
Antioxidant, α-Glucosidases and α-Amylase Inhibitory Activities of Persicaria odorata
Kanya Thongra-ar, Piyanuch Rojsanga, Savita Chewchinda, Supachoke Mangmool, and Pongtip Sithisarn*Published Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.051
Journal Issues : Number 3, July-September 2021
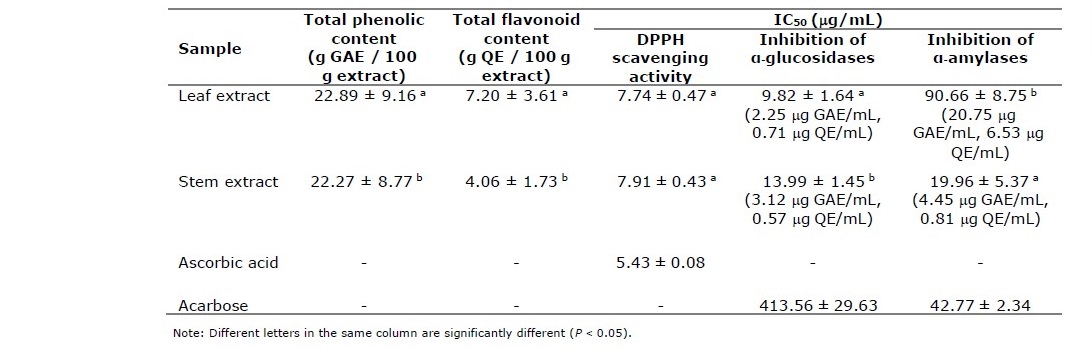
Abstract The objects of this study were to determine the effects to reactive oxygen species and antioxidant enzymes levels in HEK-293 cells and inhibition of α-glucosidases and α-amylase enzymes of extracts from Persicaria odorata or phak phaeo. The ethanol extracts from the leaves and the stems of phak phaeo were investigated for their 2,2-diphenyl-1-picryhydrazyl (DPPH) scavenging activities (IC50 were 7.74 ± 0.47 and 7.91 ± 0.43 µg/mL, respectively). Cellular antioxidant effects in human embryonic kidney-293 (HEK-293) cells with these extracts (0.1 mg/mL) also increased the mRNA expressions of manganese superoxide dismutase (Mn-SOD), glutathione peroxidase 1 (GPx-1), catalase and glutathione reductase (GRe). The leaf extract showed the higher efficacies in the induction of the mRNA expressions of Mn-SOD, GPx-1 and GRe while the stem extract exhibited a stronger effect to the induction of catalase. Phak phaeo in vitro inhibitory effects to α-glucosidase enzyme (IC50 values of 9.82 ± 1.64 and 13.99 ± 1.45 µg/mL, respectively and also strong inhibition to α-amylase with IC50 values of 90.66 ± 8.75 and 19.96 ± 5.37 µg/mL, respectively). Lineweaver-Burk plot demonstrated that phak phaeo extracts inhibited α-glucosidase and α- amylase in non-competitive manners. Total phenolic and total flavonoid contents were determined by Folin-Ciocalteu and aluminium chloride methods (the leaf and stem extracts were 22.89 ± 9.16 and 22.27 ± 8.77 g gallic acid equivalent in 100 g extract (g% GAE) and 7.20 ± 3.61 and 4.06 ± 1.73 g quercetin equivalent in 100 g extract (g% QE), respectively).
Keywords: Antioxidant enzymes, DPPH, HEK-293, MTT assay, Persicaria odorata, Reactive oxygen species, Total phenolic, Total flavonoid, α-glucosidases, α-amylase
Funding: This research project is supported by Faculty of Pharmacy, Mahidol University under the Research Cluster Grant. This research is partially supported by the 50th Anniversary of the Establishment of Faculty of Pharmacy, Mahidol University Scholarship.
Citation: Thongra-ar, K., Rojsanga, P., Chewchinda, S., Mangmool, S., and Sithisarn, P. 2021. Antioxidant, α-glucosidases and α-amylase inhibitory activities of Persicaria odorata. CMUJ. Nat. Sci. 20(3): e2021051.
INTRODUCTION
Oxidative stress is defined as an imbalance between endogenous antioxidant defense mechanisms and the production of reactive oxygen species (ROS), which at high levels can cause cell injury. Increases in oxidative stress are involved in the pathophysiology of many diseases such as cardiovascular diseases (Fearon and Faux, 2009), neurodegenerative diseases (Guo et al., 2013), and diabetes (Niedowicz and Daleke, 2005; Matough et al., 2012). Antioxidants play a major role in protecting the human body against free radical mediated diseases. Several studies found that medicinal plants and fruits are a rich source of antioxidant compounds such as phenolics and flavonoids, which can reduce the incidence of oxidative stress-related diseases (Cai et al., 2007; Zhang et al., 2011). Many researchers have been interested in the use of flavonoids as antidiabetics due to their role in decreasing the hyperglycemic effects of diabetes, by delaying hyperglycemia by inhibiting starch digestion (Ranilla et al., 2010; Tadera et al., 2010).
Diabetes mellitus (DM) are characterized by hyperglycemia, which leads to a range of adverse processes in the body such as the glycosylation of extracellular proteins, increase in body free radicals load and the generation of advanced glycation end products as a result of the Maillard Reactions (Gerich, 2003). One of the most effective methods for controlling hyperglycemia is to suppress starch digestion as it is the main contributor of glucose in the human body from diet (Jones et al., 2011). Starch digestion occurs mainly in the gastrointestinal tract, and it involves the combined action of pancreatic α-amylase and intestinal α-glucosidase to release absorbable glucose monosaccharides (Jones et al., 2011). The inhibition of α-amylase or α-glucosidase enzymes would minimize the starch digestion from our diet and decrease blood glucose levels (Gerich, 2003).
