
Production of Antioxidant Bioactive Compounds during Mycelium Growth of Schizophyllum commune on Different Cereal Media
Yossarun Boonthatui, Rewadee Chongsuwat, and Somchoke Kittisakulnam*Published Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.032
Journal Issues : Number 2, April-June 2021
Abstract Schizophyllum commune or split gill mushroom is a nutrient-rich natural food ingredient. Even during mycelium growth, S. commune could produce the bioactive compounds including phenolic and flavonoid compounds. The aim of this research was to characterize the production of bioactive compounds during mycelium growth period of S. commune cultured onto different eight edible cereal media (sorghum, corn, barley, wheat, oat, jasmine rice, Mun Poo rice and riceberry rice). The antioxidant activities and mycelial biomass were also observed during their growth. The results show that the highest mycelial growth rate was obtained onto barley and jasmine rice, while the lowest one was found in sorghum and corn. The concentration of phenolic compounds increased along with the mycelial growth. The fungal culture on wheat significantly exhibited the highest production of phenolic compounds which was 8.56 ± 1.09 mg GAE/g DW on day 8. The highest flavonoid production of 577.35 ± 29.93 µg CE/g DW, was remarkably found onto barley from day 6. The degradation of certain flavonoids in cereal materials by S. commune was also observed, particularly in riceberry rice. Antioxidant activity of cultured cereals was depended on the initial activities of materials and was mainly increased by S. commune metabolism. The DPPH• and ABTS•+ scavenging activities were sharply increased at day 4 which was early stage of log phase of S. commune. At day 8, most of cereal media exhibited high DPPH• activity with a half maximal inhibitory concentration (IC50) range of 3.83-5.80 mg DW/mL, except jasmine rice. Only wheat and oat could significantly give the highest ABTS•+ scavenging activity which was in an IC50 range of 2.38-3.27 mg DW/mL. The highest FRAP activity with a median effective concentration (EC50) of 2.14 ± 0.23 mg DW/mL, was observed in barley which corresponded with its highest flavonoid content. Antioxidant activities of S. commune culture onto cereal media were correspondent with their phenolic and flavonoid contents. Therefore, this study assured that several antioxidative substances were interestingly produced by using S. commune cultured onto the selected edible cereals, which could be possibly developed as a new supplement or active ingredient for pharmaceutical and food industries.
Keywords: Antioxidant activity, Bioactive compound, Cereal, Schizophyllum commune, Split gill mushroom
Funding: The authors are grateful to the Research and Researchers for Industries (RRi) funding from the Thailand Science Research and Innovation (TSRI) with funding No. MSD61I0014 and Chai-Yo mushroom farm for a financial support.
Citation: Boonthatui,Y., Chongsuwat, R., and Kittisakulnam, S. 2021. Production of antioxidant bioactive compounds during mycelium growth of schizophyllum commune on different cereal media. CMUJ. Nat. Sci. 20(2): e2021032.
INTRODUCTION
Schizophyllum commune or commonly known as split gill mushroom is an edible species of Basidiomycetes. This rubber wood-decaying mushroom is predominantly found in the rainforest of southern Thailand. This mushroom has been used as a medicine for a long time because its fruiting body contains several biologically active compounds, for instance, phenolic compounds, vitamins, carboxylic esters and β-glucan namely schizophyllan. These bioactive compounds could exhibit the promising health benefits such as immune system stimulation (Batbayar et al., 2012), antioxidant activity (Wanna et al., 2018), antitumor activity and suppression of inflammation (Takedatsu et al., 2012). Therefore, this mushroom or its isolated fractions have been widely applied in cosmetic, medical, pharmaceutical, nutraceuticals and food industries. Even, during the S. commune mycelial growth period in liquid bacterial media, it could produce the phenolic compounds which showed the radical scavenging activities (Dulay et al., 2016). Phenolic compounds are secondary metabolites, which found in most abundantly in plants, fungi, and bacteria. They play an important role as the defense mechanisms.
