
Effect of Enzyme Treatments on Protoplast Isolation from Leaves of Vetiver (Vetiveria spp.)
Published Date : 2021-04-01
DOI : https://doi.org/10.12982/CMUJNS.2021.048
Journal Issues : Number 3, July-September 2021
Abstract Protoplast isolation is a first and important step for establishing a new plant with desired traits through protoplast fusion technology. This experiments were conducted to evaluate various concentration of enzymes and incubation time on protoplast yield and viability in two vetiver ecotypes, Kamphaeng Phet 2 (Vetiveria zizanioides Nash) and Prachuap Khiri Khan (V. nemoralis A.Camus). The results revealed that protoplast yields were significantly affected by different enzyme treatments. The highest protoplast yield (6.12x105 protoplasts/ml) and high viability (98.61%) in Kamphaeng Phet 2 was obtained through the process of cell wall digestion when treated with enzyme solution containing 0.5% (w/v) cellulase onozuka R-10 and 0.5% (w/v) macerozyme R-10 in combination. While, the optimal enzyme solution for protoplast isolation from leaves of Prachuap Khiri Khan was the combination of 1.0% (w/v) cellulase onozuka R-10 and 0.4% (w/v) macerozyme R-10, resulting in the highest yield (6.80x105 protoplasts/ml) and viability (96.56%) of protoplasts. Meanwhile, incubation time of 24 h with the optimal enzyme solution resulted in the highest protoplast yields of both ecotypes. Our findings have the potential to generate an efficient protocol to isolate the protoplast from leaves of vetiver which can be used for further research studies in protoplast culture and fusion for vetiver improvement.
Keywords: Cellulase onozuka R-10, Macerozyme R-10, Protoplast isolation, Vetiver
Funding: This research was funded by Office of the Royal Development Projects Board (ORDPB).
Citation: Leaungthitikanchana, S., Thongdonyod, K., and Singphan, N. 2021. Effect of enzyme treatments on protoplast isolation from leaves of vetiver (Vetiveria spp.) CMUJ. Nat. Sci. 20(3): e2021048.
INTRODUCTION
Vetiver is a perennial grass of Poaceae family. It is distributed mainly in India, Southeast Asia, Tropical Africa, South Africa, and Central and South America (Lavania, 2000). In Thailand, two species of vetiver have been founded: (1) Yaa faek hom (Vetiveria zizanioides Nash), such as Kamphaeng Phet 2, Surat Thani, Songkhla 3 and Srilanka and (2) Yaa faek don (V. nemoralis A. Camus), such as Prachuap Khiri Khan, Loei, Ratchaburi and Roi Et. Both species have distinct ecological characteristics which make them adapt to different habitats. V. zizanioides can rapidly adapt to the environment. It could tolerance to diseases and to critical climatic factors. While, V. nemoralis, the local vetiver, is commonly found in dry areas or in soil conditions with good draining in all regions of Thailand. This species grows well in the areas either with strong or moderate sunlight. The tip of the clump bends over the ground like lemon grass (Ruanjaichon et al., 1995; Chusreeaeom and Roongtanakiat, 2017). The root system of vetiver is finely structured and very strong and has extensive fibrous roots. Therefore, the vetiver is an important grass and has been identified to be very effective plant for soil and water conservation, soil erosion and sediment control, for land stabilization and rehabilitation, and environmental protection (Yeboah et al., 2015).
The improvement in vetiver, such as biotic and abiotic resistance and quality, through conventional breeding is known to be difficult. It is because that the most commercial genotypes commonly used of vetiver are sterile. The usual method of propagating vetiver is to split existing plants and transplant the slips (Ruanjaichon et al., 1995; Prasertsongskun, 2004). Thus, protoplast fusion technology has a great potential for crop improvement that could be solve this limitation in vetiver. Protoplast is a plant cell that has had its cell wall removed. It can introduce agronomically important traits encoded by nuclear genomes through somatic hybridization which provides a method for overcoming the barrier of male sterility for mixing genomes of the parents (Shuro, 2018).
