
Antioxidant and Antimicrobial Properties of Moringa oleifera Leaves and Pods Extracts in Pork Meatballs During Cold Storage
Patcharee Prasajak*, Phanida Renumarn, Wichien Sriwichai, and Pakkawat DetchewaPublished Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.033
Journal Issues : Number 2, April-June 2021
Abstract Effects of M. oleifera leaves and pods extracts on physicochemical properties, free radical scavenging properties, antimicrobial activities and sensory attributes of pork meatballs were evaluated during cold storage at 4°C for 15 days. The preparation of pork meatballs was divided into eight treatments as control, 0.02% butylated hydroxytoluene (BHT), 0.2% leaves and pods aqueous extract, 0.4% leaves and pods aqueous extract, 0.8% leaves and pods aqueous extract. Aqueous leaves extract showed highest level of total phenolic (67.18 mg GAE/g extract) and flavonoid contents (5.60 mg CE/g extract) compared to those observed in aqueous pods extract as 55.17 mg GAE/g extract and 3.54 mg CE/g extract, respectively. The leaves extract had strongest antioxidant activity against DPPH radicals with IC50 49.85 μg/ml while the pods extract exhibited IC50 99.31 μg/ml. According to pork meatballs analysis, meatballs samples with addition of aqueous leaves extract exerted higher antioxidant activities in a concentration- dependent manner that were performed by higher DPPH scavenging activity and lower TBARs values in comparison with aqueous pods extract. Conversely, M. oleifera pods extract showed highest antibacterial activity against all tested foodborne bacterial strains including Staphylococcus aureus (TISTR 1466), Bacillus cereus (TISTR 678), Escherichia coli (TISTR 780), Salmonella typhimurium (ATCC 13311) with lowest MIC (1.56 mg/ml) and MBC (3.13 mg/ml) in agreement with the decrease of total microbial counts as compared to control and BHT samples. The meatballs with pods extract possessed higher sensory attributes scores than those added with the leaves extract. In conclusion, 0.8% pods extracts effectively retarded lipid oxidation as well as decreased microbial growth in pork meatballs during cold storage. However, it was point out that inferior sensory scores were affected by increasing additional the extract in the meatballs. Therefore, the use of Moringa extracts should be carefully applied in the meatballs for avoidance of lowering consumer acceptance.
Keywords: Antimicrobial, Antioxidant, M. oleifera, Pork meatballs
Funding: This work was financially supported by King Mongkut’s University of Technology North Bangkok under the grant number KMUTNB-61- NEW-013.
Citation: Prasajak, P., Renumarn,P., Sriwichai, W., and Detchewa, P. 2021. Antioxidant and antimicrobial properties of Moringa oleifera leaves and pods extracts in pork meatballs during cold storage. CMUJ. Nat. Sci. 20(2): e2021033.
INTRODUCTION
Moringa oleifera is a member of family Moringaceae. This plant is also well known in many terms such as drumstick or horseradish tree. M. oleiferais widely cultivated in tropical and subtropical regions with dry to moist tropical or subtropical climate as Middle East, Africa and Asia. Typically, all parts of this plant have been used in human both for nutritional and medicinal purposes. According to nutritive properties, M. oleifera leaves can serve as a rich source of vitamins and minerals which are necessary for supportive physical growth and development in human. These include beta-carotene, vitamins A, B, C, D and E while calcium, potassium, zinc, magnesium, iron and copper are found as essential minerals in the leaves. It has been reported that powder of M. oleifera leaves was used as diet for malnutritionin children, pregnant women and nursing mothers in Senegal and Haiti. The leaves, fruit, flowers and immature pods of M. Oleifera are used as vegetable and traditional medicine in many countries especially in India, Pakistan, Africa and Southeast Asia. In India, M. oleifera has been used as herbal remedies for treating cardiac and circular aliments (Paikra et al., 2017). Recently, M. oleifera leaves have interested as an enrich sources of several bioactive compounds which contain number of phytochemicals substances including glucosinolates, polyphenol, phenolic acids, alkaloids, flavonoids, isothiocyanates, oxalates, saponins and tannins (Dalei et al., 2016). The other parts of the tree such as seeds, flowers, pods, root bark also possess promising sources of bioactive compounds. For example, M. oleifera seed extracts have been shown to contain number of phytochemicals such as tannins, saponin, phenolics, phytate, lectins, flavonoids and terpenoids. In addition, the high phenolic and flavonoid contents were found in flower pod extract (Leone et al., 2015).
Interestingly, previous studies revealed that these bioactive compounds are relevant to various biological properties. These include antioxidant, anti-inflammation, antibacterial, hypoglycemia, hypolipidemia, hepatoprotective and anticancer (Paikra et al., 2017). It has been reported that the leaves of M. oleifera are used as folk medicine to treat several diseases including malaria, typhoid fever, arthritis, swellings, cuts, skin infections, digestive disorders, hypertension and diabetes. Over the past ten years, a number of scientific research has been conducted on the nutritional benefits of M. oleifera along with their medicinal properties. M. oleifera has been used as food additives as natural food preservatives for extending the shelf life of foods. It has been demonstrated that Moringa leaves extract significantly improves the shelf life of cooked goat meat patties by retarding lipid peroxidation under refrigerated storage (Das et al., 2012). Moringa leaves extract was reported to exhibit strong antioxidant and antimicrobial activity in chicken patties without altering the color and sensory attributes of the products (Elhadi et al., 2017). Although M. oleifera has been assessed as natural preservative for various meat products, the application in pork meatballs has not been adequately studied. Among several plant parts, leaves and pods of M. oleifera have been generally consumed as a major ingredient of traditional Thai cuisine for both of nutrition and medicine purposes. Thus, we interested to use the leaves and pods for investigation of their biological effects on the shelf life of pork meatballs product. The aim of this study was to evaluate the effect of M. oleifera leaves and pods extracts on the physicochemical, antioxidant, antimicrobial capacities and sensory attributes of pork meatballs during cold storage.
