
The Effects of Pasteurization Conditions and Storage Time on Microbial Safety, Quality and Antioxidant Properties of Cider from Rose Apple (Syzygium agueum Alston cv. Taaptimjan)
Chukwan Techakanon* and Karthikeyan VenkatachalamPublished Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.034
Journal Issues : Number 2, April-June 2021
Abstract The aims of this study were to produce rose apple cider and to compare the quality of cider following different pasteurization conditions. Rose apple (Syzygium agueum Alston cv. Taaptimjan), which is rich in bioactive compounds, was used to produce cider. Cider pasteurization was carried out at 63 ºC for 15 s, or at 71 ºC for 6 s after fermentation. Cider from each pasteurization condition was stored at room temperature (27±1 ºC). Physicochemical, microbial and sensory properties were monitored for three months in the study. The obtained cider (when not pasteurized) had initially 6% alcohol, with soluble solids in the range 4.2-4.3 ºBrix, pH 4.6, and 4.2 g/L titratable acidity. Pasteurization was effective in prolonging shelf life of the cider from 6 to 12 weeks; however, the treatment significantly decreased contents of vitamin C and antioxidants. In the sensory profile of cider pasteurized at 71 ºC, trained panelists perceived it as more sweet, less sour, with less flavor and same intensity of aftertaste, when compared to the control sample. The pasteurization conditions 71 ºC for 6 s gave desirable sensory quality and met microbiology standards for up to three months of storage in ambient conditions.
Keywords: Antioxidant, Cider, Pasteurization, Quality, Rose apple
Citation: Techakanon, C. and Venkatachalam, K. 2021.The effects of pasteurization conditions and storage time on microbial safety, quality and antioxidant properties of cider from rose apple (Syzygium agueum Alston cv. Taaptimjan). CMUJ. Nat. Sci. 20(2): e2021034.
INTRODUCTION
Rose apples are an important tropical fruit in Southeast Asia region, especially in Thailand (Supapvanich, Prathaan, and Tepsorn, 2012). The unique characters of rose apples include apple-like crispness, watery sweetness, slightly acidic taste, and aroma of roses (Yahia, 2011). Rose apples are abundant in water, carbohydrate, protein, vitamins and minerals. Additionally, they have medicinal properties, such as anti- inflammatory, antidiabetes, anticonvulsant, antihypertension, antimicrobial, antituberculosis, and antidiarrheal activities (Shü, Shiesh, and Lin, 2011; Supapvanich, Pimsaga, and Srisujan, 2011). These fruits are normally consumed in either of two ways: as a whole, or freshly cut form. They are considered one of the favorites freshly cut fruits on many occasions, in Southeast Asia (Shü, Shiesh, and Lin, 2011; Supapvanich, Pimsaga, and Srisujan, 2011). Taaptimjan cultivar is the most popular rose apple among various cultivars, due to its beautiful ruby red skin color and seedless (Supapvanich, Mitrsang, and Srinorkham, 2017). A limitation of rose apples is their short shelf life. They are highly perishable due to thin skin, which can be easily damaged. Therefore, they are not suitable for long-distance transport without proper storage conditions. Moreover, the fruits are susceptible to chilling injury in low- temperature storage (0-10 °C) (Huang, Wang, and Liou, 2005; Shü, Shiesh, and Lin, 2011). To address these issues, food processing and preservation techniques are needed to limit the quality losses. Among the various processing methods, cider fermentation could be a new approach suitable for small and large scale production, adding value to these fruit.
The outbreaks of E. coli and Salmonella sp. in the past have raised concerns about the safety of fresh fruit, juices, and other fruit products (Besser et al., 1993). Pathogenic bacteria, especially E. coli, have been reported in unpasteurized apple cider (Miller and Kaspar, 1994). Pasteurization is therefore carried out after cider fermentation to ensure safety of the cider product. This motivates developing better pasteurization methods and equipment for use by the juice industry and benefits to the consumers. According to the U.S. Food and Drug Administration regulations, juice and juice products are required to achieve 5 logs reduction (USFDA, 2001). For practical purposes, E. coli O157:H7 has been considered the target pathogen of cider pasteurization. Mild temperature short time (MTST), a heat processing method using temperature ≤ 80˚C and holding time ≤ 30 s, was reported to have minimal effect on sensory quality of juice (Caminiti et al., 2011), while the treatment can achieve 6-7 log reduction of Listeria innocua in smoothie product (Palgan et al., 2012). The pasteurization equivalent was calculated for a 5-log reduction of E. coli O157:H7, using a D-value of 23 min at 52 ºC, a z-value of 4.8 ºC, and an additional 3-fold safety factor (Splittstoesser and McLellan 1997; Splittstoesser et al. 1995). The current recommendation for flash pasteurization of apple cider is to heat the juice to 71 ºC for 6 s, or to use an equivalent time/ temperature combination (AFDO, 2003).
Thermal processing has been widely studied to ensure the safety of food products. However, such processing may induce chemical and physical changes that impair the organoleptic properties of cider and may reduce the content or bioavailability of valuable antioxidants in the product. Many research on thermal treatment of juice reported negative effects on vitamin and antioxidants (Bansal et al., 2015; Rabie et al., 2015; Surek and Nilufer-Erdil, 2014; Wilkes et al., 2014; Chaikham et al., 2013; Piasek et al., 2011; Zheng and Lu, 2011). Inactivation of microorganisms and preservation of quality attributes need to be in balance. Therefore, optimization of the process conditions is crucial to satisfy both aspects. The aim of this study was to determine the effects of pasteurization at alternative conditions (63 ºC for 15 s and 71 ºC for 6 s) on physicochemical properties, microbial safety, sensory quality and antioxidant activity of rose apple cider, and to monitor the changes in these qualities during storage for three months in ambient temperature.
MATERIALS AND METHODS
Raw material preparation
The rose apples (Syzygium agueum Alston cv. Taaptimjan) were hand harvested from a commercial orchard in Suratthani, Thailand. The fruits were selected for uniform size (7-9 cm height and 6-8 cm width), color (dark red), total soluble solids (10-14 ºBrix) and for freedom from apparent damage or disease. The rose apples were taken to the laboratory within 8 h and were cleaned with distilled water. Rose apple juice was obtained using a food processor (Philips centrifugal juicer) in a cold environment and was roughly strained.
