to it was a lignocellulosic material as a source of non-wood fibers (Zhang and Hu, 2014). However, it differed from most crop residues in its high content of silicon dioxide (SiO2). Ash content of RS was 13.73 per cent, according to previous reviews ranged from 7.8 to 15.6%, depending on the state of prevention of RS after harvesting. (Jin and Chen, 2007).
Table 1. Chemical composition of rice straw.
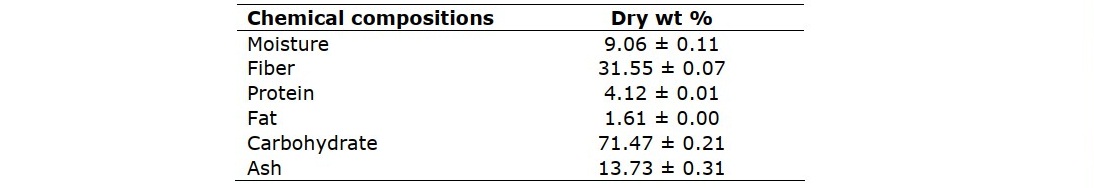
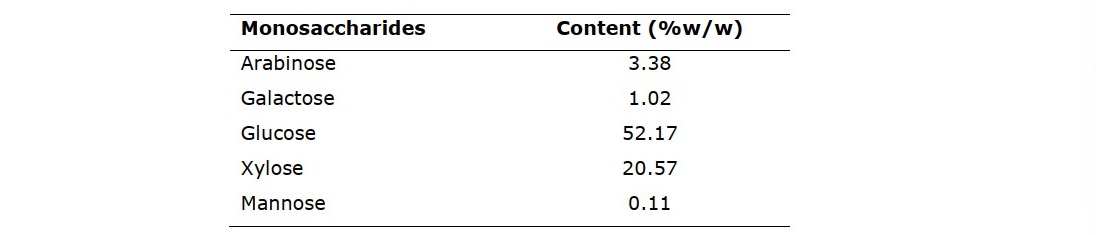
According to previous study, RS contained the lignocellulosic materials, including 30.94% of hemicellulose, 34.05% of cellulose and 3.79 of lignin (Patipong et al., 2019). The monosaccharide composition of the extractive free RS after pretreated with acetone and ethanol. The consisted of 2.69% arabinose, 1.15% galactose, 32.86% glucose, 13.49% xylose and 0.3% mannose (Table 2). Glucose is the sugar with 6 carbon atoms, mainly found in most plants structure. Glucose monomers will be absorbed in upper gastrointestinal and enhance the growth of pathogens in human microbiota which not prebiotic properties. Interestingly, RS also contained a high amount of xylose as 5 carbon atom sugar that enhance the growth of probiotics and most pathogens cannot use it as carbon source (Desai et al., 2016). Therefore, oligosaccharide consisting of xylose oligomers is an interesting functional ingredient as a prebiotic compound.
Table 2. Monosaccharides analysis results of RS after pretreatment.
Effects of microwave treatment on arabinoxylan extraction
The effects of microwave irradiation can cause fragmentation and swelling, leading to lignin and hemicellulose degradation in biomass and improving the pentose yield (Chen and Sheen, 2011). Arabinoxylans (AX) extraction from the extractive free RS was performed in microwave at 160°C for 5-15 min. Figure 1A demonstrated that reducing sugar content was dramatically increased when increasing microwave treatment time. On the other hands, total sugar content decreased when increasing treatment time. This may be caused by heat which degraded sugar (Figure 1B). Microwave treatment at 160°C for 5 and 10 min gave the highest total sugar content, while the highest of reducing sugar content was found in processing time for 10 min. Therefore, the optimum conditions for the extraction of hemicellulose were 160°C for 10 min providing the recovery of 7.73% (Figure 1C). The extracted AX or RS-AX was then further hydrolyzed by enzyme for the XOS production in next study.