MATERIALS AND METHODS
Preparation of M. oleifera leaves and pods extracts M. oleifera leaves and pods were purchased from local markets in Prachinburi provinces, Thailand. Leaves and pods were thoroughly washed with distilled water, cut into small pieces and allowed to air dry. Thereafter, dried leaves and pods were finely ground in to small particles by an electrical grinder and kept in polyethylene bags at 4°C. According to aqueous and ethanolic extraction, 25 g of dried powder were mixed with 250 ml of distilled water or 95% ethanol (QREC, Auckland, New Zealand) at room temperatures for 24 h by orbital shaker. The extract was then filtered through a Whatman No.4 filter paper and centrifuged (Labnet S0100-230V, New Jersey, USA) at 4000×g, for 10 min. The residue was re-extracted once under similar conditions. Finally, the pooled supernatant of the extract was concentrated using a rotary evaporator (Eyela, N-1000, Tokyo, Japan) at 50°C to remove the solvent. The concentrated extract was freeze-dried to obtain the crude extract (Thermo Electron Corporation, Supermodulyo-230, Massachusetts, USA) and stored at -20°C until use.
Determination of total phenolic content
Total phenolic content (TPC) was evaluated according to the Folin-Ciocalteu method (Baba and Malik, 2015). Briefly, 1 ml of crude extract (1 mg/ml) were mixed thoroughly with 0.5 ml of Folin–Ciocalteu reagent (Merck, Darmstadt, Germany) for 5 min in the dark. After that, 2 ml of 10% (w/v) Na2CO3 (Sigma Aldrich, Saint Louis, USA) was added and made up to 4 ml with distilled water. The mixture was mixed well and incubated for 30 min at room temperature in the dark, and absorbance was measured at 765 nm. The total phenolic content was calculated from the calibration curve of gallic acid (Sigma Aldrich, Saint Louis, USA) as standard. The results were expressed as mg of gallic acid equivalent per g extract (mg GAE/g extract).
Determination of total flavonoid content
The total flavonoid content (TFC) of crude extract was performed according to the aluminium chloride colorimetric method (Baba and Malik, 2015). In brief, 0.3 ml of 5% NaNO2 was added to 1 ml of crude extract (1 mg/ml) and further incubated for 5 min in the dark. Thereafter, 0.3 ml of 10% AlCl3 solution was added, and the mixture was allowed to stand for 6 min. Then, 2 ml of 1 M NaOH solution was added and the total volume was made up to 8 ml with distilled water. The mixture was mixed well and determining absorbance at 510 nm. The total flavonoid content was calculated from the calibration curve of catechin (Sigma Aldrich, Saint Louis, USA) as standard. The results were expressed as mg of catechin equivalent per g extract (mg CE/g extract).
Free radical scavenging activity by DPPH assay
The antioxidant activity of the extract was determined by the 1,1-diphenyl-2- picryl-hydrazyl or DPPH (Sigma Aldrich, Saint Louis, USA) assay as described by Ajila et al. (2010) with slight modifications. 1 ml of crude extract with a range of concentrations from 25 to 400 µg/ml was mixed with 2.5 ml of 0.1 mM DPPH solution and incubated in the dark at room temperature for 30 min. The absorbance of the mixture was then measured at 517 nm. Ascorbic acid was used as a standard control to compare an antioxidant activity of the extract. Regarding to determine DPPH antioxidant activity of pork meatballs, 8 g of sample was homogenized with 20 ml of distilled water for 5 minutes. The mixture was centrifuged at 3,000 g (Labnet International, New York, USA) for 10 min. Subsequently, 1 ml of supernatant was mixed with the reagents according to above protocol. The radical scavenging activity was calculated from following equation:
Scavenging activity (%) = [(Control OD – Sample OD)/ Control OD] x 100
From the equation, control OD is the absorbance of distilled water, and sample OD refers to the absorbance of the extract. The DPPH radical scavenging activity (%) was plotted against the extract concentration (µg/ml) in order to determine concentration of the extract which has scavenging activity enough to decrease DPPH radical by 50% (IC50).
Bacterial strains and inoculums preparation
The bacteria used in this study was relevant strains causing food poisoning diseases including Staphylococcus aureus (TISTR 1466), Bacillus cereus (TISTR 678), Escherichia coli (TISTR 780), Salmonella Typhimurium (ATCC 13311). The bacterial strains were purchased from the TISTR Culture Collection, Thailand Institute Scientific and Technological Research (TISTR), Pathum Thani, Thailand. Each bacterial strain was subcultured overnight in nutrient agar (Difco, BD, New Jersey, USA) at 37 °C. The picked colonies grown on nutrient broth (Difco, BD, New Jersey, USA) and diluted to give viable cell count of 108 CFU/ml using spectrophotometer at 600 nm.
Antimicrobial assay by agar disc diffusion
The disc diffusion method is used to evaluate antimicrobial activity of the crude extracts. The crude extracts powder was dissolved in 10% DMSO to accelerate soluble at concentration 800 mg/ml. Mueller-Hinton agar (Difco, BD, New Jersey, USA) was poured into Petri dishes and allowed to solidify under ultraviolet light for 20 min. A sterile cotton swab was dipped into bacterial suspensions which were adjusted to turbidity of 0.5 McFarland Standard (inoculum of 1.5 x 108 CFU/ml). Thereafter, the adjusted bacterial suspensions were steak on the top layer of the agar plate, then a sterile paper disk (6 mm) was loaded with stock solutions of the extracts to obtain final concentration of 16 mg/disc. Positive and negative controls used in this study were Amoxicilin (30 μg/disc) and 10% DMSO, respectively. The plates were incubated at 37°C for 24 h. The diameters of inhibition zones were measured by Vernier caliper (mm) and considering as antibacterial activity. All the tests were performed in triplicate.