The difference in nutrient composition in culture media is an important factor on the mycelial growth and antioxidant synthesis (Hoa et al., 2015). Generally, sorghum or corn is mostly used as a mushroom spawn in Thai mushroom farming because of their cost- and nutrient-effectivenesses for most mushroom fungi growing. Lee et al. (2014) documented that the characteristics of various cereal grains such as size, shape and nutrient content, have an influence on mycelial growth characteristics. Edible cereals in Thai agricultural use, for example, riceberry rice, Mun Poo rice and jasmine rice have been reported in different nutrition compositions (Wangpakapattanawong, 2010). Non-starch polysaccharides are main component in cell wall which consists of cellulose, xylose, arabinose, β-glucan and non-carbohydrate such as phenolic acids and lignin. For example, wheat and corn are rich in arabinoxylan consisting of arabinose and xylose copolymer, whereas barley and oat present a high 1,4 β-glucan content (Knudsen, 2014).
Free radicals are unstable electrons in the atomic orbitals which considered as an important factor affecting to the human health . These radicals could be generated from unhealthy behaviors and pollutions and cause oxidative damage on human cellular DNA and membrane of mitochondria, which consequently leads to the chronic diseases such as endothelium dysfunction, atherosclerosis, diabetes, cardiovascular disease and also the process of aging (Ghebre et al., 2016). Therefore, the body requires antioxidant substances to balance the free radicals and inhibit the oxidative stress. The previous studies reported that the high antioxidant phenolic and flavonoid compounds are found in the mycelium of several edible mushrooms (Vamanu, 2014). The study of Dulay et al. (2016) found that the nutritional condition in substrate had influence on mycelium growth, ability to synthesize the bioactive compounds, and antioxidant properties of S. commune mycelium Hence, this research aimed to exhibit the effect of nutrient composition in edible cereals media on production of mycelial biomass along with antioxidant properties during the mycelium growth period of S. commune, which could be possibly applied and developed in the commercial production process for pharmaceutical and food industries.
MATERIALS AND METHODS
Materials
The starter culture of S. commune was obtained from Chai-Yo farm, Suratthani, Thailand. The cereals used as culture media in this study were purchased from different local markets in Bangkok, Thailand, including whole grain sorghum (Sorghum bicolor), whole grain yellow dent corn (Zea mays var. indentata), pearl barley (Hordeum vulgare) (Raitip, Thanya Farm, Nonthaburi, Thailand), whole grain wheat (Triticum aestivum) (Dr.Green, Bangkok, Thailand), whole grain oat (Avena sativa) (Home Fresh Mart, Capmax Trading, Bangkok, Thailand), white jasmine rice (Oryza sativa) (J.P. Rice International, Surin, Thailand), partially-polished Mun-Poo rice (Naturezone, Bangkok, Thailand), and whole grain riceberry rice (Rice for Health, Shaiyo Triple A Group, Chonburi, Thailand). Potato dextrose agar (PDA) was purchased from Oxoid (Hampshire, UK). All analytical chemicals were purchased from Sigma Chemical (St Louis, MO, USA).
Cultivation of S. commune on cereal media
The preparation of cereal media, 30 g of each cereal material was immersed in water, until obtaining 50% moisture which separately measured by the gravimetric method with oven drying (AOAC, 2005). Each wet cereal was steamed until it was fully cooked in a range 20-30 min. Each cooked cereal was transferred into a flat bottle and sterilized at 121°C for 15 min. Obtained S. commune culture was isolated on PDA in a flat bottle and then incubated at 30°C for 5 days. The isolated fungal colony was cut by using an inoculation loop in triangle shape of 0.5 cm in each side and then transferred onto each sterile cereal media. Inoculated media were incubated in the dark at 30 ± 2°C.
Sample collection
The mycelium-covered cereal samples were collected in the day 0, 2, 4, 6 and 8, then freeze dried with a Christ freeze dryer model beta 2-8 LSC-plus (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode, Germany) to obtain the dried samples with the moisture less than 10%. Dried samples were separately ground into fine powders by using a Cemotec 1,090 miller (Tecator, Hoganas, Sweden) before keeping in a desiccator with no light until further analyses.
Determination of mycelium biomass
The biomass of S. commune was indirectly measured by determining the glucosamine content. A conversion method of chitin in mycelium to glucosamine was modified from Tsuji et al. (1969). Briefly, 100 mg of ground sample was added with 5 mL of 2 M HCl and then boiled (100 ± 2°C) for 2 h. The mixture was placed in room temperature for cooling down before adjusting the volume to 5 mL with distilled water. A 1 mL of the supernatant was subjected to a volumetric flask and added a drop of alcohol solution of 0.5% (w/v) phenolphthalein, then followed carefully added 3 M NaOH until the mixture color changed to pink. The back titration was performed with 1% (w/v) KHSO4 until the pink color disappeared. The titrated mixture was then adjusted with distilled water to 5 mL of volume.