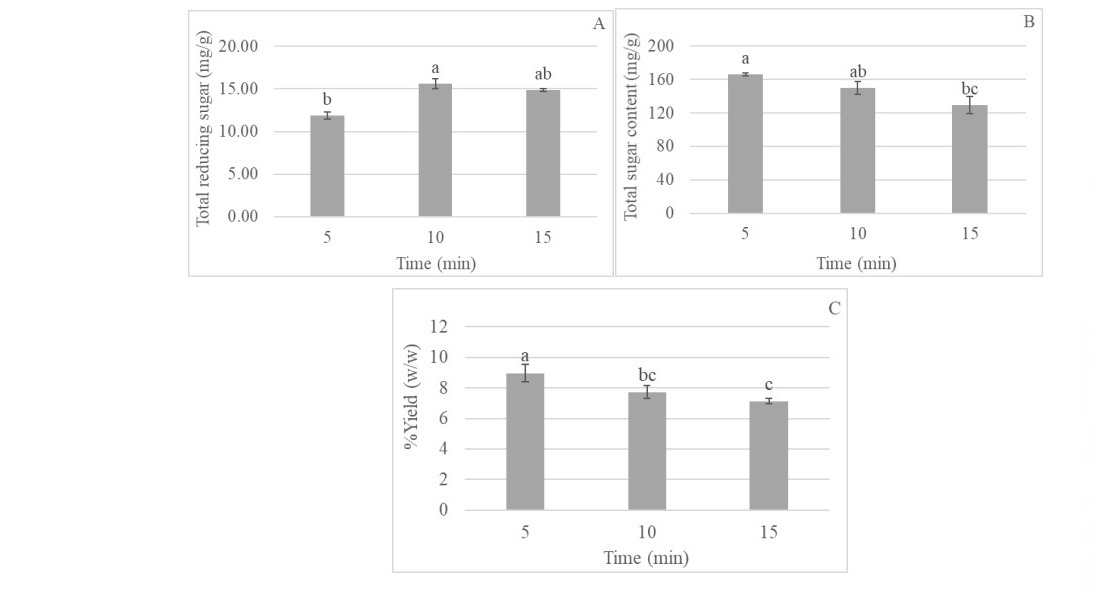
Figure 1. Total reducing sugar content (A) total sugar content (B) and yield (C) of RS after microwave processing (160 °C, 5-15 min).
Note: In each microwave treatment time without a common superscript represent significant differences (P ≤0.05).
Effects of commercial xylanases on XOS production
Commercial xylanases, BG and UM, at concentration of 50, 150, and 300 U/g in 100 mM of Sodium phosphate buffer (pH 6) were used to hydrolyze RS-AX, based on 2% of reducing sugar content. Figure 2 demonstrates the total reducing sugar content at 50°C during 0-24 h. The result showed that BG 300 U/g provided the highest reducing sugar content 17.23 mg/g at 24 h of incubation time. While 50 U/g of enzyme concentration at 12 h of incubation time provided 16.40 of reducing sugar content (Figure 2A). As depicted in Figure 2B, UM of 50 and 300 U/g at 24 h of incubation time provided the reducing sugar content as 25.46 and 25.45 mg/g, respectively. Therefore, the concentration of 50 U/g of both enzymes was considered enough for RS-XOS production.
