
Evaluation of The Antioxidant, Antidiabetic and Immunomodulatory Activity of Cydonia oblonga Fruit Extract
Fatma Zahra Sakhri*, Sakina Zerizer*, Chawki Bensouici
Published Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.052
Journal Issues :
Number 3, July-September 2021
Evaluation of The Antioxidant, Antidiabetic and Immunomodulatory Activity of Cydonia oblonga Fruit Extract
Abstract Dietary natural antioxidant consumption can protect the human body from several diseases induced by free radicals. The aim of this study was to evaluate the antioxidant, antidiabetic and immunomodulatory properties of Cydonia oblonga fruit. For this; hydroethanolic extract of Cydonia oblonga fruit (HECO) was examined for antioxidant activity using DPPH free radical sc avenging, ABTS cation radical decolorization, Cupric reducing antioxidant capacity (CUPRAC), and Metal Chelating on ferrous ions activities. The inhibitory activity of the extract against α-glucosidase enzyme was also investigated. HECO was tested in vivo for the immunomodulatory activity on non-specific immunity by the carbon clearance test. The content of the nonenzymatic antioxidant reduced glutathione (GSH) in liver tissue of used mice was estimated. in vitro studies revealed that the HECO has an inhibitory concentration (IC50) value of 249.26 ± 3.75μg/mL, 117.34 ± 1.41 μg/ml for DPPH and ABTS scavenging activity respectively. As well as the ability to reduce cupric (167.17 ± 1.15μg/mL) and iron (Fe) (417.98 ± 48.82μg/mL). The extract showed antidiabetic activity as evidenced by its capacity to inhibit the α-glucosidase enzyme (IC50: 326.48 ± 18.56 µg/mL) near the acarbose (IC50: 275.98 ± 1.57 µg/mL) used as a positive control. In addition, our results showed that HECO at the concentration of 50 and 100 mg/kg significantly increased the clearance rate of carbon from the bloodstream concomitant with increased liberation of GSH from liver cells. This study demonstrates that HECO is effective in scavenging free radicals and can serve as potent antioxidants that provide potential treatment and prevention for diabetes with benefits on the innate defense system.
Keywords: Antidiabetic, Antioxidant, Cydonia oblonga, Hydroethanolic extract, Phagocytic activity
Funding: The authors are grateful to the ministry of higher education and scientific research, Algeria for the Financial support.
Citation: Sakhri, F.Z., Zerizer, S., and Bensouici, C. 2021. Evaluation of the antioxidant, antidiabetic and immunomodulatory activity of Cydonia oblonga fruit extract. CMUJ. Nat. Sci. 20(3): e2021052.
INTRODUCTION
Cydonia Oblonga Mill. commonly known as quince, Sfarjel, a plant that belongs to the Rosaceae family. The fruit, leaf, and seeds of the quince have long been used in folk medicine for treatment and prevention of several diseases such as bronchitis, diuretic, cystitis, cardiovascular diseases, diarrhea, hepatitis, vomiting (Zhou et al., 2014, Mirmohammadlu et al., 2015, Umar et al., 2015, Ashraf et al.,2016). Pharmacological studies show a wide spectrum of health benefits of quince fruit such as antioxidant, anti-microbial, anti-ulcer, antidiabetic, antiallergic, antiproliferative, and neuroprotective activities (Silva et al., 2004, Hamauzu et al., 2005, Fattouch et al., 2007; Khoubnasabjafari and Jouyban, 2011; Aslam and Sial, 2014; Mohebi et al., 2019; Sakhri et al., 2020).
The medicinal value of the plant is related to the presence of diverse phytochemical components such as phenolic acids and flavonoids compounds (Caffeoylquinic acids, kaempferol, and quercetin glycosides), vitamins (A, C, E, riboflavin, folic acid, and K), and minerals like calcium, potassium, and phosphorus (Silva et al., 2005; Rop et al., 2011; Wojdylo et al., 2013; Umar et al.,2015).
Reactive oxygen species (ROS) are byproducts formed during aerobic metabolism and are fundamental to several signaling processes (Forrester et al., 2018). The ROS generated in humans during phagocytosis is cleared by the presence of antioxidants in the body such as enzymes and small molecules (intracellular reduced glutathione (GSH), oxidized glutathione, bilirubin, uric acid...). However, overproduction of ROS induced an imbalance between oxidative stress and the antioxidative defense system which leads to the initiation or progression of several diseases including diabetes, (Pavithra & Vadivukkarasi, 2015). Diabetes is a chronic metabolic disorder with nearly half a billion people (9.3% of adults 20–79 years) are its victim (Saeedi et al., 2019). This estimated number is expected to rise to 642 million by 2040 (Herman, 2017). Besides the higher morbidity of diabetes and adverse side-effects of currently available drugs, the onset of diabetes is accompanied by a failure in the neutrophil functions, including phagocytosis, and bacterial killing, which leads to increased risk for infection diseases (Lecube et al., 2011). Thus, the need to search for safer, more effective less costly therapy for both prevent and treat this pandemic is desired.