Glucosamine in sample was analyzed by a method of Swift (1973) with Ehrlich’s reagent. A 1 mL of the digested sample was transferred into a glass test tube. Then, a 1 mL of acetylacetone reagent (1 mL of acetylacetone mixed with 50 mL of 0.5 M Na2CO3) was added and the mixture was brought to boil for 20 min. After cooling, the mixture was added with 6 mL of ethanol and 1 mL of Ehrlich’s reagent (2.67 g of p-dimethylaminobenzaldehyde dissolved in 15 mL of ethanol and 15 mL of Conc. HCl). The solution was incubated in water bath at 65°C for 10 min and then cooled before measuring the absorbance at 530 nm with a spectrophotometer (BioTek, Vermont, U.S.).
Sample extraction
Aqueous extraction of sample was carried out. Briefly, a 3 g of sample was added with 30 mL of distilled water, then performed the extraction in a shaking water bath at 42.5°C with a shaking speed of 160 rpm for 195 min. Then, the suspension was centrifuged at 3,857 g for 10 min. The supernatant was collected and filtered through a Whatman No.1 filter paper (Camlab, UK), then kept in an amber glass bottle at 4 ± 2°C until use.
Determination of total phenolic content
Total phenolic content (TPC) was determined according to an assay of Kittisakulnam et al. (2016). A 25 µL of obtained supernatant was mixed with 125 µL of 0.2 M Folin-Ciocalteu reagent in a 96-well microplate. After leaving at room temperature (25 ± 2°C) for 5 min, the mixture was added with 100 µL of 20% (w/v) Na2CO3, and then was allowed to stand in the dark at room temperature for 30 min. The absorbance at 760 nm was measured by using a Varian Cary 50 MPR microplate reader (BioTek, Winooski, VT, USA). The total phenolic contents in all samples were expressed as mg gallic acid equivalents (GAE) per g dried extract.
Determination of total flavonoid content
Total flavonoid content (TFC) in all samples was analyzed by a modified method of Shoib et al. (2015). Briefly, a 150 µL of the supernatant was placed in a 96-well microplate and mixed with 5 µL of 5% (w/v) NaNO2. After 5 min later, a 10 µL of 10% (w/v) AlCl3 was subsequently added into the mixture. After standing for another 6 min, a 70 µL of 1 M NaOH was added and mixed well before measuring the absorbance at 510 nm. The total flavonoid content was expressed as mg catechin equivalents per g dried extract.
Antioxidant activity analyses
DPPH radical scavenging was analyzed by using an adapted method of Chen et al. (2020). Each sample supernatant was mixed with 100 µM ethanolic DPPH solution in a ratio of 1:1 in a 96-well microplate before incubating at 37°C for 30 min without light. The incubated solution was measured an absorbance at 517 nm. The result was showed as an inhibitory concentration giving 50% of radical inhibition (IC50).
ABTS cation radical scavenging was performed followed an assay of Kleekayai (2014). The ABTS stock solution was prepared by mixing 7 mM aqueous ABTS solution and 2.45 mM potassium persulfate at a ratio of 2:1, and then keeping in the dark for at least 16 h before use. The ABTS•+ solution was diluted with 5 mM phosphate buffer saline, pH 7.4 to obtain a 734 nm absorbance of 0.70 ± 0.02. A 10 µL of sample supernatant was mixed with 200 µL diluted ABTS•+ solution and followed incubated without light at room temperature for 6 min before measuring the absorbance at 734 nm. The result was shown as the IC50.
Ferric reducing antioxidant power (FRAP) was analyzed by a modified method of Kittisakulnam et al. (2016). A 26 µL of sample supernatant was mixed with 87 µL of 0.2 M phosphate buffer, pH 6.6 and 87 µL of 1% (w/v) potassium ferricyanide (in 0.2 M phosphate buffer, pH 6.6) and then incubated at 50°C for 20 min. An absorbance was measured to monitor the Perl’s Prussian blue formation at 700 nm. The result of FRAP was reported as an efficient concentration giving 50% of total reducing power (EC50).