Cider preparation
The cider fermentation was conducted based on the method suggested by Alberti et al. (2016) with some modifications. Rose apple juice extract was inoculated with the cider yeast strain, Saccharomyces bayanus (Fermentis, France), at 106 cells/ml and underwent fermentation in a fermenter. Alcoholic fermentation occurred in an anaerobic environment at 25 ºC over 10 days. At the end of fermentation, the cider was left for sedimentation then the only clear liquid was taken for the following steps.
Pasteurization and storage
The cider samples were randomly divided into three groups: control (no heat treatment); pasteurization at 63 ºC for 15 s; and pasteurization at 71 ºC for 6 s. The pasteurization was carried out in a tubular pasteurization unit. All treatment groups were bottled and stored at room temperature (27±1 ºC).
Physicochemical analysis
The color of cider samples was monitored with a Hunter Lab Ultra Scan colorimeter (Hunter Associates Laboratory, Inc. Reston, VA, USA). The color coordinates L (lightness), a (redness) and b (yellowness) were recorded. The total soluble solids were determined using a digital handheld refractometer (Atago, Tokyo, Japan), and the values are expressed in ºBrix. Ethanol content was measured with an alcoholmeter (Dujardin-Salleron, France). The pH was measured using a digital pH meter (SI Analytics, Mainz, Germany). Total acidity was expressed in equivalents tartaric acid (g/L) and was determined by volumetric neutralization with 0.1N sodium hydroxide using phenolphthalein as the indicator. The electrical conductivity of the cider was determined by a conductivity meter (YSI Incorporated, Yellow Springs, OH, USA). The values are reported in µS/cm. The kinetic viscosity was measured using a Brookfield viscometer (LVDV3T, Brookfield, USA) and is expressed as apparent viscosity (cP).
Microbial evaluation
Cider samples were plated at regular intervals for microbiological counts. Plate count agar (PCA) (Himedia, Mumbai, India) was used to determine the counts for total aerobic microbes. From each sample bag, triplicate serial dilutions were made with peptone water. Samples (1 mL) from each dilution were aseptically placed in Petri dishes, to which 20 mL of sterilized agar was poured and mixed thoroughly. Upon agar solidification, the Petri dishes were inverted and incubated for 48 h at 37ºC. Yeast and mold counts were determined using acidified Potato Dextrose Agar (PDA) (Himedia, Mumbai, India). The pH of the sterilized agar was adjusted to 3.5 with sterilized 10% (w/v) tartaric acid. Sample (0.1 mL) of each dilution was spread on solidified agar, and incubated for 5 days at 25 ºC. Colonies were counted using a Quebec colony counter and are reported as log10 CFU/mL. The E. coli tests were evaluated using 3M Petrifilm E.coli/Coliform Count Plates (3M, MN, USA).
Vitamin C, total phenols and antioxidants
The content of vitamin C was measured using titration with iodine solution, adapted from Babashahi-Kouhanestani et al. (2014). Total phenols were determined by Folin-Ciocalteu method as described by Waterhouse (2002) with some modifications. The DPPH scavenging activity was assayed according to the method of Alberti et al. (2016) and the results were expressed as percentages. The ABTS+ radical cation scavenging activity was analyzed as described by Campodonico et al. (1998). The results were presented as percentages. The hydroxyl radical scavenging ability using the deoxyribose method was applied in this study (Halliwell et al., 1987) with the results expressed as percentages. The ferric reducing ability of the cider samples was obtained by the method of Alberti et al. (2016) and the results were expressed as mmol Fe2+ per 100 ml.
Sensory evaluations
Panel training was organized for 10 two-hour sessions, training on the general characteristics of rose apple cider. Fifteen trained panelists of ages in the range of 21- 40 years evaluated the sensory profiles of the cider samples. The first set of sensory tests was carried out the following day after obtaining pathogenic results, and subsequent tests were carried out at regular intervals over 12 weeks of study. The samples from different treatments were served at room temperature in plastic cups labeled with randomly generated three-digit codes. The serving order was also randomized for each panelist. The panelists were instructed to evaluate the characteristics of the cider samples (sweetness, bitterness, sourness, sour flavor, fruit flavor and after taste). The panelists were asked to rate each sample on a 5-point scale, with 1 for “minimum intensity” and 5 for “maximum intensity”.
Statistical analysis
The experiment included three replicate processing runs. All the data are reported as means and standard deviations. Statistical analysis included analysis of variance (ANOVA) and Duncan’s multiple range test to compare the means. The significance threshold was P
RESULTS
Physicochemical properties of rose apple cider
The results from the physicochemical study of the rose apple cider during storage revealed that the control group presented a notable decreasing trend of TSS with the final value of 2.2 ºBrix (Figure 1). The sample pasteurized at 63 ºC had the same decreasing trend of TSS simultaneously with increasing alcohol content; however, with lesser changes. Pasteurization at 71 ºC gave only slight changes in TSS and ethanol content.
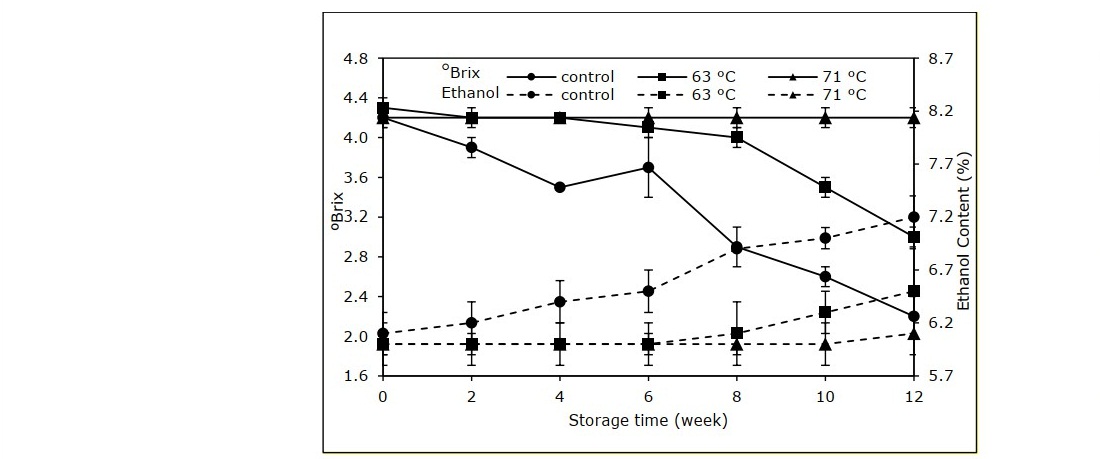
Figure 1. Total soluble solids and ethanol content in cider samples processed at alternative pasteurization conditions.