Protoplasts are isolated by using mechanical or enzymatic methods. Large amount of viable protoplast can be obtained with enzymatic methods. Less cell breakage and osmotic shrinkage occur compared with mechanical method (Chamani et al., 2012). It is often found more effective to apply a combination of enzymes, such as cellulase, hemicellulase and pectinase, to obtain optimum isolation. Cellulase and hemicellulase generally used to break the plant cell wall, while pectinase used for the separation of cell aggregates (Cove, 1979; Sija et al., 2016). The success of protoplast isolation depends on several factors that affect the release of protoplast in plants, such as the concentration and combination of enzyme, duration of enzyme incubation, pH and osmoticum of the enzyme solution, temperature and the extent of cell wall thickening (Chamani et al., 2012). These factors, especially enzyme treatments, influence different species and genotypes differently. For instances, Suzanne et al. reported the successful protoplast isolation of Gracilaria gracilis using enzyme solution containing 2% (w/v) cellulase onozuka R-10 and 1% (w/v) macerozyme R-10 with incubation time at 3 h in the dark (Huddy et al., 2013). Horvath (2009) succeeded in protoplast isolation of Solanum lycopersicum L. by using 2% cellulase R-10 and 0.5% macerozyme R-10 dissolved in 0.4 M sucrose-K3 solution and incubated for 12 h. The optimum condition of enzyme for protoplast isolation from Dendrobium crumenatum was 2% (w/v) cellulase and 2% (w/v) pectinase after incubation for 4 h (Tee et al., 2010). The high yield production of protoplast isolated from Phalaenopsis amboinensis was established by using 2% cellulase, 1% macerozyme and incubation time of 6 h (Machmudi et al., 2019).
The first and necessary step of the plant genetic improvement through somatic hybridization is an efficient protocol for protoplast isolation. Hence, this paper describes the optimum concentration of enzyme and incubation time for protoplast isolation from leaves of two vetiver ecotypes, including Kamphaeng Phet 2 and Prachuap Khiri Khan, to generate an effective method for high yields of vetiver’s protoplast production. Then, isolated protoplasts could be used for further research studies in protoplast culture and fusion for vetiver improvement.
MATERIALS AND METHODS
Plant Materials
The shoots of 2 ecotypes of vetiver, including Kamphaeng Phet 2 (V. zizanioides Nash) and Prachuap Khiri Khan (V. nemoralis A.Camus) were collected from Huai Hong Krai Royal Development Study Centre, Chiang Mai, Thailand. Shoots of vetiver were surface sterilized by shaking in 15% clorox for 15 min then cut into small pieces and cultured on MS medium (Murashige and Skoog, 1962) supplemented with 5 mg/l 6-benzyladenine (BA). The cultures were maintained in a suitable condition under a 16 h photoperiod and a temperature of 25 ± 2°C for 2 months.
Protoplast Isolation Standard Protocol
Protoplasts were isolated from 2-month-old in vitro leaves of Kamphaeng Pet 2 and Prachuap Khiri Khan. Approximately 0.5 g fresh weight of explants were cut transversely into 1-2 mm wide strips. The sliced leaves were plasmolysed in 0.5 ml of protoplast washing solution (PWS) containing 0.5 M mannitol and 2.5 mM CaCl2.2H2O (pH 5.6) for 20 min. The treated explants were removed and incubated in 5 ml of enzyme solution containing various concentration of enzymes, 0.5 M mannitol and 2.5 mM CaCl2.2H2O (pH 5.6) (Table 1). The leaf-enzyme mixtures were then incubated in the dark on orbital shaker (40 rpm) for 24 h. The solutions containing the protoplast were filtered through 40 mesh sieves to remove the undigested tissue and debris and then centrifuged at 800 rpm for 5 min. The supernatants were discarded after centrifugation and the protoplast pellets were resuspended with 10 ml of PWS and centrifuged at 800 rpm for 3 min. The protoplasts were purified by floating on a 20% sucrose solution and centrifuged at 1,000 rpm for 3 min, and then washed 3 times with PWS. The final protoplast pellets were resuspended in PWS. The protoplast yield and viability were observed by using a hemocytometer under the microscope.
Effect of Enzymes on Protoplast Isolation
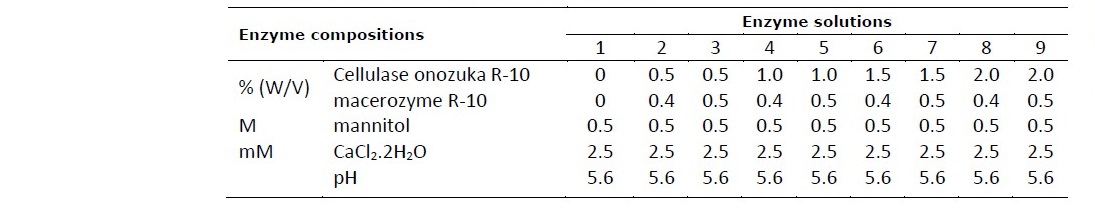
The effect of different concentration of enzymes on protoplast isolation was investigated. The various combination of 0.5, 1.0, 1.5 and 2.0% cellulase onozuka R-10 (Yakult Phamaceutical Industry Co., Ltd., Japan) and 0.4 and 0.5% macerozyme R-10 (Yakult Phamaceutical Industry Co., Ltd., Japan) which were dissolved in 0.5 M mannitol and 2.5 mM CaCl2.2H2O (pH 5.6) were used in this study as the protoplast isolation solution (enzyme solution) (Table 1). While, protoplast isolation solution without enzyme was used as the control.
