
Pollen Morphology in Various Life-form of Aquatic Macrophytes
Ratchaneekorn Sangsuk, Henrik Baslev, and Arunothai Jampeetong*Published Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.050
Journal Issues : Number 3, July-September 2021
Abstract Ability of angiosperms to produce flowers and seeds for the sexual reproduction is important also in aquatic plants. Pollination in aquatic plants is facilitated by insects, wind, and water, however, pollen morphology related to the plant’s life forms and pollen dispersal are not well described. This study investigates pollen morphology of selected aquatic macrophytes. Plants were collected and preserved as dried specimens. Mature pollen grains of each species were separated from the anthers and then placed on glass slides and mounted with distilled water. Pollen shape and size were observed under a light microscope (LM). Number of apertures and exine ornamentation were examined using scanning electron microscope (SEM). Closely related plant species had similar pollen morphology. Among the 28 species studied, pollen size varied from small to very large (range 10–200 µm) and their shapes were prolate-spheroidal, prolate, oblate, suboblate, and oblate-spheroidal. Some species had inaperturate pollen grains; the remainders were monoaperture, triaperture or polyaperture. Both colpate and porate apertures were found. The pollen surfaces were echinate, fovelate, granulate, lophate, plicate, regulate, regulate-fovelate, reticulate, striate, and verrucate, respectively.
Keywords: Aquatic plants, Palynology, Pollen ornamentation, Pollination, SEM
Funding: This research was partly supported by Chiang Mai, University, Thailand. HB acknowledges support from the Carlsberg Foundation (CF14-0245) to study the Thai flora.
Citation: Sangsuk, R., Baslev, H., and Jampeetong, A. 2021. Pollen morphology in various life-form of aquatic macrophytes. CMUJ. Nat. Sci. 20(3): e2021050.
INTRODUCTION
Pollen grains are important in the reproductive process of seed plants as they transfer the male gametes. Pollen delivery to the female targets is an initial step in the gene pool transfer to the next generation. Pollen of different species have different characteristics. Plants in the same genus or family mostly have similar pollen morphology including their shape, aperture, and exine wall pattern (Banks and Rudall, 2016). In nature, pollen can be carried by wind, animals such as insects and birds, and by water currents (Cronk and Fennessy, 2001; Tanaka et al., 2004; Cresswell et al., 2010; Zhang et al., 2010). Some pollen characteristics are matched with pollination modes. Pollen transferred by wind is usually lightweight, giving it better flotation. Some species have sticky pollen that help attaching it to the pollinators (Cook, 1988).
Aquatic macrophytes pass their whole life or part of it in or near water and have structural and physiological adaptations to the aquatic or wetland environments (Sculthorpe, 1967; Cronk and Fennessy, 2001; Chamber et al., 2008). Aquatic macrophytes live in ponds, lakes or rivers, and some grow on river banks or in the littoral zone. Aquatic macrophytes attain different life-forms such as submerged, free- floating, emerged, and floating-leaved plants which adapt them to different niches in the aquatic habitats (Cronk and Fennessy, 2001). Even though aquatic macrophytes are generally small compared to terrestrial plants, they provide essential ecosystem services such as preventing soil erosion, improving water quality, and providing food and habitats for aquatic animals (Byers et al., 2006; Chamber et al., 2008). The limited palynological literature on aquatic macrophytes includes studies of Pontederiaceae (Raj and Saxena, 1966), Haloragaceae (Aiken, 1978), Cyperaceae (Butt et al., 2018), and Nymphaea (Bhunia and Mondal, 2012). Most of the literature has focused on using pollen morphology in plant identification or in studies of aquatic plant phylogeny and evolution. Palynology provides crucial information at generic and subgeneric levels, and it can be used to understand the relationship among families and higher taxa (Stuessy, 2009). A study by Lu et al. (2015) showed that the monoaperture condition was an ancestral character of basal angiosperms which included Nymphaeaceae and Ceratophyllaceae. Luo et al. (2015) found that most monocotyledons had equatorial aperture while polypantroporate pollen was found only in Alismataceae. Moreover, the pollen aperture in basal eudicots has been derived from colpi or colpori (Yu et al., 2018). Pollen of aquatic macrophytes in India had diverse characters (Ghandi et al., 2014), but data on pollen wall and aperture were not provided in that study. On the other hand, a study by Du and Wang (2014) investigated relationships between aquatic macrophytes’ life form and pollination mode. However, there is still a lack of knowledge of pollen morphology, especially concerning size and shape of the pollen grains as well as wall structure which are related to whether the pollen is dispersed by water, wind, or insects.