The fermentation generates acids that result in sour taste and aroma. These compounds mainly determine the quality of the cider (Chanthai and Danvirutai, 2004). The pH of unpasteurized rose apple cider continuously decreased during storage, as shown in Figure 2. This matches the measured titratable acidity. At the end of 12 weeks of storage, titratable acidity of the control sample had increased significantly from an initial 4.22 to 4.30 g/L of tartaric acid (P <0.05). A small change in pH and acidity occurred in the sample treated at 63 ºC, while samples pasteurized at 71 ºC showed a more stable trend. These changes were also aligned with the perception of panelists in sensory evaluation. The panelists perceived higher intensity of sourness in control cider samples stored for 2 weeks and longer (Figure 5 A).
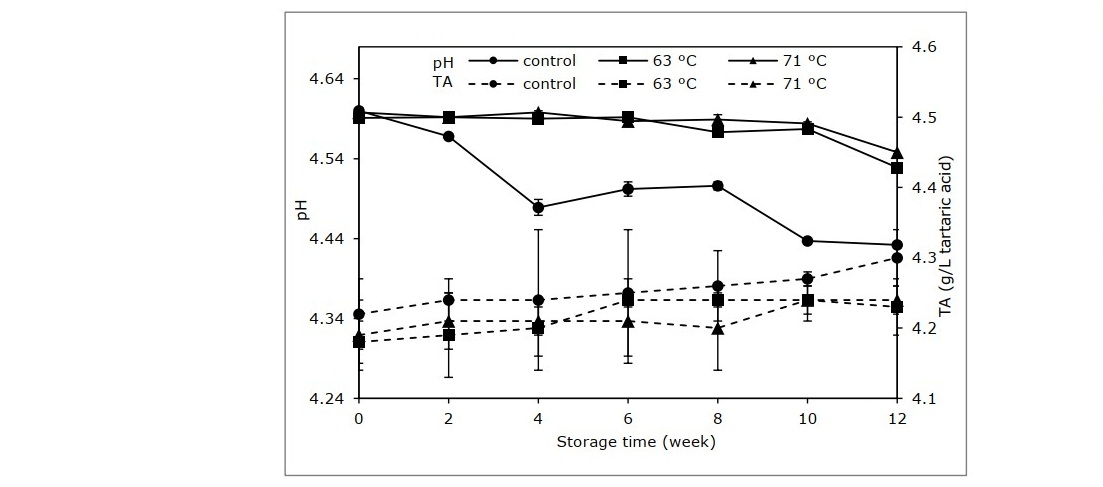
Figure 2. pH and titratable acidity of cider samples processed at different pasteurization conditions.
Table 1 shows the average values of L, a, and b from colorimeter measurements of the cider samples. The results show that the pasteurization temperature and storage time significantly affected these values (P <0.05). Lightness (L) tended to decrease during storage. According to the result, inconsistency in cider color is observed in this experiment.
Other physical properties of the cider, namely conductivity and viscosity are shown in Figures 3 and 4. Electrical conductivity of the control group were considerably elevated after week 2 until the end of storage. The cider samples treated with pasteurization had a smaller incline compared to the control group in the beginning (P <0.05), and continued with a stable trend until the end of storage. The viscosity of the control group was significantly higher compared to the pasteurized group throughout the storage period (Figure 4).
Table 1. Color of rose apple cider treated with different pasteurization conditions.
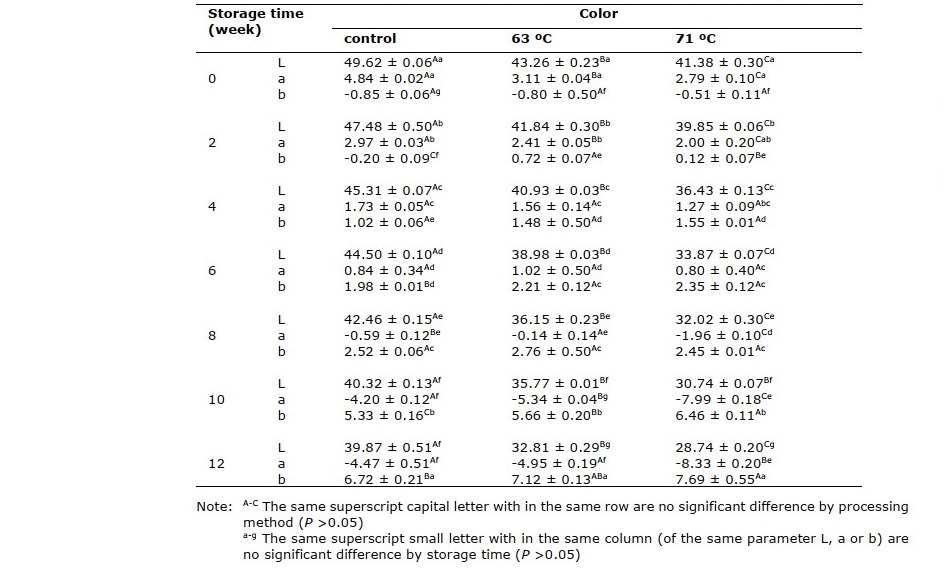
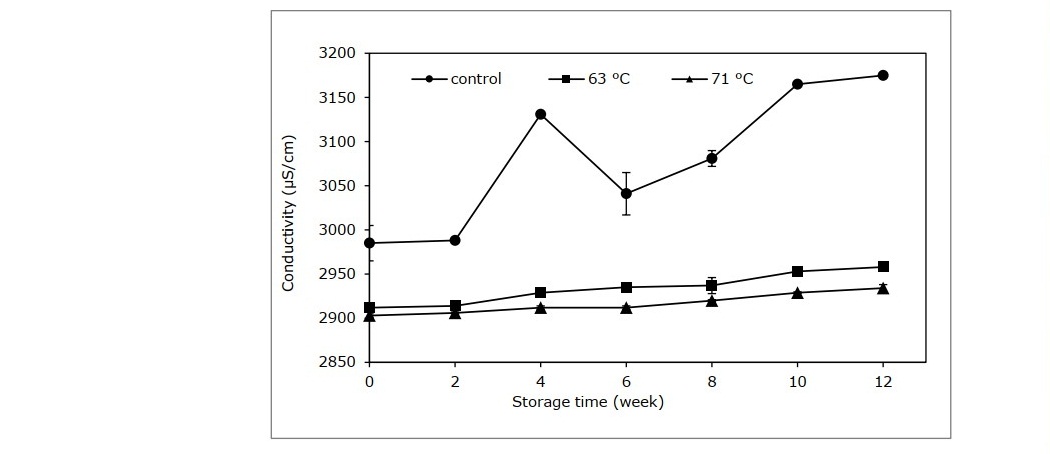
Figure 3. Conductivity of cider samples processed at different pasteurization conditions.