The main purpose of the present study is to investigate the antioxidative, antidiabetic potential of the quince fruit, and its benefits on the innate immune system.
MATERIALS AND METHODS
Reagents and solvent
1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2'-azino-bis(3-ethylbenzothiazoline- 6sulfonicacid) diammonium salt (ABTS), 3-(2-Pyridyl)-5,6-di(2-furyl)-1,2,4-triazine- 5′,5′′-disulfonic acid disodium salt (Ferene), butylatedhydroxylanisole (BHA), butylatedhydroxyltoluene (BHT), α-Tocopherol, Ethanol, Ethylenediaminetetraacetic acid (EDTA), Neocuproine, p-nitrophenyl- α-D-glucopyranoside, 5,50-dithiobis (2- nitrobenzoic) acid (DTNB), 5-sulfosalicylic acid and gelatin were obtained from Sigma Chemical Co (Sigma-Aldrich GmbH, Stern-heim, Germany). Copper (II) chloride, Potassium persulfate, Sodium Carbonate, Aluminum Nitrate, Iron (II) chloride, Potassium acetate, Ammonium acetate were purchased from Biochem Chemopharma. All other chemicals were purchased from standard local source.
Sample preparation and extraction
The quince fruits were purchased from the local market of Constantine- Algeria in the month of December 2017. Fresh fruits with uniform maturity were cleaned, the seeds were removed, fruits cut in thin slices, and dried in shade at room temperature. The fine powder was prepared from 250g of dried slices and macerated on a sufficient volume of ethanol/water (70/30) for 24 hours. This process was repeated three times. The macerate was filtered, centrifuged, and the solvent was eliminated by a rotary evaporator (Buchi R-215) at 40°C. The residue obtained was lyophilized to dry powder and stored in -20°C until use.
Evaluation of the antioxidant activity
DPPH free radical scavenging assay
The free radical-scavenging activity of the extract against stable DPPH was evaluated as previously described (Bensouici et al., 2016) with slight modifications. A methanolic solution of DPPH (0.3mM) was freshly prepared and 160µL of this solution was added to 40 µL of sample extracts at different concentrations. The resultant mixtures were left at room temperature for 20 min and the absorbance was measured at 517 nm. BHA, BHT, and α-tocopherol were used as antioxidant standards to compare the activity. The DPPH free radical dissolved in methanol solution can easily receive an electron or hydrogen radical from antioxidant compounds to become a stable diamagnetic molecule and losses its purple coloration. The results are given as 50% inhibition concentration (IC50) and the inhibition percentage was calculated using the following equation:
%Inhibition = [(Abs(c) – Abs(E))/Abs(c)] x 100
Abs(c) is the absorbance of the control reaction; Abs(E) is the absorbance of the extract or standard.
ABTS radical scavenging assay
The ABTS scavenging assay was done as described by (Khalfallah et al., 2017). The ABTS radical cations (ABTS+) were produced by the reaction between 7mM ABTS in water and 2.45 mM potassium persulphate, stored in the dark at ambient temperature for 12–16 h before use. The resulting solution was diluted to get an absorbance of 0.703 ± 0.025 at 734nm with distilled water. A mixture of 40 µL of the sample at different concentrations and 160µl of the ABTS+ radical solution was left at room temperature for 10 min and the absorbance at 734 nm was recorded immediately. BHA and BHT were used as antioxidant standards for comparison of the activity. The results were given as IC50 (µg/mL) corresponding to the concentration of 50% inhibition.
%Inhibition = [(Abs(c) – Abs(E))/Abs(c)] x 100
Abs(c) is the absorbance of the control reaction; Abs(E) is the absorbance of the extract or standard
Cupric reducing antioxidant capacity (CUPRAC)
The cupric reducing antioxidant capacity was measured according to Apak et al. (2004) with slight modifications. A mixture of 50 μL of copper (II) chloride solution (10 mM), 50 µL of neocaproine solution (7.5 mM), and 60 μL of ammonium acetate buffer (1 M, pH 7.0) was added in each well of 96 well plates. The 40 μL of the extract at different concentrations was added to the initial mixture. One hour after, the absorbance at 450nm was recorded.
BHA and BHT were used as antioxidant standards for comparison of the activity. The results were given as A0.50 (µg/mL) corresponding to the concentration indicating 0.50 absorbance intensity.
The presence of antioxidant compounds reduced the cupric ions to cuprous ions, resultant in the formation of a stable complex between neocuproine and cuprous ions. A higher absorbance indicates a higher reducing capacity of antioxidants.
Metal chelating activity on ferrous ions.