Determination of minimum inhibitory concentrations
Minimum inhibitory concentrations (MIC) of the crude extracts was performed by using broth microdilution method according to the Clinical and Laboratory Standards Institute (CLSI) protocol. The crude extracts concentration was prepared by two-fold dilutions in Mueller-Hinton broth (Difco, BD, New Jersey, USA) ranging from 1.56-800 mg/ml. The inoculums of bacterial strains were prepared from fresh overnight cultures which adjusted to 0.5 McFarland standard. The inoculums were further diluted in 1:100 ratio to give viable cells 1.5× 106 CFU/ml. The 110 μl of the crude extracts suspension was poured to each well of 96-well microplates. Then bacterial suspensions were added and mixed thoroughly into each well of volume 10 μl and incubated at 37°C for 24 h. After overnight incubation, 30 μl of 0.01% resazurin (Sigma Aldrich, Saint Louis, USA) was added to each wells and further incubation for another 4 h. A blue colored in the test wells indicated the growth inhibition while pink to colorless colored indicated bacterial growth. The lowest concentration of the crude extracts which did not observe visible growth of the bacteria was considered as MIC (mg/ml). Positive control used in this study was Amoxicilin (1.46-3000 μg/ml) while 10% DMSO and medium broth were used as negative control. All the tests were performed in triplicate.
Determination of minimum bactericidal concentrations
The minimum bactericidal concentration (MBC) was determined by directly steaking the mixture suspension of each well with concentrations higher than the MIC value, onto Mueller-Hinton agar plates. Then the plates were incubated at 37°C for 24 h. The lowest concentrations of the crude extract that did not produce any bacterial growth on the inoculated medium agar were defined as MBC (mg/ml). All the tests were performed in triplicate.
Preparation of pork meatballs
The aqueous extract of M. oleifera leaves and pods were selected for mixing ingredient in the pork meatball for further analysis. The ground pork was then formulated to contain 3 different batches as followed: test groups with additional 0.2%, 0.4%, 0.8% of the crude extract and positive control groups as supplementary with the synthetic antioxidant BHT (0.02%) including negative control groups as without BHT/the crude extract addition. Pork meatballs were prepared using 3 kg of lean ground pork, 0.25% sodium phosphate, 2% salt, 2% corn starch and 15% ice. In brief, the ground pork was mixed well with corn starch, salt and ice in a mixer for 3 minutes followed by addition of sodium phosphate and M. oleifera leaves/pods extracts or BHT into the mixture for 3 minutes to form meat emulsion. Then, the meatball batter was cooled immediately in a refrigerator at 4°C for one hour. After that, the batter was shaped by hand with gloves and cooked in hot water at 70°C for 20 minutes, then cooled in chilled water. The pork meatballs were aerobically packaged in polyethylene bag and stored at 4°C for 15 days. The meatball samples were randomly taken for analysis during storage periods. The pH, color, TBARs value and microbial growth were analyzed on day 0, 3, 6, 9, 12 and 15 of storage under refrigerated conditions (4°C). Sensory evaluation was carried out on day 0 of storage. All analyses were performed in triplicate.
pH and instrumental color evaluation
For pH measurements, 5 g of sample was homogenized with 20 ml distilled water. The mixture was filtered through Whatman No.1 filter paper to obtain a clear filtrate. The pH of the filtrate was measured using a digital pH meter (WTW, inoLab, Weilheim, Germany). All the measurements were performed in triplicate. According to color analysis, the surface color of pork meatballs was measured using the Hunter Lab system (L, a and b) with a colorimeter (Hunter Lab, Virginia, USA). All the measurements were performed in triplicate.
Microbial analysis
Total viable count (TVC) was performed as earlier description. Briefly, 25 g of each sample was homogenized with 225 ml of sterile peptone water and homogenized in a sterile stomacher bag for 1 min. A ten-fold serial dilution of the bacterial suspension was done to achieve 10-1 to 10-7 dilution. 1 ml of each dilution was inoculated on the plate count agar (Difco, BD, New Jersey, USA) by pour plate technique. The plates were further incubated at 37°C for 48 h. The number of colony count was expressed as log CFU/g sample.
Determination of TBARs values
The 2-Thio Barbituric Acid Reactive Substances (TBARs) assay is a method for quantifying lipid oxidation. 5 g of sample was homogenized in 20 ml of deionized distilled water. Meatballs homogenate (1.5 ml) was transferred into a test tube and supplemented with 75 µl of 7% BHT. Then the mixture was added with 3 ml of 0.02 M 2-thiobarbituric acid (TBA) in 15% trichloroacetic acid (TCA) (Sigma Aldrich, Saint Louis, USA) solution. The test tube was heated in water bath at 95°C for 20 min, cooled to room temperature in ice and centrifuged at 4,000 x g for 10 min. The absorbance of supernatant was measured at 532 nm. TBARs value of the sample was calculated from a standard curve of 1, 1, 3, 3-tertramethoxypropane as a precursor of malonaldehyde (MDA) which refers to end product of lipid peroxidation. The results were quantified as malonaldehyde equivalents (mg MDA/kg sample).