Table 1. Composition of enzyme solutions for protoplast isolation of vetiver, Khampheang Phet 2 and Prachuap Khiri Khan.
Effect of incubation time on protoplast isolation
Incubation time is the duration required for complete release of protoplasts. To determine the suitable duration required for obtaining the highest yield of protoplasts, the sliced leaf samples were incubated with the optimal enzyme solution for Kamphaeng Phet 2 and Prachuap Khiri Khan for 0, 2, 6, 12 and 24 h in the dark. Then the protoplasts were collected and purified as previously described. The yield and viability of protoplasts were calculated to determine the effect of incubation time.
Determination of the yield and viability of obtained protoplasts
The protoplast suspension solution was observed under the microscope. The number of protoplasts were counted using a hemocytometer. Protoplast yield was calculated as follows:
protoplast yield (protoplast/ml) = Average cell count per square x dilution factor x 104
The viability of isolated protoplasts was assessed by trypan blue staining. Ten microliters of protoplast suspension solution with 10 µl of 0.4% trypan blue (Sigma, USA) were incubated for 10 min at room temperature. Then, the total number of viable protoplasts and the number of non-viable protoplasts (blue protoplast) were counted under a light microscope with a hemocytometer. The percentage of protoplast viability was calculated as follows:
![]()
Statistical Analysis
Experimental data were statistically analyzed using analysis of variance (ANOVA) in SPSS software version 22.0. Treatment means were compared by Duncan’s multiple range tests with a 95% confidence interval (P ≤ 0.05).
RESULTS
The isolation of protoplast from leaves of two ecotypes of vetiver, Kamphaeng Phet 2 and Prachuap Khiri Khan, was optimized. The main factors affecting the protoplast isolation, concentration of enzymes and incubation time, were considered in this study. The various concentration of enzymes (0, 0.5, 1.0, 1.5 and 2.0% cellulase onozuka R-10 and 0, 0.4 and 0.5% macerozyme R-10) at different incubation time (0, 2, 6, 12 and 24 h) were investigated. Yield of protoplasts were determined using a hemocytometer. Trypan blue, a staining dye was used to observe the viability of protoplasts.
Effect of Concentration of Enzyme on Yield and Viability of Protoplast
The concentration of enzymes required for complete release of protoplast were examined. The results revealed that the concentration of enzymes had a significant effect on the protoplast yield derived from leaves of both vetiver species. The yield and viability of protoplast isolated with different concentration of enzymes were shown in Table 2. The leaves of Kamphaeng Phet 2 yielded the highest number of protoplast (6.12x105 protoplasts/ml) and high viability (98.61%) when treated with enzyme solution containing 0.5% (w/v) cellulase onozuka R-10 and 0.5% (w/v) macerozyme R- 10 with 0.5 M mannitol, 2.5 mM CaCl2.2H2O (pH 5.6). While, the highest protoplast yield (6.80x105 protoplasts/ml) and high viability (96.56%) of Prachuap Khiri Khan were obtained using enzyme solution containing 1.0% (w/v) cellualse onozyka R-10 and 0.4% (w/v) macerozyme R-10 with 0.5 M mannitol, 2.5 mM CaCl2.2H2O (pH 5.6). The isolated protoplasts of both species were green in color, spherical in shape, small in size, rich in chloroplasts and well separated (Figure 1).
Table 2. Effect of concentration of enzymes on the yield and viability of protoplasts isolated from leaves of two vetiver ecotypes, Kamphaeng Phet 2 and Prachuap Khiri Khan.