Statistical analysis
One-way analysis of variance (ANOVA) in SPSS 18 software (SPSS Inc., Chicago, IL, USA) was used to analyze the data with Duncan’s multiple range test (P <0.05) which was used to determine the significant differences between variables.
RESULTS
Mycelium biomass
Determination of mycelium biomass in the solid-state fermentation was performed by indirectly measuring the glucosamine content which indicated the growth of mushroom fungi. The results of glucosamine content during the growth of S. commune in day 0-8 onto different cereal media are given in Table 1. The fungal growth increased along with the fermentation day. The highest glucosamine content on day 8 was 7.70 ± 0.43 µg/g DW and 7.25 ± 0.12 µg/g DW in jasmine rice and barley media, respectively. There is no significant difference between day 6 and day 8 of fungal biomass in barley. The lowest glucosamine contents were observed in sorghum and yellow dent corn which gave the mycelium biomass in a range of 1.52 - 1.76 μg/g DW at day 8.
Total phenolic and flavonoid contents
The result in Table 1 shows that TPC of S. commune cultured on cereal media continuously increased along the growth period and the highest amounts were obtained on day 8. S. commune on wheat significantly exhibited the highest TPC production with 8.56 ± 1.09 mg GAE/g DW at days 8. The production of TFC was also correlated to the growing time, except in riceberry rice and sorghum which decreased in an early stage of fermentation. The highest quantity of TFC was found in barley with 577.35 ± 29.93 µg CE/g DM, while the lowest TPC and TFC were presented in jasmine rice with 2.90 ± 0.44 mg GAE/g DW and 64.33 ± 3.17 µg CE/g DW, respectively.
Antioxidant activity
The antioxidant activities of different cereal media cultured with S. commune were presented in Table 1. The results were reported in terms of IC50 and EC50 which the low value indicating to high antioxidant ability, and in contrast to high value, shows low activity. Interestingly, antioxidant properties of all fungal fermentations in 3 assays (DPPH, ABTS and FRAP) sharply increased within 2-4 d. Cultured wheat medium on day 8, which contained the highest TPC exhibited the highest antioxidant activities against DPPH• and ABTS•+ with the IC50 of 3.83 ± 0.33 mg/mL and 2.38 ± 0.45 mg/mL, respectively. At day 8, the lowest DPPH• and ABTS•+ scavenging ability were observed in jasmine rice medium, which had the lowest TPC.
Table 1. Changes of fungal biomass, TPC, TFC and antioxidant activities of mycelial S. commune on different cereal media
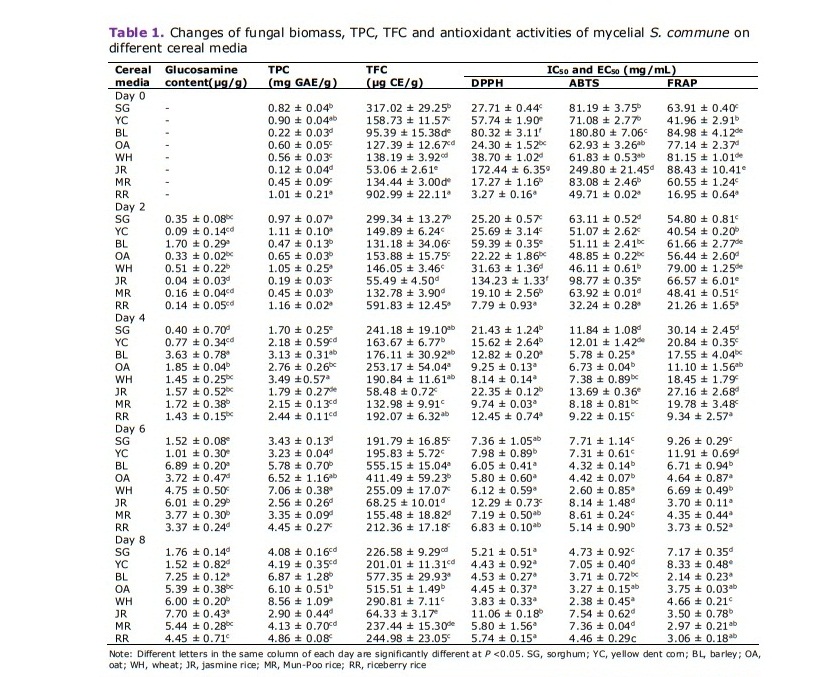
FRAP assay could represent the ability of selected sample to reduce ferric iron (Fe3+) to ferrous iron (Fe2+). Reducing capacities of all cultured media also strongly increased along with the fermentation period. The highest FRAP activities with a EC50 range of 2.14-3.75 mg/mL were obtained from barley, oat, Mun-Poo and riceberry rice media, while the lowest one found in yellow corn with an EC50 of 8.33 ± 0.48 mg/mL.