The chelating activity of the extracts on Fe2+ was measured as previously reported (Bensouici et al., 2016), Briefly, the extract solution (80 μL dissolved in ethanol in different concentrations) was added to 40 μL 0.2 mM FeCl2. The reaction was initiated by the addition of 80 μL 0.5 mM ferene. The mixture was shaken vigorously and left standing at room temperature for 10 min. After the mixture reached equilibrium, the absorbance was measured at 593 nm. EDTA was used as an antioxidant standard for comparison of the activity.
The metal chelating activity was calculated using the following equation:
%Inhibition = [(Abs(c) – Abs(E))/Abs(c)] x 100
Where (Ac)is the absorbance of control devoid of sample and (AE)is the absorbance of sample in the presence of the chelator. The results were given as IC50 (µg/mL).
Evaluation of the α-glucosidase inhibitor activity
The α-glucosidase inhibitor activity was investigated in vitro in accordance with (Lordan et al., 2013). The extract solution (50μL at different concentrations) was mixed with 50 μL of p-nitrophenyl-a-D-glucopyranoside (NPG) solution 5mM (in phosphate buffer 100mM, pH 6.9) and incubated at 37°C. After 10 min, 100μL of alpha-glucosidase (0.1 U/mL) was added to the mixture and the absorbance was recorded at 405 nm two times (0, 30min) using an ELISA microplate reader. Acarbose (1g/ml) was used as a positive control. The results were given as IC50 value (μg/mL) corresponding to the concentration shows 50% inhibition.
Evaluation of in vivo phagocytic activity
The phagocytic activity of HECO was evaluated in vivo by the carbon clearance test (Benmebarek et al., 2013). Adult Mus musculus female mice (32–40g, 2–2.5 months old) were procured from central pharmacy Algeria. The animals were kept in polyacrylic cages and maintained under standard housing conditions (room temperature 25 ± 1C with 12:12 light: dark cycles). Food was provided in the form of dry pellets (SARL Production Locale, Bouzareah, Algeria) and water ad libitum. The animal study was conducted after obtaining clearance from the ethics committee of institutional animals and conducted according to the Executive Decree n°10–90 completing the Executive Decree n°04–82 of the Algerian Government, and the ethical principles and guidelines provided by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Mice were divided into six groups of five animals each. The control group (GI) was received 0.5 ml of the vehicle (0.9% NaCl) intraperitoneally, while treatment groups GII to GV were given HECO in (12.5, 25, 50, 100, and 200 mg/kg/ i.p) doses respectively. After 48h, carbon ink suspension (3ml black carbon ink, 4ml saline, and 4ml 3% gelatin solutions) was injected intravenously to each mouse in a volume of 0.1ml/10g. Blood samples were drawn from the orbital vein at an interval of 5 and 15min after the injection of ink dispersion. Collecting blood samples (25μl) were lysed on 4ml of 0.1% Na2CO3 solution and subjected for determination of optical densities at 675 nm.
After the second blood sample had been obtained, the animal was sacrificed by rapid cervical dislocation and the liver was dissected immediately.
The phagocytic activity is expressed by the phagocytic index K that measures all the reticuloendothelial system function in the contact with the circulating blood. The clearance rate is expressed as the half-life period of the carbon in the blood (t1/2, second). Calculations were performed by the following equations:
K = (ln (OD1) – ln (OD2)) /(t2-t1)
t(1/2)= 0:693/K
Where, OD1 and OD2 are the optical densities at times t1 and t2, respectively.
Reduced Glutathione assay (GSH)
Preparation of homogenate solution
Tissue homogenates were prepared using the standard method (Messaoudi et al., 2019). From each mouse, 0,5g liver tissue was homogenized in 2ml of tris buffered saline (TBS; Tris-Cl 50 mM, NaCl 150 mM, pH 7.4) and centrifuged (9,000g/15 min) at 4˚C. The supernatant was used to measure the GSH.
Estimation of reduced glutathione level
GSH content was measured as described by Iweala et al. (2019) with slight modifications. For this, 800μl of homogenate sample was deproteinized with 200μl of 5-sulfosalicylic acid (0.25%) and centrifuged at 1000 rpm for 5min. 1ml of tris-HCL buffer (0.4 M, pH 9.61 containing 20 mM EDTA) and 25 μl of DTNB (0.01M 5,5′-dithiobis-2 nitrobenzoic acid) were mixed with 500μl of the supernatant and leave at room temperature for 5 min. The increase in absorbance due to the formation of TNB was measured at 412 nm.
Data processing:
All in vitro experiments were carried out at least in triplicate. Data were processed with SPSS 25.0 statistics software and results are given as mean ± standard errors of the mean (S.E.M). Multiple means were compared with One-Way ANOVA followed by Tukey’s multiple comparison tests. The values of P < 0.05 were considered to indicate a significant difference.