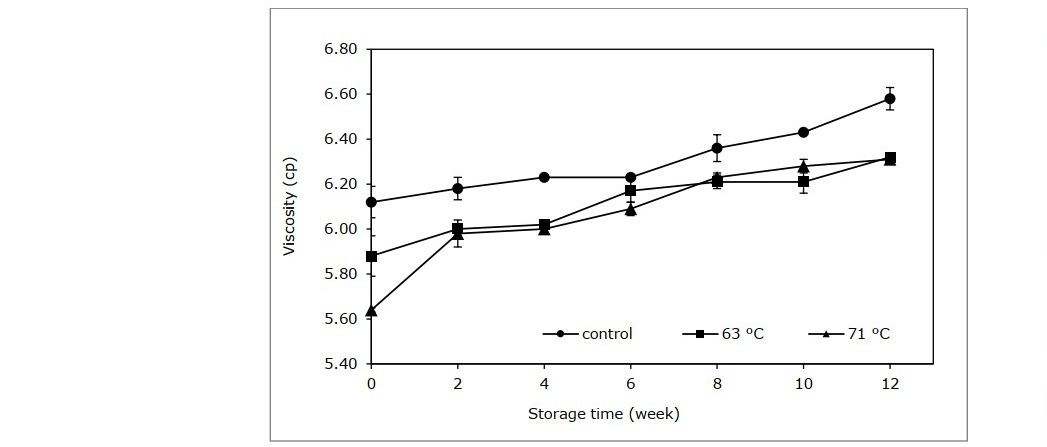
Figure 4. Viscosity of cider samples processed at different pasteurization conditions.
Microbial evaluation
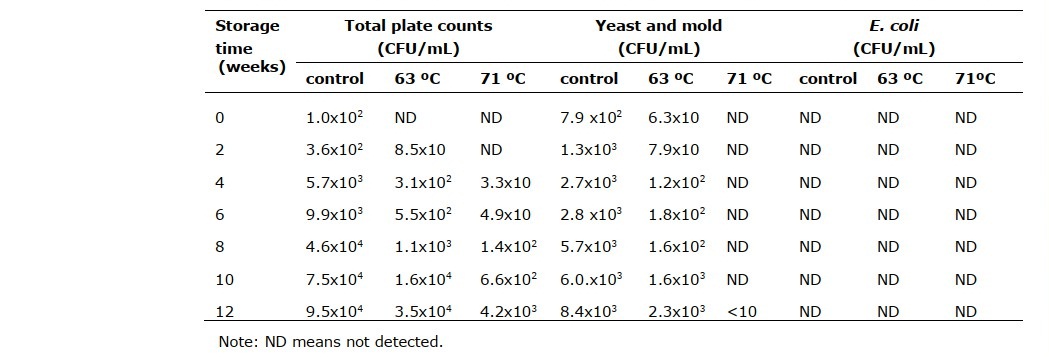
Microbial evaluation (total aerobic count, yeast and mold, and E. coli) of the cider samples were monitored for 12 weeks (Table 2). The initial aerobic count was only detectable for the control group with a microbial load of 102 CFU/g, which then increased continuously until the sample presented a sign of deterioration at week 8. The cider samples pasteurized at 63 ºC and 71 ºC had aerobic bacteria detected in week 2 and week 4 of storage. Although the microbial counts increased continuously during storage, pasteurization at 63 ºC prolonged product shelf life until 10 weeks, and the samples processed at higher temperature had no significant change in quality until the end of the storage period. Yeast and mold were detected in the unpasteurized sample and cider treated at 63 ºC at the beginning of storage at 794 CFU/mL and 63 CFU/mL, respectively. The number of yeast and mold counts continuously developed during the studied period. E. coli O157:H7 was identified as the causative agent in an outbreak of diarrhea and hemolytic uremic syndrome (HUS) associated with the consumption of apple cider. In this current study, the E. coli counts in cider samples indicate the safety of rose apple cider consumption until the end of the monitoring period of 12 weeks.
Table 2. Microbiology profiles of cider samples during storage at ambient temperature.
Vitamin C contents of cider samples
The vitamin C contents in Table 3 reveal that the pasteurization temperature and storage duration significantly affected the amount of vitamin C (P <0.05). All the pasteurized cider samples had lower vitamin C content than the control group that had not been pasteurized (P <0.05). The vitamin C content of the rose apple cider decreased significantly during storage in all treatments.
Phenolic compounds and antioxidants
Phenolic compounds in the present study were evaluated as total phenols (Table 3) and antioxidant activity of rose apple cider was determined as DPPH scavenging ability, ABTS scavenging ability, reducing power, and hydroxyl radical scavenging. According to the results, all the monitored values followed the same trend of decreasing with storage time and with pasteurization temperature. The initial total phenolic content of the unpasteurized cider sample was 81mg/L. As a result of pasteurization, the total phenols were reduced by 7% and by 21% on pasteurization at 63 ºC and 71 ºC, respectively. The findings indicate total phenols losses during the storage period of 12 weeks of 20, 22 and 28% in control, sample pasteurized at 63ºC and 71 ºC, respectively. Control cider had a higher 62-70% ABTS scavenging activity than thermally pasteurized cider at 56-64%, throughout the shelf-life period. The initial hydroxyl radical scavenging activity of the cider samples were 67 and 65% for control and pasteurized group, respectively.
Sensory quality of cider
The cider samples were monitored for sensory quality by 15 trained panelists every 2 weeks for 12 weeks. Control (unpasteurized) samples had initially level 4 of sweetness, sour flavor and fruit flavor, level 3 of sourness and bitterness; and a mild aftertaste of level 2 (Figure 5 A). This profile was stable for 4 weeks except for the sweetness and fruit flavor, which decreased to level 3. Longer storage times then induced changes in the sensory profiles as perceived by trained panelists: less sweet, less sour flavor, less fruit flavor, and sourer taste. Pasteurization at 63 ºC or 71 ºC can extend the shelf life to 10 and 12 weeks; however, the process affected the losses of flavor as shown in Figure 5 (B and C). The sample processed at lower pasteurization followed the same change in taste as the control group with further development until the end of storage life. Panelists perceived the change in the sensory profile of the control and pasteurized (63 ºC) cider as less sweet and sourer taste. The cider processed at the higher pasteurization temperature (71 ºC) had similar initial sensory profiles as that pasteurized at the lower temperature, but less intense fruit flavor and sourness. Moreover, the samples treated at 71 ºC had high level of bitterness detected by the panelists.