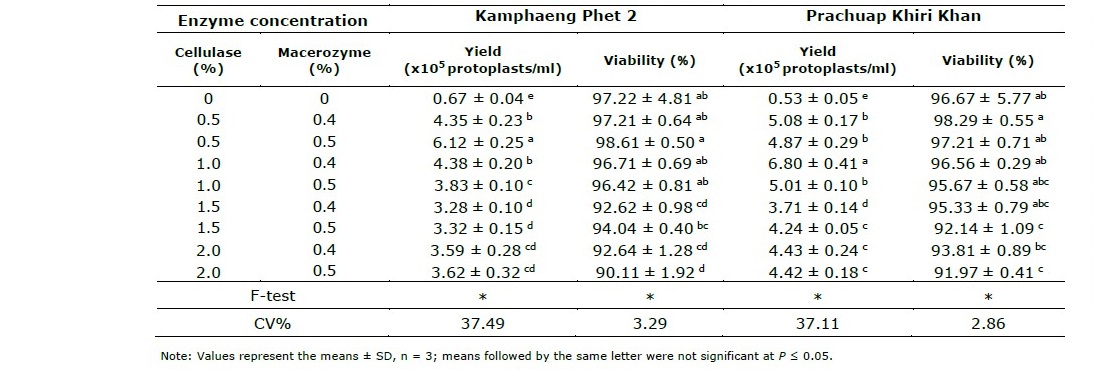

Figure 1. Isolation of protoplast from in vitro leaves of Kamphaeng Phet 2 (A) and Prachuap Khiri Khan (D) by using the optimal enzyme concentration at 24 h of incubation time. The freshly isolated protoplasts of Kamphaeng Phet 2, with 40 and 100 x objective (B and C, respectively) and Prachuap Khiri Khan, with 40 and 100 x objective (E and F, respectively) were observed under microscope. (Scale bar = 50 µm).
Effect of Incubation Time on Yield and Viability of Protoplast
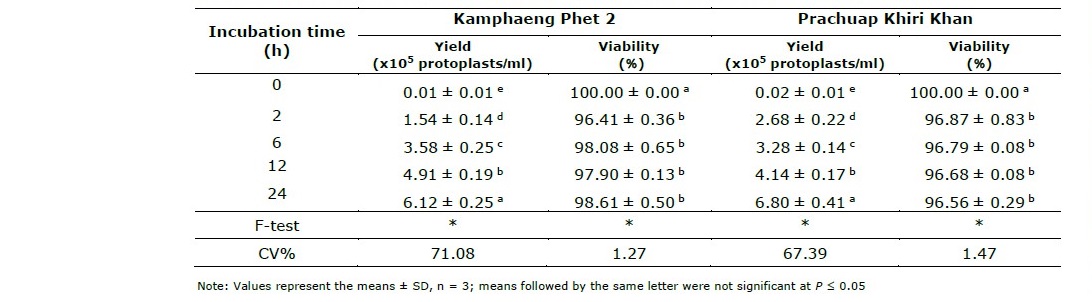
The investigation of the optimal incubation time was performed. The experiment was conducted to determine the yield and viability of protoplasts with regarding the different incubation time (0, 2, 6, 12 and 24 h). The effect of incubation time on the yield and viability of protoplasts of two vetiver ecotypes were shown in Table 3. It was clear demonstrated that the incubation time significantly influence on the yield of protoplast isolated from vetiver leaves. The results showed that 24 h of incubation gave the highest protoplast yields of both Kamphaeng Phet 2 and Prachuap Khiri Khan (6.12x105 and 6.80x105 protoplast/ml, respectively) when treated with the optimal enzyme concentration. The yield of protoplasts increased when the incubation times used were also increased from 0 h to 24 h.
The viability test with trypan blue was showing the best result with a control (0 h of incubation time) for both species which gave the same highest percentage of viability of protoplast (100%). Although, the viability of protoplasts was decreased when incubation time had longer as compared with control. However, the viability testing of each incubation time showed quite similar results which gave the high viability of protoplast around 96.41 - 98.61% for Kamphaeng Phet 2 and 96.56 - 96.87% for Prachuap Khiri Khan (Table 3). The viable protoplasts were not stained which showed spherical shape and green color, while non-viable protoplasts had a spherical shape and blue color which does not absorb the color of staining when observed under a microscope (Figure 2).