Sensory evaluation
The meatballs were evaluated sensory attributes after production by using a 9 point hedonic scale system. The sensory evaluation was performed by 30 untrained panelists. The sample was given a code number and randomly served to all volunteers. Tap water was provided to the panelists to flush their mouth after each sample testing. The sample was evaluated in terms of color, texture, flavor, odor and overall acceptability. All sensory parameters were scored by using a 9 point hedonic scale, where 1 represented dislike extremely and 9 represented like extremely.
Statistical analysis
Each experiment was performed in triplicate. Statistical analysis was evaluated in Completely Randomized design (CRD) and one way ANOVA using SPSS version 21.0 software. The significant difference between the means comparison was analyzed by Duncan’s Multiple Range Tests (DMRT) at P ≤ 0.05.
RESULTS
Total phenolic content, total flavonoid content and antioxidant activity of the crude extracts
The total phenolic content and total flavonoid contents of M. oleifera leaves and pods extracts are given in Table 1. Aqueous leaves extract contained the highest level of total phenolic (67.18 mg GAE/g extract) and flavonoid contents (5.60 mg CE/g extract) followed by aqueous pods extract and ethanolic leaves extract while ethanolic pods extract contained the lowest amounts of TPC and TFC as 37.89 mg GAE/g extract and 0.18 mg CE/g extract, respectively. Antioxidant activity of M. oleifera extracts at 25-400 μg/ml are presented in Figure 1. Aqueous leaves extract had strong antioxidant activity against DPPH radicals ranging from 49.72 to 63.60% with IC50 49.85 μg/ml. DPPH scavenging activity of aqueous pods extract was 24.86 to 59.07% (IC50 99.31 μg/ml), followed by ethanolic leaves extract, ethanolic pods extract as 42.96 to 53.07% (IC50 189.41 μg/ml) and 17.79 to 54.14%, (IC50 227.71 μg/ml) respectively. Our results revealed that M. oleifera extracts had DPPH scavenging activity increasing at a concentration-dependent manner. Although the extracts showed strong antioxidant activity, however, ascorbic acid had more percentage of scavenging activity (25.93 to 86.35%) than those observed for the extracts.
Table 1. Total phenolic, flavonoid contents and IC50 values in the crude extracts of M. oleifera leaves and pods.
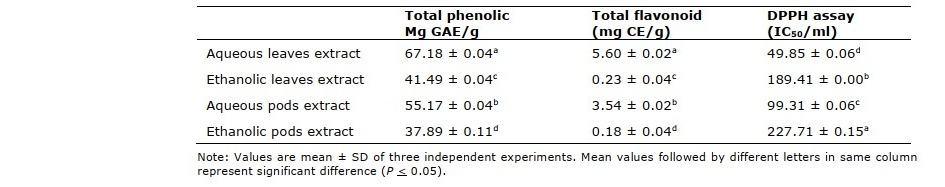
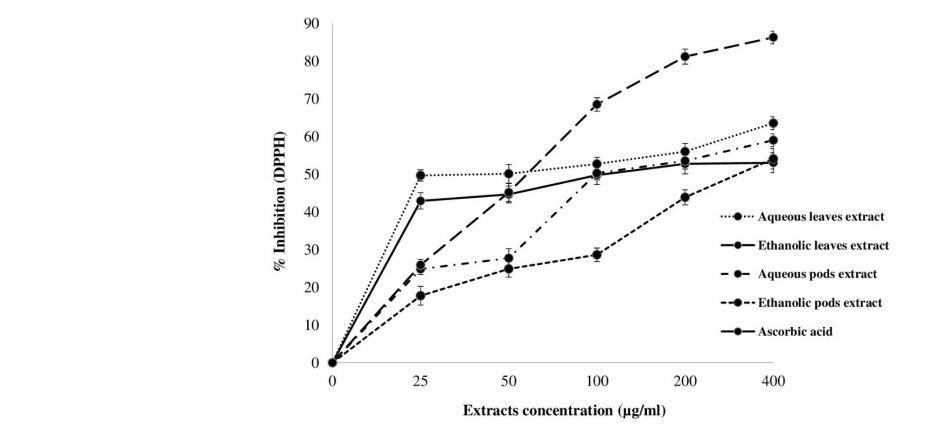
Figure 1. DPPH scavenging activities in the crude extracts of M. oleifera leaves and pods.
Antibacterial activity of the crude extracts
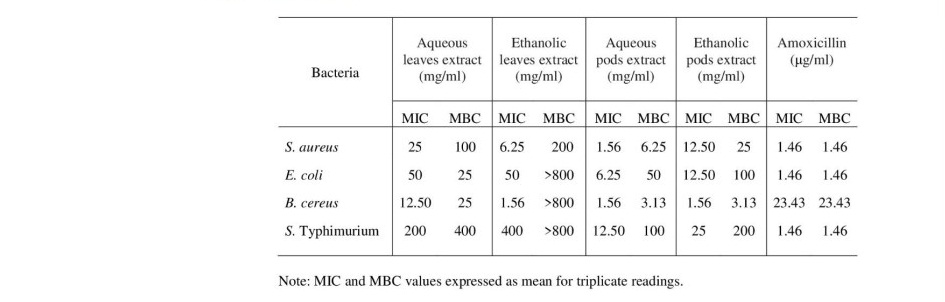
Agar disc diffusion method was used to evaluate antimicrobial activity of the crude extracts against foodborne pathogens as B. cereus, E. coli, S. aureus, S. typhimurium. The inhibition zones of M. oleifera leaves and pods extracts against different foodborne bacteria are shown in Table 2. The results revealed that M. oleifera pods extract showed highest antibacterial activity against all bacterial strains (4.67 to 19.50 mm inhibition zones) while leaves extract did not show any zone of inhibition. Both of aqueous and ethanolic pods extracts had suppressive effect on microbial growth of food poisoning bacteria. Aqueous pods extract showed the most active on inhibiting the growth of all tested pathogens at concentration of 800 mg/ml (16 mg/disc) while ethanolic pods extract exhibited inhibitory effect against only S. aureus and B. cereus. Focusing on MIC and MBC, the aqueous extract of M. oleifera pods had the lowest MIC and MBC values of 1.56 and 3.13 mg/ml, respectively followed by the other ethanolic extract (Table 3). Interestingly, although the leaves extract could not exhibit antibacterial effect on the tested bacterial cells by agar disc diffusion method, MIC and MBC values can be detected as 12.5 and 400 mg/ml of the aqueous extract, respectively. Similarity, the ethanolic leaves extract had MIC and MBC values ranging between 1.56 and >800 mg/ml, respectively. These results indicated that high concentration of the ethanolic leaves extract was required for bactericidal activity against on B. cereus, E. coli and S. typhimurium.