Persicaria odorata (Lour.) Sojak is a plant in Polygonaceae family which has been traditionally consumed as fresh vegetable in Southeast Asian cuisine. The leaves are usually used as flavoring agent in curries and hot soups (Vikram et al., 2014). In Vietnam, a poultice made from the plant is applied to relieve swelling and inflammation, while a decoction is used to treat itchy skin, diarrhea and excessive bleeding (Dash and Zakaria, 2016). The leaves are used to treat nausea, fever and to promote hair growth (Dash and Zakaria, 2016). This plant has been also used for the treatments of sores, ulcers and wounds (Dash and Zakaria, 2016). Previous studies reported that P. odorata promoted the effect on the α-glucosidase enzyme (Kee et al., 2013) while the combined extract of mango and this plant showed anticataractogenesis and antiretinopathy effects in STZ-diabetic rats (Wattanathorn et al., 2017). P. odorata has been reported to promote antibacterial and antifungal (Ridzuan et al., 2017; Nanasombat and Teckchuen, 2009; Saad et al., 2017; Ridzuan et al., 2013), antioxidant (Nanasombat and Teckchuen, 2009; Lee and Vairappan, 2011) and anticancer (Nanasombat and Teckchuen, 2009) effects and also cytotoxic and apoptotic induction effects on cancer cell lines (Putthawan et al., 2017). Some phenolic acids and flavonoids were reported from the leaves of P. odorata such as gallic acid, chlorogenic, ellagic acid, quercetin, rutin and isorhamnetin (Nanasombat and Teckchuen, 2009; Ahongshangbam et al., 2014). This study was conducted in order to evaluate the in vitro antioxidant activities of extracts from P. odorata leaves and stems, and in vitro inhibitory effects to α-amylase and α-glucosidase enzymes. Phytochemical study of the extracts from this plant was also done using chromatographic and spectrophotometric techniques.
MATERIALS AND METHODS
Plant sample preparation
Fresh Persicaria odorata leaves and stem were collected from Chonburi province, Thailand in November, 2019. Plant samples were identified by the botanist at Bangkok herbarium (BK), Department of Agriculture, Bangkok, Thailand (Voucher number BK-071403). Plant samples were dried in a hot air oven at 60°C, powdered using an electric mill and passed through a sieve with mesh number 20.
Plant Extract preparation
The dried powders of P. odorata leaves and stems were separately macerated with 75% ethanol (plant:solvent ratio, 1:15 w/v). The mixtures were allowed to stand at room temperature for 24 hours with occasional agitation. The aqueous extract of each sample was filtered through a Whatman no.1 filter paper while the residue was extracted under the same conditions another two times. The filtrates were collected and were evaporated to dryness under reduced pressure at temperature not higher than 40°C using a rotary vacuum evaporator. The dried extracts of the leaves and stems of P. odorata were kept in a refrigerator until use.
Thin layer chromatography (TLC) analysis
P. odorata leaf and stem extracts were phytochemically analyzed by thin layer chromatography (TLC) under the following conditions:
Stationary phase : silica gel 60 F254
Mobile phase : ethyl acetate: formic acid: glacial acetic acid: water (100:11:11:26 v/v/v/v)
Detector : UV cabinet at wavelengths of 254 and 366 nm
Spray reagent : natural product/polyethylene glycol reagent (NP/PEG)
Determination of total phenolic content
Folin-Ciocalteu reagent (25 μL) was added to P. odorata leaf or the stem extract solution (25 μL) in 96-well plate; then 75 μL of distilled water and 100 μL of 20% w/v sodium carbonate solution was added. The absorbance of the resulting blue colored solution was measured at the wavelength of 765 nm after 60 minutes using a microplate reader. Each sample was done in triplicate. Total phenolic content was calculated from the standard curve of gallic acid and was expressed as g gallic acid equivalent in 100 g extract (g% GAE). (Sithisarn et al., 2015)
Determination of total flavonoids content
P. odorata leaf and stem extract (100 μL) was separately reacted with 2% w/v aluminium chloride solution in the same volume. The absorbance was measured at the wavelength of 415 nm after 10 minutes using a microplate reader. Total flavonoid content was calculated from the standard curve of quercetin and was expressed as g quercetin equivalent in 100 g of plant extracts (g% QE). (Sunthudlakhar et al., 2019)
Determination of antioxidant activity by DPPH scavenging assay
The antioxidant activities of P. odorata leaf and stem extracts were investigated using a 2,2-diphenyl-1-picryhydrazyl (DPPH) assay. DPPH was dissolved in methanol to prepare the DPPH solution at a concentration of 207 µM. The DPPH solution (100 µL) was added to each plant extract solution with various concentrations ranging from 5 – 1,000 µg/mL in the same volume. The mixture was mixed and kept in the dark for 15 minutes. The absorbance of each reaction solution was determined at the wavelength of 515 nm using a microplate reader. The percentage of inhibition for each reaction was calculated, and EC50 values (µg/mL) were be calculated from the linear equation from the curve between the percentage of inhibition and the solution’s concentration. Each experiment was conduct in triplicate. The EC50 value of each extract was expressed as mean ± SD. (Sunthudlakhar et al., 2019)
Cell culture
Human embryonic kidney-293 (HEK-293) cells (from ATCC®) were cultured using Dulbecco’s modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 1% v/v penicillin-streptomycin solution in a humidified atmosphere of 5% Carbon Dioxide at 37°C as previously described (Anantachoke et al., 2016).