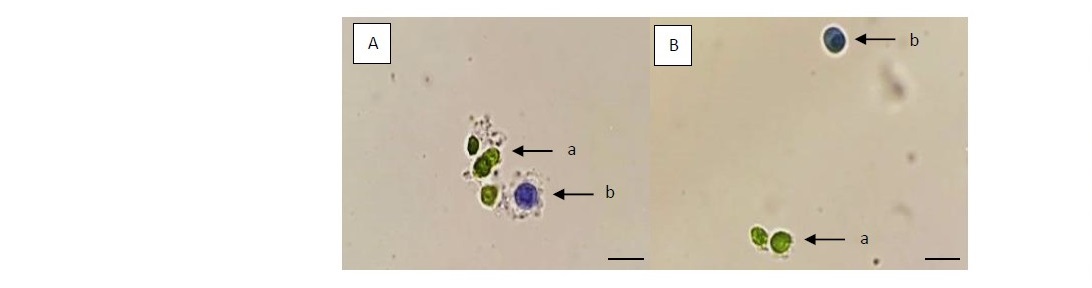
Figure 2. The viability of protoplasts isolated from in vitro leaves of Kamphaeng Phet 2 (A) and Prachuap Khiri Khan (B). The leaf mesophyll cells were digested for 24 h in optimal enzyme solution. The viable protoplasts were not stained (a), while the only non-viable protoplasts were stained with trypan blue (b). (Scale bar = 50 µm, with 100 x objective).
Table 3. Effect of incubation time on the yield and viability of protoplasts isolated from leaves of two vetiver ecotypes, Kamphaeng Phet 2 and Prachuap Khiri Khan.
DISCUSSION
The suitable condition of enzyme is a critical step in the optimization of protoplast isolation. The success of protoplast isolation is especially dependent on the concentration of enzyme used (Tahami et al., 2014). In this study, the results indicated that all concentration of enzyme treatments were effective method when compared to the control (the isolation solution without enzyme). However, they showed in varying numbers of protoplast yield and viability. The combination of cellulase, i.e. cellulase onozuka R-10, and pectinase, i.e. macerozyme R-10, is normally used to digest the cell walls and separate protoplasts to a single cell. The cellulose, hemicellulose and pectin in the cell wall were removed by enzymatic hydrolysis with those enzymes inducing, the protoplasts were then released and individually separated (Power and Cocking, 1970; Tang et al., 2019). This study suggested that the combination of 0.5-1.0% (W/V) cellulase onozuka R-10 and 0.4-0.5% (W/V) macerozyme R-10 was found to be suitable for protoplast isolation from leaves of Kamphaeng Phet 2 and Prachuap Khiri Khan. The yields of protoplasts were much lower, 3.28x105 - 4.43x105 protoplasts/ml, when the concentration of cellulase onozuka R-10 were used higher than 1.5% (W/V) of that in both species. It is possible that the higher cellulase concentration used as over-digestion of explants resulted in the decreasing of protoplast yield (Zhu et al, 2005). Moreover, higher concentrations of enzymes might negatively influence the viable protoplasts (Chamani et al., 2012). Similar observations were obtained by Yao et al. (2016) who reported that 1.0% cellulase R-10, 0.5% pectolyase Y-23 and 0.6 M mannitol (pH 5.8) was the optimum concentration used to obtain high yield of protoplast for sweet cherry (Prunus avium L.), 4.3x106 protoplasts/g FW of explants and 0.5% celluase onozuka RS10 and 0.5% macerozyme R-10 in combination was the suitable enzyme concentration for obtaining high protoplast yields of Citrus reticulata L. (Wulandari et al., 2018).
Furthermore, this study found that Prachuap Khiri Khan required the higher concentration of cellulase onozuka R-10 as compared with Kamphaeng Phet 2. This was probably because they had differently cell structure. Khanema (2009) reported that internal leaf structures of both species were different in particularly. For instances, the angle of leaf wings was steeply about 45° with curve wings (from middle to end) in Prachuap Khiri Khan, while that in Kamphaeng Phet 2 was about 60° without curve wings. The leaf of Prachuap Khiri Khan has more thickness of bundle caps from phloem to lower epidermis than that of Kamphaeng Phet 2, resulting to harder cell digestion and separation. Each plant has different respond toward enzyme composition and concentration. Generally, different plant species required different cell wall degrading enzymes. The optimal enzyme condition for protoplast isolation is very much species specific and depend on various factors varying from complexity of explants, cell wall composition, age and source of plant (Tee et al., 2010; Yeong et al., 2008).