Knowledge about the pollen of aquatic macrophytes is needed to understand whether the species of the four dominant life-forms of aquatic macrophytes share morphological features. By employing light microscopy and electron microscopy, this study aims to describe the pollen grain morphology of a number of aquatic macrophytes. The results from this study will be helpful for plant identification and provide useful information for plant ecology, paleoecology, archaeology and forensic botany.
MATERIALS AND METHODS
Plant materials
We collected mature flowers of 28 species of aquatic macrophytes belonging to 22 genera and 18 families (Table 1) from ponds, lakes, canal banks in northern Thailand. For each species, at least three samples were selected for herbarium specimen preparation. Plant nomenclature follows World Flora Online (http://www.worldfloraonline.org/) and plant vouchers were deposited at the Herbarium of Biology Department of Chiang Mai University (CMUB), Thailand.
Pollen study
Pollen grains were dissected out of the anthers, then placed on a glass slide and mounted in distilled water. Pollen morphology was observed and photographed under a light microscope (LM). The polar (P) and equatorial (E) diameters were measured in 10 samples per species. Then, the size and shape of the pollen grains were examined and their P/E ratios were calculated (Erdtman, 1969). Micrographs were obtained with a scanning electron microscope (LV-SEM, JSM 5910, Tokyo, Japan). Pollen grains were separated from the anthers and placed on aluminum stubs with double-adhesive tape. The samples were coated with gold-palladium for two minutes using a SPI-Module sputter coater, then the pollen wall was observed under LV-SEM, using an accelerating voltage of 15 kV. Exine sculpturing and aperture descriptions follow standard terminology (Erdtman, 1969; Moore et al., 1991; Punt et al., 2007; Halbritter et al., 2018).
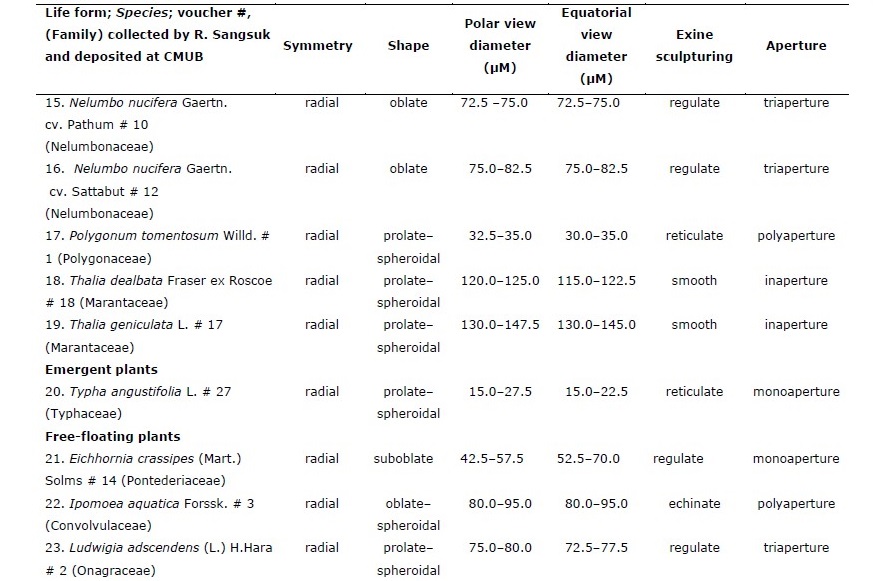
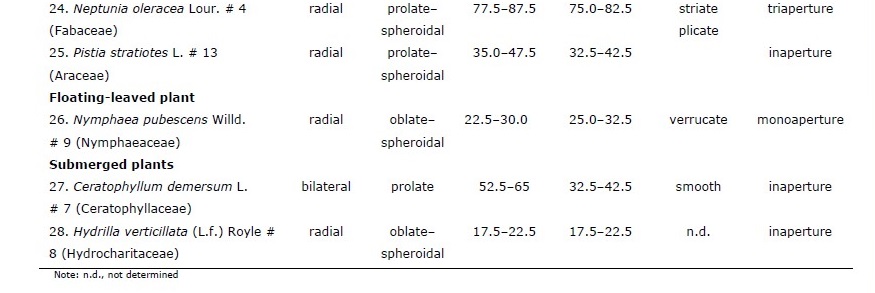
Table 1. Pollen characteristics of 28 species of aquatic macrophytes from northern Thailand arranged by life-form and then alphabetically by species.