DISCUSSIONS
Mycelium biomass
The difference of mycelium growth depended on the specific nutrient content in culture media. The most important factor is an adequate amount of carbohydrate and nitrogen sources in the media. Beloshapka et al. (2016) reported that jasmine rice and pearl barley consisted of high starch proportion up to 88% and 73% DM which could be digested into dextrin by glucoamylase of S. commune (Shimazaki et al., 1984). According to the reports of Ibrahim et al. (2011) suggested that dextrin is a good carbon source and positively promotes the growth of S. commune more than other carbohydrates such as monosaccharides, disaccharides, sugar alcohols and soluble starch.
In addition, Alam et al. (2010) found that organic nitrogen sources are more effective on the mycelium growth than inorganic nitrogen sources. This results were supported by a report of Hoa et al. (2015) suggested that mycelium density of Pleurotus ostreatus and P. cystidiosus cultured in the organic nitrogen containing medium was denser than that in the inorganic nitrogen containing medium. Several cereal grains are the sources of amino acids which considered as the organic nitrogen compounds (Cervantes-Pahm et al., 2014). Mosse (1990) reported that barley and oat consisted of high nitrogen content up to 4.01% and 3.80%, respectively which supported mycelium growth in this result. Nasreen et al. (2015) exhibited that the gram powder was a suitable nitrogen source for the growth of S. commune. Adebayo-Tayo et al. (2011) also reported that glycine was the most suitable nitrogen source for mycelium growth of mushroom Macrolepiota procera and P. ostreatus.
The digestion resistance of full-grain cereal considerably retards the fungal catabolism on conversion of the nutrients to the consumable energy. Jasmine rice and barley are normally passed the milling process which removes the hardly digestible polysaccharides containing parts including husk and bran. The starch digestion was rapidly occurred in barley medium causing S. commune mycelium sharply grew with significant difference among other cereals from day 2 until day 6. In contrast, the slowest growth was observed in corn which has a thick seed coat consisting of cellulose, xylose, and lignin (Knudsen, 2014). Similarly, Tripathy et al. (2009) observed that thickness of seed coat have effect to mycelium growth.
Interestingly, the phenolic compounds derived from cereal could slightly restrain the mycelium growth. High phenolic compounds containing cereal like riceberry rice, yellow dent corn and red sorghum significantly showed less fungal biomass than the low phenolic compounds containing cereals (jasmine rice and pearl barley). Normally, phenolic compounds are abundant in cereal bran fraction, therefore the cereals passed the bran removing procedure, contain lower in them. Upadhyay et al. (2016) reported that phenol addition in media inhibited the mycelial biomass production of basidiomycetous fungi. With an agreement of Yildiz et al. (2017), they found that there was an inverse relation between concentration of antioxidant compounds and growth rate of P. ostreatus and P. citrinopileatus (oyster mushroom).
Total phenolic and flavonoid contents
The increases of TPC and TFC were closely related to the extracellular enzyme activity that digested the culture materials and released the bound nutrients which consequently were used for antioxidant synthesis. Mostly, cell walls of cereal grains were digested with extracellular enzymes such as depolymerase, glycosidase, esterase, xylanase, laccase and peroxidases, and then released the free phenol compounds (Bonnin et al., 2002; McCUE et al., 2003). Whole grain wheat medium was rich in lignin which was in bran fraction, after lignin digestion by the fungus enzymes, the cinnamic acid derivatives were released resulting in the increment of TPC (Knudsen, 2014).