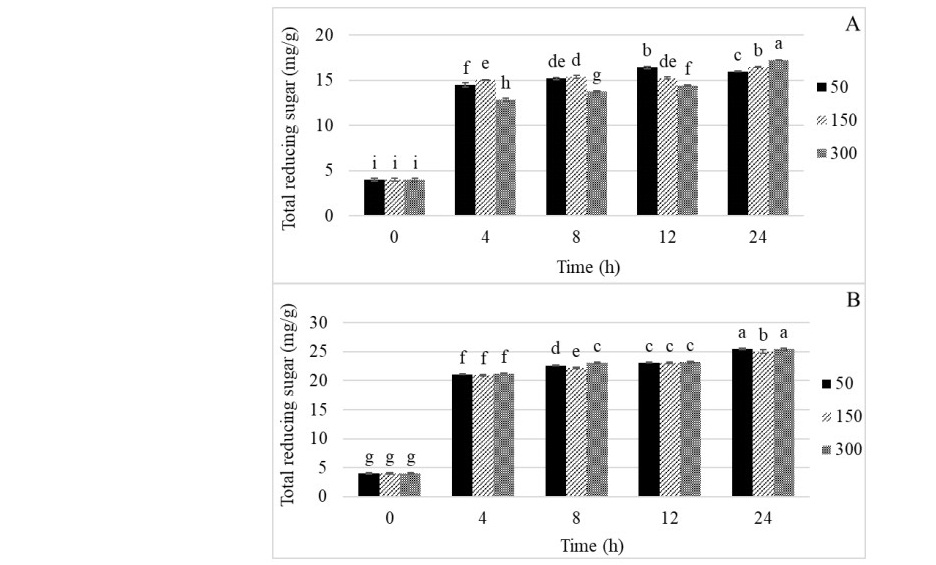
Figure 2. Total reducing sugar contents of RS hydrolyzed by BG (A) and UM (B) as commercial xylanases (50 U/g, 50°C, pH 6, 0-4 h)
Note: In each enzyme concentration and incubation time without a common superscript represent significant differences (P ≤ 0.05).
The oligosaccharides profile was primarily observed by TLC during 0-4 h of incubation, as compared to mixtures of xylose (X1), xylobiose (X2), xylotriose (X3), and xylotetraose (X4) as standards (Figure 3). Before the incubation of RS-AX with xylanases, the profiles of oligosaccharides demonstrated DP greater than 5. The commercial xylanase (UM10) seemed to greater release a small DP (X1, X2, X3, and X4) than BG. Moreover, XOS produced by BG provided various kind of oligosaccharides included X1, X2, X3 X4, and X5. As a result, BG was to the suitable enzyme for production of RS-XOS.
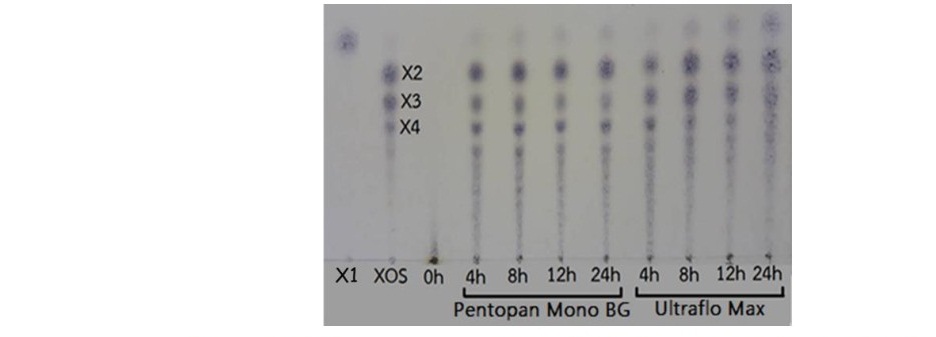
Figure 3. TLC chromatograms of XOS obtained from RS-AX after hydrolyzed with two commercial xylanases, 50 U/g, 50°C, pH 6.0 for 0-24 h. Mixture of xylose (X1), xylobiose (X2), xylotriose (X3) and, xylotetraose (X4) were used as standard.
Bacterial utilization of XOS
The growth promoting properties of XOS derived from RS were investigated using L. brevis. The growth ability by this lactic acid bacterium on RS-XOS utilization was compared to that in glucose, and commercial XOS at a concentration of 2% w/v in MRS individually. The results showed that the RS-XOS was able to promote the growth of L. brevis as the same number of populations as glucose, particularly better than commercial XOS. However, the fastest growth rate of L. brevis within 16 h in glucose while RS-XOS promoted within 30 h (Figure 4).