Table 2. The inhibition zones of M. oleifera leaves and pods extracts against different foodborne bacteria.
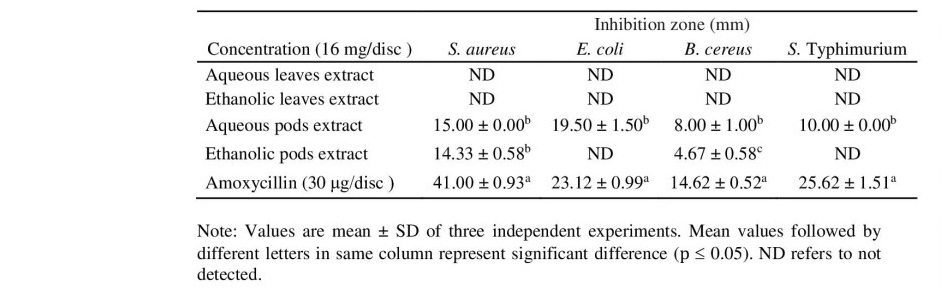
Table 3. MIC and MBC values of M. oleifera leaves and pods extracts against different foodborne bacteria.
Antioxidant activity evaluation of pork meatballs
Aqueous extracts from leaves and pods were selected to incorporate in pork meatballs based on the highest DPPH scavenging activity. The results showed that antioxidant activity were significantly high in 0.2%, 0.4% and 0.8% leaves and pods extracts incorporated pork meatballs samples compared to the control and BHT groups during storage at 4°C for 15 days (Table 4). Interestingly, leaves extracts added sample exhibited superior in antioxidant capacity than the pork meatballs supplemented with pods extracts in a concentration-dependent manner. Moreover, 0.8% leaves extracts sample has shown to prolong antioxidant capacity throughout the storage period ranging from 70.44 to 84.08%. Thus, leaves and pods extracts of Moringa had remain strongest antioxidant activity even addition to the pork meatballs.
Table 4. DPPH scavenging activities in pork meatballs incorporated M. oleifera leaves and pods extracts.
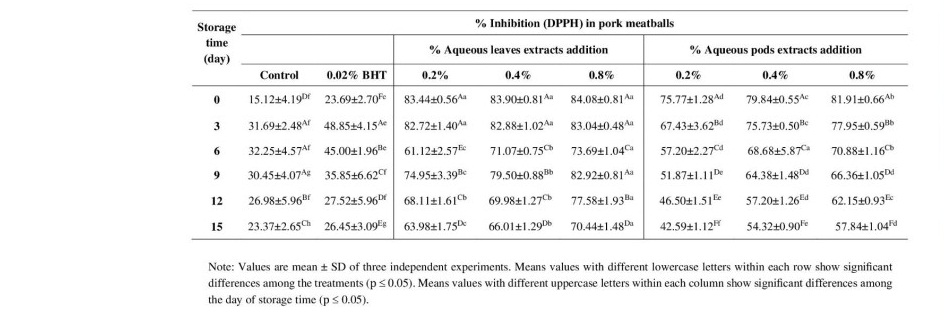
TBARs values determination of pork meatballs
TBARs values was used to evaluate lipid oxidation in pork meatballs added leaves and pods extracts during storage at 4°C for 15 days. The effects of adding the extracts on TBARs values of pork meatballs are shown in Table 5. The control sample showed a higher level of TBARs values than samples treated with leaves and pods extracts at all storage times. In addition, 0.8% and 0.4% both of treated samples showed significantly lower TBARs values throughout the storage period whereas the BHT added sample exhibited progressive increasing in a time-dependent manner. These results indicate that leaves and pods extracts of Moringa could accelerate retarding lipid oxidation in the pork meatballs at refrigerator temperatures. Moreover, it has been shown that aerobically packaged pork meatballs had effect on development of lipid oxidation which showed by increasing TBARs values over storage period.
Table 5. TBARs values in pork meatballs incorporated M. oleifera leaves and pods extracts.