Moreover, S. commune could produce the antioxidant compounds by using shikimate pathway. This pathway generally was used for generating precursor chorismate from a central metabolic pathway, to eventually form antioxidative secondary metabolites, such as coumarin derivatives, isoflavones, flavones, flavonols, anthocyanins, tannins and other phenolic compounds. The carbon substrates particularly glucose could flow into this pathway via glycolysis. Barros et al. (2008) reported that glucose was the best carbon source for synthesizing phenol and flavonoid compounds in Leucopaxillus giganteus mycelium. Additionally, Tsujiyama (2009) also documented that the increase in phenolic compound production of S. commune was positively supported by increment of arabinoxylan content in solid state media. Arabinoxylan is mostly found in wheat, barley and oat (Knudsen, 2014).
The result was remarkably found that the reduction of TFC in riceberry rice and sorghum at the early period of the fungal growth. The most of flavonoid compounds in riceberry rice and red sorghum are anthocyanins belonging to flavonoids which characterized with heavy pigmented outer layer and they are attached to the cell wall sugars like arabinose, xylose, and glucose with the glycosidic bonds (Sivamaruthi et al., 2018). After cleaving these bonds by fungal β-glucosidases, the free anthocyanins are released and possibly oxidized by the extracellular phenol oxidase (laccase) and peroxidase of S. commune resulting in the decrease of TFC (Tovar-Herrera et al., 2018).
Antioxidant activity
DPPH, ABTS and FRAP have a positive correlation between antioxidant activity and TPC was observed in all cultured cereal media. There have been various studies establishing that the antioxidative activities were correlated to TPC. Mirfat et al. (2010) found that the fruiting body extracts of S. commune had the DPPH• scavenging activity with strong correlation to TPC. This also was in an agreement in other edible mushrooms (Cheung et al., 2003).
The results from our observation suggested that the antioxidant activities of S. commune mycelium onto edible cereals mostly came from the phenolic compounds. In addition, other bioactive compounds that formed during fermentation period beside phenolic and flavonoid compounds, such as schizophyllan (Li et al., 2011), amino acids (Anraku et al., 2015) and vitamins (Adejoye et al., 2007), were also reported to involve in antioxidant mechanisms.
CONCLUSIONS
The S. commune mycelium growth and its antioxidant properties were strongly affected by the nutrient compositions derived from each edible cereal. The starch-rich cereal sources like barley and jasmine rice were the most suitable media for S. commune mycelial growth. For the antioxidant production, wheat had the highest TPC which was corresponded with its highest DPPH• and ABTS•+ scavenging activities. The highest FRAP was obtained from barley which probable have other bioactive compounds from production of S. commune that show antioxidant activity. Therefore, solid-state fermentation of S. commune onto edible cereals could be effectively performed for antioxidative substance production. This finding has a potential to further develop for pharmaceutical and value-added foodstuffs in the future.
ACKNOWLEDGEMENTS
We also would like to thank the Department of Nutrition, Faculty of Public health, Mahidol University for a technical support to complete this research work.
REFERENCES
Adejoye, O., Adebayo-Tayo, B., Ogunjobi, A., and Afolabi, O. 2007. Physicochemical studies on Schizophyllum commune (Fries) a Nigerian edible fungus. World Applied Sciences Journal. 2: 73-76.
Adebayo-Tayo, B., Jonathan, G., Popoola, O.O., and Egbomuche, R.C. 2011. Optimization of growth conditions for mycelial yield and exopolysaccharride production by Pleurotus ostreatus cultivated in Nigeria. African Journal of Microbiology Research. 5: 2130-2138.
Alam, N., Cha, Y.J., Shim, M.J., Lee, T.S., and Lee, U.Y. 2010. Cultural conditions for mycelial growth and molecular phylogenetic relationship in different wild strains of Schizophyllum commune. Mycobiology. 38: 17-25.
Anraku, M., Shintomo, R., Taguchi, K., Kragh-Hansen, U., Kai, T., Maruyama, T., and Otagiri, M. 2015. Amino acids of importance for the antioxidant activity of human serum albumin as revealed by recombinant mutants and genetic variants. Life Sciences. 134: 36-41.
Association of official analytical chemist (AOAC). 2005. Office methods of analysis of the AOAC. 18th ed. Washington DC.