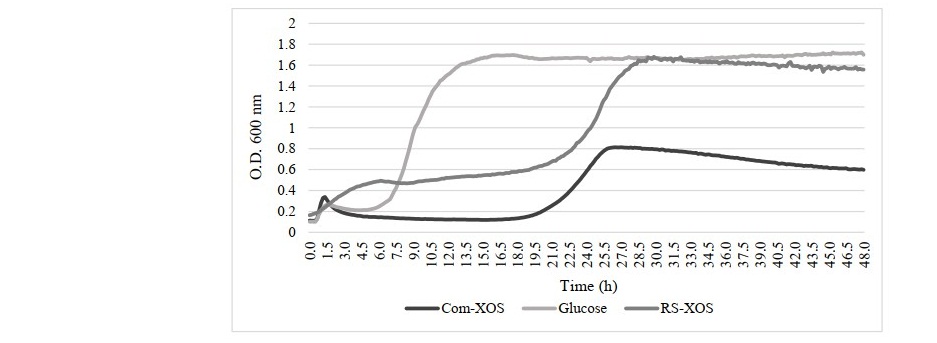
Figure 4. Growth promotion of Lactobacillus brevis by RS-XOS compared with commercial XOS and glucose.
DISCUSSION
Microwave irradiation of biomass is a promising pretreatment process because it utilizes thermal and specific effects generated by microwaves in aqueous environments (Keshwani and Cheng, 2010). The previous study conducted on low temperature pretreatment is normally ideal to avoid hemicellulose degradation and to improve the yield of pentose (Verma et al., 2011). Microwave heating at 160°C, the extracted hemicellulose as AX contained the highest total reducing sugar and total sugar content. As non-thermal and thermal effects of microwave can cause fragmentation and swelling, leading to lignin, and hemicellulose degradation in biomass, the reducing sugar content increased (Puligundla et al., 2016).
RS-AX hydrolyzed by two commercial xylanases namely BG and UM, which are belong to glycoside hydrolase family 10 and 11, shown some differences in reducing sugar content and sugar profiles (Cheng et al., 2010). As UM has been classified in the glycoside hydrolase family 10, while BG has been classified as the glycoside hydrolase family GH11. The result showed that UM provided higher reducing sugar content compared to BG. However, the oligosaccharides profile observed by TLC showed that UM tends to increase monosaccharide in RS-XOS. Because family 10 tends to demonstrate preference for groups at the terminal of xylan bonds, have low substrate specificity, and can degrade xylan backbones with many hydrolyzing substitutions at the side branches. Whereas BG provided DP of XOS higher than UM, because Family 11 xylanases hydrolyzes only internal xylan bonds, acting principally on the xylose unit at the center of the oligosaccharide chain, and unsubstituted xylan chains (Morgan et al., 2017).
Utilization of XOS by the microorganism generally requires the action of three important enzymes, namely, xylosidases (EC 3.2.1.37), exo-oligoxylanases (EC 3.2.1.156) and α-L-arabinofuranosidases (EC 3.2.1.55). In this study, the result demonstrated that RS-XOS was able to promote L. brevis better than commercial XOS. However, glucose was fastest promoted L. brevis, due to glucose is monosaccharide that most bacteria are easily catabolized as carbon source to convert into energy (Desai et al., 2016). Nonetheless, glucose can also utilize by pathogenic bacteria which is not prebiotics. L. brevis is one of the fewest lactobacilli in the literature capable of growing and using low molecular weight XOS because it contains intracellular ß-D-xylosidase. In the previous study, ß-D-xylosidase from L. brevis NCDC01 grown on XOS was purified and characterized. The activity of the enzyme was 12.3 U in the dialyzed cell free extract prepared by ultrasonication of cell pellets in lysis solution (intracellular ß-D-xylosidase). The apparent molecular weight of ß-d-xylosidase of L. brevis NCDC01 was ∼58.0 kDa (Moura et al., 2007; Lasrado and Gudipati, 2013). Nevertheless, the degradation and utilization of XOS is strain-specific and also affects the different DP of oligomers present in the XOS mixture (Aachary and Prapulla, 2011). In this study, XOS mixtures mainly constituted by xylobiose and xylotriose as seen on TLC, similar to many research studies which prepared XOS by enzymatic treatment from xylan-rich materials (Gullon et al. 2008). In vitro assays proved that Bifidobacterium spp. and B. adolescentis are able to utilize both xylobiose and xylotriose, whereas a mixture containing xylobiose as the major component was utilized by B. adolescentis, B. infantis, and B. longum (Vazquez et al., 2000).