RESULTS
Extraction yield and antioxidant activity
The extract yield of Cydonia oblonga fruit was 28.74% and the results of the antioxidant activity tests are shown in table 1. In DPPH the HECO exhibited a fairly well activity (249.26 ± 3.75 μg/mL). The extract displayed a good activity in ABTS (IC50 = 117.84 ± 1.41 μg/mL), and in CUPRAC (A0.5 = 167.17 ± 1.15 μg/mL). The antioxidant activity in metal chelating of ferrous ions was (417.98 ± 48.82 μg/mL). However, the extract had not compared results with the standards.
Table 1. Antioxidant activity of Hydroethanolic extract of Cydonia oblonga fruit (HECO).
α-Glucosidase inhibitory activity
The standard curve of the inhibitory effects of acarbose (12.5–200 mg/mL) against α -Glucosidase showed the dose dependent activity (r2 = 0.9813). The HECO showed good inhibitory activity of α-Glucosidase (IC50: 326.48 ± 18.56 µg/mL) near the acarbose (IC50: 275.98 ± 1.57 µg/mL) used as a standard.
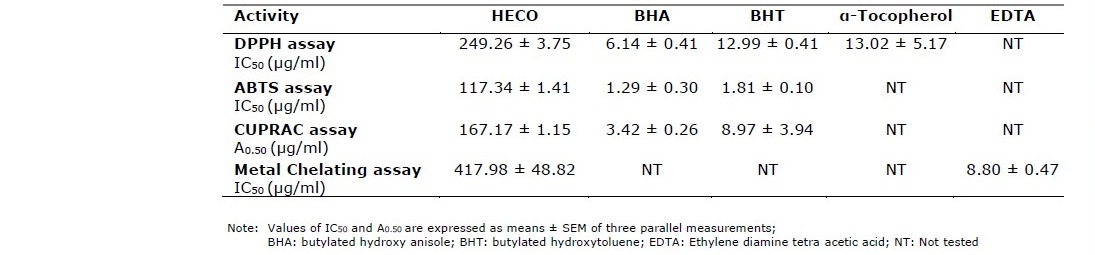
Phagocytic index
Results obtained showed an increase in the phagocytic index in all groups pretreated with HECO (12.5, 25, =50, 100, 200 mg/kg b.w) as compared to the control group (Figure 2). The groups pretreated with HECO at the dose (50-100 mg/kg) had the highest phagocytic index comparing to other groups as well as the shorter half time of the carbon in the bloodstream (P ˂ 0.01vs control group; Figure 1).
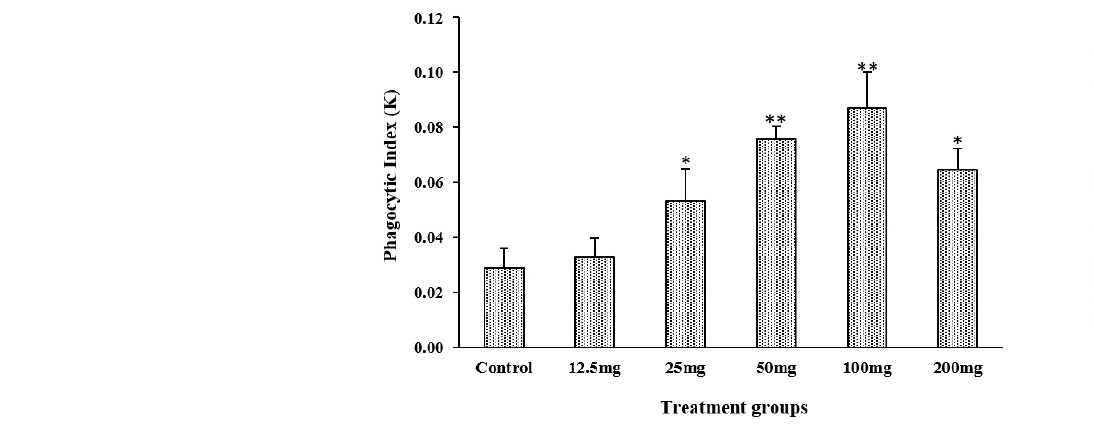
Figure 1, 2. Effect of HECO on the half time of carbon in the bloodstream. n = 5, values are the mean ± SEM, *P ˂ 0.05, **P ˂ 0.01 vs control group.
Liver GSH contents in the pretreated mice with HECO (25, 50, 100 and 200 mg/kg b.w) were significantly decreased, compared to the control group (P ˂ 0.01 (mice treated with HECO (25, 200 mg/kg) vs control group), and P ˂ 0.001(mice treated with HECO (50, 100 mg/kg) vs control group), Figure 3). No difference was noted between groups pretreated with HECO at the doses (50, 100, 200 mg/kg b.w).