DISCUSSION
In the present study, pasteurization conditions strongly affected the quality of rose apple cider. The decrease in TSS of cider samples was due to the conversion of sugar to alcohol manifested by yeast. This is consistent with the amount of alcohol generated. The yeast uses dissolved solids as a substrate for growth and alcohol production (Wanapu et al., 2004). Pasteurization at 71 ºC gave only slight changes in TSS and ethanol content because the treatment effectively inhibited the growth of yeast.
Regarding physicochemical properties, the control group demonstrated a decreasing trend of pH. Malolactic fermentation possibly occurred as a secondary fermentation in this group, which contributed the decreased pH. After fermentation was completed, lactic acid bacteria that can tolerate low pH and high alcohol content converted malic acid to lactic acid and CO2 (Brizuela et al., 2019). The reduction of pH could also be caused by protons generated from nitrogen consumption by the yeast (Akin et al., 2008). Lightness (L) of all samples tended to decrease during storage possibly as a result of oxidation reactions, because of light exposure and heat during pasteurization (Maneerat et al., 2007). In addition, inconsistency in cider color may be due to the short fermentation period, which affects both color and taste (Chumpookam, Subnum and Jaruwattanaphan, 2014). Fellows (2009) mentioned that natural pigments are destroyed by heat, changes in pH, or can be oxidized during storage. The color of the rose apple cider comes from the color of the peel, which contains anthocyanins. The cider production and storage involve exposures to elevated temperature, oxygen, pH, light, and can trigger isomerization and oxidation reactions. These factors are responsible for the loss of anthocyanins, affecting the color of cider (Shi and Maguer, 2000). The electrical conductivity of the control group considerably increased. It was affected by the degradation of yeast cells, since protons released from dead cells contribute to the increasing conductivity. Accordingly, the group with the most microbial multiplication (control) presented also the highest conductivity. Viscosity is temperature dependent; therefore, the samples following thermal treatment tend to have lower viscosity. This result was in agreement with the data of pasteurized cactus juice (Deboni et al., 2014) and carrot juice (Vandresen et. al, 2009).
The data from microbial evaluation showed that pasteurization effectively reduce the number of microorganisms in the cider samples as a result of microbial cell membrane disruption induced by heat (Walton and Pringle, 1980). The number of yeast and mold counts in control samples and the samples treated at 63 ºC continuously developed during the studied period. This result is in accordance with an increase of ethanol and reduction of TSS demonstrated earlier in figure 1. Although the microbial counts increased continuously during storage, pasteurization at 63 ºC prolonged product shelf life until 10 weeks, and the samples processed at higher temperature had no significant change in quality until the end of the storage period. Tandon et al. (2003) reported that pasteurization at 63 ºC could extend the shelf life of apple cider to 14 weeks in refrigerated storage.
The thermal processing had a destructive influence on vitamin C, in agreement with the previous studies (Laslo et al., 2018; Sadecka et al., 2014; Narwojsz and Borowska, 2010; Pérez-Conesa et al., 2009). Factors contributing to the loss of vitamin C in drinks are pasteurization temperature, duration of storage, and permeability to oxygen of the container (Farnworth et al., 2001). In the present study, vitamin C content was found to decrease during storage in all cider samples. The oxygen- containing environment in the packaging will result in the loss of vitamin C during storage (Yeom et al., 2000; Odriozola-Serrano et al., 2009). Davey et al. (2000) suggested that vitamin C loss was caused by the oxidative mechanism resulting from the presence of not just oxygen but also exposure to light, heat, peroxides and enzymes. In this study, the cider samples were bottled at 60-70 ºC, at which time the air (oxygen) in the container was flushed out. However, loss of vitamin C was still noticed during storage for all samples.
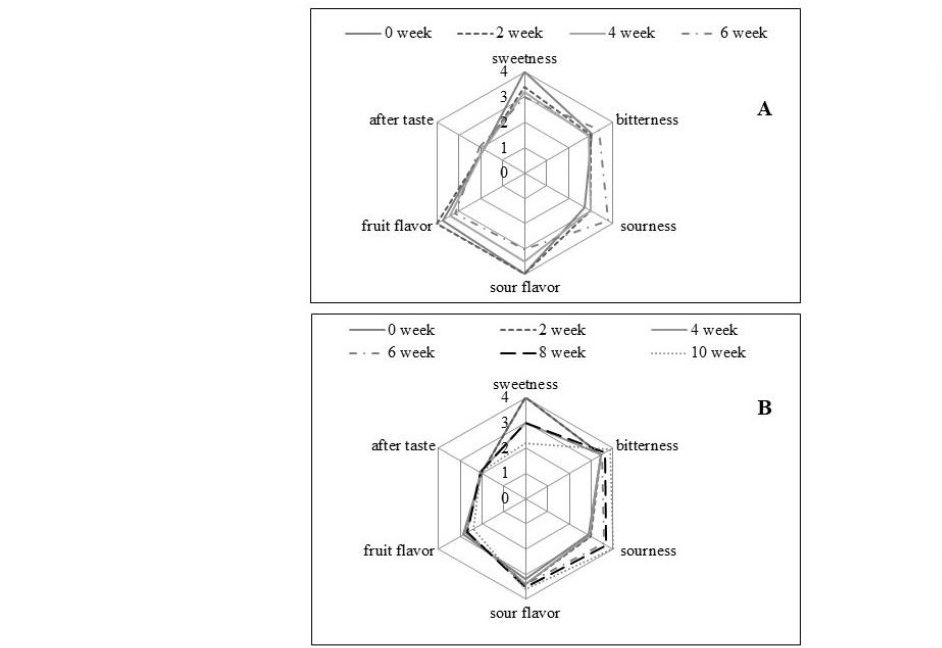
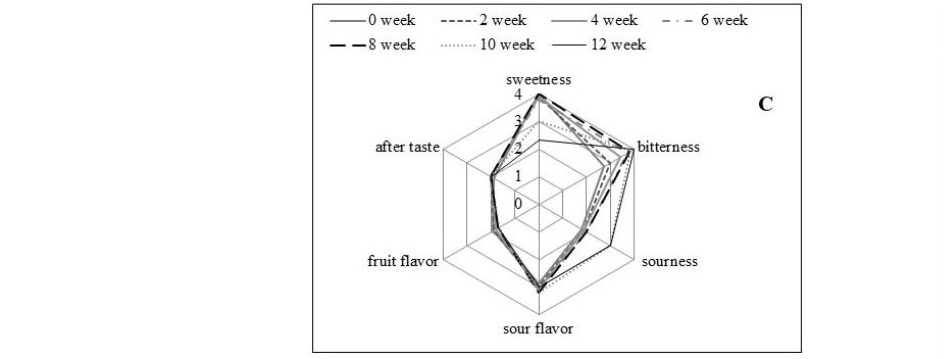
Figure 5. Sensory profile of cider sample pasteurized at (a) control, (b) 63 ºC and (c) 71 ºC
Table 3. Vitamin C, total phenols and antioxidant activity of cider samples.