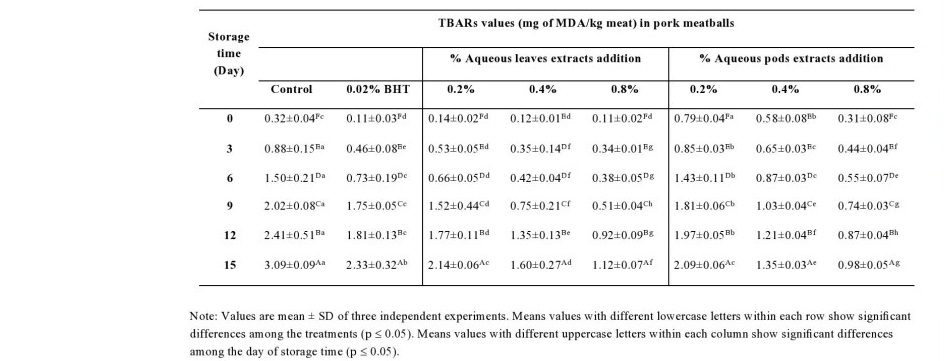
Color and pH changes in pork meatballs
The pH of pork meatballs was decreased with increased storage period (Table 6). pH values were not significantly (P > 0.05) different among the treatments during the first week. However, pH was significantly (P ≤ 0.05) lower in the pork meatballs incorporated with various percentage of leaves extracts when compared with the other samples including control throughout the second weeks of storage. Moreover, the highest reduction of the pH was observed in the sample with Moringa leaves extracts ranging from 6.19 to 6.23 at the end of storage.
Table 6. pH changes in pork meatballs incorporated M. oleifera leaves and pods extracts.
Color values of the pork meatballs incorporated leaves and pods extracts were evaluated (data not shown). There was no significant (P > 0.05) different in L, a and b values among all samples during the first weeks of storage period. Regarding the increase of storage time, however, the higher both of a and b values were observed in the samples added leaves extracts whereas those L values showed lower than the other treatments. Additionally, the pork meatballs supplemented with Moringa extracts had trend to decrease lightness (L values) compared with control sample. These findings may be involved in darkness color of the crude extracts that affected to the physical appearance of the pork meatballs.
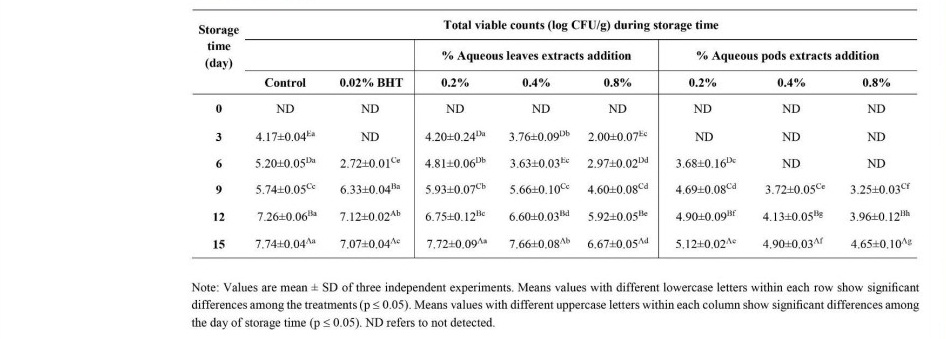
The microbiological analysis of pork meatballs
The total viable count of pork meatballs incorporated with Moringa leaves and pods extracts are shown in Table 7. The results showed that all treated samples with 0.2 to 0.8% extracts significantly inhibited microbial growth as compared with the control sample during cold storage. The control pork meatballs had higher total aerobes counts (4.17 to 7.74 log CFU/g) than those treated with the extracts at all time of storage. Among all treatments, 0.8% pods extracts gave superior antimicrobial efficacy to inhibit the growth of total aerobes in the meatballs (3.25 to 4.65 log CFU/g) during storage period for 15 days. In addition, the decrease of microbial growth was shown in accordance with increasing concentration of the extracts. These results showed that addition of Moringa leaves and pods extracts had significant effects on decreasing microbial growth in the pork meatballs under cold storage for 15 days.
Table 7. Microbiological analysis in pork meatballs incorporated M. oleifera leaves and pods extracts.
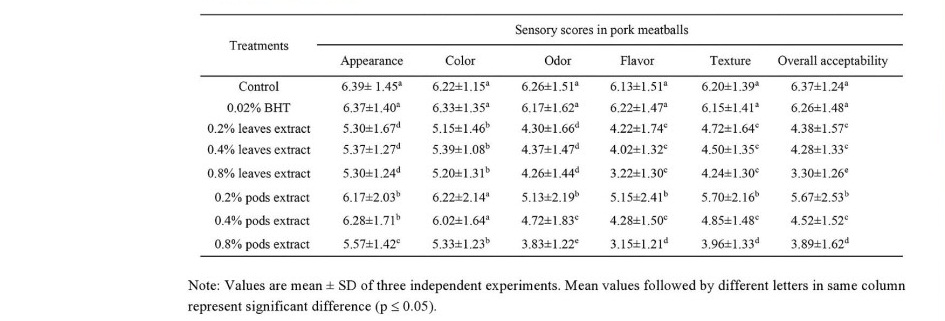
The sensory qualities of pork meatballs
The sensory scores of pork meatballs with or without the leaves and pods extracts are given in Table 8. The results showed that control samples gave higher all the sensory scores (P ≤ 0.05) than the extracts incorporated meatballs samples. Among the treatments supplemented the extracts, however, it was shown that 0.2% pods extracts samples had the highest scores of appearance, color, odor, flavor, texture and overall acceptability while the lowest sensory scores were presented in those meatballs with 0.8% pods extracts. Moreover, the addition of Moringa extracts had significant effect on reducing sensory properties in the pork meatballs. Accordingly, the use of Moringa extracts in the meatballs should be carefully considered for the suitable amount of supplementation.
Table 8. Sensory qualities analysis in pork meatballs incorporated M. oleifera leaves and pods extracts.