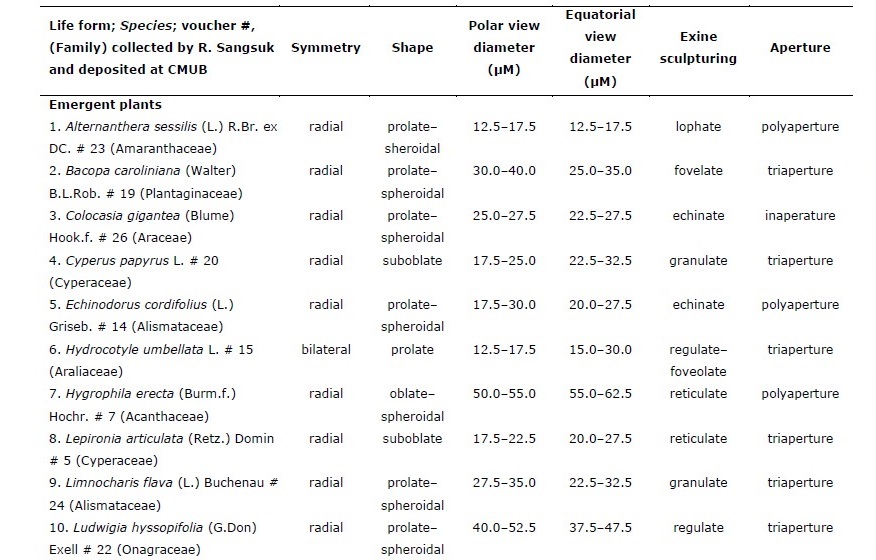
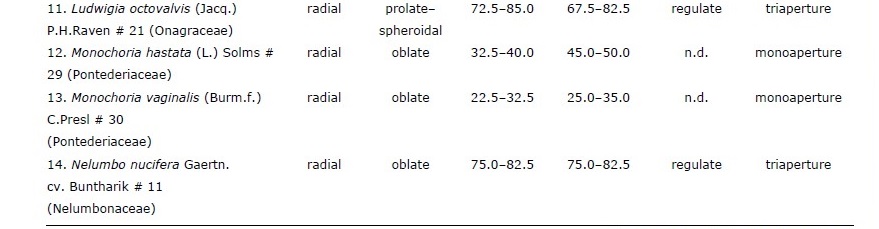
Table 1. Continued.
RESULTS
Of the 28 sampled species, 20 were emergent, five were free-floating, two were submerged, and one had floating-leaves. Pollen of congeneric species had similar morphology whereas those of plants in different genera or families had different morpho-palynological characteristics (Table 1).
Overall, 50% of studied species had prolate-spheroidal pollen grain (P/E = 1.00– 1.14) while approximately 18%, 11%, 14% and 7% of the studied species were oblate (P/E = 0.50–0.75), suboblate (P/E = 0.75–0.88), oblate-spheroidal (P/E = 0.88–1.00) and prolate (P/E = 1.33–2.00), respectively (Figure 1). Pollen size was classified into four categories including nine species with small grains (10–25 µm), ten species with medium sized grains (25–50 µm), seven species with large grains (50–100 µm), and two species with very large grains (100–200 µm).