Determination of antioxidant activity in HEK-293 cells Cytotoxic test by MTT Assay
HEK-293 cell viability was performed by MTT assay according to the method previously described (Anantachoke et al., 2016). HEK-293 cells were seeded in 96-well culture plates at a density of 1 × 104 cells/well, in a total volume of 200 µL of DMEM supplemented with 1% FBS plus 1% penicillin/streptomycin. Cells were allowed to adhere to plate for 24 hours, before being treated with solvent (control) and crude extracts at various concentrations (0.005 – 5 mg/mL) for 24 hours. The experiments were performed in triplicate wells. The relative number of viable cells was then determined at 24 hours after incubation, by adding 2 mg/mL of MTT solution and further incubating the cell for 4 hours. The formazan crystals formed were then solubilized with DMSO. The absorbance of the plate at the wavelength of 570 nm which directly represented the relative cell numbers was read. The percentage of cell viability was calculated according to the following equation;
The percentage of cell viability = [Absorbance of treated cells / Absorbance of control cells] × 100
Measurement of intracellular ROS level in HEK-293 cells
The intracellular antioxidant activities of the leaf and stem extracts of P. odorata were measured using a fluorescent probe dichlorodihydrofluorescein diacetate (DCFH- DA) to estimate the intracellular ROS production in the cells as previously described (Anantachoke et al., 2016). HEK-293 cells were seeded in a 12-well plate (1 × 105 cells/well) and treated with P. odorata extracts (0.01 and 0.1 mg/mL) for 12 hours. After 12 hours, the cells were then incubated with 100 µM of H2O2 for 2 hours and washed once with phosphate buffered saline (PBS), pH 7.4. Thereafter, 10 µM DCFH- DA was added and incubated with the cells at 37°C in the dark for 30 minutes. The fluorescence intensity of dichlorofluorescein (DCF) was determined using a Multi- detection microplate reader (BioTek Instruments) with an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
mRNA expression analysis of antioxidant genes by realtime RT-qPCR
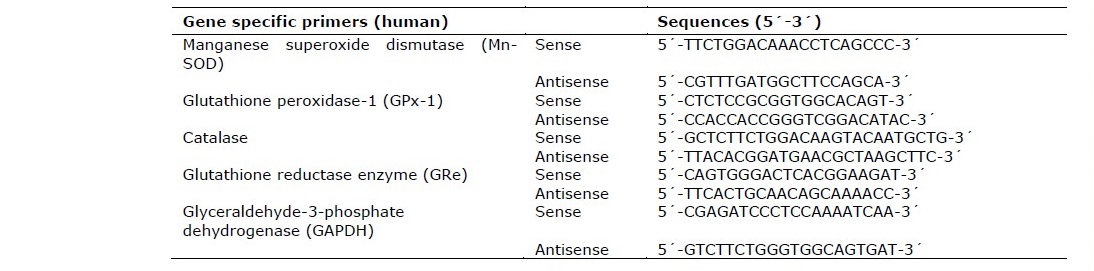
The mRNA extraction of HEK-293 cells was modified from the previous study (Anantachoke et al., 2016). The extraction of RNA was performed using the GeneJET™ mRNA extraction Kit (Thermo Scientific). RT-qPCRs for RNA expression were performed using the KAPA SYBR FAST One-Step RT-qPCR Kit according to the manufacturer’s instructions. RT-qPCRs were performed on an AriaMx Real-Time PCR System (Agilent). The primers of human antioxidant genes are shown in Table 1 as follows: manganese superoxide dismutase (Mn-SOD), Glutathione peroxidase-1 (GPx-1), Catalase (CAT), glutathione reductase enzyme (GRe) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Data was presented as specific ratio between the gene of interest and the reference gene (GAPDH) normalized to vehicle-treated group (control). Table 1 shows the sequences of the primers used in this study.
Table 1. Sequences of primers used in mRNA expression analysis of antioxidant genes affected by the treatment of the leaf and stem extracts of P. odorata.
Determination of α-glucosidase inhibitory activity
The α-glucosidase inhibitory activity was determined according to the spectrophotometric method described by Supasuteekul et al., 2016 with some modifications (Supasuteekul et al., 2016). Briefly, 10 μL of P. odorata extracts or acarbose (positive control) was gently combined with 40 μL of α-glucosidase enzyme (0.1 U/mL) in an 0.1 M phosphate buffer (pH 6.9). After incubation at 37°C for 10 minutes, 50 μL of 2 mM p-nitrophenyl α-D-glucopyranoside (pNPG) was added in order to initiate the reaction and further incubated at 37°C for 20 minutes. The reaction mixture was terminated by adding 100 μL of 1 M Na2CO3. p-nitrophenol released from the substrate (pNPG) turned yellow color which was then detected at the wavelength of 405 nm by microplate reader. The measurements were done in triplicate. The percentage of inhibition was calculated using the equation below. The IC50 value obtained from the linear curve plotting between the concentrations of test substance and percentage of inhibition was then calculated and expressed as mean ± SD.
α-glucosidase inhibitory activity (%) = [(Ac – As) / Ac] × 100
Where Ac and As are the absorbance of control and P. odorata extract treatments, respectively.
Enzyme kinetics of α-glucosidase inhibition
Inhibition type of P. odorata leaf and stem extracts on α-glucosidase were measured using the assay conditions described above. The experimental data were observed at initial time and at 20 min of reaction time by varying the concentrations of substrate pNPG (0.25, 0.5, 1.0, 2.0, and 4.0 mM) in the absence and presence of P. odorata leaf and stem extracts (5, 10, 15 μg/mL and 7, 14, 21 μg/mL). Kinetic parameters including the inhibition constant (Ki), the Michaelis-Menten constant (Km), and the maximum velocity of enzyme activity (Vmax) were determined by the different types of Michaelis-Menten equations, using Solver add-in equipped with Microsoft Excel 2010. Mode of enzyme inhibition was identified according to Lineweaver-Burk plot between the reciprocal value (1/v and 1/[S]), where v was reaction velocity and [S] was the substrate concentration.
Determination of α-amylase inhibitory activity
The α-amylase inhibitory capacities of P. odorata leaf and stem extracts were investigated using the 3, 5-dinitrosalicylic acid (DNSA) method (Wickramaratne et al., 2016; Tan et al., 2017). A buffer solution (20 mM Na2HPO4/NaH2PO4 with 6.7 mM NaCl, pH 6.9) was prepared for dissolving the P. odorata extracts and α-amylase. The DNSA color reagent was obtained from mixing 96 mM 3, 5-DNSA (25 mL), and 5.31 M sodium potassium tartrate in 2 M NaOH (8 mL). P. odorata extract solutions (200 μL) and were pre-incubated with 200 μL of α-amylase solution (2 U/mL) at 30°C for 10 minutes. Then, 200 μL of 1% w/v starch solution was added and mixed. The mixture was incubated at 30°C for 3 minutes. The reaction was terminated by adding 200 μL of DNSA reagent and boiling in a water bath (85 – 90 °C) for 10 minutes. Then, the mixture was cooled at room temperature before 200 μL of the mixture was transferred into a 96-well plate. The absorbance of the mixture was measured at the wavelength of 540 nm using a microplate reader. Each determination was done in triplicate. The percentage of α-amylase inhibitory activity was calculated by the formula below. The IC50 value was obtained from the linear curve plotting between the concentrations of test substance and percentage of inhibition was then calculated and expressed as mean ± SD.
α-amylase inhibitory activity (%) = [(Ac – As) / Ac] × 100
Where Ac and As are the absorbance of control and P. odorata extract treatments, respectively.