DISCUSSIONS
In this study, we determined the effects of M. oleifera leaves and pods extracts on physicochemical properties, free radical scavenging properties, antimicrobial activities and sensory quantities of pork meatballs during cold storage at 4°C for 15 days. Remarkably, we observed that the crude aqueous extracts of leaves and pods had DPPH scavenging activity with the increase of total phenolic and flavonoid compounds. Phenolic and flavonoid contents have been reported responsible for antioxidant activity of the crude extract. Vongsak et al. (2013) reported the correlation between total flavonoid and phenolic contents with the DPPH scarvenging activity from ethanolic leaves extract of M. oleifera. They also revealed that the high amounts of chlorogenic acid and isoquercetin in Moringa leaves corresponded to their high antioxidant activity. Similarity, the extract of M. oleifera ripe seed also showed to have significant antioxidant capacities relevant to their phenolic contents. In addition, GC-MS results revealed the highest amount of identified phenolic compound from the aqueous residue of the seeds extract (Adebayo et al., 2018). Corresponding the results of Wangcharoen and Gomolmanee (2011), we found that M. oleifera leaves extracts had higher antioxidant capacity and total phenolic compounds than the pods extract. Moreover, they reported the antioxidant and phenolic substances derived from these extracts were water-soluble rather than ethanol-soluble. Phenolic and flavonoid compounds are secondary plant metabolites that represent as common natural antioxidants from the plant. It has been reported that phenolic and flavonoid compounds exhibit as free radical scavenger to exert anti-inflammatory, anti- allergy, antidiabetic, antimicrobial which are render to protect against diseases with oxidative stress such as cancer and cardiovascular diseases (Shahidi and Ambigaipalan, 2015). Phenolic and flavonoid compounds act as hydrogen donor to convert purple color DPPH radicals into yellow color reduced form which can be detected by absorbance decrease (Huyut et al., 2017).
Regarding antibacterial properties, although the leaves extract could not exhibit any antibacterial zone of inhibition, low MIC and MBC values can be detected in these aqueous extract. These findings may be explained by the diffusion capacities of phytochemical compounds in the extracts that trend to have more opportunity to direct exposure with microorganism in suspension system obtained by broth microdilution method. In contrast to this study, Tekle et al. (2015) reported the suppressive effect of Moringa leaves extracts on bacterial growth. This discrepancy might be affected by various factors that have effect on phytochemical profile of the extracts such as geographic region, cultivation and procedure of extraction. Previous study has been found the correlation of microbial inhibition potency of M. oleifera seed extracted with increasing amounts of total phenolic compounds (Gaafar et. al., 2016). Similarly, Singh et al. (2013) reported that the antibacterial activity of M. oleifera seed flour correlated well with total phenol and flavonoid contents in the extracts. Phenolic compounds are low-molecular weight plant secondary metabolites that have shown to possess antimicrobial properties against pathogenic bacteria. It has been demonstrated that low-molecular weight proteins and peptides compounds were active agents of M. oleifera extracts that responded for antimicrobial activities (Fahey, 2005). Additionally, interaction of secondary metabolites with SH-groups of microbial proteins have been purposed as antimicrobial mechanism to induce conformation changes and modulating microbial bioactivities (Wink, 2015). Thus, these active compounds seem to interfere protein function of microbes which ultimately leads to cells death.
Based on the strongest antioxidant activity and highest total phenolic compounds, aqueous leaves and pods extracts were selected to incorporate in pork meatballs. Our findings are in agreement with several previous studies which reported the effect of polyphenol-enriched leaves and seeds extracts on reducing lipid oxidation in meat products (Das et al., 2012; Muthukumar et al., 2014; Shah et al., 2015). Similar to the results of crude extracts, Moringa leaves powders were found to retain their high antioxidant activities in the treated meatballs even after boiling process for 20 minutes. Generally, lipid oxidation is commonly related to the sensory qualities of meat products. It has been reported that the maximum level of TBARs value in meat products was 1 mg MDA/kg sample (Wu et al., 1991). Accordingly, the meatballs with 0.8% both of leaves and pods extracts could be kept for approximately 12 days with minimal defect of lipid oxidation occurrence. Regarding the restriction of Thai FDA, the standard for acceptable microbial counts should not be detected more than 5 log CFU/g sample. Based on this regulation, addition of Moringa leaves and pods extracts significantly reduced the increasing microbial growth in the pork meatballs under cold storage for at least one week. Moreover, we observed that addition of the leaves and pods extracts had significant adverse effect on sensory scores of the pork meatball samples. Our findings are similar to other studies, Jayawardana et al. (2015) reported negative effect on sensory attributes of chicken sausages treated with Moringa leaf powder above 0.5%. In addition, sensory attributes of beef patties were reduced with increasing percentage of Moringa seed powder (Juhaimi et al., 2016). Thus, applying Moringa extracts in the pork meatballs should be carefully considered for their adverse effect on consumer acceptance.
CONCLUSIONS
Our results demonstrate that addition of pork meatballs with Moringa leaves and pods extracts successfully improve shelf life while still maintain quality and safety of the product throughout refrigerated storage for 15 days. Moringa leaves and pods extracts possessed functional antioxidant and antimicrobial properties which were clearly clarified in the crude extracts and even applying in meat product. Additionally, 0.8% leaves and pods extracts effectively retarded lipid oxidation as well as decreased microbial growth in pork meatballs during cold storage period. However, it was point out that inferior sensory scores were affected by increasing additional the extract in the meatballs. Therefore, the use of Moringa extracts should be carefully applied in the meatballs for avoidance of lowering consumer acceptance. Altogether, Moringa leaves and pods extracts can be used as a natural preservative ingredient in meat products with extending shelf life and maintaining product quality.