Four categories of apertures were observed; six species had inaperturate pollen (no opened channel), five species had monoaperturate grains (1 opened channel), twelve species had triaperturate grains (3 opened channels), and five species had polyaperturate pollen grains (more than 3 opened channel). Approximately 43% of studied pollen grains were triaperturate. The exine showed variation in ornamentation (Figure 1, 2). Three species had smooth exine, three species had echinate exine, two species had granulate exine, one species had fovelate exine, one species had lophate exine, one species had plicate pollen, four species had reticulate, seven species had regulate, one species had regulate-fovelate pollen, one species had verrucate pollen and one species had pollen with striate exine structure. In Hydrilla verticillata (L.f.) Royle, Monochoria hastata (L.) Solms, and M. vaginalis (Burm.f.) C.Presl we could not determine the nature of the exine surface.
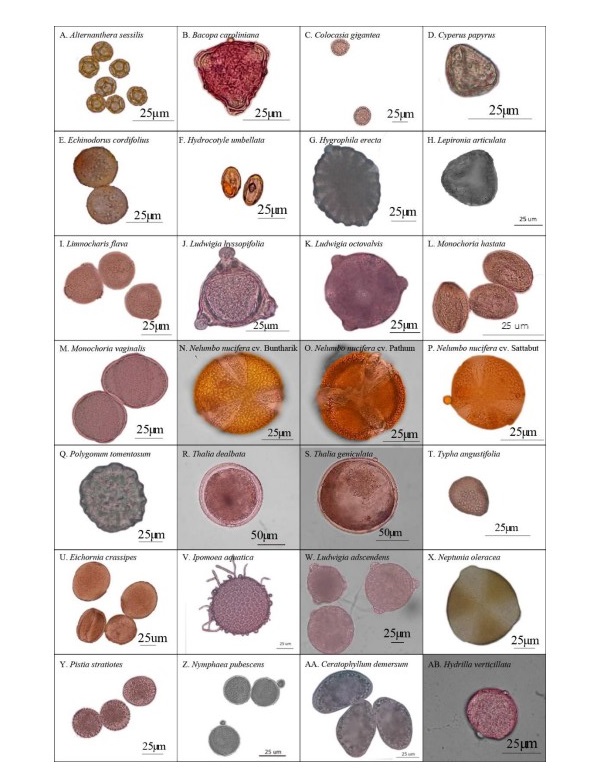
Figure 1. Light microscope photographs of pollen grains arranged by life-form and then alphabetically by species. Emergent plants: A. Alternanthera sessilis; B. Bacopa caroliniana; C. Colocasia gigantea; D. Cyperus papyrus; E. Echinodorus cordifolius; F. Hydrocotyle umbellata; G. Hygrophila erecta; H. Lepironia articulata; I. Limnocharis flava; J. Ludwigia hyssopifolia; K. Ludwigia octovalvis; L. Monochoria hastata; M. Monochoria vaginalis; N. Nelumbo nucifera cv. Buntharik; O. Nelumbo nucifera cv. Pathum; P. Nelumbo nucifera cv. Sattabut; Q. Polygonum tomentosum; R. Thalia dealbata; S. Thalia geniculata; T. Typha angustifolia; Free-floating plants: U. Eichhornia crassipes; V. Ipomoea aquatica; W. Ludwigia adscendens; X. Neptunia oleracea; Y. Pistia stratiotes; Floating-leaved plant: Z. Nymphaea pubescens; Submerged plants: AA. Ceratophyllum demersum; AB. Hydrilla verticillata.