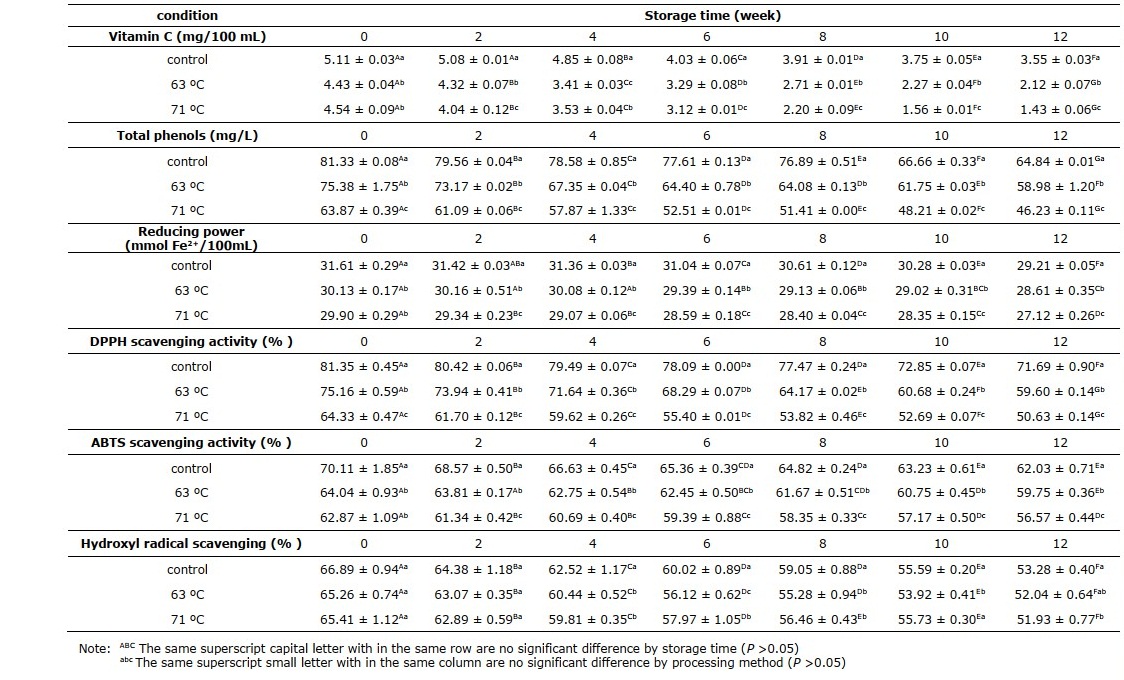
There are various antioxidant types found in cider such as flavonoids, tannin, vitamin C, vitamin E, and ß-carotene (Jirumarn and Srihanam, 2011). As a result of pasteurization, the total phenols were reduced for both conditions at 63 ºC and 71 ºC. This is in agreement with the study on physalis juice heated to 90 ºC for 2 min (Rabie et al., 2015). To determine the antioxidant capacity of food and beverage, numerous methods based on different reaction mechanisms have been available (Roginsky and Lissi, 2005). The results from the present study indicated a decreasing trend of all monitored antioxidant capacity with pasteurization temperature and with storage time. DPPH scavenging activity of cider samples decreased more sharply during storage than the reducing power. Apparently, the cider had a stronger ability to donate H-protons than to reduce free radicals. The reduction of antioxidant activity on pasteurization is also aligned with the loss of vitamin C, as it acts as an antioxidant. Odriozola-Serrano et al. (2009) suggested that heating greatly affects the loss of ascorbic acid through the aerobic pathway because ascorbic acid is a heat-sensitive bioactive compound. The hydroxyl radical scavenging activity of samples suggested that the cider exhibit a scavenging effect on hydroxyl radicals that could help to protect biological molecules from free radical attacking (Abirami, Nagarani, and Siddhuraju, 2014). Data obtained in this study suggest that low-temperature pasteurization maintained higher antioxidant content and higher levels of antioxidant capacity.
Sensory profile of the cider samples followed physicochemical results. Panelists perceived the change in the sensory profile of the control and pasteurized (63 ºC) cider as less sweet and sourer taste. The development of sour taste and reduction in sweetness, resulting from a continuing fermentation, was not observed in the sample treated with higher pasteurization level (71 ºC for 6 s). Although pasteurization at this condition was able to ensure product safety as regards microorganisms until 12 weeks, most sensory characteristics dramatically changed at week 10. The cider samples had high level of bitterness but less intense fruit flavor detected by the panelists. The loss of flavor is possibly due to thermal processing stimulate evaporation of volatile compounds and enhance kinetic reactions that accelerated the loss of flavor compounds (Abirami, Nagarani, and Siddhuraju, 2014). Therefore, to determine the practical storage life of rose apple cider processed at different conditions, consumer acceptance tests should be carefully included in the criteria.