Enzyme kinetics of α-amylase inhibition
For kinetic analysis, the experimental data were observed at initial time (t0) and at 3 min of reaction time (t3). For t3 series, three concentrations of P. odorata leaf and stem extract (45.5, 91, 136.5 μg/mL and 10, 20, and 30 μg/mL) were tested with different concentrations of starch solution (2.5, 5, 10, 20, and 40 mg/mL) using the same procedure as described above. In initial time series (t0), the same method was conducted but the reaction was stopped immediately after adding starch solution. Enzyme kinetic parameters (Km, Ki, and Vmax) were calculated using the same method as described in enzyme kinetics of α-glucosidase inhibition section.
Statistical Analysis.
Data were presented as mean ± SEM. The statistical analysis was determined using one-way analysis of variance (ANOVA) and Student’s t-test, and values of P
RESULTS
Yields of P. odorata leaf and stem extracts
Leaf and stem extracts of P. odorata appeared as dark green semi-solids with the yields of 9.27 and 9.27 % w/w of dried plant powders
TLC analysis of P. odorata leaf and stem extracts
As shown in Figure 1, leaf and stem extracts of P. odorata showed specific chromatographic fingerprints with the chromatographic bands corresponding to standard gallic acid, chlorogenic and isoquercetin at Rf values of 0.87, 0.47 and 0.60, respectively.
Determination of total phenolic and total flavonoid contents of P. odorata leaf and stem extracts
Total phenolic contents of P. odorata leaf and stem extracts were 22.89 ± 9.16 and 22.27 ± 8.77 g gallic acid equivalent/ 100 g extract (g GAE/ 100 g extract), respectively while total flavonoid contents were 7.20 ± 3.61 and 4.06 ± 1.73 g quercetin equivalent/ 100 g extract (g QE/ 100 g extract), respectively (Table 2).
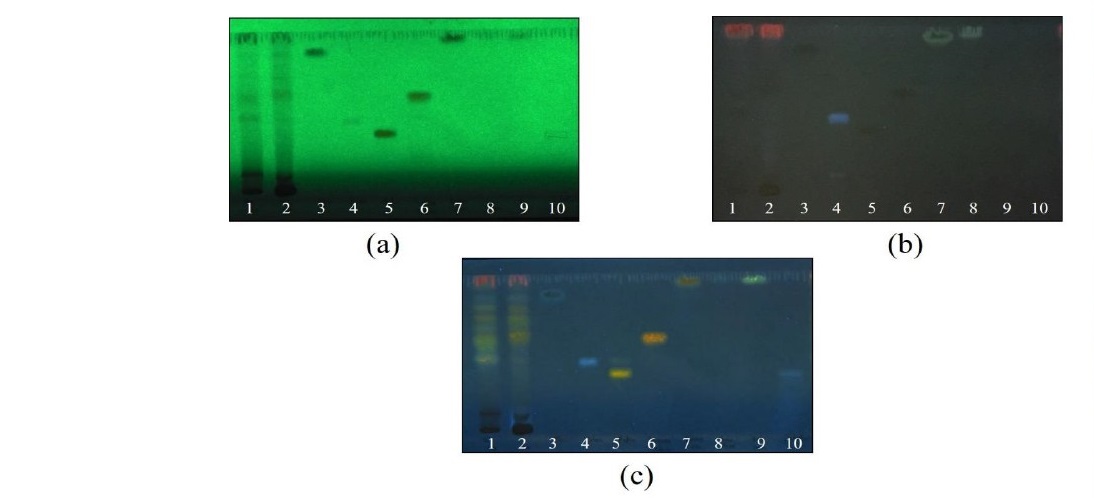
Figure 1. Thin layer chromagography (TLC) chromatogram of P. odorata leaf and stem extracts; 1 = P. odorata leaf extract, 2 = P. odorata stem extract, 3 = gallic acid, 4 = chlorogenic acid, 5 = rutin, 6 = isoquercetin, 7 = quercetin, 8 = kaempferol, 9 = apigenin, 10 = scutellarin. Adsorbent: silica gel GF254. solvent system: detection: (a) = UV 254 nm, (b) = UV 366 nm, and (c) = NP/PEG under UV 366 nm.
Table 2. DPPH scavenging activities, inhibitory effects to α-glucosidase and α-amylase enzymes and total phenolic and total flavonoid contents of extracts from the leaves and stems of P. odorata.
Determination of antioxidant activity in HEK-293 cells Cytotoxic test by MTT Assay
At various concentrations ranged from 0.005 – 5 mg/mL, P. odorata leaf and stem extracts showed steady cell viability at around 100% at concentrations ranging from 0.005 – 0.1 mg/mL (Figure 2a, b) suggesting no toxicity. The higher concentrations of these two extracts caused a reduction of cell viability to around 40%. Therefore, the concentrations of 0.1 and 0.01 mg/mL were selected for further study with an intracellular ROS production assay and the determination of mRNA expression of the antioxidant genes.
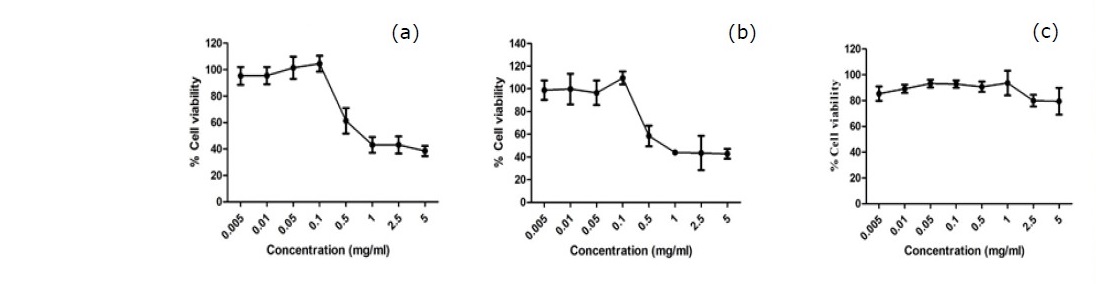
Figure 2. Cytotoxicity profile of P. odorata leaf and stem extracts in HEK-293 cells. HEK-293 cells were treated with either P. odorata leaf (a) and stem (b) extracts (0.005 – 5 mg/mL) or vehicle (c) for 24 h. Cell viability was quantified, expressed as a percentage of cell viability, and shown as the mean ± SEM (n = 4).