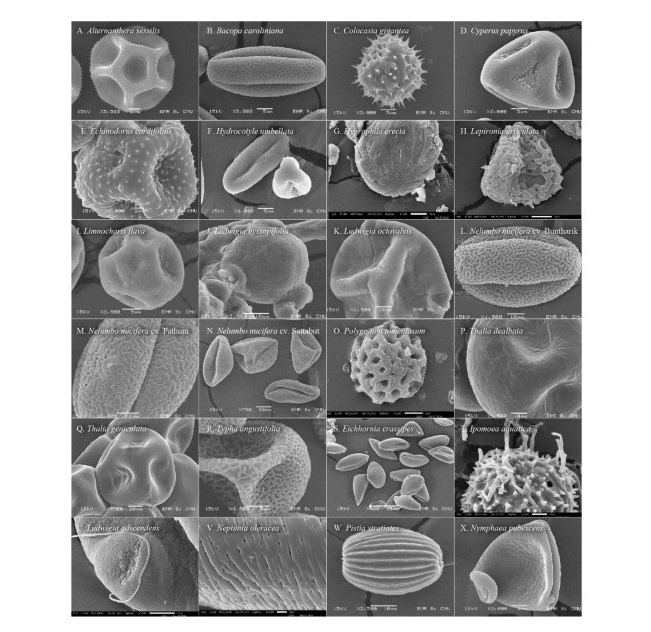
Figure 2. Scanning electron microscope photographs of pollen grains arranged by life-form and then alphabetically by species. Emergent plants:
A. Alternanthera sessilis; B. Bacopa caroliniana; C. Colocasia gigantea; D. Cyperus papyrus; E. Echinodorus cordifolius; F. Hydrocotyle umbellata; G. Hygrophila erecta;
H. Lepironia articulata; I. Limnocharis flava; J. Ludwigia hyssopifolia; K. Ludwigia octovalvis; L. Nelumbo nucifera cv. Buntharik; M. Nelumbo nucifera cv. Pathum;
N. Nelumbo nucifera cv. Sattabut; O. Polygonum tomentosum; P. Thalia dealbata;
Q. Thalia geniculata; R. Typha angustifolia; Free-floating plants: S. Eichhornia crassipes; T. Ipomoea aquatica; U. Ludwigia adscendens; V. Neptunia oleracea;
W. Pistia stratiotes; Floating-leaved plant: X. Nymphaea pubescens.
DISCUSSION
In general, pollen morphology, especially characters of apertures and pollen’s wall structure, have been widely used as an important tool to serve taxonomic purposes. External characters of pollen grains are valuable for lower levels of the taxonomic hierarchy while internal ones are valuable for higher levels. Moreover, palynological data may suggest relationship at the specific level (Stuessy, 2009). Pollen grains of Nymphaea pubescens Willd. were categorized by its ring-like aperture while pollen grains of other species of Nymphaea had other kinds of apertures, i.e., colpate with obtuse ends in N. nouchali Burm.f., and boat like sulcate aperture in N. stellata Willd. and N. rubra Roxb. ex Andrews. Psilate or scabrate to verrucate, fossulate with irregularly shaped grove appears as exine ornamentation of insects pollinated species of Nymphaea (Bhunia and Mondal, 2012). However, distal apertures located at the pole were observed in Nymphaeaceae and the monoaperture type was considered to be the ancestral type for the earliest angiosperms (Lu et al., 2015). Pollen evolution in monocots is displayed by pollen apertures. The aperture characters in early seed plants shifted from a proximal to a distal position whereas equatorial position shifting appeared in basal eudicot (Luo et al., 2015). Zavada (1983) identified the evolutionary trend of monocot pollen which changed by losing sulcus characters and by increasing types and numbers of apertures. The monosulcate type was considered the most primitive character in monocots while other derivative characters (i.e., disulcate and multiaperturate) are common in the advanced monocots (Zavada, 1983). Nevertheless, inaperturate pollen commonly occurs in primitive dicotyledons and among early branches of monocot taxa such as Araceae and Hydrocharitaceae. This aperture type, surprisingly, is absent from Araceae’s closely related family Alismataceae (Furness and Rudal, 1999). On the other hand, a common character in the dicotyledons, which is the triaperturate type, occurs in only few monocot taxa (Zavada, 1983). As shown in this study, pollen of Cyperus papyrus L. was of the triaperturate type which is incongruent with other species of Cyperus (Nagels et al., 2009). The number of apertures in Cyperaceae varies from zero to more than eight. Increasing numbers of apertures may offer more germination sites (Nagels et al., 2009). Three-colpate apertures arranged around the equatorial plane of the grains is the most common type in most eudicots. The colpi are occasionally reduced to pores and sometimes increase in number as in Amaranthaceae, in which multiple pores are globally distributed over the pollen surface. This qualification might increase the fertilization rate in the plants (Furness and Rudal, 2004). However, palynological information obviously must be integrated with other information such as pollination biology (Stuessy, 2009). Pollination modes and pollen features are correlated to the plants’ environment and conditions (Luo et al., 2015).