CONCLUSION
The present study provided information regarding the effects of alternative pasteurization conditions on physicochemical, microbial and sensory quality and antioxidant profile of rose apple cider during storage for up to three months. Cider pasteurized at 63 ºC had 10 weeks of storage life with some quality changes. Although this condition can better preserve antioxidant content and antioxidant capacity, the process was not sufficient to inactivate further fermentation. Thermal treatment at 71 ºC for 6 s resulted in shelf-life extension to 12 weeks; however, this treatment induced changes in color, degradation of ascorbic acid, and reductions in total phenols and antioxidant activities. The decrease of vitamin C content, as well as total polyphenols and antioxidant activities, indicate the effect of thermal processes and oxidation taking place in the rose apple cider during storage. Therefore, suitable packaging and storage condition would be the key to retain vitamin C content, total polyphenols and antioxidant activities. In terms of microbial safety, the E. coli evaluation result guaranteed the safety of rose apple cider consumption throughout the storage period. The sensory evaluations revealed that pasteurization causes flavor loss in cider samples; however, other sensory parameters were comparable to the control group. Moreover, the samples heat- treated at 71 ºC presented more desirable profile, detected by the panelists as a higher level of sweetness and less sour taste.
ACKNOWLEDGEMENTS
This research was financially supported by Prince of Songkla University, Hatyai Campus (project grant no. SIT590720S) and Prince of Songkla University, Surat Thani Campus, 2018. The authors would like to thank Assoc. Prof. Seppo Karrila for his kind support. Furthermore, the Food Innovation and Product Development (FIPD) Laboratory is strongly acknowledged here for the provided lab space and equipment support.
REFERENCES
Abirami, A., Nagarani, G., and Siddhuraju, P. 2014. In vitro antioxidant, anti-diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C. maxima fruits. Food Science and Human Wellness, 3: 16-25.
AFDO. 2003. Apple cider processing operations requirements and guidelines.
Association of Food and Drug Officials, York.
Akin, H., Brandam, C., Meyer, X.M., and Strehaiano, P.A. 2008. Model for pH determination during alcoholic fermentation of a grape must by Saccharomyces cerevisiae. Chem. Eng. Process Process Intensif. 47: 1986– 1993.
Alberti, A., Santos, T.P.M., Zielinski, A.A.F., Santos, C.M.E., Braga, C.M., and Demiate, I.M. 2016. Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. Food Science and Technology. 65: 436-443.
Babashahi-Kouhanestani, M., Salehi, M., Mazloomi, S.M., and Almasi-Hashyani, A. 2014. Quantitative evaluation of vitamin C in industrial lemon juice by titration method. Journal of Biology and Today’s World. 3: 139-141.
Bansal, V., Sharma, A., Ghanshyam, C., Singla, M.L., and Kim, K.H. 2015. Influence of pulsed electric field and heat treatment on Emblica officinalis juice inoculated with Zygosaccharomyces bailii. Food and bioproducts processing, 95: 146-154.
Besser, R.E., Lett, S.M., Weber, J.T., Doyle, M.P., Barrett, T.J., Wells, J.G., and Griffin, P.M. 1993. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. The Journal of the American Medical Association. 269: 2217-2220.
Brizuela, N., Tymczyszyn, E.E., Semorile, L.C., Hens, D.V.L., Delfederico, L., Hollmann, A., and Ferrada, B.B. 2019. Lactobacillus plantarum as a malolactic starter culture in winemaking: a new (old) player. Electronic Journal of Biotechnology. 38: 10-18.
Caminiti, I.M., Noci, F., Muñoz, A., Whyte, P., Morgan, D.J., Cronin, D.A., and Lyng, J.G. 2011. Impact of selected combinations of non-thermal processing technologies on the quality of an apple and cranberry juice blend. Food Chemistry. 124: 1387-1392.
Campodonico, P., Barbieri, E., Pizarro, M., Sotomayor, C.P., and Lissi, E.A. 1998. A comparison between total phenol content of wines and their trap values measured by the bleaching of ABTS radical cations. Boletin de la Sociedad Chilena de Quimica. 43: 281-285.
Chaikham, P., Chunthanom, P., and Apichartsrangkoon, A. 2013. Storage stability of pennywort juice as affected by high pressure and thermal processing. International Food Research Journal. 20: 3069.
Chanthai, S. and Danvirutai, P. 2004. Chemical analysis of locally produced wines. Part I. Acidity and organic compounds. Journal Academic Service Center Khon Kaen University. 12: 23-29. (in Thai).
Chumpookam, J., Subnum, V., and Jaruwattanaphan, T. 2014. Effect of component ratio on coffee pulp wine quality and consumer's satisfaction. Khon Kaen Agriculture Journal. 42(Suppl.3): 415-420.
Davey, M.W., Montagu, M.V., Inze, D., Sanmartin, M., Kanellis, A., Smirnoff, N., Benzie, I. JJ., Strain, J.J., Favell, D., and Fletcher, J. 2000. Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. Journal of the Science of Food and Agriculture. 80: 825-860.
Deboni, T.M., Bündchen, M., Junior, C.V., Hotza, D., Piletti, R., and Quadri, M.G.N. 2014. Effect of the processing steps on cactus juice production. Food and bioprocess technology. 7: 990-1000.
Farnworth, E.R., Lagace, M., Couture, R., Yaylayan, V., and Stewart, B. 2001. Thermal processing, storage conditions, and the composition and physical properties of orange juice. Food Research International. 34: 25-30.
Fellows, P.J. 2009. Food processing technology: principles and practice. Elsevier. Amsterdam.
Halliwell, B., Gutteridge, J.M.C., and Aruoma, O.I. 1987. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Analytical Biochemistry. 165: 215-219.
Huang, C.C., Wang, D.N., and Liou, D.T. 2005. Reduction of prevention of chilling injury by pruning and covering treatments on wax apple. Crop, Environment and Bioinformatics. 2: 73-80.
Jarvis, B. 2014. Cider: cyder and hard cider. In C. A. B. L. tortorello (Ed.). Encyclopedia of Food Microbiology. Academic Press, Oxford.
Jirumarn, J. and Srihanam, P. 2011. Oxidants and antioxidants: sources and mechanism. Acadamic Journal of Karasin Rajabhat University. 1: 59-70.
Laslo, V., Socaci, S., Teusdea, A., Timar, A., Tofana, M., and Ioana-Vicas, S. 2018. The effect of pasteurization time on phytochemical composition and antioxidant capacity of two apple cultivars juices. Bulletin UASVM Food Science and Technology. 75: 67-77.
Maneerat, S., Hanpongkittikun, A., and Musa, V. 2007. Comparison of wine clarification by pectinase enzyme and other methods. Songkhla Rajabhat University, Songkhla.
Miller, L.G. and Kaspar, C.W. 1994. Escherichia coli O157: H7 acid tolerance and survival in apple cider. Journal of Food Protection. 57: 460-464.
Narwojsz, A. and Borowska, E.J. 2010. Cranberry and strawberry juices-influence of method production on antioxidants content and antioxidative capacity. Polish Journal of Natural Sciences. 25: 209-214.