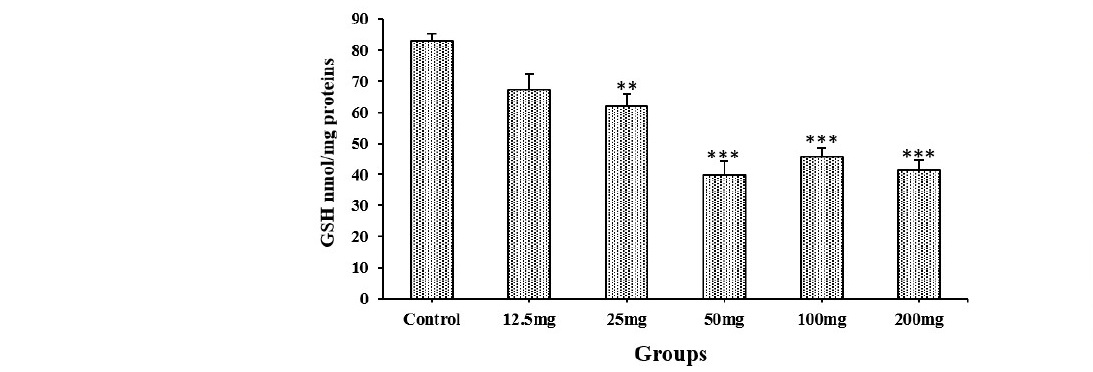
Figure 3. Reduced glutathione levels in the liver of control and mice pretreated with HECO after Carbon ink injection. n = 5, values are the mean ± SEM, **P ˂ 0.01, ***P ˂ 0.001 vs control group.
DISCUSSION
Cydonia oblonga fruit is one of the fruits that has not been exploring well as fresh fruit, our experiment differs from studies of (Silva et al., 2004; Hamauzi et al.,2005; Silva et al., 2005; Fattouch et al.,2007; Rop et al., 2011, Sut et al., 2019) that we did not separate the peel to the pulp of the fruit. The extraction of quince fruit was carried out with ethanol/water (70%) which is suitable for extracting bioactive compounds with strong polarity and antioxidant properties (Sun et al., 2015). The result of extract yield was higher than that obtained by Sut et al. (2019) who recorded 6.8% and 8.5% for quince peels and pulps respectively. The obtained extract (HECO) was examined for its in vitro antioxidant, anti-diabetic activities, and in vivo phagocytic activity.
Due to the presence of a mixture of compounds with different functional groups, resultant different kinds of defense systems in HECO, the evaluation of the antioxidant activity was carried out using four different methods DPPH, ABTS, CUPRAC, and metal chelating on ferrous ions, which allowed us to identify an antioxidant profile of the extract on the basis of its reactivity towards radical species, and its ability to reduce transition metals.
As it was expected, HECO antioxidant capacities increased in a concentration- dependent manner and the ABTS radical was found to be higher than that of DPPH radical. The capacity of ABTS to react with hydrophilic and lipophilic compounds main explains this difference.
HECO exhibited better scavenging of DPPH activity than that reported by Silva et al (2004) for peel and pulp extracts (IC50= 0.6 mg/mL; 1.7 mg/mL, respectively). Hamauzu et al. (2005) evaluated the antioxidant properties of Chinese quince, quince, and apple fruits using DPPH method. They found that Chinese quince and quince fruit showed slightly higher activities than apple fruit, which had mainly low polymerized procyanidins as flavan-3-ol series.
Regarding the CUPRAC method, it is a redox assay, based on the reduction of Cu(II) to Cu(I) by antioxidants present in the plant extracts using copper(II)- neocuproine reagent as the chromogenic oxidizing agent (Apak et al 2004). According to table 1, HECO exerted an interesting capacity to reduce copper ions. Reducting property is one of the mechanisms which can refer to the capacity of compounds to regenerate another compound already oxidized by free radicals or to stop the free radical chain reaction (Jayaprakasha et al., 2001).
Among the transition metals, iron is commonly found in food systems and considered to be the most effective pro-oxidants due to its high reactivity. The ferrous ions are well known as an initiator for the unwanted production of free radicals by breaking down hydrogen and lipid peroxides via the Fenton reaction. Therefore, the minimization of these ions affords protection against oxidative damage (KÖksal et al., 2009). According to the metal chelating data, HECO may offer protection to the cells against oxidative damage induced by an excess of Fe+2 ions in normal physiology.
Wojdylo et al. (2013) evaluated the antioxidant properties of 13 different varieties of quince fruit using the DPPH, ABTS, and FRAP methods. They found a significant variation in antioxidant activity among the studied varieties which ranged between 0.9 and 2.4 μmol trolox/g dry matter for ABTS, 0.9 and 2.5 for DPPH as well as 0.4 and 1.5 for the FRAP method. In addition, the total antioxidant in the hydrophilic fraction is higher than lipophilic in both the pulp and peel parts of the fruit (Silva et al., 2004).