Note: the concentrations used in further experiments in order to prevent the cytotoxic effect allow at least 80% cells survival.
Measurement of intracellular ROS levels in HEK-293 cells
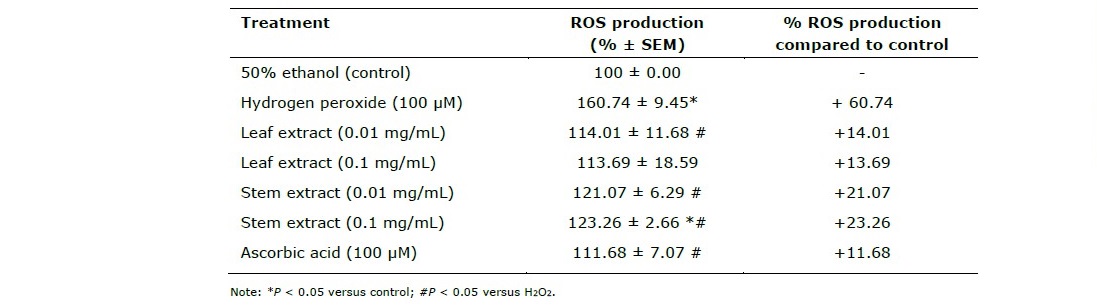
Shown in Table 3, extracts from the leaves and stems of P. odorata exhibited the antioxidant effects by preventing the production of intracellular reactive oxygen species (ROS) induced by H2O2.
Table 3. Effects of extracts from the leaves and stems of P. odorata on H2O2-induced ROS production in HEK-293 cells.
mRNA expression analysis of antioxidant genes by realtime RT- qPCR
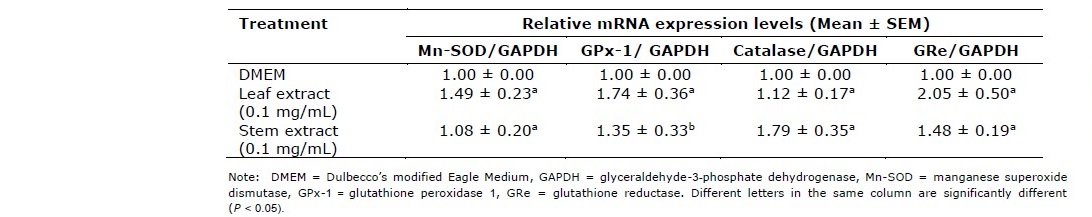
Persicaria odorata leaf and steam extracts at the concentrations of 0.1 mg/mL increased mRNA expressions of manganese superoxide dismutase (Mn-SOD), glutathione peroxidase 1 (GPx-1), catalase and glutathione reductase (GRe) enzymes (Table 4).
Table 4. Effects of extracts from the leaves and stems of P. odorata on the mRNA expression of antioxidant enzymes.
Evaluation of inhibitory effects to α-glucosidase and α-amylase enzymes to P. odorata extracts
From Table 2, P. odorata leaf and stem extracts promoted strong inhibitory effects to yeast α-glucosidase enzyme with IC50 values of 9.82 ± 1.64 and 13.99 ± 1.45 μg/mL. Lineweaver-Burk plot (Figure 3) demonstrated that P. odorata leaf and stem extracts inhibited α-glucosidase in a non-competitive manner, in contrast to acarbose which competitively inhibits α-glucosidase. The kinetic parameters, Km, and Ki were calculated to be 1.34 mM, and 0.97 μg/mL for the leaf extract, and 1.35 mM, and 0.98 μg/mL for the stem extract and promoted inhibitory effects on α-amylase enzyme from a porcine pancreas with IC50 values of 90.66 ± 8.75 and 19.96 ± 5.37 μg/mL. The Lineweaver-Burk plot for determination of kinetic parameters demonstrated that the mode of α-amylase inhibition by P. odorata leaf and stem extracts was non-competitive manner, Km, and Ki were calculated to be 6.76 mM, and 11.08 µg/mL for the leaf extract, 7.97 mM, and 7.54 µg/mL for the stem extract.
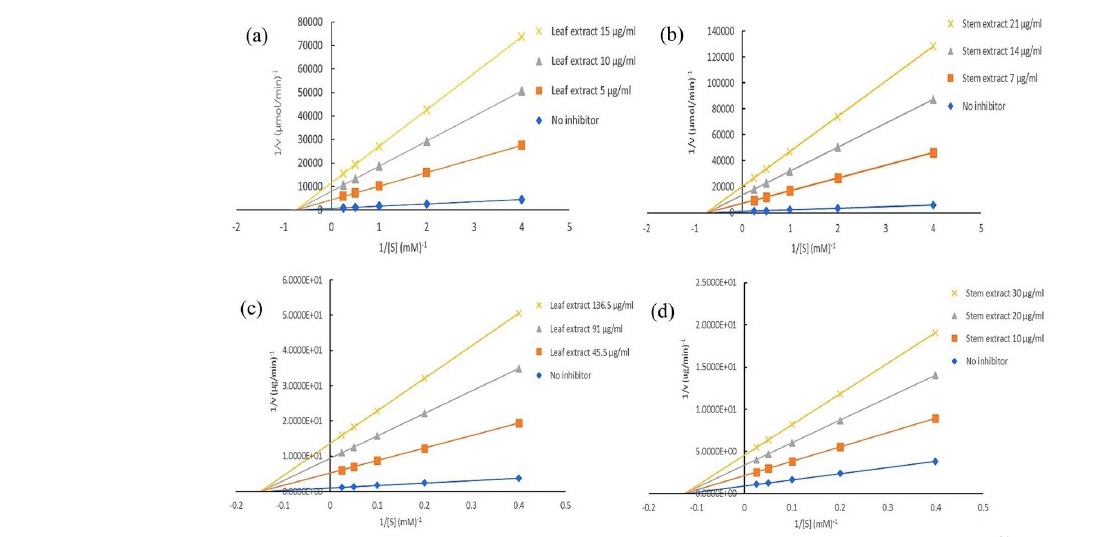
Figure 3. Lineweaver-Burk plot of α-glucosidase inhibitory activities of P. odorata leaf (a) and stem (b) extract. Lineweaver-Burk plot of α-amylase inhibitory activities of P. odorata leaf (c) and stem (d) extract.