CONCLUSION
The results from this study showed that microwave treatment is a promising method for AX extraction from RS, a by-products of rice farming. The commercial xylanases, BG and UM were able to hydrolyze RS-AX into RS-XOS. Nevertheless, BG provided less monosaccharide and various kind of oligosaccharides compared to UM. RS-XOS prepared by BG was able to promote the growth of a probiotic as L. brevis better than that of commercial XOS, indicating high potential of prebiotics property. For further study, the purification process of crude RS-AX should be performed to eliminate the monosaccharides in order to improve the quality and prebiotics property of the purified XOS products.
ACKNOWLEDGEMENTS
Authors would like to thank the Thailand research fund for supporting this research grant (Grant No. TRG6180290) and Naresuan University for providing instrument.
REFERENCES
Aachary, A.A., and Prapulla, S.G. 2011. Xylooligosaccharides (XOS) as an emerging prebiotic: microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Comprehensive Reviews in Food Science and Food Safety. 10: 2-16.
Aguilar-Reynosa, A., Romani, A., Rodriguez-Jasso, R. M., Aguilar, C. N., Garrote, G., and Ruiz, H. A. 2017. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: an overview. Energy Conversion and Management. 136: 50-65.
Akpinar, O., Erdogan, K., and Bostanci, S. 2009. Enzymatic production of Xylooligosaccharide from selected agricultural wastes. Food and Bioproducts Processing. 87: 145-151.
Azwanida, N.N. 2015. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Medicinal and Aromatic Plants. 4: 1-6.
Chen, W.H., Tu, Y.J., and Sheen, H.K. 2011. Disruption of sugarcane bagasse lignocellulosic structure by means of dilute sulfuric acid pretreatment with microwave-assisted heating. Applied energy. 88: 2726-2734.
Cheng, Y.S., Zheng, Y., Yu, C.W., Dooley, T.M., Jenkins, B.M., and VanderGheynst, J.S. 2010. Evaluation of high solids alkaline pretreatment of rice straw. Applied Biochemistry and Biotechnology. 162: 1768-1784.
Desai, M.S., Seekatz, A.M., Koropatkin, N.M., Kamada, N., Hickey, C.A., Wolter, M., and Young, V.B. 2016. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 167: 1339- 1353.
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.T., and Smith, F. 1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 28: 350-356.
Gibson, G.R. and Roberfroid, M.B. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. The Journal of Nutrition. 125: 1401-1412.
Gullon, P., Moura, P., Esteves, M. P., Girio, F. M., Domínguez, H., and Parajó, J. C. 2008. Assessment on the fermentability of xylooligosaccharides from rice husks by probiotic bacteria. Journal of agricultural and food chemistry. 56: 7482-7487.
Handa, S.S., Khanuja, S.P.S., Longo, G., and Rakesh, D.D. 2008. Extraction technologies for medicinal and aromatic plants (United Nations Industrial Development Organisation and the International Centre for Science and High Technology). International Centre for Science and High Technology-United Nations Industrial Development Organization, area Science Park Padriciano. 99: 34012.
Jaichakan P., Dang Thi HN., Nakphaichit M., and Klangphetch, W. 2019. The Effect of alkali pretreatment and acid debranching on rice husk, rice straw and defatted rice bran for xylobiose production by commercial xylanases. Journal of Science and Technology. 11: 91-103.