Several studies (Silva et al., 2002; Silva et al., 2004; Legua et al., 2013; Szychowski et al., 2014) reported a higher positive correlation between antioxidant activity and total polyphenols compounds content in quince fruit with greater activity exhibited in the peel part than the corresponding pulp part and with the most active antioxidants are quercetin and quercetin 3-O-rutinoside in quince peels (Khoubnasabjafari and Jouyban, 2011). The presence of thirteen phenolics compounds in the skin part including the six compounds identified in the pulp part (3-O-, 4-O-, and 5-O-caffeoylquinic acids, 3, 5-Odicaffeoylquinic acid, quercetin 3-galactoside, and rutin) mainly explain this difference (Silva et al., 2005). Meanwhile, the presence of carotenoids, tocopherols, and vitamin C in the fruit may also contribute to the antioxidant activity (Hamauzu et al., 2005; Magalhães et al., 2009; Legua et al., 2013; Szychowski et al., 2014).
It is well known that oxidative stress is considered a key factor in the pathogenesis of several diseases, including diabetes. Hence, we investigate the capacity of HECO to inhibit α-glucosidase activity, which is a useful method to control hyperglycemia. α-glucosidase inhibitor delays carbohydrate digestion, which consequently reduces the postprandial plasma glucose level. It was reported that the use of α-glucosidase inhibitor drugs such as acarbose, miglitol, voglibose can induce many side effects, such as renal tumors, adverse gastrointestinal disturbance, and liver toxicity (Fujisawa et al., 2005). For this reason, the prevention and use of natural antidiabetic are important.
The presence of antioxidant molecules such as 5-Hydroxymethyl-2- furancarboxaldehyde (5-HMF), caffeoylquinic acids, kaempferol 3-glucoside, rutin, kaempferol-3-rutinoside, and quercetin 3-galactoside (Mohebbi et al., 2019) in the fruit mainly explain this activity and justifying the traditional use of C.oblonga in the prevention and the treatment of diabetes.
Prophylactic treatment of mice with HECO enhanced the rate of carbon clearance from the blood when compared with the control group. It was reported that some fruits and plant extracts (Bin-Hafeez et al., 2003; Benmebarek et al., 2013; Chan-Zapata et al., 2018, Alves et al., 2020) ameliorated the nonspecific immunity mechanism by increasing the phagocytic activity of macrophages. In our study, the phagocytosis was expressed as the phagocytic index, in which the rate of clearance of carbon from the blood is governed by an exponential equation. HECO extract was found to stimulate phagocytic activity in a dose-dependent manner. Furthermore, the HECO at the doses of (50 and 100 mg/kg.bw) showed the maximum phagocytic index concomitant with the lowest GSH contents in the liver of treated mice. GSH is a powerful non-enzymatic antioxidant which considered a therapeutic agent for several diseases. The carbon ink injection increased oxidative stress and altered antioxidant status in treated animals. it seems reasonable to suppose that pre-treatment with HECO improves antioxidant status altered by carbon ink injection by enhancing the liberation of GSH from liver cells which in turn stimulates the phagocytic activity through nuclear factor kappa B (Kwon et al., 2019). However, following the diversity of antioxidant compounds in HECO, it is most likely that improving phagocytic activity exhibited by the extract may be mediated by more than a single mechanism. Our findings suggest that HECO increases the capacity of the detoxification ability of the organism.
CONCLUSION
Our findings showed that the HECO demonstrated good antioxidant and antidiabetic activities with a capacity to increase the phagocytic activity by enhancing the liberation of reduced glutathione. The quince fruit may be selected as a useful approach to increase the human innate defense system.
REFERENCES
Alves, S.F., Gomes, C.M., de Oliveira, M.G., de Andrade, W.M., Moreira, L.C., Borges, L.L., et al. 2020. Cytotoxicity, phagocytic activity, and leishmanicidal potential of extract standardized in geranylgeraniol obtained from the fruit of Pterodon emarginatus vogel. Pharmacognosy Magazine. 16: 140.
Apak, R., Güçlü, K., Özyürek, M., Karademir, S.E. 2004. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. Journal of Agricultural and Food Chemistry. 52: 7970-7981.
Ashraf, M.U., Muhammad, G., Hussain, M.A., Bukhari, S.N. 2016. Cydonia oblonga M., a medicinal plant rich in phytonutrients for pharmaceuticals. Frontiers in Pharmacology. 7: 163.
Aslam M., Sial A. A. 2014. Effect of hydroalcoholic extract of Cydonia oblonga Miller (Quince) on sexual behaviour of wistar rats. Advances in Pharmacological Sciences, 2014.
Benmebarek, A., Zerizer, S., Laggoune, S., Kabouche, Z. 2013. Immunostimulatory activity of Stachys mialhesi de Noé. Allergy Asthma & Clinical Immunology. 9: 1-4.