Although, the isolation protoplasts from cell suspension derived from inflorescence of vetiver, Surat Thani ecotype, had been reported by Prasertsongskun (2004) by using the combination of 2% cellulase onozuka R10, 2% macerozyme R10 and 0.5% pectinase, resulting maximum protoplast yields (8.4x104 protoplasts/ml). However, our study is the first report for the isolating of protoplast from leaves of vetiver which required the lower concentration of enzymes (0.5-1.0% cellulase onozuka R-10 and 0.4- 0.5% macerozyme R-10 in combination) and obtained the higher protoplast yield (6.12x105 - 6.80x105 protoplasts/ml) compared to the previous study. It indicated that the leaves are good source of protoplasts isolation obtaining the high number of yields from vetiver. The different responses in yield might result from the differences in the physiological status and extent of cell wall thickening of the explants. The different protoplast sources require different enzymes to isolate protoplasts because they have different intra and intercellular tissue compositions (Machmudi et al., 2019). Additionally, the use of leaf mesophyll cells as a protoplast source had been a more successful to achieve a high yield of viability protoplast of a wide range of plants. This could be because mesophyll tissues are loosely arranged, therefore the enzyme solution has easy access to the cell wall (Tudses et al., 2014; Ayeleso, 2015).
The incubation time, duration of incubating explants in enzyme solution, required to breakdown the cell wall by enzyme and release protoplast varied among different plant species. The appropriate duration for isolating protoplasts dependent on the complexity of the cell wall, enzyme composition and incubation temperature. The enzyme treatment period generally used in protoplast isolation varies including short-term duration (2-6 h) or slower long-term duration (16-24 h) (Navratilova, 2004; Tudses et al., 2014). Our study revealed that the suitable incubation time for protoplast isolation from leaves of both vetiver species were a longer time (24 h), when treated with enzyme solution. It indicated that increased incubation time had also increased the yield of protoplasts with high viability. This was because that a longer incubation time should potentially lead to more cell walls being degraded due to the enzymes working, and thus more protoplasts being released (Selga, 2017). Similarly, Kim et al. (2005) reported that 24 h of incubation time gave the optimum protoplast yield, 19.2x105 protoplast/g FW in Alstroemeria as compared with 4, 8, 12, 16 and 20 h. Hakman and Arnoid (1983) studied on protoplast isolation of Pinus contorta Dougl.ex Loud. They found that 0.5% macerase and 1% cellulysin with 24 h of incubation time was the suitable condition. Chamani et al. (2012) reported that a digestion period of 24 h resulted in the highest yield of protoplasts (6.65x105 protoplasts/g FW) from Lilium ledebeourii. In addition, incubation time of 24 h was the appropriate for isolation of protoplast from pine (Pinus Iambertiana) when treated with the combination of 0.15% onozuka, 0.05% rhozyme and 0.08% pectinase (David and David, 1979).
Several studies reported that the incubation time could be affected by the concentration of enzyme solution used. The lower enzyme concentration required longer duration as the optimum incubation time for protoplast isolation (Tee et al., 2010). Whereas, the longer incubation time reduced yield of protoplast which caused the cells to be over digested and then reduced the protoplast yields (Nassour and Dorion, 2002), for instances, the time required by Gracilaria changii was 3 h, the amount of protoplast yield decreased when treated with 4% (w/v) cellulase onozuka R-10, 2% (w/v) macerozyme R-10 and 1 U/ml agarase after incubation more than 3 h (Yeong et al., 2008). Ratanasanobon and Seaton (2013) studied on protoplast isolation in Chamelaucium group plants with longer than 6 h of incubation time, protoplasts could not be released when treated with 2% cellulase and 1% macerozyme. Zhou et al. (2019) reported that period of incubation significantly affects the yield and viability of protoplast in Platycladus orientalis, yield of viable protoplasts decreased after 16 h of incubation time. Different species have different requirements which influence the success of protoplast isolation. Therefore, to increase the yield of viable protoplasts, it is necessary to optimize the length of incubation for each individual genotype.
CONCLUSION
The present study is successful protoplast isolation and thus establishing an efficient protocol for isolating the large number of protoplasts from leaves of vetiver, Kamphaeng Phet 2 and Prachuap Khiri Khan. In this research, the yield and viability of protoplasts were greatly influenced by the concentration of the combination enzymes and the time of enzymatic digestion. Based on the results, the combination of 0.5% (w/v) cellulase onozuka R-10 and 0.5% (w/v) macerozyme R-10 was the optimal enzyme solution for releasing Kamphaeng Phet 2 protoplast. While, 1.0% (w/v) cellulase onozuka R-10 and 0.4% (w/v) macerozyme R-10 in combination was the optimal enzyme solution for Prachuap Khiri Khan. The suitable incubation time yielded the highest protoplasts of both ecotypes were found at 24 h. The obtained protoplast could be used for advanced research studies in vetiver improvement through protoplast fusion technology.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the following for their advice and kind assistance: Asst. Prof. Dr. Aphichat Chidburee and Asst. Prof. Phongyuth Nualbunruang from Agricultural Technology Research Institute, Rajamangala University of Technology Lanna, Lampang.