Note: [V] and [S] are the reaction velocity and the concentration of substrate, respectively.
DISCUSSION
This study demonstrates for ethanol extracts from P. odorata leaves and stems exhibits antioxidant. As shown in Table 3, extracts from the leaves and stems of P. odorata exhibited the antioxidant effects by preventing the production of intracellular reactive oxygen species (ROS) induced by H2O2. By induction of H2O2, ROS productionincreased from 100% of the control (50% ethanol) to 160.74 ± 9.45%. The leaf extract at the concentration of 0.01 mg/mL significantly decreased the ROS production induced by H2O2 in HEK-293 cells into the normal level which was as effective as the effect from ascorbic acid while the stem extract at the concentration of 0.1 mg/mL significantly decreased the ROS production compared to H2O2 treated group but the ROS production was still significantly higher than the control group. The in vitro results from both DPPH scavenging assay and this measurement of intracellular ROS level indicated that the extracts from P. odorata leaves and stems had strong potential for their antioxidant effects.
P. odorata leaf and steam extracts at the concentrations of 0.1 mg/mL increased mRNA expressions of manganese superoxide dismutase (Mn-SOD), glutathione peroxidase 1 (GPx-1), catalase and glutathione reductase (GRe) enzymes. As three key antioxidant enzymes, GPx and catalase act as enzymes for the elimination of hydrogen peroxide (H2O2) while SOD alternately catalyzes the dismutation of the superoxide radical into oxygen (O2) and H2O2, which is then degraded by GPx and catalase (Anantachoke et al., 2016). These three enzymes function connectively; their malfunction could lead to an accumulation of ROS production and increased oxidative stress in the cells (Anantachoke et al., 2016). GRe catalyzes the reduction of glutathione disulfide into the sulfhydryl form, glutathione (GSH), which is a critical molecule in resisting oxidative stress and maintaining the reducing environment of the cell (Mannervik, 1987; Deponte, 2013). From our study, both Persicaria odorata leaf and stem extracts at the concentration of 0.1 mg/mL exhibited induction effects on mRNA expressions of these 4 antioxidant enzymes. However, the leaf extract exhibited a more effective induction of mRNA expressions of Mn-SOD, GPx-1 and GRe while the stem extract exhibited greater effect to catalase relative mRNA expression (Table 4). They contain quite high amounts of total phenolic and total flavonoid (> 20 g% GAE and > 4 g% QE in dried extracts) with the presence of gallic acid and chlorogenic acid according to the results from TLC analysis. Previous experiment reported that the administration of some phenolic acids such as gallic acid, ferulic acid and p-coumaric acid at a concentration of 100 mg/kg for 2 weeks promoted an induction of hepatic antioxidant enzymes such as SOD, GPx and catalase in animal model (Yeh and Yen, 2006). The results from our study corresponded to the previous experiment reported that the combined extract of Mangifera indica seeds and P. odorata aerial parts prepared at a ratio of 1 : 5 (2.286 mg quercetin equivalent (QE)/ 100 mg extract and 0.636 mg gallic acid/ 100 mg extract) at the doses of 2, 10 and 50 mg/ kg body weight for 10 weeks elevated the activities of antioxidant enzymes, especially catalase in a diabetic rat model (Wattanathorn et al., 2017). This information suggests that P. odorata leaf and stem extracts could be beneficial in the reduction of oxidative stress in diabetics.
Alpha-glucosidase is a significant enzyme that catalyses the last step in carbohydrate digestion. The inhibition of this enzyme can reduce glucose absorption in the small intestine, leading to the control of blood glucose level (Kim et al., 2000; Xiao et al., 2013; Supasuteekul et al., 2016). From Table 2, P. odorata leaf and stem extracts promoted strong inhibitory effects to yeast α-glucosidase enzyme about thirty times stronger than acarbose. The leaf extract promoted slightly stronger inhibitory effect on the α-glucosidase enzyme than the stem extract. The α-amylase inhibitory studies also demonstrated that the stem extract of P. odorata promoted strong inhibitory effects on α-amylase enzyme from a porcine pancreas which was stronger than the effect from acarbose. The inhibition of α- amylase has been called a starch blocking effect as the substance prevents or slow the absorption of starch into the body mainly by blocking the hydrolysis of 1,4-glycosidic linkages of starch and other oligosaccharides into maltose, maltriose and other simple sugars (Kumar et al., 2010; Wickramaratne et al., 2016). However, P. odorata leaf extract showed lower inhibitory effects on this enzyme. The results from this study corresponded to a previous report that indicated that among the tested herbs, P. odorata or Vietnamese mint leaf tea promoted the most potent inhibitory effect on the yeast α-glucosidase enzyme, and was of similar potency to the effect of green tea (Camellia sinensis) (Kee et al., 2013). This report also suggested that P. odorata leaf tea promoted strong inhibition of the yeast α-glucosidase but weak inhibition of the rat intestinal α-glucosidases because there are other enzyme activities in the rat α-glucosidases complex, inhibiting maltase activity alone does not result in the ‘complete’ inhibition of activities as the other nonmaltase activities can still hydrolyse the pNPG substrate in the reaction (Hogan et al., 2010; Kee et al., 2013). For α-amylase enzymes, P. odorata stem extract promoted strong inhibitory effects similar to the effect of methanol extract of Adenanthera pavonina leaves (Wickramaratne et al., 2016). The study of two black legumes of different genera showed that fractions from black soybean and black turtle bean promoted effective inhibitory effects against α-amylase and α-glucosidase enzymes (Tan et al., 2017) suggesting the same trend as this study in that plant extracts acted more effectively on α-glucosidase than α-amylase enzymes. This previous study also demonstrated that among the tested phenolics and flavonoids, myricetin showed the highest inhibition of α-amylase and α-glucosidase. Kim et.al (Kim et al., 2000) studied the effects of 21 flavonoids against α-glucosidase and reported that luteolin, amentoflavone, luteolin-7-O-glucoside and daidzein all exhibited strong inhibition. There is a report suggesting the molecular structures of phenolics and flavonoids that influence the inhibition against α-glucosidase such as hydroxylation and galloylation of flavonoids improve the inhibitory activity and the glycosylation of the hydroxyl group and the hydrogenation of the C2=C3 double bond on flavonoids both weaken the inhibition (Xiao et al., 2013). From our phytochemical study, P. odorata leaf and stem extracts showed the chromatographic bands corresponded to some phenolic acids including gallic acid and chlorogenic acid and a flavonoid, isorhamnetin. These compounds could responsible for both antioxidant activity and inhibitory effects on α-amylase and α-glucosidase enzymes. Moreover, some studies suggest that diabetic complications occur as a result of the oxidative stress due to the formation of free radicals with glucose oxidation and the subsequent oxidative degradation of glycated proteins (Wickramaratne et al., 2016; Mehta et al., 2006). To avoid these complications, the uses of antioxidants together with anti-diabetic drugs should be recommended (Wickramaratne et al., 2016). The combined extract of Mangifera indica seeds and P. odorata aerial parts improved oxidative stress, and improved cataracts and retinopathy in STZ-diabetic rats (Wattanathorn et al., 2017). This combined extract, at a dose of 10 mg/kg body weight, decreased blood sugar levels in diabetic rats after they received the treatment for 5 weeks while in prolonged treatment (10 weeks), only diabetic rats which received the combined extract at a dose of 2 mg/kg body weight, showed a decrease in blood sugar levels (Wattanathorn et al., 2017). The results from enzyme kinetic studies obtained from Lineweaver-Burk plot demonstrated that P. odorata extracts inhibited α-glucosidase and α-amylase in non-competitive manners.