ACKNOWLEDGEMENTS
The authors would like to thank Suthimon Thongpramoon and Warisara Rakchat for their assistance in this study and also acknowledge to the faculty of Agro-Industry, King Mongkut’s University of Technology North Bangkok for providing instruments.
CONFLICT OF INTEREST
The Authors declare no conflict of interest.
REFERENCES
Adebayo, I.A., Arsad, H., and Samian, M.R. 2018. Total phenolics, total flavonoids, antioxidant capacities, and volatile compounds Gas chromatography-mass spectrometry profiling of Moringa oleifera ripe seed polar fractions. Pharmacognosy Magazine. 14: 191-194.
Ajila, C.M., Aalami, M., Krishnarau, L., and Prasada, R.U. 2010. Mango peel powder: a potential source of antioxidant and dietary fiber in macaroni preparations. Innovative Food Science and Emerging Technologies. 11: 219-224
Baba, S.A. and Malik, S.A. 2015. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. Journal of Taibah University for Science. 9: 449-454.
Dalei, J., Rao, V.M., Sahoo, D., Rukmini, M., and Ray, R. 2016. Review on nutritional and pharmacological potencies of Moringa oleifera. European Journal of Pharmaceutical and Medical Research. 3: 150-155.
Das, A.K., Rajkumar, V., Verma, A.K., and Swarup, D. 2012. Moringa oleiferia leaves extract: a natural antioxidant for retarding lipid peroxidation in cooked goat meat patties. International Journal of Food Science and Technology. 47: 585- 591.
Elhadi, A.E., Elgasim, E.A., and Mohamed Ahmed, I.A. 2017. Microbial and oxidation characteristics of refrigerated chicken patty incorporated with moringa (Moringa oleifera) leaf powder. CyTA-Journal of Food. 15: 234-240.
Fahey, J.W. 2005. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Trees for Life Journal. 1.
Gaafar, A.A., Ibrahim, E.A., Asker, M.S., Moustafa, F.A., and Salama, Z.A. 2016. Characterization of polyphenols, polysaccharides by HPLC and their antioxidant, antimicrobial and antiinflammatory activities of defatted Moringa (Moringa oleifera L.) meal extract. International Journal of Pharmaceutical and Clinical Research. 8: 565-573.
Huyut, Z., Beydemir, S. and Gulcin, E. 2017. Antioxidant and antiradical properties of selected flavonoids and phenolic compounds. Biochemistry Research International. 2017: Article ID 7616791.
Jayawardana, B.C., Liyanage, R., Lalantha, N., Iddamalgoda, S., and Weththasinghe, 2015. Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT Food Science and Technology. 64: 1204- 1208.
Juhaimi, F., Ghafoor, K., Hawashin, M.D., Alsawmahi, O.N., and Babiker, E.E. 2016. Effects of different levels of Moringa (Moringa oleifera) seed flour on quality attributes of beef burgers. CyTA-Journal of Food. 14: 1-9.
Leone, A., Spada, A., Battezzati, A., Schiraldi, A., Aristil, J., and Bertoli, S. 2015. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. International Journal of Molecular Sciences. 16: 12791-12835.
Muthukumar, M., Naveena, B.M., Vaithiyanathan, S., Sen, A.R., and Sureshkumar, K. 2014. Effect of incorporation of Moringa oleifera leaves extract on quality of ground pork patties. Journal of Food Science and Technology. 51: 3172-3180.
Paikra, B.K., Dhongade, H.K., and Gidwani, B. 2017. Phytochemistry and pharmacology of Moringa oleifera Lam. Journal of Pharmacopuncture. 20: 194-200.
Shah, M.A., Bosco, J.D., and Mir, S.A. 2015. Effect of Moringa oleifera leaf extract on the physicochemical properties of modified atmosphere packaged raw beef. Food Packaging and Shelf Life. 3: 31–38.
Shahidi, F. and Ambigaipalan, P. 2015. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects-A review. Journal of Functional Foods. 18: 820-897.
Singh, R.S.G, Negi, P.S., and Radha, C. 2013. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. Journal of Functional Foods. 5: 1883-1891.
Tekle, E.W., Sahu, N.P., and Makesh, M. 2015. Antioxidative and antimicrobial activities of different solvent extracts of Moringa oleifera: an in vitro evaluation. International Journal of Scientific and Research Publications. 5.
Vongsak, B., Sithisarn, P., Mangmool, S., Thongpraditchote, S., Wongkrajang, Y., and Gritsanapan, S. 2013. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Industrial Crop s and Products. 44: 566-571.
Wangcharoen, W. and Gomolmanee, S. 2011. Antioxidant capacity and total phenolic content of Moringa oleifera grown in Chiang Mai, Thailand. Thai Journal of Agricultural Science. 44: 118-124.
Wink, M. 2015. Modes of action of herbal medicines and plant secondary metabolites.
Medicines. 2: 251-286.
Wu, W., Rule, D., Busboom, J., Field, R., and Ray, B. 1991. Starter culture and time/temperature of storage influences on quality of fermented mutton sausage. Journal of Food Science. 56: 916-919.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Patcharee Prasajak1,*, Phanida Renumarn2, Wichien Sriwichai2, and Pakkawat Detchewa1
1 Department of Agro-Industry Technology and Management, Faculty of Agro-Industry, King Mongkut’s University of Technology North Bangkok, Prachinburi 25230, Thailand
2 Department of Innovation and Technology of Product Development, Faculty of Agro-Industry, King Mongkut’s University of Technology North Bangkok, Prachinburi 25230, Thailand
Corresponding author: Patcharee Prasajak, E-mail: patcharee.p@agro.kmutnb.ac.th
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: May 12, 2020;
Revised: July 1, 2020;
Accepted: October 12, 2020