REFERENCES
Ayeleso, T.B. 2015. Protoplast isolation and plant regeneration in bambara groundnut: A platform for transient gene expression [dissertation]. Cape Town: Cape Peninsula University of Technology.
Chamani, E., Tahami, S.K., Zare, N., Zakaria, R.A., Mohebodini, and M., Joyce, D. 2012. Effect of different cellulase and pectinase enzyme treatments on protoplast isolation and viability in Lilium ledebeourii Bioss. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 40: 123–128.
Chusreeaeom, K. and Roongtanakiat, N. 2017. Plant development and nutrient content in Thai vetiver grass under flooded condition. In: Proceeding of the 55th Kasetsart University annual conference. Bangkok, 31 Jan-3 Feb 2017. Kasetsart University, Bangkhen. p.965–972.
Cove, D.J. 1979. The uses of isolated protoplasts in plant genetics. Heredity. 43: 295– 314.
Lavania, U.C. 2000. Primary and secondary center of organ of vetiver and its dispersion. In: Chomechalow, N., and Barang, N., editors. Proceeding of the 2nd International Conference of Vetiver: Vetiver and Environment. Bangkok, 28-29 Nov 2002. Rama Gardens Hotel, Laksi. p.224–427.
David, A. and David, H. 1979. Isolation and callus formation from cotyledon protoplasts of Pine (Pinus pinaster). Zeitschrift für Pflanzenphysiologie. 94: 173–177.
Hakman, I. and Arnold, S. 1983. Isolation and growth of protoplasts from cell suspensions of Pinus contorta Dougl. ex Loud. Plant Cell Reports. 2: 92–94.
Horváth, E. 2009. Protoplast isolation from Solanum lycopersicum L. leaf tissues and their response to short-term NaCl treatment. Acta Biologica Szegediensis. 53: 83–86.
Huddy, S.M., Meyers, A.E., and Coyne, V.E. 2013. Protoplast isolation optimization and regeneration of cell wall in Gracilaria gracilis (Gracilariales, Rhodophyta). Journal of Applied Phycology. 25: 433–443.
Khanema, P. 2009. Carbon sequestration and turnover by vetiver (Chrysopogon spp.) [dissertation]. Nakhon Ratchasima: Suranaree University of Technology.
Kim, J.B., Bergervoet, J.E.M., Raemakers, J.J.M., Jacobsen, E., and Visser, R.G.F. 2005. Isolation of protoplasts, and culture and regeneration into plants in Alstroemeria. In Vitro Cellular and Developmental Biology–Plant. 41: 505–510.
Machmudi, M., Purnobasuki, H., and Utami, E.S.W. 2019. The optimization mesophyll protoplast isolation for Phalaenopsis amboinensis. Bulgarian Journal of Agricultural Science. 25: 737–743.
Murashige, T., and Skoog, F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiology Journal. 15: 473–497.
Nassour, M., and Dorion, M. 2002. Plant regeneration from protoplasts of micropropagated Pelargonium x hortorum ‘Alain’: Effect of some environmental and medium factors on protoplast system efficiency. Plant Science. 163: 169– 173.
Navratilova, B. 2004. Protoplast cultures and protoplast fusion focused on Brassicaceae: A review. Horticultural Science. 31: 140–157.
Power, J.B. and Cocking, E.C. 1970. Isolation of leaf protoplast: Macromolecule uptake and growth substance response. Journal of Experimental Botany. 21: 64–70.
Prasertsongskun, S. 2004. Isolation and culture of suspension protoplasts of vetiver. Songklanakarin Journal of Science and Technology. 26: 411–416.
Ratanasanobon, K. and Seaton, K.A. 2013. Protoplast isolation for species in the Chamelaucium group and the effect of antioxidant enzymes (superoxide dismutase and catalase) on protoplast viability. In vitro Cellular and Developmental Biology– Plant. 49: 593–598.