Jaichakan, P., Nhung, D.T.H., Nakphaichit, M., and Klangpetch, W. 2019. Intensification of Cellulolytic Hydrolysis of Rice Husk, Rice Straw, and Defatted Rice Bran by Sodium Hydroxide Pretreatment. Food and Applied Bioscience Journal. 7: 172- 183.
Jin, S. and Chen, H. 2007. Near-infrared analysis of the chemical composition of rice straw. Industrial Crops and Products, 26: 207-211.
Keshwani, D.R. and Cheng, J.J. 2010. Microwave‐based alkali pretreatment of switchgrass and coastal Bermudagrass for bioethanol production. Biotechnology Progress. 26: 644-652.
Kulkarni, N., Shendye, A., and Rao, M. 1999. Molecular and biotechnological aspects of xylanases. FEMS Microbiology Reviews. 23: 411-456.
Lasrado, L.D. and Gudipati, M. 2013. Purification and characterization of β-D-xylosidase from Lactobacillus brevis grown on xylo-oligosaccharides. Carbohydrate polymers. 92: 1978-1983.
Miller, G.L. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Analytical Chemistry. 31: 426-428.
Morgan, N.K., Wallace, A., Bedford, M.R., and Choct, M. 2017. Efficiency of xylanases from families 10 and 11 in production of xylo-oligosaccharides from wheat arabinoxylans. Carbohydrate polymers. 167: 290-296.
Moura, P., Barata, R., Carvalheiro, F., Gírio, F., Loureiro-Dias, M.C., and Esteves, M.P. 2007. In vitro fermentation of xylo-oligosaccharides from corn cobs autohydrolysis by Bifidobacterium and Lactobacillus strains. LWT-Food Science and Technology. 40: 963-972.
Nakphaichit, M., Thanomwongwattana, S., Phraephaisarn, C., Sakamoto, N., Keawsompong, S., Nakayama, J., and Nitisinprasert, S. 2011. The effect of including Lactobacillus reuteri KUB-AC5 during post-hatch feeding on the growth and ileum microbiota of broiler chickens. Poultry Science. 90: 2753-2765.
Patipong, T., Lotrakul, P., Padungros, P., Punnapayak, H., Bankeeree, W., and Prasongsuk, S. 2019. Enzymatic hydrolysis of tropical weed xylans using xylanase from Aureobasidium melanogenum PBUAP46 for xylooligosaccharide production. 3 Biotech. 9: 56.
Puligundla, P., Oh, S.E., and Mok, C. 2016. Microwave-assisted pretreatment technologies for the conversion of lignocellulosic biomass to sugars and ethanol: a review. Carbon Letters. 17: 1-10.
Vazquez, M.J., Alonso, J.L., Domınguez, H., and Parajo, J.C. 2000. Xylooligosaccharides: manufacture and applications. Trends in Food Science and Technology. 11: 387- 393.
Verma, A., Kumar, S., and Jain, P.K. 2011. Key pretreatment technologies on cellulosic ethanol production. Journal of Scientific Research, 55: 57-63.
Zhang, L. and Hu, Y. 2014. Novel lignocellulosic hybrid particleboard composites made from rice straws and coir fibers. Materials and Design. 55: 19-26.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Alisa Pattarapisitporn1, Nonglak Thiangthong1, Pakorn Inthajak1, Pannapapol Jaichakan1, Wantana Panpa1, and Wannaporn Klangpetch1,2,*
1 Department of Agro-Industry, Faculty of Agriculture, Natural Resources and Environment, Naresuan University, Phitsanulok 65000, Thailand
2 Centre of Excellence in Fats and oils, Faculty of Agriculture Natural Resources and Environment, Naresuan University, Phitsanulok 65000, Thailand
Corresponding author: Wannaporn Klangpetch, E-mail: wannapornk@nu.ac.th
Total Article Views
Editor:
Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: May 12, 2020;
Revised: July 1, 2020;
Accepted: October 12, 2020