Barros, L., Ferreira, I.C. and Baptista, P. 2008. Phenolics and antioxidant activity of mushroom Leucopaxillus giganteus mycelium at different carbon sources. Food Science and Technology International. 14: 47-55.
Batbayar, S., Lee, D.H., and Kim, H.W. 2012. Immunomodulation of fungal β-glucan in host defense signaling by dectin-1. Biomolecules and Therapeutics. 20: 433- 445.
Beloshapka, A., Buff, P., Fahey, G., and Swanson, K. 2016. Compositional analysis of whole grains, processed grains, grain co-products, and other carbohydrate sources with applicability to pet animal nutrition. Foods, 5: 23.
Bonnin, E., Saulnier, L., Brunel, M., Marot, C., Lesage-Meessen, L., Asther, M., and Thibault, J.F. 2002. Release of ferulic acid from agroindustrial by-products by the cell wall-degrading enzymes produced by Aspergillus niger I-1472. Enzyme and Microbial Technology. 31: 1000-1005.
Cervantes-Pahm, S.K., Liu, Y., and Stein, H.H. 2014. Digestible indispensable amino acid score and digestible amino acids in eight cereal grains. British Journal of Nutrition. 111: 1663-1672.
Chen, X., Liang, L., and Han, C. 2020. Borate suppresses the scavenging activity of gallic acid and plant polyphenol extracts on DPPH radical: a potential interference to DPPH assay. LWT. 131: 109769.
Cheung, L., Cheung, P.C., and Ooi, V.E. 2003. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chemistry. 81: 249-255.
Dulay, R.M.R., Vicente, J.J.A., Cruz, A.D., Gagarin, J.M., Fernando, W., Kalaw, S.P. and Reyes, R.G. 2016. Antioxidant activity and total phenolic content of Volvariella volvacea and Schizophyllum commune mycelia cultured in indigenous liquid media. Mycosphere. 7: 131-138.
Ghebre, Y.T., Yakubov, E., Wong, W.T., Krishnamurthy, P., Sayed, N., Sikora, A.G., and Bonnen, M.D. 2016. Vascular aging: implications for cardiovascular disease and therapy. Translational Medicine (Sunnyvale, Calif.). 6: 183.
Hoa, H.T. and Wang, C.L. 2015. The effects of temperature and nutritional conditions on mycelium growth of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology. 43: 14-23.
Ibrahim, S., Mel, M., and Mohd Nor Rashid, N. 2011. Carbon and nitrogen effect on exopolysaccharides production of Schizophyllum commune. International Congress of the Malaysian Society for Microbiology, Penang, Malaysia. December 8-11 2011.
Kittisakulnam, S., Saetae, D., and Suntornsuk, W. 2016. Antioxidant and antibacterial activities of spices traditionally used in fermented meat products. Journal of Food Processing and Preservation. 41: e13004.
Kleekayai, T., Saetae, D., Wattanachaiyingyong, W., Tachibana S., Yasuda, M., and Suntornsuk, W. 2014. Characterization and in vitro biological activities of Thaitraditional fermented shrimp pastes. Journal of Food Science and Technology. 52.
Knudsen, K.E.B. 2014. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poultry Science. 93: 2380-2393.
Lee, B.J., Lee, M.A., Kim, Y.G., Lee, K.W., Lee, B.E., and Seo, G.S. 2014. Characteristics and suitability of various cereal grains in spawn production of button mushroom. Journal of Mushroom. 12: 237-243.
Li, W., Wang, G., Zhou, P., Yu, L., and Zhang, Z. 2011. Optimization of conditions for schizophyllan production in submerged culture of Schizophyllum commune. doi: 10.1109/HHBE.2011.6027945. In: Proceedings 2011, the International Conference on Human Health and Biomedical Engineering. Jilin, China, 19-22 Aug 2011. Institute of Electrical and Electronics Engineers, New York.
McCue, P., Horii, A., and Shetty, K. 2003. Solid‐state bioconversion of phenolic antioxidants from defatted soybean powders by Rhizopus oligosporus: role of carbohydrate‐cleaving enzymes. Journal of Food Biochemistry. 27: 501-514.
Mirfat, A., Noorlidah, A., and Vikineswary, S. 2010. Scavenging activity of Schizophyllum commune extracts and its correlation to total phenolic content. Journal of Tropical Agriculture and Food Science. 38: 231-238.