In this study, we show that pollen morphology differs between aquatic macrophyte life forms. In emergent plants, free-floating plants and floating-leaved plants, most species are entomophilous. These plants attract pollinators with colorful petals or anthers, providing nectar or releasing scent. The pollen wall, particularly the exine sculpturing had ridges, spines, and papillae that help the grains attach to the pollinators such as bees, other insects, or other animals. The exine sculpturing facilitates pollen grain delivery to the stigma, and the pollen surface is also important in protecting the pollen from undesirable environments and in pollen-stigma interactions at the initial step of sexual reproduction within the same species (Heslop-Harrison, 1979; Edlund et al., 2004, Wang and Dobritsa, 2018). Thalia was an exception, in that its exine wall was smooth and apparently ineffective in pollen-pollinators attachment. Previous studies have revealed that pollination in Thalia had a close relationship to its floral structure and its pollination mechanism. Before anthesis, the pollen is presented in a depression behind the stigma. At anthesis, the style and cucullate staminode are under tension being positioned opposite to each other to maintain equilibrium. When the pollinator inserts its organs into the flower causing a depression to the appendage on the cucullate staminode, the style is released and moves forward, guided by the stigma to contact with the foreign pollen from the previously visited flowers on the visitor’s body. When contacting the visitor, the flower places its own pollen onto the specific organ of the visitor in the form of sticky paste (Classen-Bockhoff, 1991; Kennedy, 2000). This mechanism was called “explosive” and it was found in Thalia dealbata Fraser ex Roscoe, T. geniculata L., and T. trichocalyx Gagnep. (Aruli and Reddi, 1995). It is expected that the large size of the pollen grains may increase the surface area that eventually will touch the stigma. In contrast, wind pollinated plants such as C. papyrus, Lepironia articulata (Retz.) Domin, and Typha angustifolia L. had inflorescences and flower structures that helped the wind to carry the pollen grains. For example, the inflorescences of C. papyrus and T. angustifolia had long peduncles that caused most flowers to raise into the air, or even in L. articulata in which the inflorescence is very short, but the plants produce long filaments bringing the anthers in contact with the air. Among the 28 species studied here, those with wind pollination all had small pollen grains. This characteristic helps the pollen to be easily carried by the wind (Cook, 1988). In submerged species, especially Ceratophyllum demersum L., the pollen was produced under the water and its pollination relied on water currents. The pollen grains were smooth and thin-walled and the exine was thin and inaperturate (Takahashi, 1995). Thin-walled inaperturate pollen is common in submerged species. Because of the thin wall, we were not able to prepare the sample for SEM study. Similarly, pollen grains of H. verticillata were inaperturate and with undefined exine structure. Another study of Hydrilla showed that it has tightly arranged small spinous pollen grains (Tanaka et al., 2004). The delicate nature of the exine in this submerged species may be related to its pollination mechanism, which is considered to be intermediate between hydrophily and wind pollination. After Hydrilla’s staminate flowers are released and float on the water surface, the pollen grains are released into the air and then transferred to the open pistillate flowers (Cox, 1988; Du and Wang, 2014). If the pollen wall was smooth like in other submerged plants, the chance that the pollen grains would attach themselves to the stigma would be small. Thus, we interpret the tiny spinous wall as an adaptation to the attachment of the pollen when contacting the stigmas.
Pollen apertures, which are thin areas of the exine wall, are the sites where the pollen germinates, and their shape and position can influence the success of the pollination (Heslop-Harrison, 1979; Edlund et al., 2016). These thin areas of the wall also permit water to enter and exist pollen in response to surrounding hydration (Edlund et al., 2004; Halbritter and Hesse, 2004; Katifori et al., 2010). Among the four aquatic macrophyte life forms, the submerged species (C. demersum and H. verticillata) did not have pollen apertures which may be because of the non-hydrodynamic change inside the pollen grains. So, this characteristic may adapt the species to living submerged.