Odriozola-Serrano, I., Soliva-Fortuny, R., Hernández-Jover, T., and Martín Belloso, O. 2009. Carotenoid and phenolic profile of tomato juices processed by high intensity pulsed electric fields compared with conventional thermal treatments. Food Chemistry. 112: 258-266.
Palgan, I., Muñoz, A., Noci, F., Whyte, P., Morgan, D.J., Cronin, D.A., and Lyng, J.G. 2012. Effectiveness of combined Pulsed Electric Field (PEF) and Manothermosonication (MTS) for the control of Listeria innocua in a smoothie type beverage. Food Control. 25: 621-625.
Pérez-Conesa, D., García-Alonso, J., García-Valverde, V., Iniesta, M.D., Jacob, K., Sánchez-Siles, L.M., and Periago, M.J. 2009. Changes in bioactive compounds and antioxidant activity during homogenization and thermal processing of tomato puree. Innovative Food Science and Emerging Technologies. 10: 179- 188.
Piasek, A., Kusznierewicz, B., Grzybowska, I., Malinowska-Pańczyk, E., Piekarska, A., Azqueta, A., and Bartoszek, A. 2011. The influence of sterilization with EnbioJet® Microwave Flow Pasteurizer on composition and bioactivity of aronia and blue-berried honeysuckle juices. Journal of Food Composition and Analysis. 24: 880-888.
Rabie, M.A., Soliman, A.Z., Diaconeasa, Z.S., and Constantin, B. 2015. Effect of pasteurization and shelf life on the physicochemical properties of physalis (Physalis peruviana L.) Juice. Journal of Food Processing and Preservation. 39: 1051-1060.
Roginsky, V. and Lissi, E.A. 2005. Review of methods to determine chain-breaking antioxidant activity in food. Food Chemistry. 92: 235-254.
Sadecka, J., Polovka, M., Kolek, E., Belajova, E., Tobolkova, B., DAŠKO, Ľ., and Durec, J. 2014. Orange juice with pulp: impact of pasteurization and storage on flavour, polyphenols, ascorbic acid and antioxidant activity. Journal of Food & Nutrition Research. 53: 371-388.
Shi, J. and Maguer, M.L. 2000. Lycopene in tomatoes: chemical and physical properties affected by food processing. Critical Reviews in Food Science and Nutrition. 40: 1-42.
Shü, Z.H., Shiesh, C.C., and Lin, H.L. 2011. Wax apple (Syzygium samarangense (Blume) Merr. and L.M. Perry) and related species. In E.M. Yahia (Ed.). Postharvest biology and technology of tropical and subtropical fruits. Woodhead Publishing, Cambridge.
Splittstoesser, D.F. and Mclellan, M.R. 1997. Destruction of Escherichia coli O157:H7 in cider. Kansas Apple Cider Seminar, Topeka, KS.
Splittstoesser, D.F., Mclellan, M.R., and Churey, J.J. 1995. Heat resistance of Escherichia coli O157:H7 in apple juice. Journal of Food Protection. 59: 226- 229.
Supapvanich, S., Pimsaga, J., and Srisujan, P. 2011. Physicochemical changes in fresh-cut wax apple (Syzygium samarangenese [Blume] Merrill & L.M. Perry) during storage. Food Chemistry. 127: 912-917.
Supapvanich, S., Prathaan, P., and Tepsorn, R. 2012. Browning inhibition in fresh- cut rose apple fruit cv. Taaptimjaan using konjac glucomannan coating incorporated with pineapple fruit extract. Postharvest Biology and Technology. 73: 46-49.
Supapvanich, S., Mitrsang, P., and Srinorkham, P. 2017. Effects of ‘Queen’and ‘Smooth cayenne’pineapple fruit core extracts on browning inhibition of fresh- cut wax apple fruit during storage. International Food Research Journal, 24: 559-564.
Surek, E., and Nilufer-Erdil, D. 2014. Changes in phenolics and antioxidant activity at each step of processing from pomegranate into nectar. International journal of food sciences and nutrition, 65: 194-202.
Tandon, K., Worobo, R.W., Churey, J.J., and Padilla-Zakour, O.I. 2003. Storage quality of pasteurized and UV treated apple cider. Journal of Food Processing and Preservation. 27: 21-35.
U.S.FDA. 2001. Hazard analysis and critical control point (HACCP). Procedures for the safe and sanitary processing and importing of juice. Federal Register. 66: 6137-6202.
Vandresen, S., Quadri, M.G., de Souza, J.A., and Hotza, D. 2009. Temperature effect on the rheological behavior of carrot juices. Journal of Food Engineering. 92: 269-274.
Walton, E.F. and Pringle, J.R. 1980. Effect of growth temperature upon heat sensitivity in Saccharomyces cerevisiae. Archives of Microbiology.124: 285– 287.
Wanapu C., Boonkerd N., and Dithavibool L. 2004. Winemaker1. Sombun Printing House, Nakhon Ratchasima.
Waterhouse, A.L. 2002. Determination of total phenolics In: current protocols in food analytical chemistry. John Wiley & Son Inc, New Jersey.
Wilkes, K., Howard, L.R., Brownmiller, C., and Prior, R.L. 2014. Changes in chokeberry (Aronia melanocarpa L.) polyphenols during juice processing and storage. Journal of Agricultural and Food Chemistry. 62: 4018-4025.
Yahia, E. 2011. Watson B. Cider, Hard and Sweet: History, Traditions, and Making Your Own. (3rd ed). The Countryman Press, Vermont.
Yeom, H.W., Streaker, C.B., Zhang, Q.H., and Min, D.B. 2000. Effects of pulsed electric fields on the quality of orange juice and comparison with heat pasteurization. Journal of Agricultural and Food Chemistry. 48: 4597-4605.
Zheng, H., and Lu, H. 2011. Use of kinetic, Weibull and PLSR models to predict the retention of ascorbic acid, total phenols and antioxidant activity during storage of pasteurized pineapple juice. LWT-Food Science and Technology, 44: 1273- 1281.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Chukwan Techakanon* and Karthikeyan Venkatachalam
Faculty of Innovative Agriculture and Fishery Establishment Project, Prince of Songkla University Surat Thani campus, Surat Thani 84000, Thailand
Corresponding author: Chukwan Techakanon, E-mail: chukwan.t@psu.ac.th
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: May 12, 2020;
Revised: July 1, 2020;
Accepted: October 12, 2020