Bensouici, C., Kabouche, A., Karioti, A., Öztürk, M., Duru, M. E., Bilia, A. R., Kabouche, Z. 2016. Compounds from Sedum caeruleum with antioxidant, anticholinesterase, and antibacterial activities. Pharmaceutical Biology. 54: 174- 179.
Bin-Hafeez, B., Haque, R., Pervez, S., Pandey, S., Sayeed, I., Raisuddin, S. 2003. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. International Immunopharmacology. 3: 257-265.
Chan-Zapata, I., Canul-Canche, J., Fernández-Martín, K., Martín-Quintal, Z., Torres- Romero, J.C., Lara-Riegos, et al. 2018. Immunomodulatory effects of the methanolic extract from Pouteria campechiana leaves in macrophage functions. Food and Agricultural Immunology. 29: 386-399.
Fattouch, S., Caboni, P., Coroneo, V., Tuberoso, C.I., Angioni, A., Dessi, S., ... and Cabras, P. 2007. Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. Journal of Agricultural and Food Chemistry. 55: 963-969.
Forrester, S.J., Kikuchi, D.S., Hernandes, M.S., Xu, Q., Griendling, K.K. 2018. Reactive oxygen species in metabolic and inflammatory signaling. Circulation Research. 122: 877-902.
Fujisawa T., Ikegami H., Inoue K., Kawabata Y., and Ogihara T. 2005. Effect of two α- glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism. 54: 387-390.
Hamauzu, Y., Yasui, H., Inno, T., Kume, C., and Omanyuda, M. 2005. Phenolic profile, antioxidant property, and anti-influenza viral activity of Chinese quince (Pseudocydonia sinensis Schneid.), quince (Cydonia oblonga Mill.), and apple (Malus domestica Mill.) fruits. Journal of Agricultural and Food Chemistry. 53: 928-934.
Herman, W.H. 2017. The global burden of diabetes: an overview. In Diabetes mellitus in developing countries and underserved communities (pp. 1-5). Springer, Cham.
Iweala, E.E.J., Evbakhavbokun, W.O., and Maduagwu, E.N. 2019. Antioxidant and hepatoprotective effect of Cajanus cajan in N-nitrosodiethylamine-induced liver damage. Scientia Pharmaceutica. 87: 24.
Jayaprakasha, G.K., Singh, R.P., and Sakariah, K.K., 2001. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chemistry. 73, 285–290.
Khalfallah, A., Berrehal, D., Bensouici, C., Kabouche, A., Semra, Z., Voutquenne- Nazabadioko, L., ... and Kabouche, Z. 2017. Flavonoids, cytotoxic, antioxidant and antibacterial activities of Evax pygmaea. Pharmaceutical biology. 55: 2292- 2296.
Khoubnasabjafari, M., and Jouyban, A. 2011. A review of phytochemistry and bioactivity of quince (Cydonia oblonga Mill.). Journal of Medicinal Plants Research. 5: 3577- 3594.
KÖksal, E., GÜLÇİN, İ., Beyza, S., Sarikaya, O., & Bursal, E. 2009. In vitro antioxidant activity of silymarin. Journal of Enzyme Inhibition and Medicinal Chemistry. 24: 395-405.
Kwon, D.H., Lee, H., Park, C., Hong, S.H., Hong, S.H., Kim, G.Y., Cha, H.J., Kim, S., Kim, H.S., Hwang, H.J., et al. 2019. Glutathione induced immune-stimulatory activity by promoting m1-like macrophages polarization via potential ROS scavenging capacity. Antioxidants (Basel, Switzerland). 8: 413.
Lecube, A., Pachón, G., Petriz, J., Hernández, C., and Simó, R. 2011. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PloS one. 6: e23366.
Legua, P., Serrano, M., Melgarejo, P.M., Valero, D., Martínez, J.J., Martínez, R., et al. 2013. Quality parameters, biocompounds and antioxidant activity in fruits of nine quince (Cydonia oblonga Miller) accessions. Scientia Horticulturea. 154: 61–65.
Lordan, S., Smyth, T.J., Soler-Vila, A., Stanton, C., and Ross, R.P. 2013. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extract. Food Chemistry. 141: 2170-2176.
Magalhães, A.S., Silva, B.M., Pereira, J.A., Andrade, P.B., Valentão, P., and Carvalho, M. 2009. Protective effect of quince (Cydonia oblonga Miller) fruit against oxidative hemolysis of human erythrocytes. Food Chem Toxicology. 47: 1372-1377.
Messaoudi, S., Tebibel, S., Beladjila, A.K., Touhami, F.K., and Kabouche, Z. 2019. Anti- hyperlipidemic, Anti-inflammatory and antioxidant activities of Citrullus lanatus. World Journal of Environmental Biosciences. 8: 100-106.