CONCLUSION
Pollen morphology of aquatic macrophytes varies among species. In submerged species, the exine wall was less developed and without aperture. This differs from the pollen morphology found in other life forms (free-floating, floating-leaved and emergent plants) most of which had exine patterns with ridges, spines, and papilla that could help in the attachment of pollen to pollinators and enhance the pollen delivery to the female target. Pollen exine sculpturing also plays an important role in pollen-stigma interaction. However, T. dealbata and T. geniculata with smooth exine wall were an exception.
REFERENCES
Aiken, S.G. 1978. Pollen Morphology in the genus Myriophyllum (Haloragaceae). Canadian Journal of Botany. 56: 976-982.
Aruli, J.S.R. and Reddi, S.C. 1995. Explosive pollen release and pollination in flowering plants. Proceeding of Indian National Science Academy. 61: 323-332.
Banks, H. and Rudall, P.J. 2016. Pollen structure and function in Caesalpinioideae legumes. American Journal of Botany. 103: 423-436.
Bhunia D. and Mondal A.K. 2012. Studies on production, morphology, and free amino acid of pollen of four members in the genus Nymphaea L. (Nymphaeaceae). International Journal of Science and Nature. 3: 705-718.
Butt, M.A., Zafar, M., Ahmad, M., Sultana, S., Ullah, F., Jan, J., Irfan, A., and Naqvi, S.A.Z. 2018. Morpho-palynological study of Cyperaceae from wetlands of Azad Jammu and Kashmir using SEM and LM. Microscopy Research and Technique. 81: 458-468.
Byers, E.J., Cuddington, K., Jones, C.G., Talley, T.S., Hastings, A., Lambrinos, J.G., Crooks, J.A., and Wilson, W.G. 2006. Using ecosystem engineers to restore ecological systems. Trends in Ecology and Evolution. 21: 493-500.
Chambers, P.A., Lacoul, P., Murphy K.J., and Thomaz, S.M. 2008. Global diversity of aquatic macrophytes in freshwater. Hydrobiologia. 595: 9-26.
Classen-Bockhoff, R. 1991. Investigations on the construction of the pollination apparatus of Thalia geniculata (Marantaceae). Botanica Acta. 104: 183-193.
Cook, C.D.K. 1988. Wind pollination in aquatic angiosperms. Annals of the Missouri Botanical Garden. 75: 768-777.
Cox, P.A. 1988. Hydrophilous pollination. Annual Review of Ecology and Systematics. 19: 261–279.
Cresswell, J.E., Krick, J., Patrick, M.A., and Lahoubi, M. 2010. The aerodynamics and efficiency of wind pollination in grasses. Functional Ecology. 24: 706-713.
Cronk, J.K. and Fennessy, M.S. 2001. Wetland Plants: Biology and Ecology. CRC Press, New York.
Du, Z.Y. and Wang, Q.F. 2014. Correlations of life form, pollination mode and sexual system in aquatic angiosperms. PLOS ONE 9: 1-15.
Edlund, A.F., Swanson, R., and Preuss, D. 2004. Pollen and stigma structure and function: The role of diversity in pollination. Plant and Cell. 16: S84-S97.
Edlund, A.F., Zheng, Q., Lowe, N., Kuseryk, S., Ainsworth, K.L., Lyles, R.H., Sibener, S.J., and Preuss, D. 2016. Pollen from Arabidopsis thaliana and other Brassicaceae are functionally omniaperturate. American Journal of Botany. 103: 1006-1019.
Erdtman, G. 1969. Handbook of palynology: morphology, taxonomy, ecology. An introduction to the study of pollen grains and spores. Munksgaard, Copenhagen. Furness, C.A. and Rudal, P.J. 1999. Inaperture pollen in monocotyledons. International Journal of Plant Science. 160: 395-414.
Furness, C.A. and Rudal, P.J. 2004. Pollen aperture evolution - a crucial factor for eudicots success. Trends in Plant Science. 9: 154-158.
Gandhi, A.K., Pragasam, A. and Ponnuchamy, R. 2014. Distribution and pollen characters of selected aquatic angiosperms of Pondicherry region, South India. Elixir Bio Diversity. 67: 21631-21636.
Halbritter, H. and Hesse, M. 2004. Principal modes of infoldings in tricolp (or) ate Angiosperm pollen. Grana. 43: 1-14.