The results of our study indicated that not only P. odorata leaf extract but also the stem extract showed the potential for the antidiabetic treatments through the mechanisms of inhibition of α-glucosidase and α-amylase. These extracts promoted in vitro inhibitory effects to free radicals and H2O2-induced ROS production in HEK-293 cells. One key mechanism of action is induction of the mRNA expressions of antioxidant enzymes. The potential active compound responsible for both antioxidant activity and inhibitory effects on α-amylase and α-glucosidase enzymes could be gallic acid, chlorogenic acid and isorhamnetin.
CONCLUSION
The leaf and stem extracts from P. odorata exhibited strong in vitro free radical scavenging activities. At the concentration of 0.01 and 0.1 mg/mL, they showed strong inhibitory effects on H2O2 -induced ROS production in HEK-293 cells. The antioxidant effects of these extracts are mediated through the upregulation of antioxidant enzyme synthesis by increasing in mRNA expression. The leaf extract showed higher effectiveness on the induction of Mn-SOD, GPx-1 and GRe synthesis while the stem extract showed higher tendency to catalase. The leaf and stem extracts from P. odorata also showed strong in vitro inhibitory effects on α-glucosidase and α-amylase enzymes. Phytochemical analysis of the leaf and stem extracts from P. odorata showed that they contained high total phenolic and total flavonoid contents with the presences of some bioactive phytochemicals including gallic acid, chlorogenic acid and isoquercetin. From this study, P. odorata leaf and stem extracts showed a strong antioxidant effect with an inhibitory effect on enzymes related to carbohydrate digestion, which could be beneficial for the treatment of diabetes and diabetic complications. Further phytochemical study and a quantitative analysis of active compounds along with the in vivo antidiabetic effects and toxicity tests should be performed in the future.
ACKNOWLEDGEMENT
This research project is supported by Center of Innovative Pharmacy, Faculty of Pharmacy, Mahidol University under the Research Cluster Grant (Grant number PY- CIP6302).
REFERENCES
Ahongshangbam, S., Guruaribam, S., and Chattopadhyay, S. 2014. Bioactive compounds and antioxidant activity of Polygonum odoratum Lour. International Journal of Basic and Applied Biology. 2: 94-97.
Anantachoke, N., Lomarat, P., Praserttirachai, W., Khammanit, R., and Mangmool, S. 2016. Thai fruits exhibit antioxidant activity and induction of antioxidant enzymes in HEK-293 cells. Evidence-Based Complementary and Alternative Medicine. 2016: 1-14.
Cai, Y., Luo, Q., Sun, M., and Corke, H. 2004. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sciences. 74: 2157-2184.
Dash, G.K., and Zakaria, B. 2016. Pharmacognostic studies on Persicaria odorata (Lour.) Sojak. Journal of Pharmacy Research. 10, 377-380.)
Deponte, M. 2013. Glutathione catalysis and the reaction mechanisms of glutathione- dependent enzymes. Biochimica et Biophysica Acta. 1830: 3217-3266.
Fearon, I.M., and Faux, S.P. 2009. Oxidative stress and cardiovascular disease: Novel tools give (free) radical insight. Journal of Molecular and Cellular Cardiology. 47: 372-381. 013
Gerich, J.E. 2003. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. JAMA Internal Medicine. 163: 1306-1316.
Guo, C., Sun, L., Chen, X., and Zhang, D. 2013. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regeneration Research. 8: 2003-2014. Hogan, S., Zhang, L., Li, J, Sun, S., Canning, C., and Zhou, K. 2010. Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutrition and Metabolism. 7: 1-9.
Jones, K., Sim, L., Mohan, S., Kumarasamy, J., Liu, H., Avery, S., Naim, H.Y., Quezada- Calvillo, R., Nichols, B.L., Pinto, B.M., and Rose, D.R. 2011. Mapping the intestinal alpha-glucogenic enzyme specificities of starch digesting maltase- glucoamylase and sucrase-isomaltase. Bioorganic & Medicinal Chemistry. 19: 3929-3934.
Kee, K.T., Koh, M., Oong, L.X., and Ng, K. 2013. Screening culinary herbs for antioxidant and α-glucosidase inhibitory activities. International Journal of Food Science & Technology. 48: 1884-1891.
Kim, J.S., Kwon, C.S., and Son, K.H. 2000. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Bioscience Biotechnology and Biochemistry. 64: 2458- 2461.
Kumar, B.D., Mitra, A., and Manjunatha, M. 2010. A comparative study of alpha- amylase inhibitory activities of common antidiabetic plants of Kharagpur 1 block. International Journal of Green Pharmacy. 4: 115-121.
Lee, T., and Vairappan, C. 2011. Antioxidant, antibacterial and cytotoxic activities of essential oils and ethanol extracts of selected South East Asian herbs. Journal of Medicinal Plants Research. 5: 2584-2590.
Mannervik, B. 1987. The enzymes of glutathione metabolism: an overview. Biochemical Society Transactions. 15: 717-718.