Mosse, J. 1990. Nitrogen-to-protein conversion factor for ten cereals and six legumes or oilseeds. A reappraisal of its definition and determination. Variation according to species and to seed protein content. Journal of Agricultural and Food Chemistry. 38: 18-24.
Nasreen, Z., Khan, S.J, Yasmeen, A., Shafique, M., Usman, S., and Ali, S. 2015. Optimization of sub-merged culture conditions for biomass production in Schizophyllum commune, a medicinal mushroom. International Journal of Current Microbiology and Applied Sciences. 4: 258-266.
Shimazaki, T., Hara, S., and Sato, M. 1984. Production, purification and some properties of extracellular amylase of Schizophyllum commune. Journal of Fermentation Technology. 62: 165-170.
Shoib, BA. and Shahid, MA. 2015. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. Journal of Taibah University for Science. 9: 449-454.
Sivamaruthi, B., Kesika, P., and Chaiyasut, C. 2018. Anthocyanins in Thai rice varieties: Distribution and pharmacological significance. International Food Research Journal. 25: 2024-2032.
Swift, M.J. 1973. The estimation of mycelial biomass by determination of the hexosamine content of wood tissue decayed by fungi. Soil Biology and Biochemistry. 5: 321-332.
Takedatsu, H., Mitsuyama, K., Mochizuki, S., Kobayashi, T., Sakurai, K., Takeda, H., and Sata, M. 2012. A new therapeutic approach using a schizophyllan-based drug delivery system for inflammatory bowel disease. Molecular therapy : Journal of the American Society of Gene Therapy. 20: 1234-1241.
Tovar-Herrera, O.E., Martha-Paz, A.M., Pérez-Lano, Y., Aranda, E., Tacoronte-Morales, J.E., Pedroso-Cabrera, M.T., Arévalo-Niño, K., Folch-Mallol, J.L., and Batista-García,
R.A. 2018. Schizophyllum commune: an unexploited source for lignocellulose degrading enzymes. MicrobiologyOpen. 7: e00637.
Tripathy, A., Patel, A., and Sahoo, T. 2009. Effect of various substrates on linear mycelial growth and fructification of Volvariella diplasia. Asian Journal of Plant Sciences. 8: 566-569.
Tsuji, A., Kinoshta, T., and Hosino, M. 1969. Analytical-chemical studies of amino sugars
- Determination of hexosamines using 3-methyl-2-benzothiazolone-hydrazone hydrochloride. Chemical and Pharmaceutical Bulletin. 17: 1505-1510.
Tsujiyama, S.I. and Ueno, M. 2008. Formation of 4-vinyl guaiacol as an intermediate in bioconversion of ferulic acid by Schizophyllum commune. Bioscience, Biotechnology, and Biochemistry. 72: 212-215.
Upadhyay, P., Shrivastava, R., and Agrawal, P.K. 2016. Bioprospecting and biotechnological applications of fungal laccase. 3 Biotech, 6: 15.
Vamanu, E. 2014. Antioxidant properties of mushroom mycelia obtained by batch cultivation and tocopherol content affected by extraction procedures. BioMed Research International. 2014: 974804.
Wangpakapattanawong, P. 2010. Nutrition of rice and innovative utilization. Thai Journal of Clinical Nutrition. 4: 32-40.
Wanna, C. and Sudhadham, M. 2018. The effect of coconut water and boiling on antioxidant activity and total phenolic contents in Schizophyllum commune. Pharmacognosy Journal. 10: 925-931.
Yıldız, S., Yılmaz, A., Can, Z., Kılıç, C. and Yıldız, Ü. 2017. Total phenolic, flavonoid, tannin contents and antioxidant properties of Pleurotus ostreatus and Pleurotus citrinopileatus cultivated on various sawdust. The Journal of Food. 42: 315-323.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Yossarun Boonthatui, Rewadee Chongsuwat, and Somchoke Kittisakulnam*
Department of Nutrition, Faculty of Public Health, Mahidol University, Bangkok 10400, Thailand
Corresponding author: Somchoke Kittisakulnam, E-mail: somchoke.kit@mahidol.ac.th
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: May 12, 2020;
Revised: July 1, 2020;
Accepted: October 12, 2020