Halbritter, H., Ulrich, S., Grimsson, F., Weber, M., Zetter, R., Hesse, H. Buchner, R., Svojtka, M., and Radivo, A.F. 2018. Illustrated Pollen Terminology. 2nd ed. Cham.
Heslop-Harrison, J. 1979. Aspects of the structure, cytochemistry and germination of the pollen of rye. Annals of Botany. 44: 1-47.
Katifori, E., Alben, S., Cerda, E., Nelson, D.R., and Dumais, J. 2010. Foldable structures and the natural design of pollen grains. Proceedings of the National Academy of Sciences of the United States of America. 107: 7635-7639.
Kennedy, H. 2000. Diversification in pollination mechanism in the Marantaceae. Monocot: Systematic and Evolution. CSIRO publishing. Collingwood.
Lu, L., Wortley, A.H., Li, D., Wang, H. and Blackmore, S. 2015. Evolution of angiosperm pollen. 2. the basal angiosperms. Annals of the Missouri Botanical Garden. 100: 227-269.
Luo, Y., Lu, L., Wortley, A.H., Li, D., Wang, H., and Blackmore, S. 2015. Evolution of angiosperm pollen. 3. monocots. Annals of the Missouri Botanical Garden. 101: 406-455.
Moore, P.D., Webb, J.A., and Collinson, M.E. 1991. Pollen analysis. 2nd ed. Blackwell Scientific Publication. Vienna.
Nagels, A., Muasya, A.M., Huysmans, S., Vrijdaghs, A., Smets, E., and Vinckier, S. 2009. Palynological diversity and major evolutionary trends in Cyperaceae. Plant Systematics and Evolution. 277: 117-142.
Punt, W., Hoen, P.P., Blackmore, S., Nilsson, S., and Le Thomas, A. 2007. Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology. 143: 1-81.
Raj, B. and Saxena, M.R. 1966. Pollen Morphology of Aquatic Angiosperms. Pollen et Spores. 8: 49-55.
Sculthorpe C.D. 1967. The Biology of Aquatic Vascular Plants. Edward Arnold Publishers, London, pp 3-10.
Stuessy, T.F. 2009. Plant taxonomy: the systematic evaluation of comparative data. 2nd ed. Columbia University Press. West Sussex.
Takahashi, M. 1995. Development of Structure-less Pollen Wall in Ceratophyllum demersum L. (Ceratophyllaceae). Journal of Plant Research. 108: 205-208.
Tanaka, N., Uehara, K., and Murata, J. 2004. Correlation between pollen morphology and pollination mechanisms in the Hydrocharitaceae. Journal of Plant Research. 117: 265-276.
Wang, R. and Dobritsa, A.A. 2018. Exine and aperture patterns on the pollen surface: Their formation and roles in plant reproduction. Annual Plant Reviews. 1: 1-40.
Yu, Y., Wortley, A.H., Lu, L., Li, D., Wang, H., and Blackmore, A. 2018. Evolution of angiosperm pollen. 5. Early diverging Superasteridae (Berberiopsidales, Caryophyllales, Cornales, Ericales, and Santalales) plus Dilleniales. Annals of the Missouri Botanical Garden. 103:106-161.
Zavada, M.S. 1983. Comparative morphology of monocot pollen and evolutionary trends of apertures and wall structures. The Botanical Review. 49: 331-379.
Zhang, X.L., Gituru, R.W., and Yang, C.F. 2010. Exposure to water increased pollen longevity of pondweed (Potamogeton spp.) indicates different mechanisms ensuring pollination success of angiosperms in aquatic habitat. Evolutionary Ecology. 24: 939-953.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Ratchaneekorn Sangsuk1 , Henrik Baslev2, and Arunothai Jampeetong1,*
1 Department of Biology, Faculty of Science, Chiang Mai University, Mueang, Chiang Mai, 50200, Thailand
2 Department of Biology, Aarhus University, Ny Munkegade 114-116, DK-8000 Aarhus C., Denmark
Corresponding author: Arunothai Jampeetong, E-mail: arunothai.2519@gmail.com
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: October 7, 2020;
Revised: December 22, 2020;
Accepted: December 25, 2020;
Published online: March 18, 2021