Mirmohammadlu, M., Hosseini, S.H., Kamalinejad, M., Gavgani, M.E., Noubarani, M., and Eskandari, M.R. 2015. Hypolipidemic, hepatoprotective and renoprotective effects of Cydonia oblonga Mill. fruit in streptozotocin-induced diabetic rats. Iranian Journal of Pharmaceutical Research. 14: 1207.
Mohebbi, S., Naserkheil, M., Kamalinejad, M., Hosseini, S.H., Noubarani, M., Mirmohammadlu, M., and Eskandari, M.R. 2019. Antihyperglycemic activity of quince (Cydonia oblonga Mill.) fruit extract and its fractions in the rat model of diabetes. International Pharmacy Acta. 2: 2-7.
Pavithra, K., and Vadivukkarasi, S. 2015. Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima (Jacq.) Cogn. Food Science and Human Wellness. 4: 42-46.
Rop, O., Balik, J., Řezníček, V., Jurikova, T., Škardová, P., Salaš, P., et al. 2011. Chemical characteristics of fruits of some selected quince (Cydonia oblonga Mill.) cultivars. Czech Journal of Food Science. 29: 65-73.
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., et al. 2019. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Research and Clinical Practice. 157.
Sakhri, F.Z., Adachi, N., Zerizer, S., Ohashi, Y., Ikemoto, H., Tsukada, M., et al. 2020. Behavioral and neurological improvement by Cydonia oblonga fruit extract in chronic immobilization stress rats. Phytotherapy Research.
Silva, B.M., Andrade, P.B., Ferreres, F., Domingues, A.L., Seabra, R.M., and Ferreira, M.A. 2002. Phenolic profile of quince fruit (Cydonia oblonga Miller) (pulp and peel). Journal of Agricultural and Food Chemistry. 50: 4615-4618.
Silva, B.M., Andrade, P.B., Martins, R.C., Valentão, P., Ferreres, F., Seabra, R.M., et al. 2005. Quince (Cydonia oblonga Miller) fruit characterization using principal component analysis. Journal of Agricultural and Food Chemistry. 53: 111-122.
Silva, B.M., Andrade, P.B., Valentão, P., Ferreres, F., Seabra, R.M., Ferreira, M.A. 2004. Quince (Cydonia oblonga Miller) fruit (pulp, peel, and seed) and jam: antioxidant activity. Journal of Agricultural and Food Chemistry. 52: 4705-4712.
Sun, C., Wu, Z., Wang, Z., and Zhang, H. 2015. Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. Evidence-Based Complementary and Alternative Medicine, 2015.
Sut, S., Dall'Acqua, S., Poloniato, G., Maggi, F., and Malagoli, M. 2019. Preliminary evaluation of quince (Cydonia oblonga Mill.) fruit as extraction source of antioxidant phytoconstituents for nutraceutical and functional food applications. Journal of the Science of Food and Agriculture. 99: 1046-1054.
Szychowski, P.J., Munera-Picazo, S., Szumny, A., Carbonell-Barrachina, Á.A., Hernández, F. 2014. Quality parameters, bio-compounds, antioxidant activity and sensory attributes of Spanish quinces (Cydonia oblonga Miller). Scientia Horticulturae. 165: 163-170.
Umar, A., Iskandar, G., Aikemu, A., Yiming, W., Zhou, W., Berké, B., et al. 2015. Effects of Cydonia oblonga Miller leaf and fruit flavonoids on blood lipids and antioxidant potential in hyperlipidemia rats. Journal of Ethnopharmacology. 169: 239-243.
Wojdylo, A., Oszmiański, J., Bielicki, P. 2013. Polyphenolic composition, antioxidant activity, and polyphenol oxidase (PPO) activity of quince (Cydonia oblonga Miller) varieties. Journal of Agricultural and Food Chemistry. 61: 2762-2772.
Zhou, W., Abdusalam. E., Abliz, P., Reyim, N., Tian, S., Aji, Q., et al. 2014. Effect of Cydonia oblonga Mill. fruit and leaf extracts on blood pressure and blood rheology in renal hypertensive rats. Journal of Ethnopharmacology. 152: 464– 469.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Fatma Zahra Sakhri1, 2,*, Sakina Zerizer1, *, and Chawki Bensouici3
1 Université Des Frères Mentouri-Constantine 1, Laboratoire d’Immunologie, 25000 Constantine, Algeria,
2 Université Des Frères Mentouri-Constantine 1, Laboratoire d’Obtention de Substances Thérapeutiques, 25000 Constantine, Algeria
3 Centre de Recherche en Biotechnologie Ali Mendjli Nouvelle Ville UV 03 BP E73 Constantine, Algeria
Corresponding author: Fatma Zahra Sakhri, E-mail: fatimazohra.sakhri@umc.edu.dz
Sakina Zerizer, E-mail: zerizer.sakina@umc.edu.dz
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: October 6, 2020;
Revised: January 8, 2021;
Accepted: January 15, 2021;
Published online: April 7, 2021