Matough, F.A., Budin, S.B., Hamid, Z.A., Alwahaibi, N., and Mohamed, J. 2012. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos University Medical Journal. 12: 5-18.
Mehta, J.L., Rasouli, N., Sinha, A.K., and Molavi, B. 2006. Oxidative stress in diabetes: a mechanistic overview of its effects on atherogenesis and myocardial dysfunction. The International Journal of Biochemistry and Cell Biology. 38: 794- 803.
Nanasombat, S., and Teckchuen, N. 2009. Antimicrobial, antioxidant and anticancer activities of Thai local vegetables. Journal of Medicinal Plants Research. 3: 443- 449.
Niedowicz, D.M., and Daleke, D.L. 2005. The role of oxidative stress in diabetic complications. Cell Biochemistry and Biophysics. 43: 289-330.
Putthawan, P., Poeaim, S., and Areekul, V. 2017. Cytotoxic activity and apoptotic induction of some edible Thai local plant extracts against colon and liver cancer cell lines. Tropical Journal of Pharmaceutical Research. 16: 2927-2933.
Ranilla, L.G., Kwon, Y-I., Apostolidis, E., and Shetty, K. 2010. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresource Technology. 101: 4676-4689.
Ridzuan, P.M., Aini, H.H., Norazian, M.H., Shah, A., Roesnita., and Aminah, K.S. 2013. Antibacterial and antifungal properties of Persicaria odorata leaf against pathogenic bacteria and fungi. The Open Conference Proceedings Journal. 4: 71- 74.
Ridzuan, P.M., Hamzah, H.A., Shah, A., Hassan, N.M., and Roesnita, B. 2017. Synergistic effects of Persicaria odorata (Daun Kesom) leaf extracts with standard antibiotics on pathogenic bacteria. International Medical Journal Malaysia. 16: 27-32.
Saad, R., Khan, J., Krishnanmurthi, V., Asmani, F., and Yusuf, E. Effect of different extraction techniques of Persicaria odorata extracts utilizing anti-bacterial bioassay. British Journal of Pharmaceutical Research. 4: 2146-2154.
Sithisarn, P., Rojsanga, P., Sithisarn, P., and Kongkiatpaiboon, S. 2015. Antioxidant activity and antibacterial effects on clinical isolated Streptococcus suis and Staphylococcus intermedius of extracts from several parts of Cladogynos orientalis and their phytochemical screenings. Evidence-Based Complementary and Alternative Medicine. 2015: 1-8.
Sunthudlakhar, P., Sithisarn, P., Wannissorn, B., Jarikasem, S., and Rojsanga, P. 2019. Phytochemical profiles, antioxidant and antibacterial activities of 11 Phellinus mushrooms collected in Thailand. The Natural Products Journal. 9: 144-156.
Supasuteekul, C., Nonthitipong, W., Tadtong, S., Likhitwitayawuid, K., Tengamnuay, P., and Sritularak, B. 2016. Antioxidant, DNA damage protective, neuroprotective, and α-glucosidase inhibitory activities of a flavonoid glycoside from leaves of Garcinia gracilis. Revista Brasileira de Farmacognosia. 26: 312-320.
Tadera, K., Minami, Y., Takamatsu, K., and Matsuoka, T. 2006. Inhibition of alpha- glucosidase and alpha-amylase by flavonoids. Journal of Nutritional Science and Vitaminology. 52: 149-153.
Tan, Y., Chang, S.K.C., and Zhang, Y. 2017. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chemistry. 214: 259-268.
Type 2 diabetes (homepage on the Internet). International Diabetes Federation. (cited 30 Oct 2019). Available from: https://www.idf.org/aboutdiabetes/type-2- diabetes.html
Vikram, P., Chiruvella, K.K., Ripain, I.H.A., and Arifullah, M. 2014. A recent review on phytochemical constituents and medicinal properties of kesum (Polygonum minus Huds.). Asian Pacific Journal of Tropical Biomedicine. 4: 430-435.
Wattanathorn, J., Thiraphatthanavong, P., Thukham-Mee, W., Muchimapura, S., Wannanond, P., and Tong-Un, T. 2017. Anticataractogenesis and antiretinopathy effects of the novel protective agent containing the combined extract of mango and vietnamese coriander in STZ-diabetic rats. Oxidative Medicine and Cellular Longevity. 2017: 1-13.
Wickramaratne, M.N., Punchihewa, J.C., and Wickramaratne, D.B. 2016. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complementary and Alternative Medicine. 16: 1-5.
Xiao, J., Kai, G., Yamamoto, K., and Chen, X. 2013. Advance in dietary polyphenols as a-glucosidases inhibitors: a review on structure-activity relationship aspect. Critical reviews in food science and nutrition. 53: 818-836.
Yeh, C.T., and Yen, G.C. 2006. Induction of hepatic antioxidant enzymes by phenolic acids in rats is accompanied by increased levels of multidrug resistance- associated protein 3 RNA expression. The Journal of Nutrition. 136: 11–15.
Zhang, L., Ravipati, A.S., Koyyalamudi, S.R., Jeong, S.C., Reddy, N., Smith, P.T., Bartlett, J., Shanmugam, K., Münch, G., and Wu, M.J. 2011. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. Journal of Agricultural and Food Chemistry. 59: 12361- 12367.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Kanya Thongra-ar1, Piyanuch Rojsanga2,5, Savita Chewchinda3, Supachoke Mangmool4, and Pongtip Sithisarn1,5,*
1 Department of Pharmacognosy, Faculty of Pharmacy, Mahidol University, Bangkok 10400, Thailand
2 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Mahidol University, Bangkok 10400, Thailand
3 Department of Food Chemistry, Faculty of Pharmacy, Mahidol University, Bangkok 10400, Thailand
4 Department of Pharmacology, Faculty of Science, Mahidol University, Bangkok 10400, Thailand
5 Center of Innovative Pharmacy for Pharmaceutical and Herbal Product Development, Faculty of Pharmacy, Mahidol University, Bangkok 10400, Thailand
Corresponding author: Pongtip Sithisarn, E-mail: pongtip.sit@mahidol.ac.th
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: September 19, 2020;
Revised: December 5, 2020;
Accepted: January 4, 2021;
Published online: March 5, 2021