Ruanjaichon, V., Sangduen, N., Thiraporn, R., and Srifah, P. 1995. The use of random amplified polymorphic DNA technique for the identification of vetiveria grass ecotypes in Thailand. In: Proceeding of the 33rd Kasetsart University annual conference. Bangkok, 30 Jan-1 Feb 1995. Kasetsart University, Bangkhen. p.164–173.
Selga, L. 2017. Optimization of protoplast methods suitable for transient CRISPR/Cas9 expression in Lepidium campestre [dissertation]. Uppsala: Swedish University of Agricultural Sciences.
Shuro, A.R. 2018. Review paper on the role of somatic hybridization in crop improvement. International Journal of Research Studies in Agricultural Sciences. 4: 1–8.
Sija, S.L, Potty, V.P, and Santhoshlal, P.S. 2016. Optimization of protoplast isolation protocols from callus culture of Anacardium occidentale L. International Journal of Agriculture, Environment and Bioresearch. 9: 917–924.
Tahami, S.K., Chamani, E., and Zare, N. 2014. Plant regeneration from protoplasts of Lilium ledebourii (Baker) Boiss. Journal of Agricultural Science and Technology. 16: 1133–1144.
Tang, J., Liu, B., Chen, M., Xue, Y., Liu, Y., and Wu, Z. 2019. Isolation of protoplast from leaves of castor (Ricinus communis L.). In: Proceeding of the 7th Annual International Conference on Materials Science and Engineering. Hubei, 19-20 Apr 2019. Wuhan. P.1–5.
Tee, C.S., Lee, P.S., Kiong, A.L.P., and Mahmood, M. 2010. Optimisation of protoplast isolation protocols using in vitro leaves of Dendrobium crumenatum (pigeon orchid). African Journal of Agricultural Research. 5: 2685–2693.
Tudses, N., Siripong, P., and Duangporn, P. 2014. Optimal conditions for high-yield protoplast isolations of Jatropha curcas L. and Ricinus communis L. American- Eurasian Journal of Agricultural and Environmental Sciences. 14: 221–230.
Wulandari, D., Purwito, A., Susanto, S., Husni, A., and Ermayanti, T. 2018. Protoplast fusion between Indonesian Citrus maxima (Burm.) Merr. and Citrus reticulata L.: A preliminary report. Agrivita Journal of Agricultural Science. 40: 233–241.
Yao, L., Liao, X., Gan, Z., Peng, X., Wang, P., Li, S., and Li, T. 2016. Protoplast isolation and development of a transient expression system for sweet cherry (Prunus avium L.). Scientia Horticulturae. 209: 14–21.
Yeboah, S., Allotey, A.N.M., and Biney, E. 2015. Purification of industrial wastewater with vetiver grasses (Vetiveria zizanioides): The case of food and beverages wastewater in Ghana. Asian Journal of Basic and Applied Sciences. 2: 1–14.
Yeong, H., Khalid, N., and Phang, S. 2008. Protoplast isolation and regeneration from Gracilaria changii (Gracilariales, Rhodophyta). Journal of Applied Phycology. 20: 641–651.
Zhu, L., Wang, B.C., Zhou, J., Chen, L.X., Dai, C.Y., and Duan, C.R. 2005. Protoplast isolation of callus in Echinacea augustifolia. Colloids and Surfaces B: Biointerfaces. 44: 1–5.
Zhou, Q., Jiang, Z., Li, Y., Zhang, T., Zhu, H., Zhao, F., and Zhao, Z. 2019. Mesophyll protoplast isolation technique and flow cytometry analysis of ancient Platycladus orientalis (Cupressaceae). Turkish Journal of Agriculture and Forestry. 43: 275– 287.
OPEN access freely available online
Chiang Mai University Journal of Natural Sciences [ISSN 16851994]
Chiang Mai University, Thailand https://cmuj.cmu.ac.th
Sumana Leaungthitikanchana1,*, Khachapohn Thongdonyod1, and Nootjaree Singphan2
1 Department of Biotechnology, School of Agriculture and Natural Resources, University of Phayao, Phayao 56000, Thailand.
2 Department of Plant Science, Faculty of Agricultural Technology and Industrial Technology, Phetchabun Rajabhat University, Phetchabun 67000, Thailand.
Corresponding author: Sumana Leaungthitikanchana, E-mail: sumana.le@up.ac.th
Total Article Views
Editor: Wasu Pathom-aree,
Chiang Mai University, Thailand
Article history:
Received: September 11, 2020;
Revised: November 12, 2020;
Accepted: November 26, 2020;
Published online: March 5, 2021

